Abstract
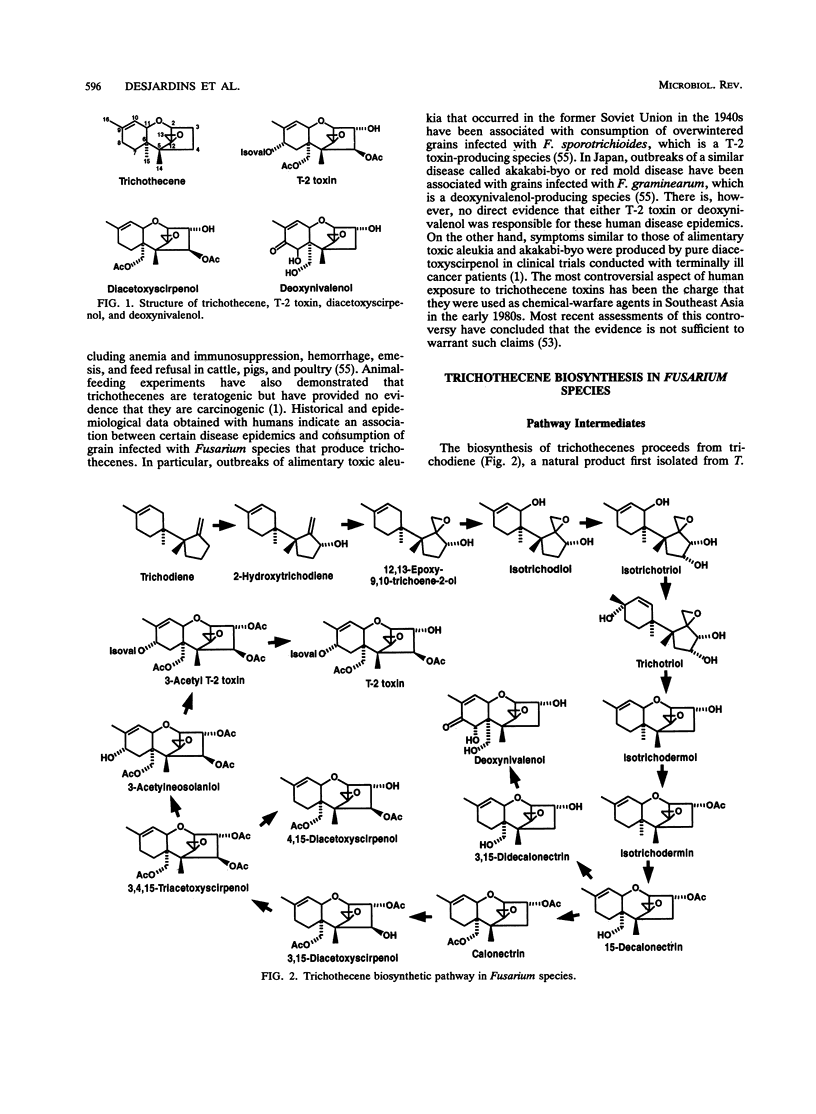
Several species of the genus Fusarium and related fungi produce trichothecenes which are sesquiterpenoid epoxides that act as potent inhibitors of eukaryotic protein synthesis. Interest in the trichothecenes is due primarily to their widespread contamination of agricultural commodities and their adverse effects on human and animal health. In this review, we describe the trichothecene biosynthetic pathway in Fusarium species and discuss genetic evidence that several trichothecene biosynthetic genes are organized in a gene cluster. Trichothecenes are highly toxic to a wide range of eukaryotes, but their specific function, if any, in the survival of the fungi that produce them is not obvious. Trichothecene gene disruption experiments indicate that production of trichothecenes can enhance the severity of disease caused by Fusarium species on some plant hosts. Understanding the regulation and function of trichothecene biosynthesis may aid in development of new strategies for controlling their production in food and feed products.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean G. A., Fernando T., Jarvis B. B., Bruton B. The isolation and identification of trichothecene metabolites from a plant pathogenic strain of Myrothecium roridum. J Nat Prod. 1984 Jul-Aug;47(4):727–729. doi: 10.1021/np50034a031. [DOI] [PubMed] [Google Scholar]
- Beremand M. N. Isolation and characterization of mutants blocked in T-2 toxin biosynthesis. Appl Environ Microbiol. 1987 Aug;53(8):1855–1859. doi: 10.1128/aem.53.8.1855-1859.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cane D. E., Wu Z., Oliver J. S., Hohn T. M. Overproduction of soluble trichodiene synthase from Fusarium sporotrichioides in Escherichia coli. Arch Biochem Biophys. 1993 Jan;300(1):416–422. doi: 10.1006/abbi.1993.1056. [DOI] [PubMed] [Google Scholar]
- Carr L. G., Skatrud P. L., Scheetz M. E., 2nd, Queener S. W., Ingolia T. D. Cloning and expression of the isopenicillin N synthetase gene from Penicillium chrysogenum. Gene. 1986;48(2-3):257–266. doi: 10.1016/0378-1119(86)90084-3. [DOI] [PubMed] [Google Scholar]
- Coque J. J., Martín J. F., Calzada J. G., Liras P. The cephamycin biosynthetic genes pcbAB, encoding a large multidomain peptide synthetase, and pcbC of Nocardia lactamdurans are clustered together in an organization different from the same genes in Acremonium chrysogenum and Penicillium chrysogenum. Mol Microbiol. 1991 May;5(5):1125–1133. doi: 10.1111/j.1365-2958.1991.tb01885.x. [DOI] [PubMed] [Google Scholar]
- Corley D. G., Rottinghaus G. E., Tempesta M. S. Secondary metabolites from Fusarium. Two new modified trichothecenes from Fusarium sporotrichioides MC-72083. J Nat Prod. 1987 Sep-Oct;50(5):897–902. doi: 10.1021/np50053a021. [DOI] [PubMed] [Google Scholar]
- Desjardins A. E., Plattner R. D., Beremand M. N. Ancymidol blocks trichothecene biosynthesis and leads to accumulation of trichodiene in Fusarium sporotrichioides and Gibberella pulicaris. Appl Environ Microbiol. 1987 Aug;53(8):1860–1865. doi: 10.1128/aem.53.8.1860-1865.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins A. E., Plattner R. D., Vanmiddlesworth F. Trichothecene Biosynthesis in Fusarium sporotrichioides: Origin of the Oxygen Atoms of T-2 Toxin. Appl Environ Microbiol. 1986 Mar;51(3):493–497. doi: 10.1128/aem.51.3.493-497.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gledhill L., Hesketh A. R., Bycroft B. W., Dewick P. M., Gilbert J. Biosynthesis of trichothecene mycotoxins: cell-free epoxidation of a trichodiene derivative. FEMS Microbiol Lett. 1991 Jul 1;65(3):241–245. doi: 10.1016/0378-1097(91)90220-5. [DOI] [PubMed] [Google Scholar]
- Hobden A. N., Cundliffe E. Ribosomal resistance to the 12,13-epoxytrichothecene antibiotics in the producing organism Myrothecium verrucaria. Biochem J. 1980 Sep 15;190(3):765–770. doi: 10.1042/bj1900765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn T. M., Beremand M. N. Regulation of Trichodiene Synthase in Fusarium sporotrichioides and Gibberella pulicaris (Fusarium sambucinum). Appl Environ Microbiol. 1989 Jun;55(6):1500–1503. doi: 10.1128/aem.55.6.1500-1503.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn T. M., Beremand P. D. Isolation and nucleotide sequence of a sesquiterpene cyclase gene from the trichothecene-producing fungus Fusarium sporotrichioides. Gene. 1989 Jun 30;79(1):131–138. doi: 10.1016/0378-1119(89)90098-x. [DOI] [PubMed] [Google Scholar]
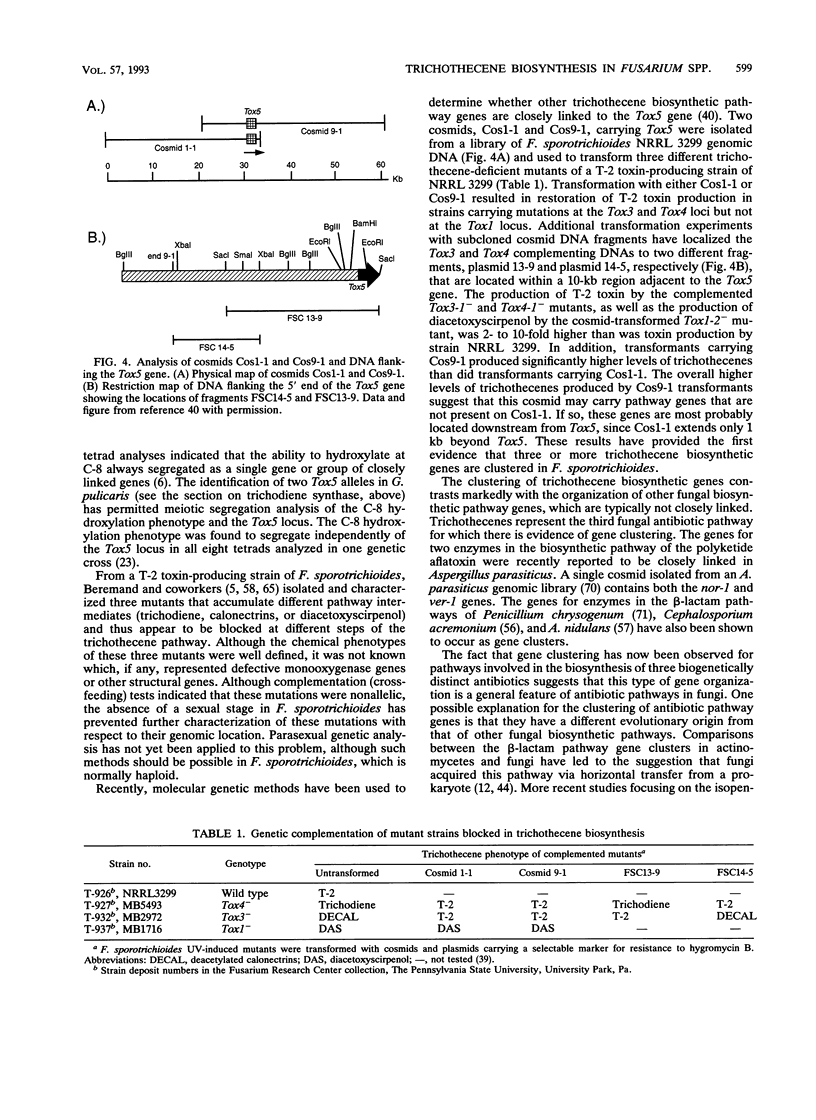
- Hohn T. M., Desjardins A. E. Isolation and gene disruption of the Tox5 gene encoding trichodiene synthase in Gibberella pulicaris. Mol Plant Microbe Interact. 1992 May-Jun;5(3):249–256. doi: 10.1094/mpmi-5-249. [DOI] [PubMed] [Google Scholar]
- Hohn T. M., Desjardins A. E., McCormick S. P. Analysis of Tox5 gene expression in Gibberella pulicaris strains with different trichothecene production phenotypes. Appl Environ Microbiol. 1993 Aug;59(8):2359–2363. doi: 10.1128/aem.59.8.2359-2363.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn T. M., Ohlrogge J. B. Expression of a fungal sesquiterpene cyclase gene in transgenic tobacco. Plant Physiol. 1991 Sep;97(1):460–462. doi: 10.1104/pp.97.1.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn T. M., Plattner R. D. Expression of the trichodiene synthase gene of Fusarium sporotrichioides in Escherichia coli results in sesquiterpene production. Arch Biochem Biophys. 1989 Nov 15;275(1):92–97. doi: 10.1016/0003-9861(89)90353-6. [DOI] [PubMed] [Google Scholar]
- Hohn T. M., Vanmiddlesworth F. Purification and characterization of the sesquiterpene cyclase trichodiene synthetase from Fusarium sporotrichioides. Arch Biochem Biophys. 1986 Dec;251(2):756–761. doi: 10.1016/0003-9861(86)90386-3. [DOI] [PubMed] [Google Scholar]
- Hoskins J. A., O'Callaghan N., Queener S. W., Cantwell C. A., Wood J. S., Chen V. J., Skatrud P. L. Gene disruption of the pcbAB gene encoding ACV synthetase in Cephalosporium acremonium. Curr Genet. 1990 Dec;18(6):523–530. doi: 10.1007/BF00327023. [DOI] [PubMed] [Google Scholar]
- Jarvis B. B., Midiwo J. O., Bean G. A., Aboul-Nasr M. B., Barros C. S. The mystery of trichothecene antibiotics in Baccharis species. J Nat Prod. 1988 Jul-Aug;51(4):736–744. doi: 10.1021/np50058a012. [DOI] [PubMed] [Google Scholar]
- Johal G. S., Briggs S. P. Reductase activity encoded by the HM1 disease resistance gene in maize. Science. 1992 Nov 6;258(5084):985–987. doi: 10.1126/science.1359642. [DOI] [PubMed] [Google Scholar]
- MacCabe A. P., Riach M. B., Unkles S. E., Kinghorn J. R. The Aspergillus nidulans npeA locus consists of three contiguous genes required for penicillin biosynthesis. EMBO J. 1990 Jan;9(1):279–287. doi: 10.1002/j.1460-2075.1990.tb08106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox J. Natural history of yellow rain. Nature. 1984 May 17;309(5965):207–207. doi: 10.1038/309207a0. [DOI] [PubMed] [Google Scholar]
- Mathison L., Soliday C., Stepan T., Aldrich T., Rambosek J. Cloning, characterization, and use in strain improvement of the Cephalosporium acremonium gene cefG encoding acetyl transferase. Curr Genet. 1993 Jan;23(1):33–41. doi: 10.1007/BF00336747. [DOI] [PubMed] [Google Scholar]
- McCormick S. P., Taylor S. L., Plattner R. D., Beremand M. N. Bioconversion of possible T-2 toxin precursors by a mutant strain of Fusarium sporotrichioides NRRL 3299. Appl Environ Microbiol. 1990 Mar;56(3):702–706. doi: 10.1128/aem.56.3.702-706.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick S. P., Taylor S. L., Plattner R. D., Beremand M. N. New modified trichothecenes accumulated in solid culture by mutant strains of Fusarium sporotrichioides. Appl Environ Microbiol. 1989 Sep;55(9):2195–2199. doi: 10.1128/aem.55.9.2195-2199.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner R. D., Tjarks L. W., Beremand M. N. Trichothecenes accumulated in liquid culture of a mutant of Fusarium sporotrichioides NRRL 3299. Appl Environ Microbiol. 1989 Sep;55(9):2190–2194. doi: 10.1128/aem.55.9.2190-2194.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P. M., Nelson K., Kanhere S. R., Karpinski K. F., Hayward S., Neish G. A., Teich A. H. Decline in deoxynivalenol (vomitoxin) concentrations in 1983 ontario winter wheat before harvest. Appl Environ Microbiol. 1984 Oct;48(4):884–886. doi: 10.1128/aem.48.4.884-886.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skory C. D., Chang P. K., Cary J., Linz J. E. Isolation and characterization of a gene from Aspergillus parasiticus associated with the conversion of versicolorin A to sterigmatocystin in aflatoxin biosynthesis. Appl Environ Microbiol. 1992 Nov;58(11):3527–3537. doi: 10.1128/aem.58.11.3527-3537.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Burnham M. K., Edwards J., Earl A. J., Turner G. Cloning and heterologous expression of the penicillin biosynthetic gene cluster from penicillum chrysogenum. Biotechnology (N Y) 1990 Jan;8(1):39–41. doi: 10.1038/nbt0190-39. [DOI] [PubMed] [Google Scholar]
- Smith M. W., Feng D. F., Doolittle R. F. Evolution by acquisition: the case for horizontal gene transfers. Trends Biochem Sci. 1992 Dec;17(12):489–493. doi: 10.1016/0968-0004(92)90335-7. [DOI] [PubMed] [Google Scholar]
- Striph G. G., Hart W. M., Jr, Olk R. J. Modified grid laser photocoagulation for diabetic macular edema. The effect on the central visual field. Ophthalmology. 1988 Dec;95(12):1673–1679. doi: 10.1016/s0161-6420(88)32957-x. [DOI] [PubMed] [Google Scholar]
- Zamir L. O., Devor K. A., Sauriol F. Biosynthesis of the trichothecene 3-acetyldeoxynivalenol. Identification of the oxygenation steps after isotrichodermin. J Biol Chem. 1991 Aug 15;266(23):14992–15000. [PubMed] [Google Scholar]



