Abstract
Mammals, including humans, can synthesize the vitamin nicotinamide from tryptophan in the liver. The resultant nicotinamide is distributed to non-hepatic tissues. We have studied the effects of changes in tryptophan–nicotinamide metabolism on niacin nutritional status. The liver plays a critical role in nicotinamide supply. Animal studies showed that the tryptophan–nicotinamide pathway is affected by physiological conditions, the presence of disease, nutrients, hormones, and chemicals. Human studies have shown that 1 mg of nicotinamide is produced from 67 mg of tryptophan intake, and that the conversion ratio of tryptophan to nicotinamide is enhanced from mid to late pregnancy. These findings have contributed to the determination of dietary reference intakes for niacin recommended in the Dietary Reference Intakes for Japanese 2010. Our findings suggest that the conversion of nicotinamide from tryptophan is important in maintaining niacin nutrition.
Keywords: tryptophan, nicotinamide, nutrition
Introduction
Niacin, also called vitamin B3, refers to both nicotinamide and nicotinic acid. The only route by which nicotinamide is biosynthesized is from tryptophan.1 Niacin in the form of coenzymes NAD and NADP functions in many reactions such as redox reactions, ADP ribosylation, and sirtuin activation. Niacin deficiency leads to pellagra, the typical symptoms of which are diarrhea, dermatitis, dementia, and death. Pellagra was common in the United States and parts of Europe in the early 20th century among people consuming foods containing low levels of niacin and tryptophan, such as corn. Pellagra epidemics have been documented in several areas associated with a high dietary dependence on unfortified corn.2
Nicotinamide can be synthesized from tryptophan in mammals, and the resultant nicotinamide is distributed to non-hepatic tissues.1 Dietary surveys have shown that the amount of nicotinamide biosynthesized from tryptophan is equal to the amount of pre-formed niacin from food intake in Japan, and matches the niacin requirement in humans.1,3,4 Although niacin can be supplied from amino acid tryptophan, nicotinamide biosynthesized from tryptophan is considered to be a byproduct of the kynurenine pathway, and the amount is thought not to be sufficient to meet the requirement. Thus niacin has been recognized as a vitamin. Disorders resulting from abnormal tryptophan metabolism, such as Hartnup disease, show symptoms similar to those of pellagra.5 These facts suggest that conversion of nicotinamide from tryptophan is important in maintaining niacin nutrition.
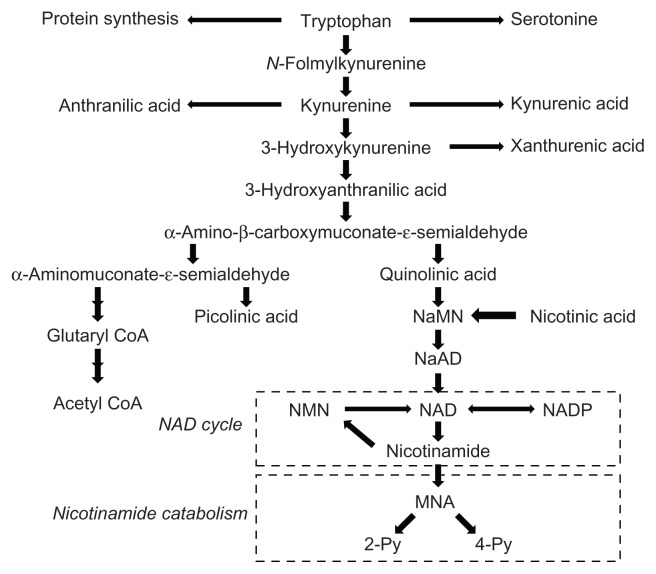
Conversion from tryptophan to nicotinamide requires nine steps, and this route is called the tryptophan–nicotinamide pathway (Fig. 1). The tryptophan–nicotinamide pathway is divided into two parts: the first part involves metabolic conversion of tryptophan to α-amino-β-carboxymuconate-ɛ-semialdehyde (ACMS), also called the kynurenine pathway, while the second part involves metabolic conversion of ACMS to nicotinamide. Most ACMS is metabolized to α-aminomuconate-ɛ-semialdehyde by ACMS decarboxylase (ACMSD), leading to picolinic acid or the tricarboxylic acid (TCA) cycle via glutaryl-CoA and acetyl-CoA, while some ACMS is spontaneously cyclized to quinolinic acid. Therefore, quinolinic acid formation shows an inverse correlation with ACMSD. The second part consists of quinolinic acid formation, the NAD cycle, and nicotinamide catabolism (Fig. 1). In this part, quinolinic acid is converted to nicotinic acid mononucleotide in the presence of 5-phosphoribosyl-1- pyrophosphate by quinolinic acid phosphoribosyltransferase (QPRT). Nicotinic acid mononucleotide is synthesized to NAD via nicotinic acid adenine dinucleotide. NAD is hydrolyzed to nicotinamide, which enters the NAD cycle and is catabolized. Nicotinamide is methylated to N1-methylnicotinamide (MNA), and MNA is then oxidized to either N1-methyl-2-pyridone-5- carboxamide (2-Py) or N1-methyl-4- pyridone-3-carboxamide (4-Py). Since nicotinamide and these nicotinamide metabolites are excreted in urine, the amounts of urinary nicotinamide and its metabolites can be used as indices to evaluate the amount of nicotinamide biosynthesized from tryptophan.6 Of three rate- limiting or key enzymes, the initial enzyme tryptophan 2,3-dioxygenase (TDO), ACMSD and QPRT in the tryptophan–nicotinamide pathway, ACMSD is considered to play a crucial role in the formation of nicotinamide.
Figure 1.
The tryptophan-nicotinamide pathway.
Notes: The pathway consists of the two parts: the first part is from tryptophan to quinolinic acid, and the second is from quinolinic acid to 2-Py and 4-Py, which includes the NAD cycle and the nicotinamide catabolism.
Abbreviations: NaMN, nicotinic acid mononucleotide; NMN, nicotinamide mononucleotide; MNA, N1-methylnicotinamide; 2-Py, N1-methyl-2- pridone-5-carboxamide; 4-Py, N1-methyl-4-pridone-3-carboxamide.
In the present article, recent findings from our animal experiments and human studies are described to elucidate the importance of the tryptophan–nicotinamide pathway for maintaining niacin nutrition.
Necessity of tryptophan intake for niacin nutrition
In animal experiments, a 14%–20% casein diet containing 30 mg/kg nicotinic acid has been generally used. This diet contains enough niacin and tryptophan to maintain niacin nutrition. We investigated whether rats can maintain their niacin nutrition from nicotinamide supplied only from tryptophan with no dietary niacin.7 Rats were fed a niacin-free 20% casein diet starting from the age of 3 weeks until the age of 80 weeks. Blood NAD and liver nicotinamide levels as nutritional biomarkers for niacin in aged rats were the same as those in young rats. Although urinary excretion of nicotinamide and its metabolites, used as indices of the conversion of nicotinamide from tryptophan, gradually decreased to 60% from 30 weeks of age, these results show that enough dietary tryptophan can sustain niacin nutrition without niacin intake in rats.
Human studies have shown that urinary excretion of nicotinamide metabolites and blood NAD levels in elderly Japanese are not different from those in young Japanese, suggesting a similar conversion ratio in the elderly and young.8,9 Although many studies have shown the effects of age on the tryptophan metabolism in animals and humans,10–12 little is known about the tryptophan–nicotinamide metabolism from nutritional aspects in the elderly people.
Primary tissue converting tryptophan to nicotinamide
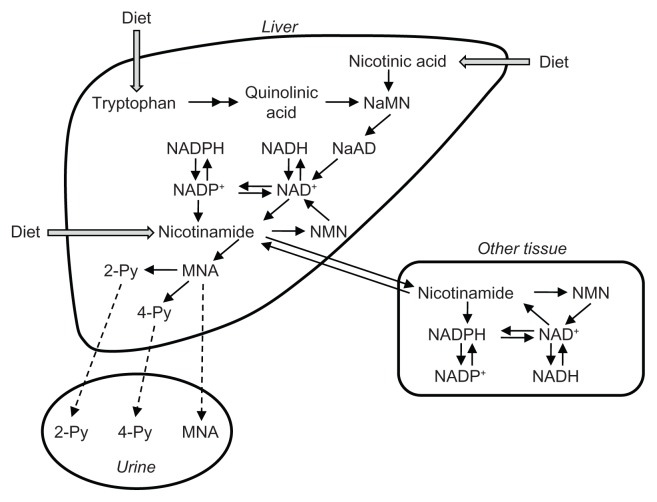
The processes involved in the tryptophan–nicotinamide pathway occur in the liver and kidneys. Only the liver has all the pathway enzymes. The kidneys lack several enzymes, such as TDO and kynureninase.13 To determine the tissue that plays a critical role in the biosynthesize of nicotinamide, we created animal models of renal failure and liver injury, and investigated the relationships between tissue enzyme activities and niacin nutritional status.13,14 Rats were fed a diet containing orotic acid, which induces fatty liver.14 In the fatty liver, TDO activity was decreased to 40%, the conversion ratio of tryptophan to nicotinamide to 30%, and liver NAD levels to 60% of the values in control animals. Chronic renal failure was induced by feeding an adenine-containing diet.13 Renal failure led to decreases in liver TDO, liver QPRT, and kidney ACMSD activities, and to an increase in liver ACMSD activity, causing the liver enzymes to suppress the conversion of nicotinamide to tryptophan and the kidney enzymes to enhance it. The renal failure rats showed lower blood and liver NAD levels, and conversion of tryptophan to nicotinamide, corresponding to the changes in liver enzyme activities but not in the kidney enzyme activities. These results show that the liver plays a critical role in nicotinamide supply to peripheral tissues (Fig. 2). Since the kidneys have higher ACMSD activity than the liver, and the rats with renal insufficiency showed higher serum quinolinic acid levels,15 the kidney may have the pathway to eliminate tryptophan metabolites rather than to product nicotinamide.
Figure 2.
Schematic representation of tryptophan dynamics and nicotinamide supply in the tryptophan-nicotinamide pathway.
Abbreviations: NaMN, nicotinic acid mononucleotide; NMN, nicotinamide mononucleotide; MNA, N1-methylnicotinamide; 2-Py, N1-methyl- 2-pridone-5-carboxamide; 4-Py, N1-methyl-4-pridone-3-carboxamide.
Factors affecting tryptophan–nicotinamide conversion
The processes involved in tryptophan–nicotinamide metabolism can be elucidated by measurement of tryptophan metabolites in urine as several urinary tryptophan metabolites increase following tryptophan intake.16 Changes in urinary tryptophan–nicotinamide metabolites reflect their enzyme activities, and thus can be used as biomarkers to evaluate changes in the pathway.9,17,18 Furthermore, apparent tryptophan–nicotinamide conversion can be calculated using nicotinamide and its metabolites in urine as output versus tryptophan intake as input in animals fed a niacinfree diet.19 Using these techniques, many factors have been found to affect the tryptophan–nicotinamide pathway. Table 1 summarizes the factors affecting tryptophan–nicotinamide conversion. Animal studies have shown that the tryptophan–nicotinamide pathway is affected by conditions such as diabetes, renal failure, and pregnancy,13,20,21 as well as by nutrients such as protein and fatty acids,22,23 hormones such as thyroxin and adrenaline,24,25 and chemicals such as the anti-tuberculosis drug pyrazinamide, the peroxisomal proliferator clofibrate, and phthalate ester plasticizers.18,26,27
Table 1.
Factors affecting tryptophan-nicotinamide conversion.
| Enhance | Suppress |
|---|---|
| Nutrients | Nutrients |
| High-quality protein38 | Low tryptophan diet39 |
| Unsaturated fatty acids23 | Low-molecular peptides38 |
| Hormones | Less vitamin B1 intake40 |
| Thyroxine24 | Less vitamin B6 intake17 |
| Chemicals | Excess protein intake41 |
| Antihyperlipidemic drug26 | Hormones |
| Antitubercular drugs27 | Adrenaline25 |
| Phthalate esters18 | Estrogen42 |
| Physiological conditions | Diseases |
| Pregnancy21 | Type-I diabetes20 |
| Renal failure13 |
Note: Superscript numbers reflect reference number.
Analysis of mice deficient in the tryptophan–nicotinamide pathway gene
To investigate niacin deficiency, niacin-free and tryptophan-imbalanced diets have been used to create a niacin deficiency animal model.28–31 These diets contain low casein levels, high sucrose levels and additional amino acids without niacin, and the rodents show reductions in body weight gain and tissue NAD levels which can be prevented by feeding niacin-containing diets. However, many factors affect tryptophan–nicotinamide metabolism, and elucidation of the effects of factors that affect niacin deficiency, is sometimes difficult in animals fed niacin-free and tryptophan-imbalanced diets. To study the niacin nutritional status regardless of tryptophan status, we generated mice that are missing the QPRT gene.32 Growth retardation, lower niacin nutritional status including blood and liver NAD levels, and no detection of urinary nicotinamide or its metabolites were observed in the qprt-deficient mice fed a niacin-free diet, but not in mice fed a niacin- containing diet. Histological observation of tissues from the qprt−/− mice fed a niacin-free diet revealed disappearance of the inner circular layer of smooth muscle cells in the small intestine. These results indicate that the qprt-deficient mice may be a useful animal model to study niacin deficiency.
Determination of the conversion ratio of tryptophan to nicotinamide in Japanese
As previously mentioned, dietary surveys of Japanese populations have shown that half of the niacin utilized is nicotinamide biosynthesized from tryptophan.4 Estimates of how much tryptophan is convertible to nicotinamide are important in deciding niacin requirements in humans. In 1956, a human study first showed that in men approximately 60 mg of tryptophan is equivalent to 1 mg of niacin calculated using supplemented intake of the free form of tryptophan and urinary MNA, one of the metabolites of nicotinamide.33 Various conversion ratios of tryptophan to nicotinamide were subsequently reported.34,35 One of the reasons for this might be related to the methodologies used: calculations were based on supplemental tryptophan intake and not on proteinous tryptophan intake, not all urinary nicotinamide metabolite was measured, and urinary nicotinamide metabolites are derived from both tryptophan and dietary niacin.
Therefore, we performed a study in humans to determine the conversion ratio of tryptophan to nicotinamide in Japanese women fed a niacin-free purified diet not supplemented with the free form of tryptophan.36 Young Japanese women consumed the niacin-free purified diet for 7 days; the diet contained 0.67 g/day of tryptophan corresponding to habitual tryptophan intake in Japanese women. The conversion ratio was calculated from dietary tryptophan and urinary nicotinamide and its metabolites, and 67 mg of tryptophan was found to be equivalent to 1 mg of nicotinamide. This finding was adopted as part of the evidence for setting the conversion ratio for tryptophan to nicotinamide in the Dietary Reference Intakes for Japanese 2010.3 The Ministry of Health, Labor, and Welfare of Japan releases dietary reference intakes for Japanese every 5 years. The Dietary Reference Intakes for Japanese 2010 was published in 2009 and was developed to provide reference values for the intake of energy and 34 nutrients for the maintenance and promotion of health and the primary prevention of lifestyle-related diseases in healthy individuals and groups.
Changes in tryptophan–nicotinamide metabolism during pregnancy
The dietary habits of pregnant women are important for meeting the nutritional needs of the women and their unborn children, especially in the early stages of the growth of the fetus. Although increased excretion of urinary nicotinamide metabolites during pregnancy was reported as early as the 1940s,37 no evidence has been found as to how tryptophan– nicotinamide metabolism is altered in pregnancy. Since understanding tryptophan–nicotinamide metabolism during pregnancy is important in maintaining niacin nutrition in pregnant women and their unborn children, we investigated the urinary excretion of metabolites of the tryptophan–nicotinamide pathway from spot urine samples taken from pregnant women.21 Spot urine samples were collected from a total of 434 pregnant Japanese women who were between 5 to 40 weeks of gestation, and the intermediates and metabolites of the tryptophan–nicotinamide pathway in the urine samples were measured. Urinary excretion of nicotinamide metabolites increased from 20 weeks of gestation during the second trimester, reached a peak of 2.3 times normal at 33 weeks during the third trimester, and declined to control levels postpartum. The changes in urinary excretion of 3-hydroxyanthranilic acid and quinolinic acid showed similar patterns to those of all nicotinamide metabolites such as MNA, 2-Py and 4-Py, increasing from 15–20 weeks of gestation, reaching a peak at 30–35 weeks, and then declining postpartum.
These results show that tryptophan–nicotinamide metabolism is enhanced during mid and late pregnancy. Energy and nutrient requirements increase during pregnancy to allow for fetal growth, and these findings suggest that nicotinamide production is also enhanced during pregnancy to compensate for the increase in niacin requirement. These findings also contributed to the Dietary Reference Intakes for Japanese 2010 for niacin in pregnant women, which notes that “the amount of nicotinamide biosynthesized from tryptophan increases during pregnancy, and this compensates for the increase in niacin requirement.” Thus, pregnant women do not require additional niacin intake.3
Conclusion
We conclude that the tryptophan–nicotinamide pathway plays a critical role in maintaining niacin nutrition via supply of biosynthesized nicotinamide to the peripheral. In addition, many factors affect tryptophan–nicotinamide metabolism, and these changes should be considered in maintaining niacin nutrition. However, precise mechanisms for these changes have not been fully elucidated. We have recently generated qprt-deficient mice, and hope that these mice will contribute to the elucidation of the tryptophan–nicotinamide pathway at the molecular level.
Footnotes
Author Contributions
Conceived and designed the concept: TF. Wrote the first draft of the manuscript: TF. Agree with manuscript results and conclusions: TF, KS. Made critical revisions and approved final version: KS. All authors reviewed and approved of the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
Funding
Part of this work was supported by a Grants-in-Aid for Scientific Research grant from the Japan Society for the Promotion of Science, and by research funding from the International Council on Amino Acid Science (ICAAS). 13th ISTRY Satellite Symposium and the publication fee of this proceeding were supported by ICAAS.
References
- 1.Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington (DC): National Academies Press (US); 1998. [PubMed] [Google Scholar]
- 2.Seal AJ, Creeke PI, Dibari F, et al. Low and deficient niacin status and pellagra are endemic in postwar Angola. Am J Clin Nutr. 2007;85(1):218–24. doi: 10.1093/ajcn/85.1.218. [DOI] [PubMed] [Google Scholar]
- 3.Ministry of Health, Labor, and Welfare of Japan. Dietary reference Intakes for Japanese, 2010. Tokyo: 2009. [Google Scholar]
- 4.Ministry of Health, Labor, and Welfare of Japan. The National Health and Nutrition Survey, 2010. Tokyo: 2012. [Google Scholar]
- 5.DesGroseilliers JP, Shiffman NJ. Pellagra. Can Med Assoc J. 1976;115(8):768–70. [PMC free article] [PubMed] [Google Scholar]
- 6.Shibata K, Matsuo H. Effect of dietary tryptophan levels on the urinary excretion of nicotinamide and its metabolites in rats fed a niacin-free diet or a constant total protein level. J Nutr. 1990;120(10):1191–7. doi: 10.1093/jn/120.10.1191. [DOI] [PubMed] [Google Scholar]
- 7.Fukuwatari T, Wada H, Shibata K. Age-related alterations of B-group vitamin contents in urine, blood and liver from rats. J Nutr Sci Vitaminol. 2008;54(5):347–52. doi: 10.3177/jnsv.54.357. [DOI] [PubMed] [Google Scholar]
- 8.Shibata K, Sanada H, Yuyama S, Suzuki T. Evaluation of niacin nutrition in persons of advanced age supposed by the urinary excretion of niacin metabolites. Vitamins (Japan) 1994;68:365–72. [Google Scholar]
- 9.Wada H, Fukuwatari T, Sasaki R, et al. Blood NAD and NADP levels in the elderly. Vitamins (Japan) 2006;80(3):125–7. [Google Scholar]
- 10.Yoshida R, Nukiwa T, Watanabe Y, Fujiwara M, Hirata F, Hayaishi O. Regulation of indoleamine 2,3-dioxygenase activity in the small intestine and the epididymis of mice. Arch Biochem Biophys. 1980;203(1):343–51. doi: 10.1016/0003-9861(80)90185-x. [DOI] [PubMed] [Google Scholar]
- 11.Truscott RJ, Elderfield AJ. Relationship between serum tryptophan and tryptophan metabolite levels after tryptophan ingestion in normal subjects and age-related cataract patients. Clin Sci (Lond) 1995;89(6):591–9. doi: 10.1042/cs0890591. [DOI] [PubMed] [Google Scholar]
- 12.Comai S, Costa CV, Ragazzi E, Bertazzo A, Allegri G. The effect of age on the enzyme activities of tryptophan metabolism along the kynurenine pathway in rats. Clin Chim Acta. 2005;360(1–2):67–80. doi: 10.1016/j.cccn.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Fukuwatari T, Morikawa Y, Sugimoto E, Shibata K. Effects of fatty liver induced by niacin-free diet with orotic acid on the metabolism of tryptophan to niacin in rats. Biosci Biotechnol Biochem. 2002;66(6):1196–204. doi: 10.1271/bbb.66.1196. [DOI] [PubMed] [Google Scholar]
- 14.Fukuwatari T, Morikawa Y, Hayakawa F, Sugimoto E, Shibata K. Influence of adenine-induced renal failure on tryptophan-niacin metabolism in rats. Biosci Biotechnol Biochem. 2001;65(10):2154–61. doi: 10.1271/bbb.65.2154. [DOI] [PubMed] [Google Scholar]
- 15.Saito K, Fujigaki S, Heyes MP, et al. Mechanism of increases in l- kynurenine and quinolinic acid in renal insufficiency. Am J Physiol Renal Physiol. 2000;279(3):F565–72. doi: 10.1152/ajprenal.2000.279.3.F565. [DOI] [PubMed] [Google Scholar]
- 16.Okuno A, Fukuwatari T, Shibata K. Urinary excretory ratio of anthranilic acid/kynurenic acid as an index of the tolerable amount of tryptophan. Biosci Biotechnol Biochem. 2008;72(7):1667–72. doi: 10.1271/bbb.70630. [DOI] [PubMed] [Google Scholar]
- 17.Shibata K, Mushiage M, Kondo T, Hayakawa T, Tsuge H. Effects of vitamin B6 deficiency on the conversion ratio of tryptophan to niacin. Biosci Biotechnol Biochem. 1995;59(11):2060–3. doi: 10.1271/bbb.59.2060. [DOI] [PubMed] [Google Scholar]
- 18.Fukuwatari T, Ohsaki S, Fukuoka S, Sasaki R, Shibata K. Phthalate esters enhance quinolinate production by inhibiting α-amino-β-carboxymuconate-ɛ-semialdehyde decarboxylase (ACMSD), a key enzyme of the tryptophan pathway. Toxicol Sci. 2004;81(2):302–8. doi: 10.1093/toxsci/kfh204. [DOI] [PubMed] [Google Scholar]
- 19.Shibata K, Murotani M, Onodera M. Difference in tryptophan-nicotinamide conversion according to dietary nitrogen sources; casein, casein hydrolysate, and mixtures of amino acids. Biosci Biotech Biochem. 1992;56:665–9. doi: 10.1271/bbb.56.665. [DOI] [PubMed] [Google Scholar]
- 20.Tanabe A, Egashira Y, Fukuoka S, Shibata K, Sanada H. Expression of rat hepatic 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase is affected by a high protein diet and by streptozotocin-induced diabetes. J Nutr. 2002;132(6):1153–9. doi: 10.1093/jn/132.6.1153. [DOI] [PubMed] [Google Scholar]
- 21.Fukuwatari T, Murakami M, Ohta M, et al. Changes in the urinary excretion of the metabolites of the tryptophan-niacin pathway during pregnancy in Japanese women and rats. J Nutr Sci Vitaminol. 2004;50(6):392–8. doi: 10.3177/jnsv.50.392. [DOI] [PubMed] [Google Scholar]
- 22.Shibata K, Matsuo H. Effect of dietary tryptophan levels on the urinary excretion of nicotinamide and its metabolites in rats fed a niacin-free diet or a constant total protein level. J Nutr. 1990;120(10):1191–7. doi: 10.1093/jn/120.10.1191. [DOI] [PubMed] [Google Scholar]
- 23.Egashira Y, Murotani G, Tanabe A, et al. Differential effects of dietary fatty acids on rat liver α-amino-β-carboxymuconate-ɛ-semialdehyde decarboxylase activity and gene expression. Biochim Biophys Acta. 2004;1686(1–2):118–24. doi: 10.1016/j.bbalip.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Shibata K, Toda S. Effect of thyroxine on the metabolism of tryptophan to nicotinamide in rats. Biosci Biotechnol Biochem. 1994;58:1757–62. [Google Scholar]
- 25.Shibata K. Effects of adrenalin on the conversion ratio of tryptophan to niacin in rats. Biosci Biotechnol Biochem. 1995;59(11):2127–9. doi: 10.1271/bbb.59.2127. [DOI] [PubMed] [Google Scholar]
- 26.Shin M, Mori Y, Kimura A, et al. NAD+ biosynthesis and metabolic fluxes of tryptophan in hepatocytes isolated from rats fed a clofibrate-containing diet. Biochem Pharmacol. 1996;52(2):247–52. doi: 10.1016/0006-2952(96)00201-8. [DOI] [PubMed] [Google Scholar]
- 27.Shibata K, Fukuwatari T, Sugimoto E. Effects of dietary pyrazinamide, an antituberculosis agent, on the metabolism of tryptophan to niacin and of tryptophan to serotonin in rats. Biosci Biotechnol Biochem. 2001;65(6):1339–46. doi: 10.1271/bbb.65.1339. [DOI] [PubMed] [Google Scholar]
- 28.Krehl WA, Teply LJ, Sarma PS, Elvehjem CA. Corn as an etiological factor in the production of a nicotinic acid deficiency in the rat. Science. 1945;101(2620):489–90. doi: 10.1126/science.101.2620.283. [DOI] [PubMed] [Google Scholar]
- 29.Henderson LM, Deoder T, Krehl WA, Elevehjem CA. Factors of affecting the growth of rats receiving niacin-tryptophan-deficient diets. J Biol Chem. 1947;170(1):261–8. [Google Scholar]
- 30.Zhang JZ, Henning SM, Swendseid ME. Poly(ADP-ribose) polymerase activity and DNA strand breaks are affected in tissues of niacin-deficient rats. J Nutr. 1993;123(8):E1349–55. doi: 10.1093/jn/123.8.1349. [DOI] [PubMed] [Google Scholar]
- 31.Jean MR, Jackson TM, Driscoll ER, Kirkland JB. Niacin deficiency lowers tissue poly (ADP-ribose) and NAD concentrations in Fischer-344 rats. J Nutr. 1994;124:1597–603. doi: 10.1093/jn/124.9.1597. [DOI] [PubMed] [Google Scholar]
- 32.Terakata M, Fukuwatari T, Sano M, et al. Establishment of true niacin deficiency in quinolinic acid phosphoribosyltransferase knockout mice. J Nutr. 2012;142(12):2148–53. doi: 10.3945/jn.112.167569. [DOI] [PubMed] [Google Scholar]
- 33.Horwitt MK, Harvey CC, Rothwell WS, Cutler JL, Haffron D. Tryptophanniacin relationships in man: Studies with diets deficient in riboflavin and niacin, together with observations on the excretion of nitrogen and niacin metabolites. J Nutr. 1956;60:1–43. [Google Scholar]
- 34.Goldsmith GA, Miller ON, Unglaub WG. Efficiency of tryptophan as a niacin precursor in man. J Nutr. 1961;73:172–6. [Google Scholar]
- 35.Nakagawa I, Takahashi T, Suzuki T, Masana Y. Effect in man of the addition of tryptophan or niacin to the diet on the excretion of their metabolites. J Nutr. 1969;99:325–30. doi: 10.1093/jn/99.3.325. [DOI] [PubMed] [Google Scholar]
- 36.Fukuwatari T, Ohta M, Kimura N, Sasaki R, Shibata K. Conversion ratio of tryptophan to niacin in Japanese women fed on a purified diet conforming to the Japanese Dietary Reference Intakes. J Nutr Sci Vitaminol. 2004;50(6):385–91. doi: 10.3177/jnsv.50.385. [DOI] [PubMed] [Google Scholar]
- 37.Moore MC, Purdy MB, Gibbens EJ, Hollinger ME, Goldsmith G. Food habits of women during pregnancy. J Am Diet Ass. 1947;23:847–53. [PubMed] [Google Scholar]
- 38.Shibata K, Onodera M. Comparison of tryptophan-niacin conversion in rats fed with a nicotinic acid-free diet containing egg white, egg white proteolysate, or mixtures of amino acids. Agric Biol Chem. 1991;55:1291–8. [Google Scholar]
- 39.Shibata K. Effect of adding the limiting amino acids to an amino acid diet simulating rice protein on the conversion of tryptophan to nicotinamide in rat. Biosci Biotech Biochem. 1994;58(2):442–3. [Google Scholar]
- 40.Shibata K, Kondo T, Yonejima M. Conversion ratio of tryptophan to niacin in rats fed a vitamin B1-free diet. J Nutr Sci Vitaminol. 1997;43(4):479–83. doi: 10.3177/jnsv.43.479. [DOI] [PubMed] [Google Scholar]
- 41.Kimura N, Fukuwatari T, Sasaki R, Shibata K. The necessity of niacin in rats fed on a high protein diet. Biosci Biotechnol Biochem. 2005;69(2):273–9. doi: 10.1271/bbb.69.273. [DOI] [PubMed] [Google Scholar]
- 42.Shibata K, Kondo T. Effect of progesterone and estrone on the conversion of tryptophan to nicotinamide in rats. Biosci Biotech Biochem. 1993;57:1890–3. [Google Scholar]