Abstract
Among adults with sickle cell disease (SCD), pulmonary complications are a leading cause of death. Yet, the natural history of lung function in adults with SCD is not well established. We conducted a retrospective cohort study of adults with SCD who had repeated pulmonary function tests performed over 20 years of age. Ninety-two adults were included in this cohort. Rate of decline in FEV1 for men and women with SCD was 49 cc/year (compared with 20–26 cc/year in the general population). Further studies are needed to identify factors which impact the rate of lung function decline in adults with SCD.
Introduction
Pulmonary disease is a common cause of morbidity and mortality among individuals with sickle cell disease (SCD) [1]. Acute pulmonary complications, such as acute chest syndrome (ACS), predominate in children, while chronic lung disease is more common in adults with SCD. ACS occurs in ~50% of individuals with SCD over the course of their lifetime [2]. Repetitive episodes of ACS may lead to sickle chronic lung disease, which is characterized by a restrictive pattern on pulmonary function testing [3]. Sickle chronic lung disease is the cause of death for 20% of adults with SCD [4].
Despite the significant contribution of pulmonary disease to SCD-related morbidity and mortality, pulmonary function test (PFT) abnormalities are not well described in adults with SCD. Klings et al. reports PFT results in a large cohort of adults with SCD (n = 310) who participated in the Cooperative Study of Sickle Cell Disease [5]. Abnormal PFTs are noted in 90% of individuals assessed. Restrictive physiology is the most common abnormality, present in 74% of adults. Other investigators also report restrictive lung disease in adults with SCD [3,6,7]; however, no study to date describes the longitudinal course of lung function among adults with SCD.
The primary objective of our study was to describe the changes in pulmonary function over time in adults with SCD. Further, we sought to describe the prevalence of abnormal patterns of pulmonary function in this cohort and the relationship of these abnormal patterns to SCD-related morbidity.
Results
Ninety-two adults with SCD who completed at least one PFT after 20 years of age were included in our cohort (Table I). The cohort consisted of 48% males and the mean age at last PFT was 36 years (range, 20–68 years). Of the 92 adults in the cohort, 49 individuals underwent repeated PFTs. Those with repeated measurements completed a mean of 2.6 PFTs. Participants were followed for a mean of 13 years. This cohort accrued 1330 patient-years of follow up. Twenty-three percent of the cohort smoked cigarettes at the time of pulmonary function testing. No participant was known to have a diagnosis of HIV.
TABLE I.
Demographic and Morbidity Data for Total Cohort and Subgroup with Repeat Pulmonary Function Tests (PFT)
| Cohort | Total | ≥2 PFTs |
|---|---|---|
| No. Patients | 92 | 49 |
| Gender, % | ||
| Male | 48 | 43 |
| Age last PFT, mean, y ± SD | 36.2 ± 10.6 | 37.1 ± 9.2 |
| Follow-up, mean, y ± SD | 13.3 ± 10.2 | 13.5 ± 9.1 |
| Phenotype, % | ||
| SS | 72 | 80 |
| SC | 16 | 12 |
| Sβ-thalassemia0 | 2 | 2 |
| Sβ-thalassemia+ | 7 | 4 |
| SHPFH | 2 | 2 |
| SD | 1 | 0 |
| Smoked cigarettes, % | 23 | 20 |
| Pain admissions, no. #/year ± SD | 0.9 ± 0.9 | 1.0 ± 0.9 |
| ACS admissions, no. #/year ± SD | 0.4 ± 1.3 | 0.2 ± 0.3 |
| History of asthma, % | 13 | 22 |
SHPFH, S-hereditary persistence of fetal hemoglobin; ACS, acute chest syndrome.
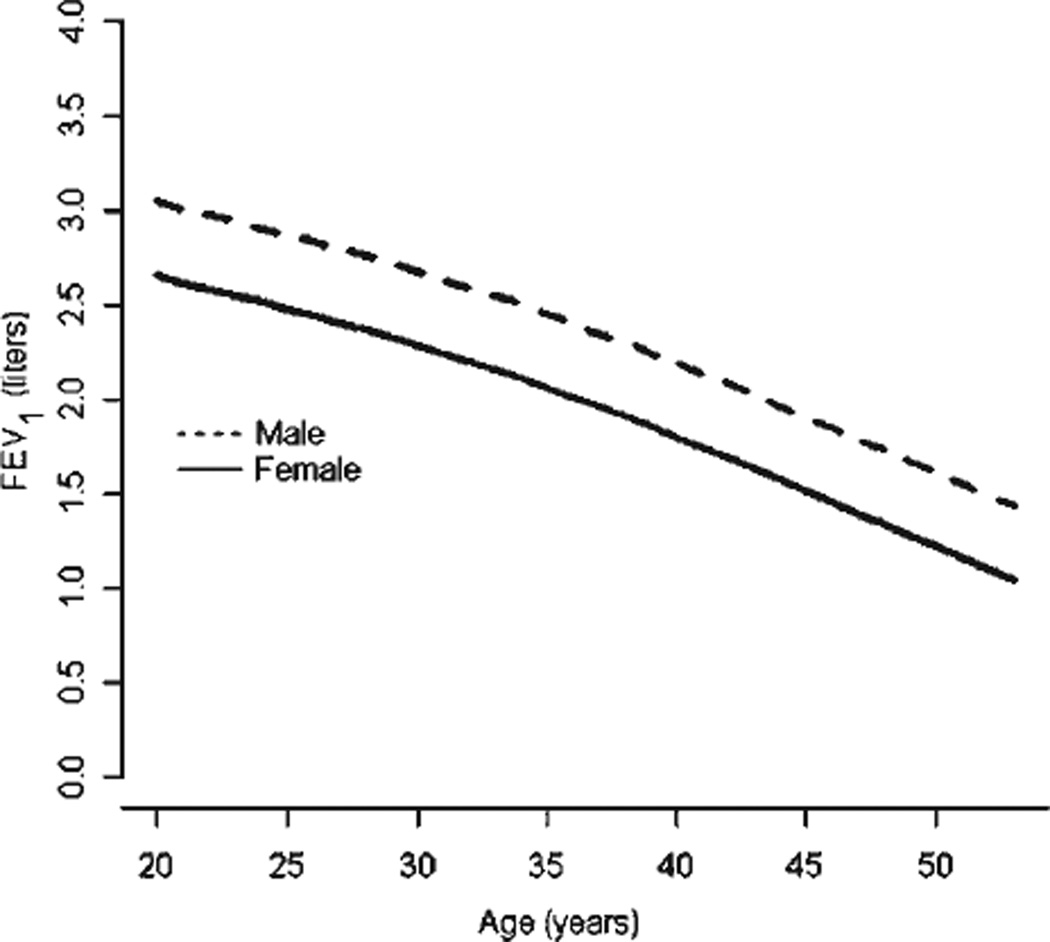
Lung function significantly declined over time in adults with SCD (Fig. 1). After age 20 years, the rate of decline in FEV1 among men and women with SCD was 49 cc/year. There was no difference in the decline of FEV1 between individuals with HbSS compared to a group comprised of the phenotypes HbSC, HbSβ-thalassemia+, HbSβ-thalassemia0, HbS-hereditary persistence of fetal hemoglobin, and HbSD (P = 0.29). Smoking cigarettes also did not significantly affect the decline in FEV1 (P = 0.07). Similar to previous findings in adults with SCD [5], restrictive was more common than obstructive physiology (Table II). There was no association between increased rate of pain and subsequent restrictive (odds ratio 1.0, 95% CI 0.6–1.6, P = 0.97), or obstructive physiology (OR 0.5, 95% CI 0.2–1.1, P = 0.09), or abnormal FEV1 (OR 1.4, 95% CI 0.9–2.3, P = 0.14); there was also no association between increased rate of ACS and subsequent restrictive (OR 1.9, 95% CI 0.6–6.1, P = 0.28), or obstructive physiology (OR 1.1, 95% CI 0.8–1.5, P = 0.67), or abnormal FEV1 (OR 1.0, 95% CI 0.7–1.4, P = 0.93).
Figure 1.
Longitudinal forced expiratory volume in 1 second adjusted for height in adults with sickle cell disease.
TABLE II.
Pulmonary Function Tests in Adults with Sickle Cell Disease
| PFT results, mean ± SD | |
| FEV1 (n = 92) | 78.9 ± 15.4 |
| FVC (n = 92) | 71.1 ± 15.6 |
| FEV1/FVC (%) (n = 92) | 84.6 ± 8.0 |
| RV (n = 85) | 97.3 ± 61.5 |
| TLC (n = 84) | 86.7 ± 20.6 |
| Below normal, % | |
| FEV1 | 41.3 |
| FVC | 70.7 |
| FEV1/FVC (obstruction) | 18.5 |
| TLC (restriction) | 35.7 |
FEV1, Forced expiratory volume in 1 sec, FVC, forced vital capacity; RV, residual volume; TLC, total lung capacity.
Last pulmonary function measurement was analyzed.
Discussion
Our study demonstrates that among adults with SCD, the rate of decline in lung function is greater than would be expected from historical controls without SCD [8]. Also, we have shown that the prevalence of restrictive lung disease in adults may be less than previously thought.
Cross-sectional studies of pulmonary function in adults with SCD demonstrate a reduced FEV1 [3,5–7]. However, longitudinal assessment was not done to determine the rate of decline. In a nonsmoking individual without SCD, pulmonary function increases in childhood until about age 20 years, plateaus until age 35 years and then begins to decline at a rate of 20–26 cc/year [8–10]. An abnormal FEV1 in adults could be due to abnormal lung growth, a blunted plateau phase, an increased rate of decline or any combination of these factors. In our study, we could not assess lung growth because we did not include children in our cohort, and the plateau phase was difficult to assess due to our small sample size. The rate of decline in FEV1 after 20 years of age (49 cc/year for men and women with SCD) was significantly greater than the rate of decline described in nonsmoking, non-SCD adults, 20–26 cc/year [8,10]. In the general population, poor lung function is an independent predictor of mortality [11]. The contribution of abnormal lung function to mortality among individuals with SCD is not known.
As with any retrospective study, our study has limitations. PFTs were obtained for clinical purposes and, thus, selection bias may have influenced our results. If this bias were significant, we would expect our adults to be more severely affected compared to those described in other studies. However, the percentage of adults in our study with restrictive lung disease (36%) is far less than described in the largest cross-sectional study of pulmonary function in adults with SCD (74%) [5]. Another limitation of our study was a small sample size for the purposes of studying factors that influence longitudinal changes in lung function, such as SCD phenotype. Although phenotype did not affect the rate of FEV1 decline in our study, our small sample is prone to a type II error and thus our results must be interpreted with caution. Future studies are needed to better characterize the changes in lung function over time, and to identify factors that influence lung function decline.
In conclusion, the rate of decline in FEV1 among adults with SCD is increased compared to the general population. Large, prospective studies of longitudinal pulmonary function are needed in children and adults with SCD so that risks of lung functions decline and its association with morbidity and mortality may be determined.
Methods
This study was approved by both Washington University School of Medicine Human Research Protection Office (St. Louis, MO) and the Research Ethics Committee of Central Middlesex Hospital (London, UK). From November 1, 1999 through March 1, 2005 individuals with SCD were enrolled in the European Haemoglobinopathy Registry (EHR). Informed consent to participate in the EHR allowed investigators to use participant’s medical data for research purposes. Consent was obtained in accordance with the requirements and guidelines of the Research Ethics Committee at Central Middlesex Hospital.
Inclusion/exclusion
All patients at Central Middlesex Hospital with SCD who provided informed consent and underwent at least one outpatient pulmonary function assessment for clinical purposes were included. Lung volumes were done in a clinical laboratory using standard techniques with helium dilution. Individuals were required to have at least one PFT performed at 20 years of age or older to be included in the cohort. Carbon monoxide diffusing capacity (DLCO) was not included in this analysis because simultaneous hemoglobin values were not obtained and therefore adjustments for hemoglobin could not be performed. For cross-sectional analysis of PFT data, the last measurement was analyzed. Persons were excluded if inadequate follow up data existed, defined as less than 1 year of follow up at Central Middlesex Hospital.
Predicted pulmonary function values
Predicted values for FEV1, FVC, FEV1/FVC were obtained using the equations of Hankinson, et al, according to American Thoracic Society (ATS) criteria [12]. Predicted values for total lung capacity (TLC) and residual volume (RV) were obtained using the equations of Stocks and Quanjer, adjusting for race, according to ATS criteria [13]. Lower limit of normal was defined as values lower than the 5th percentile for an individual based on age, gender, race, and height.
Definitions
Restrictive lung disease
Restrictive lung disease was defined as a TLC below the 5th percentile for an individual based on age, gender, race, and height [13].
Obstructive lung disease
Obstructive lung disease was defined as an FEV1/FVC ratio below the 5th percentile for an individual based on age, gender, race, and height [12].
Pain episodes
A painful episode was defined as pain in the arms and legs, back, abdomen, chest or head that lasted at least 2 hr, lead to an admission to the hospital, and for which no other explanation was found (e.g., osteomyelitis or appendicitis) [14].
ACS episodes
An ACS episode was defined as new infiltrate on chest radiograph or a defect on radionuclide imaging of the chest, in combination with fever or respiratory symptoms [15].
Statistical data analysis
We used a multiple logistic regression model to study the relationship of restrictive and obstructive lung disease and abnormal FEV1 with the number of prior pain and ACS episodes, adjusting for age. Longitudinal analysis of FEV1 was performed using the generalized estimating equations method to model the association of FEV1 with age and height, taking into account of correlations among repeated measurements within each study subject. To accommodate nonlinear relationships of age with the pulmonary functions, the restricted cubic spline functions were used. Empirical robust variances of parameter estimates were used for statistical inference. The Wald-type statistic was used for statistical inference of individual parameter, and the score statistic was used for the statistical inference of one variable in the presence of other variables. In our study sample, 87% of individuals with repeated FEV1 measurements had between 2 and 4 measurements. We restricted the longitudinal analysis of FEV1 to individuals with 2–4 repeated measurements to avoid forcing the FEV1 trend of most participants (those with 2–4 measurements) to follow the trend of a few individuals (those with 5 or more measurements). Data were analyzed using SAS software v 9.1.
Acknowledgments
Contract grant sponsors: National Heart, Lung, Blood Institute; Contract grant numbers: K12HL08710, HL079937; Contract grant sponsor: Doris Duke Foundation.
References
- 1.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. New Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 2.Castro O, Brambilla DJ, Thorington B, et al. The acute chest syndrome in sickle cell disease: Incidence and risk factors. The Cooperative Study of Sickle Cell Disease. Blood. 1994;84:643–649. [PubMed] [Google Scholar]
- 3.Powars D, Weidman JA, Odom-Maryon T, et al. Sickle cell chronic lung disease: prior morbidity and the risk of pulmonary failure. Medicine (Baltimore) 1988;67:66–76. [PubMed] [Google Scholar]
- 4.Powars DR, Chan LS, Hiti A, et al. Outcome of sickle cell anemia: A 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005;84:363–376. doi: 10.1097/01.md.0000189089.45003.52. [DOI] [PubMed] [Google Scholar]
- 5.Klings ES, Wyszynski DF, Nolan VG, Steinberg MH. Abnormal pulmonary function in adults with sickle cell anemia. Am J Respir Crit Care Med. 2006;173:1264–1269. doi: 10.1164/rccm.200601-125OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anthi A, Machado RF, Jison ML, et al. Hemodynamic and functional assessment of patients with sickle cell disease and pulmonary hypertension. Am J Respir Crit Care Med. 2007;175:1272–1279. doi: 10.1164/rccm.200610-1498OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vendramini EC, Vianna EO, De Lucena Angulo I, et al. Lung function and air-way hyperresponsiveness in adult patients with sickle cell disease. Am J Med Sci. 2006;332:68–72. doi: 10.1097/00000441-200608000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Lange P, Parner J, Vestbo J, et al. A 15-year follow-up study of ventilatory function in adults with asthma. New Engl J Med. 1998;339:1194–1200. doi: 10.1056/NEJM199810223391703. [DOI] [PubMed] [Google Scholar]
- 9.Tager IB, Segal MR, Speizer FE, Weiss ST. The natural history of forced expiratory volumes. Effect of cigarette smoking and respiratory symptoms. Am Rev Respir Dis. 1988;138:837–849. doi: 10.1164/ajrccm/138.4.837. [DOI] [PubMed] [Google Scholar]
- 10.Apostol GG, Jacobs DR, Jr, Tsai AW, et al. Early life factors contribute to the decrease in lung function between ages 18 and 40: The Coronary Artery Risk Development in Young Adults study. Am J Respir Crit Care Med. 2002;166:166–172. doi: 10.1164/rccm.2007035. [DOI] [PubMed] [Google Scholar]
- 11.Ryan G, Knuiman MW, Divitini ML, et al. Decline in lung function and mortality: The Busselton Health Study. J Epidemiol Commun Health. 1999;53:230–234. doi: 10.1136/jech.53.4.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 13.Stocks J, Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Official Statement of The European Respiratory Society. Eur Respir J. 1995;8:492–506. doi: 10.1183/09031936.95.08030492. [DOI] [PubMed] [Google Scholar]
- 14.Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. Rates and risk factors. New Engl J Med. 1991;325:11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- 15.Vichinsky EP, Styles LA, Colangelo LH, et al. The Cooperative Study of Sickle Cell Disease. Acute chest syndrome in sickle cell disease: Clinical presentation and course. Cooperative Study of Sickle Cell Disease. Blood. 1997;89:1787–1792. [PubMed] [Google Scholar]