Abstract
Low-grade and chronic inflammation is elicited in white adipose tissue in human obesity. The presence of inflammatory molecules leads to an increased tryptophan catabolism through the induction of indoleamine-2,3-dioxygenase-1 (IDO1). In order to characterize the mechanisms underlying this dysregulation, we have studied 2 mouse models of obesity. Unexpectedly, we did not detect any IDO1 expression in obese or lean mice adipose tissue. In a previous study, we did not find any significant difference in the liver for IDO2 and tryptophan-2,3-dioxygenase (TDO2) gene expression between normal weight and obese patients. IDO2 and TDO2 expression was increased in the liver of high-fat fed mice, but not in ob/ob mice, and was strongly correlated with hydroxysteroid-(11-beta) dehydrogenase-1 (HSD11B1) expression, an enzyme that generates active cortisol within tissues. In conclusion, despite a dysregulation of tryptophan metabolism, obese mice display discrepancies with human obesity metabolism, rendering them inappropriate for further investigations in this animal model.
Keywords: tryptophan 2, 3-dioxygenase, indoleamine 2, 3-dioxygenase 2, obesity, high fat diet
Introduction
Obesity and the associated metabolic disorders are characterized by the presence of a chronic “low-grade” inflammatory response in insulin-sensitive tissues, particularly adipose tissue and liver. The different adipose depots of the organism are enlarged and infiltrated with macrophages and diverse immune cells that promote a proinflammatory state.1 In this context, epidemiological studies have demonstrated that the visceral adipose compartment was the more deleterious in terms of the risk of developing associated comorbidities, due to its higher inflammatory features.2
We have recently demonstrated that tryptophan metabolism is activated in human obesity3 and likely results from the inflammatory response linked to this pathology. In particular, in indoleamine 2,3-dioxygenase (IDO1) the rate-limiting enzyme of the tryptophan–kynurenine pathway4,5 is induced in white adipose tissue and liver. The kynurenine pathway is the main degradative pathway for the essential amino acid tryptophan. The first step in the conversion of tryptophan to kynurenine can be carried out by 3 different enzymes: indoleamine 2,3 dioxygenase-1 (IDO1), tryptophan 2,3-dioxygenase (TDO2) and indoleamine 2,3-dioxygenase-2 (IDO2), a recently described paralog of IDO1.6 IDO1 is known to be ubiquitous whereas IDO2 and TDO2 are both expressed in the liver, brain (TDO2), kidney or epididymis (IDO2). IDO1 is induced by pro-inflammatory cytokines while TDO2 is induced by tryptophan and cortisol.5,7 Inductors of IDO2 expression might be the same as IDO1’s, but few are known and the data are still controversial.6
The kynurenine/tryptophan ratio, used as an indicator of IDO1/TDO2 activity, is enhanced in the sera of obese patients, demonstrating a dysregulation of this pathway which is not re-equilibrated by weight loss.8 Several studies have suggested that IDO1 regulates the balance of TH17 cells (proinflammatory) relative to regulatory T-cells (Treg, a lymphocyte subset with immunosuppressive attributes) in mice9,10 and in humans.11 We further demonstrated that the ratio between TH17 cells and regulatory T cells correlates with IDO1 activation in subcutaneous adipose tissue.3 Despite IDO1 enhancement, the TH17/Treg ratio remained high, especially in the omental compartment, which we demonstrated to be more inflammatory.12 This suggests an impairment of the pathway in this compartment. The ability of regulatory T-cells to decrease adipose inflammation and to counteract insulin resistance has been demonstrated in obese, leptin-deficient ob/ob mice.13 Since visceral adipose inflammation in obesity is a major pathophysiological risk factor for the development of whole body insulin-resistance and metabolic abnormalities,14 our main objective was to further understand mechanisms underlying inability of IDO1 to regulate TH17/Treg ratio in the omental compartment.
For this purpose, we studied the high-fat diet induced-obesity model and the leptin-deficiency model (ob/ob), the two main mouse models that have been extensively studied in this field.15,16 We first explored tryptophan catabolism and production of kynurenine in these models both in the white adipose tissue and in the liver, which are the 2 main organs prone to inflammation in obesity.17,18 In this study, we have monitored weight gain in mice during 16 weeks with a high fat diet, as well as inflammation in the white adipose tissue. The tryptophan degradation pathway was investigated by the analysis of the expression of 3 degrading enzymes and by the measurement of metabolite concentrations in sera. Unexpectedly, we found a dysregulation of tryptophan metabolism in obese mice, but with very different features than in human obesity, that render mouse models inappropriate for further mechanistic investigations of this pathway.
Materials and Methods
Animals, diet and treatment
Two common mice obesity models have been used in this study: mice fed a high fat diet and ob/ob mice. For diet-induced obesity, 6-week-old C57BL/6J male mice (Charles River Laboratories, L’Arbresle, France) were fed either with a standard diet or with a high-fat diet (Research Diets, INC, New Brunswick, NJ) containing 10% or 60% in kcal of fat, respectively, but produced with ingredients of the same origin. Food intake and body weight were measured weekly for up to 16 weeks of diet. As another model of obesity with metabolic abnormalities,16 we used 7-week-old ob/ob male mice (JAX® Mice Strain: B6.V-Lep(ob)/J, Charles River Laboratories) deficient in the hormone leptin and used littermates C57BL/6J male mice as control as recommended.
As a positive control of inflammation we used a model of acute inflammation obtained by lipopolysaccharide (LPS) administration. LPS (E. coli 0127:B8, Sigma, Steinheim, Germany) was intraperitoneally administrated to 6-week-old C57BL/6J male mice at 830 μg/kg body weight in PBS. Animals were sacrificed 6 hours (h) after injection. All animals were kept on a 12:12 h light–darkness cycle and were given free access to food and water. Breeding, housing and experimentations were carried out according to the French and European guidelines of laboratory animal care (European Communities Council Directive of 430 1986, 86/609/EEC) and approved by the Departmental Direction of Veterinary Services (Prefecture of Lille, France; authorization number: 59-350172).
Intraperitoneal glucose tolerance test
Glucose tolerance tests (GTT) were performed on 6 h-fasted mice injected intraperitoneally with D-glucose (1g/kg body weight, Sigma-Aldrich). Glucose levels were measured by tail tip bleeding with an automatic glucometer (ACCU-CHEK Performa, Roche, Meylan, France) immediately before and 15, 30, 60 and 180 minutes (min) after the glucose injection.
Adipose and liver tissue sampling and RNA extraction
Tissue samples (liver, epidydimal fat and subcutaneous fat) were retrieved from each animal immediately after cervical dislocation. They were stored at −85 °C in Qiazol reagent. Total ribonucleic acid (RNA) was extracted from each sample by homogenization with TissueLyser II (QIAGEN Inc., Valencia, CA) using Qiazol reagent and carbide beads ( QIAGEN). These extracts were further automatically processed in a Qiacube machine using RNeasy Lipid Tissue MiniKit (QIAGEN). The RNA concentration was determined by absorbance at 260 nm (A260), and the purity was estimated by determining the A260/A280 ratio using a Nanodrop spectrophotometer (Nanodrop Technologies, Wilmington, DE).
Relative quantitative real-time PCR
The relative quantification of messenger RNA (mRNA) was performed with a quantitative RT-PCR assay. 1 μg of total RNA was transcribed into cDNA using the cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA). Each cDNA sample was analyzed for gene expression by quantitative real-time PCR (qPCR) using the fluorescent TaqMan 5′-nuclease assay with an Applied Biosystems 7900HT sequence detection system. The TaqMan RT-PCR was performed using 2X TaqMan Master Mix and 20X premade TaqMan gene expression assays (Applied Biosystems). Analysis was performed with the ABI 7900HT SDS 2.2 Software. The mRNA levels were normalized to that of the 60S acidic ribosomal protein P0 (RPLP0), which was chosen for its stability in these samples. The data are given as the ratio of the level of the target gene mRNA to that of RPLP0 mRNA.
Determination of serum concentrations of tryptophan and kynurenine
An analytical procedure based on liquid chromatography-tandem mass spectrometry, employing electrospray ionization (LC-ESI/MS/MS), has been developed according to previously published methods, with slight modifications.19,20 100 μL of pooled sera was analyzed after the addition of 50 μL methanol containing methyl-clonazepam (Sigma-Aldrich, Saint-Quentin Fallavier, France) at 1.25 mg/L, as an internal standard, and 50 μL acetonitrile. The samples were mixed and centrifuged (11200 g, 6 min) and the supernatant (100 μL) was added to deionized water (500 μL). 15 μL of this mixture was injected onto an UPLC-MS-MS system (Acquity TQ Detector, Waters, Milford, MA) equipped with a HSS C18 column. Ions of each analyzed compound were detected in a positive ion mode using multiple reaction monitoring. Chromalinks software (Waters) was used for data acquisition and processing.
Statistical analysis
Statistical analysis was performed with the GraphPad Prism version 5.00 for Windows, GraphPad Software (San Diego, California, USA). Circulating metabolite concentrations and mRNA levels were compared using U Mann-Whitney’s test. Correlation analyses were performed using Pearson analysis. The threshold of significance was set to P < 0.05.
Results
Lack of IDO1 expression in adipose tissue of obese mice
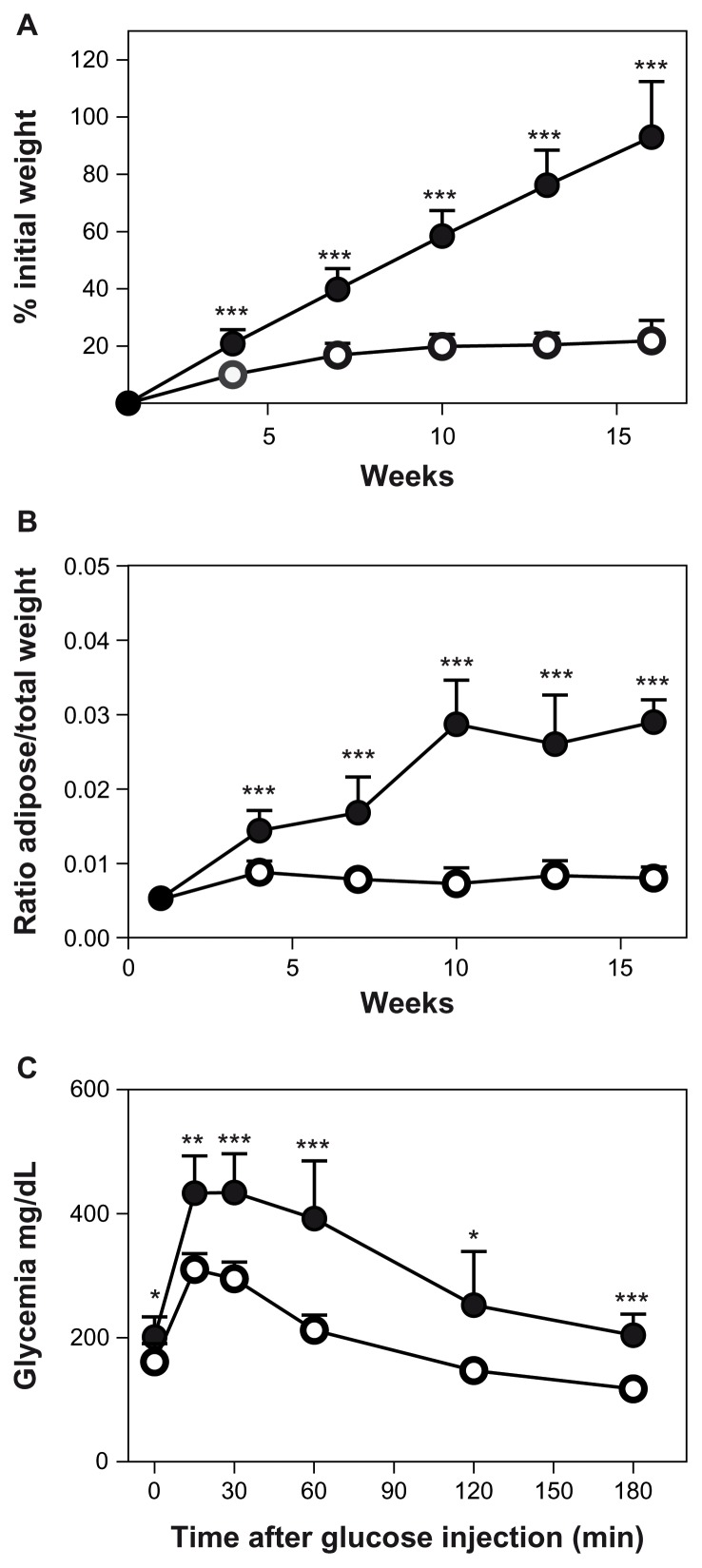
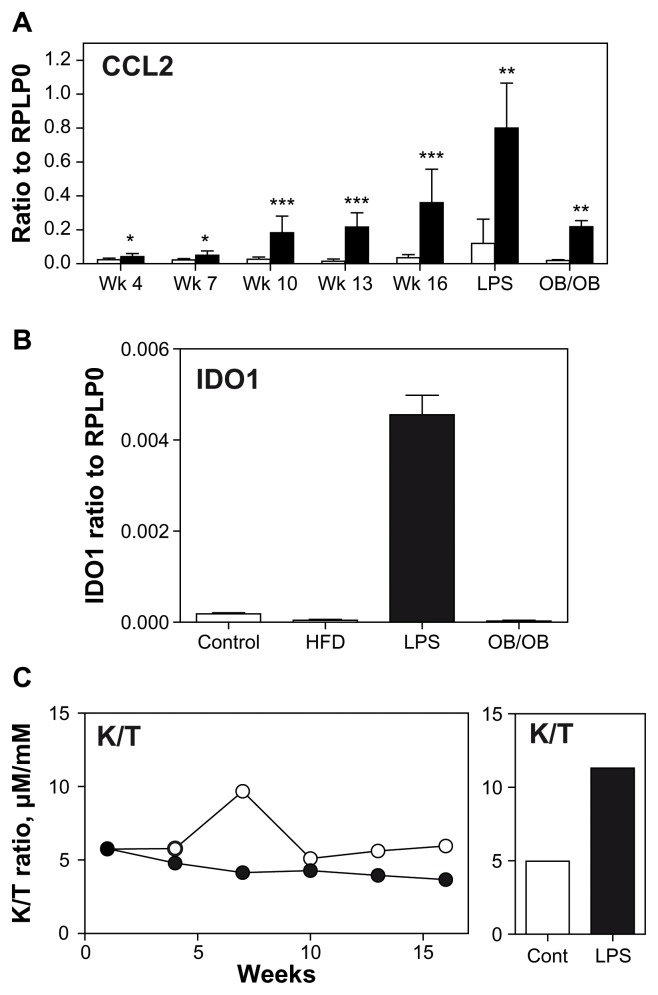
Compared to standard diet fed animals, high-fat fed animals displayed, as expected, a significant increase of their body weight from first week of diet, to an over 100% increase after 15 weeks (Fig. 1A). This weight increase was mainly associated with an increase in adipose tissue mass as visualized by the analysis of epididymal fat mass (Fig. 1B). Upon feeding of a high fat diet, animals became glucose resistant and displayed a higher fasting glycemia (Fig. 1C). Inflammation was monitored in adipose tissue by analyzing CCL2 expression. In accordance with the literature,21 CCL2 is highly induced in adipose tissue by high fat feeding while it remains low in control mice (Fig. 2A). This chemokine is also induced in adipose tissue by LPS administration and in ob/ob mice (Fig. 2A). Interferon-gamma (IFNG) expression in adipose tissue remained below detection limits in obese mice but was significantly induced 6 h after LPS administration (data not shown).
Figure 1.
Six-week-old C57Bl6J male mice were fed either a standard diet (○) or a high-fat diet (●); n = 40/group. N = 8 in each group were sacrificed every 3 weeks. (A) Body weight was expressed as a percentage of initial mass. (B) Left epidydimal adipose tissue mass was expressed as a percentage of total body weight (C) Intraperitoneal glucose tolerance test with 1 g glucose/kg body mass after 6 hr of fasting performed on animals after 16 weeks of diet.
Notes: ***P < 0.001; **P < 0.005; *P < 0.05.
Figure 2.
(A) Expression of CCL2 in epidymal adipose tissue of mice: C57Bl/6J fed either a standard diet (white) or a high fat diet (black) n = 40/group. N = 8 in each group were sacrificed every 3 weeks (week 4 to week 16)—or 6 hours after an intraperitoneal administration of LPS (vehicle: white; LPS: black; n = 8)—or of ob/ob mice (control littermate: white; OB/OB: black; n = 5). (B) Expression of IDO1 in epidydimal adipose tissue of mice: C57Bl/6J fed either a standard diet (control) or a high fat diet (HFD) for 16 weeks (n = 8)—or 6 hours after an intraperitoneal administration of LPS (LPS; n = 8)—or of ob/ob mice (OB/OB; n = 5). (C) Kynurenine/tryptophan ratio in pooled sera of C57Bl/6J fed either a standard diet (white) or a high fat diet (black) for 16 weeks; n = 40/group. N = 8 in each group were sacrificed every 3 weeks and corresponding sera were pooled—or 6 hours after an intraperitoneal administration of LPS (vehicle: white; LPS: black; n = 8).
Notes: ***P < 0.001; **P < 0.005; *P < 0.05.
Despite inflammatory and glucose resistance features similar to those encountered in human obesity, we did not detect any significant IDO1 expression in epididymal adipose tissue of either control or high-fat diet fed animals (Fig. 2B). Furthermore, in the ob/ob mice, no IDO1 expression was detected in the adipose tissue (Fig. 2B). Subcutaneous adipose tissue was also studied and never displayed any IDO1 expression (data not shown). Intraperitoneal administration of LPS induced a weak but significant expression of IDO1 in adipose tissue, six hours after administration (Fig. 2B).
Finally, in order to detect a potential activation of the kynurenine pathway in our animals, we analyzed the ratio of circulating kynurenine/tryptophan in high fat-fed mice. Serum was harvested from sacrificed animals and pooled for spectrometry analysis. This ratio was slightly lower in high fat fed mice than in corresponding controls (Fig. 2C), and this was associated with a decrease in kynurenine concentration while tryptophan concentration was not different between high fat-fed and control animals (data not shown). However, an acute inflammation induced by LPS administration is able to shift twice this ratio (Fig. 2C) and this shift was the result of a strong increase in kynurenine concentration, while tryptophan variation was low (data not shown). Low levels of IDO1 expression were detected in spleens of mice, but no significant difference was demonstrated between control and high fat diet animals (ratio to RPLP0; week 16; control = 1.13 10−4 ± 1.78 10−5 and high fat diet = 1.22 10−4 ± 2.51 10−5). Hepatic IDO1 expression was also very weak or undetectable either in normal or in high fat fed mice.
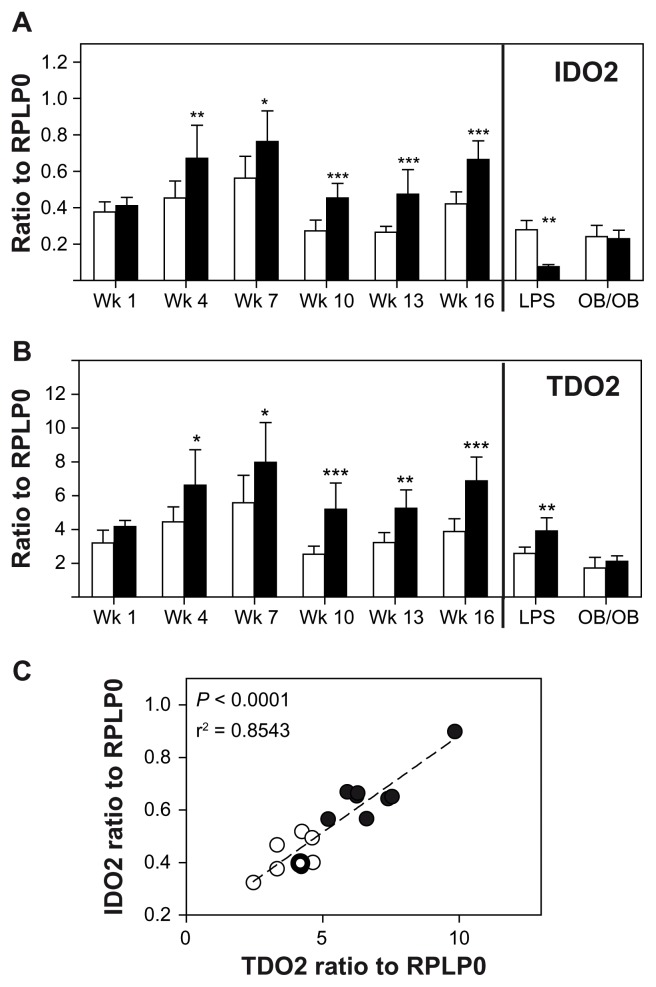
Induction of TDO2 and IDO2 in liver of high-fat diet mice
We further evaluated the expression of TDO2 and IDO2 in the liver of high fat diet mice. An enhancement of IDO2 expression became significant after 4 weeks of high fat diet and reached two-fold after 13 weeks (Fig. 3A). Acute inflammation induced decreased IDO2 expression after 6 hours using intraperitoneal injection of LPS. In contrast, the ob/ob mice did not display any variation in IDO2 expression when compared to wild-type littermates (Fig. 3A). TDO2 displayed an expression pattern very similar to that of IDO2 except for the acute inflammation experiment where TDO2 was significantly induced by LPS administration (Fig. 3B).
Figure 3.
Expression of IDO2 (A) and of TDO2 (B) in hepatic tissue of mice: C57Bl/6J fed either a standard diet (white) or a high fat diet (black) n = 40/group. N = 8 in each group were sacrificed every 3 weeks (week 4 to week 16)—or 6 hours after an intraperitoneal administration of LPS (vehicle: white; LPS: black; n = 8)—or of ob/ob mice (control littermate: white; OB/OB: black; n = 5). (C) Correlation between TDO2 and IDO2 expression in hepatic tissue of C57Bl/6J mice after 16 weeks of standard diet (○) or high-fat diet (●); Pearson analysis.
Notes: ***P < 0.001; **P < 0.005; *P < 0.05.
For this reason, we assessed the correlation between IDO2 and TDO2 expressions in hepatic tissue of control and high fat-fed mice after 16 weeks of feeding (Fig. 3C). Pearson analysis showed very low P-value and correlation coefficient (P < 0.0001 and r2 = 0.8543) suggesting that these 2 genes are induced by the same factor. To note, IDO2 and TDO2 expressions in adipose tissues were weak in all studied groups and were, on average, 10000-fold less than in the liver (data not shown).
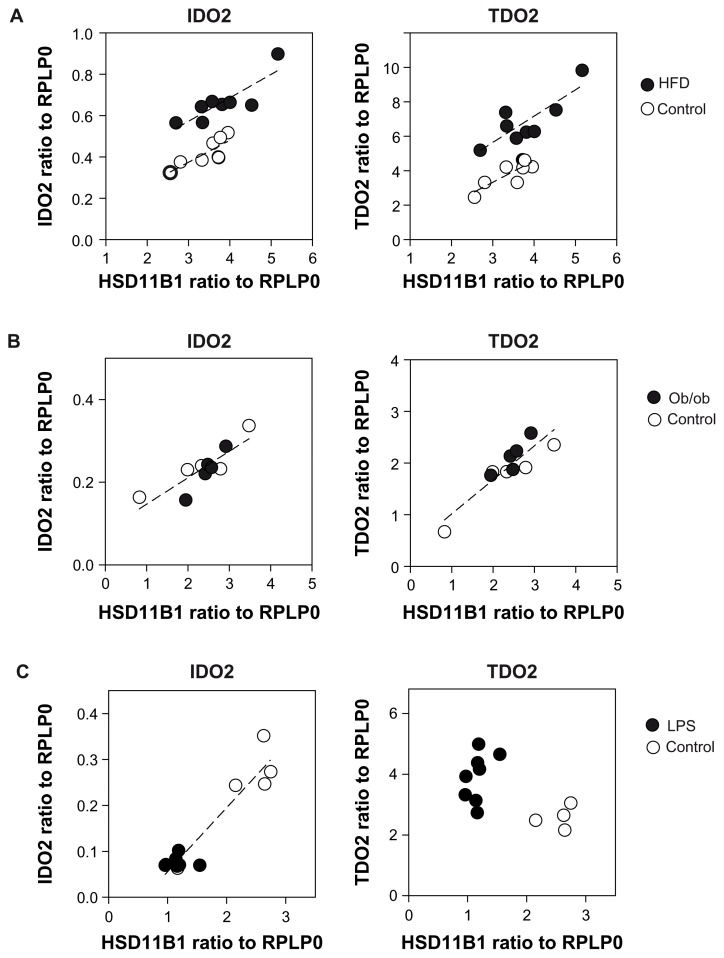
Hydroxysteroid (11-beta) dehydrogenase 1 (HSD11B1) is a microsomal enzyme that catalyzes the conversion of the inactive metabolite cortisone to the stress hormone cortisol22 and its activation is known to promote obesity.23 TDO2 can be regulated by glucocorticoids5,7 therefore we analyzed HSD11B1 expression in the liver of our animals. No significant difference in expression was demonstrated between control and high fat fed animals (data not shown). We then studied correlations of TDO2 and IDO2 expression with HSD11B1 expression in liver of mice fed during 16 weeks a standard or a high fat diet. A global Pearson analysis of all the liver samples demonstrated a positive correlation of TDO2 (P = 0.003; r2 = 0.696) and of IDO2 (P = 0.003; r2 = 0.696) expression with HSD11B1 expression. When high fat-fed and control group were analyzed separately, the correlation coefficients were higher. For TDO2 expression, r2 = 0.825 (P = 0.012) for high fat diet and r2 = 0.829 (P = 0.011) for control. For IDO2 expression r2 = 0.806 (P = 0.016) for high fat diet and r2 = 0.847 (P = 0.008) for control (Fig. 4A). We performed the same analysis with hepatic samples of ob/ob mice and littermate controls and found a positive correlation between TDO2 and IDO2 expression and HSD11B expression, but the groups could not be split into 2 unconnected populations (Fig. 4B). Furthermore, LPS administration induced a decrease in HSD11B1 expression in the liver 6 hours after injection (data not shown). IDO2 and TDO2 expression were inversely regulated after this interval (Fig. 3). We found a strong correlation between IDO2 and HSD11B1, while the association between TDO2 and HSD11B1 was no more significant (Fig. 4C).
Figure 4.
Correlation between IDO2 or TDO2 and HSD11B1 expression in hepatic tissue (Pearson analysis). (A) C57Bl/6J fed during 16 weeks either a standard diet (○) or a high fat diet (●) IDO2: r2 = 0.806 (P = 0.016) for high fat diet and r2 = 0,847 (P = 0.008) for control TDO2: r2 = 0.825 (P = 0.012) for high fat diet and r2 = 0.829 (P = 0.011) for control. (B) ob/ob (●) or littermate control (○) IDO2: r2 = 0.742 (P = 0.0014) TDO2: r2 = 0.816 (P = 0.0003). (C) 6 hours after an intraperitoneal administration of LPS (●) or vehicle (○); IDO2 expression r2 = 0.943 (P < 0.0001).
Discussion
In the obese state, the different adipose depots in the body are enlarged and often infiltrated with macrophages and other diverse immune cells that promote a proinflammatory state.1 We have recently demonstrated that tryptophan metabolism is activated in human obesity.3 In order to further investigate biological mechanisms involved in obesity, we analyzed the tryptophan metabolism in mouse models of obesity such as animals fed a high fat diet15 and genetically deficient in leptin,16 a protein implied in the regulation of food intake and energy expenditure. We demonstrated here that despite the presence of inflammation in adipose depots, there is no detectable IDO1 mRNA expression in mice subcutaneous and visceral adipose tissues, in contrast to what we previously found in human obese samples.3 We observed other striking differences between the 2 species: the kynurenine/tryptophan ratio was significantly increased in obese human subjects due to an increase level of circulating kynurenine. In mice this ratio was always lower in mice fed a high fat diet in comparison with mice fed a control diet. Nevertheless, an acute inflammation induced by LPS administration was able to shift this ratio, as expected. Furthermore, in the human liver IDO2 and TDO2 expression was not significantly different between normal weight and obese patients.3 In high fat-fed mice we observed an early induction of these 2 enzymes, from 4 weeks after the change in diet, with an increasing difference between obese and control mice until the end of the experiment. We found that the expression of IDO2 and TDO2 was strongly correlated. TDO2 is regulated by tryptophan and stress hormones, such as corticoids.5,24 Little is known about IDO2 regulators. We analyzed expression of HSD11B1, an enzyme that generates active glucocorticoid compounds within tissues. Circulating cortisol levels are not elevated in idiopathic obesity, but overexpression of HSD11B1 in mice causes obesity by local increase of glucocorticoid concentrations, especially in adipose tissue.25,26 We did not observe any significant differences between high fat-fed and control-fed mice in HSD11B1 expression in liver, as previously described in high fat-fed mice.27 However, a significant correlation between HSD11B1 and IDO2 or TDO2 was found, demonstrating that intracellular cortisol is likely to be a strong inducer of these two enzymes. 2 earlier studies have described that TDO2 can be upregulated by steroids.28,29 Nevertheless, our results suggest that leptin could be involved in the regulation of IDO2 and TDO2, since we observed in high fat fed mice an increased level in expression of TDO2 and IDO2, which was not observed in leptin-deficient obese mice. Furthermore, the induction of acute inflammation with LPS administration triggers an opposite effect on IDO2 and TDO2 induction, demonstrating that these two enzymes can be differentially regulated by pro-inflammatory factors. In addition, we noticed a quite fluctuating baseline in tryptophan concentrations and in IDO2 and TDO2 expression for control mice during weeks of feeding. We have no rational explanation for these fluctuations. Altogether, these results deserve further investigations on the molecular mechanisms involved in IDO2 and TDO2 regulation.
Our data led us to conclude that there is a limitation to the use of mouse models to study dysregulation of tryptophan metabolism in human obesity, especially to try to find different mechanisms in subcutaneous and visceral adipose tissue. But, even if the characteristics are different between human and mouse, tryptophan degradation pathway is obviously affected by obesity. A recent metabolomic analysis performed on C57BL/6 mice fed with a high fat diet (safflower oil and menhaden oil, 59% calories), demonstrated a decrease in kynurenine concentration corroborating our results.30 Discrepancies between species could have been awaited since variations between species or strains in expression and activity of enzymes of the kynurenine degradation pathway have already been documented.31–33
Correlations between the tryptophan degradation pathway and other metabolic diseases have recently been found. Tryptophan and some of its catabolites were recently associated with an adverse metabolic profile in individuals who are prone to developing diabetes mellitus and cardiovascular disease.34 The gene expression profile of tryptophan metabolism is altered in the liver of mice undergoing a high fat diet.35 In addition, the obesity-induced increase in circulating free fatty acids can result in a displacement of serum-protein-bound tryptophan and reduce availability of circulating free tryptophan.36 This suggests a likely involvement of the kynurenine pathway in lipid metabolism in the liver. In addition, serotonin, another tryptophan metabolite, is also involved in the development of steatosis and steatohepatitis.37,38 Inflammation, which is an underlying feature of most the metabolic diseases, is directly connected with increased tryptophan metabolism. IDO1 is implicated in the endogenous induction of peripheral tolerance and immunosuppression,39,40 particularly through the control of the balance between TH17/Treg cells.10 Recent evidence suggests that both IDO2 and TDO2 could also be involved in immunomodulation via their downstream metabolites. Kynurenine, produced after TDO2 activation, has been shown to suppress antitumor immune responses and to promote tumor-cell survival.41 Both IDO1 and TDO2 are able to block T cell proliferation.42
In conclusion, we identified here a new link between obesity and tryptophan metabolism in mice, despite its discrepancies with human metabolism. The dysregulation of tryptophan degrading enzymes in human and mouse obesity point out the kynurenine pathway as a new potential target for the prevention and treatment of metabolic diseases.
Acknowledgements
We thank Isabelle Wolowczuk and Stéphanie Lucas for helpful discussion and Solenne Taront for technical help.
Footnotes
Author Contributions
Conceived and designed the experiments: OPG, EE, DA, AL, BH. Analyzed the data: OPG, EE, DA, AL, BH. Wrote the first draft of the manuscript: OPG. Contributed to the writing of the manuscript: DA, GJG, OPG, PF. Agree with manuscript results and conclusions: EE, BH, AL, OPG, GJG, DA, PF. Jointly developed the structure and arguments for the paper: DA, OPG. Made critical revisions and approved final version: OPG, GJG, DA, PF. All authors reviewed and approved of the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
Funding
We thank the “Conseil Régional du Nord pas de Calais” (CPER 2007–2013) for its financial support.
References
- 1.Harford KA, Reynolds CM, McGillicuddy FC, Roche HM. Fats, inflammation and insulin resistance: insights to the role of macrophage and T-cell accumulation in adipose tissue. Proc Nutr Soc. 2011;70(4):408–17. doi: 10.1017/S0029665111000565. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Fox CS, Hickson DA, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab. 2010;95(12):5419–26. doi: 10.1210/jc.2010-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolowczuk I, Hennart B, Leloire A, et al. Tryptophan metabolism activation by indoleamine 2,3-dioxygenase in adipose tissue of obese women: an attempt to maintain immune homeostasis and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2012;303(2):R135–43. doi: 10.1152/ajpregu.00373.2011. [DOI] [PubMed] [Google Scholar]
- 4.Takikawa O, Yoshida R, Kido R, Hayaishi O. Tryptophan degradation in mice initiated by indoleamine 2,3-dioxygenase. J Biol Chem. 1986;261(8):3648–53. [PubMed] [Google Scholar]
- 5.Oxenkrug GF. Metabolic syndrome, age-associated neuroendocrine disorders, and dysregulation of tryptophan-kynurenine metabolism. Ann N Y Acad Sci. 2010;1199:1–14. doi: 10.1111/j.1749-6632.2009.05356.x. [DOI] [PubMed] [Google Scholar]
- 6.Ball H, Yuasa H, Austin C, Weiser S, Hunt N. Indoleamine 2,3-dioxygenase-2; a new enzyme in the kynurenine pathway. Int J Biochem Cell Biol. 2009;41:467–71. doi: 10.1016/j.biocel.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Niimi S, Nakamura T, Nawa K, Ichihara A. Hormonal regulation of translatable mRNA of tryptophan 2,3-dioxygenase in primary cultures of adult rat hepatocytes. J Biochem. 1983;94(5):1697–706. [PubMed] [Google Scholar]
- 8.Brandacher G, Winkler C, Aigner F, et al. Bariatric surgery cannot prevent tryptophan depletion due to chronic immune activation in morbidly obese patients. Obes Surg. 2006;16:541–8. doi: 10.1381/096089206776945066. [DOI] [PubMed] [Google Scholar]
- 9.Romani L, Fallarino F, De Luca A, et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451(7175):211–5. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 10.Baban B, Chandler PR, Sharma MD, et al. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009;183(4):2475–83. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Favre D, Mold J, Hunt PW, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2(32):32ra36. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poulain-Godefroy O, Lecoeur C, Pattou F, Fruhbeck G, Froguel P. Inflammation is associated with a decrease of lipogenic factors in omental fat in women. Am J Physiol Regul Integr Comp Physiol. 2008;295(1):R1–7. doi: 10.1152/ajpregu.00926.2007. [DOI] [PubMed] [Google Scholar]
- 13.Ilan Y, Maron R, Tukpah AM, et al. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci U S A. 2010;107(21):9765–70. doi: 10.1073/pnas.0908771107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross R, Aru J, Freeman J, Hudson R, Janssen I. Abdominal adiposity and insulin resistance in obese men. Am J Physiol Endocrinol Metab. 2002;282(3):E657–63. doi: 10.1152/ajpendo.00469.2001. [DOI] [PubMed] [Google Scholar]
- 15.Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr Res Rev. 2010;23(2):270–99. doi: 10.1017/S0954422410000168. [DOI] [PubMed] [Google Scholar]
- 16.Lindstrom P. The physiology of obese-hyperglycemic mice [ob/ob mice] ScientificWorldJournal. 2007;7:666–85. doi: 10.1100/tsw.2007.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kintscher U, Hartge M, Hess K, et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28(7):1304–10. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- 18.Peng Y, Rideout D, Rakita S, Lee J, Murr M. Diet-induced obesity associated with steatosis, oxidative stress, and inflammation in liver. Surg Obes Relat Dis. 2012;8(1):73–81. doi: 10.1016/j.soard.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 19.Yamada K, Miyazaki T, Shibata T, Hara N, Tsuchiya M. Simultaneous measurement of tryptophan and related compounds by liquid chromatography/electrospray ionization tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;867(1):57–61. doi: 10.1016/j.jchromb.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Schefold JC, Zeden JP, Fotopoulou C, et al. Increased indoleamine 2,3-dioxygenase (IDO) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: a possible link between chronic inflammation and uraemic symptoms. Nephrol Dial Transplant. 2009;24(6):1901–8. doi: 10.1093/ndt/gfn739. [DOI] [PubMed] [Google Scholar]
- 21.Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116(1):115–24. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomlinson JW, Walker EA, Bujalska IJ, et al. 11beta-hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr Rev. 2004;25(5):831–66. doi: 10.1210/er.2003-0031. [DOI] [PubMed] [Google Scholar]
- 23.Morton NM. Obesity and corticosteroids: 11beta-hydroxysteroid type 1 as a cause and therapeutic target in metabolic disease. Mol Cell Endocrinol. 2010;316(2):154–64. doi: 10.1016/j.mce.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 24.Salter M, Pogson CI. The role of tryptophan 2,3-dioxygenase in the hormonal control of tryptophan metabolism in isolated rat liver cells. Effects of glucocorticoids and experimental diabetes. Biochem J. 1985;229(2):499–504. doi: 10.1042/bj2290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staab CA, Maser E. 11beta-Hydroxysteroid dehydrogenase type 1 is an important regulator at the interface of obesity and inflammation. J Steroid Biochem Mol Biol. 2010;119(1–2):56–72. doi: 10.1016/j.jsbmb.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 26.Wake DJ, Walker BR. Inhibition of 11beta-hydroxysteroid dehydrogenase type 1 in obesity. Endocrine. 2006;29(1):101–8. doi: 10.1385/ENDO:29:1:101. [DOI] [PubMed] [Google Scholar]
- 27.Morton NM, Ramage L, Seckl JR. Down-regulation of adipose 11beta-hydroxysteroid dehydrogenase type 1 by high-fat feeding in mice: a potential adaptive mechanism counteracting metabolic disease. Endocrinology. 2004;145(6):2707–12. doi: 10.1210/en.2003-1674. [DOI] [PubMed] [Google Scholar]
- 28.Danesch U, Gloss B, Schmid W, Schutz G, Schule R, Renkawitz R. Glucocorticoid induction of the rat tryptophan oxygenase gene is mediated by two widely separated glucocorticoid-responsive elements. EMBO J. 1987;6(3):625–30. doi: 10.1002/j.1460-2075.1987.tb04800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura T, Niimi S, Nawa K, et al. Multihormonal regulation of transcription of the tryptophan 2,3-dioxygenase gene in primary cultures of adult rat hepatocytes with special reference to the presence of a transcriptional protein mediating the action of glucocorticoids. J Biol Chem. 1987;262(2):727–33. [PubMed] [Google Scholar]
- 30.Li LO, Hu YF, Wang L, Mitchell M, Berger A, Coleman RA. Early hepatic insulin resistance in mice: a metabolomics analysis. Mol Endocrinol. 2010;24(3):657–66. doi: 10.1210/me.2009-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allegri G, Bertazzo A, Biasiolo M, Costa CV, Ragazzi E. Kynurenine pathway enzymes in different species of animals. Adv Exp Med Biol. 2003;527:455–63. doi: 10.1007/978-1-4615-0135-0_53. [DOI] [PubMed] [Google Scholar]
- 32.Badawy AA, Evans M. The role of free serum tryptophan in the biphasic effect of acute ethanol administration on the concentrations of rat brain tryptophan, 5-hydroxytryptamine and 5-hydroxyindol-3-ylacetic acid. Biochem J. 1976;160(2):315–24. doi: 10.1042/bj1600315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monroe C. Induction of tryptophan oxygenase and tyrosine aminotransferase in mice. Am J Physiol. 1968;214:1410–4. doi: 10.1152/ajplegacy.1968.214.6.1410. [DOI] [PubMed] [Google Scholar]
- 34.Cheng S, Rhee EP, Larson MG, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation. 2012;125(18):2222–31. doi: 10.1161/CIRCULATIONAHA.111.067827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toye AA, Dumas ME, Blancher C, et al. Subtle metabolic and liver gene transcriptional changes underlie diet-induced fatty liver susceptibility in insulin-resistant mice. Diabetologia. 2007;50(9):1867–79. doi: 10.1007/s00125-007-0738-5. [DOI] [PubMed] [Google Scholar]
- 36.Badawi A, Morgan C, Davis N, Dacey A. High-fat diets increase tryptophan availability to the brain: importance of choice of the control diet. Biochem J. 1983;217:863–4. doi: 10.1042/bj2170863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nocito A, Dahm F, Jochum W, et al. Serotonin mediates oxidative stress and mitochondrial toxicity in a murine model of nonalcoholic steatohepatitis. Gastroenterology. 2007;133(2):608–18. doi: 10.1053/j.gastro.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 38.Osawa Y, Kanamori H, Seki E, et al. L-tryptophan-mediated enhancement of susceptibility to nonalcoholic fatty liver disease is dependent on the mammalian target of rapamycin. J Biol Chem. 2011;286(40):34800–8. doi: 10.1074/jbc.M111.235473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117(5):1147–54. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fallarino F, Grohmann U. Using an ancient tool for igniting and propagating immune tolerance: IDO as an inducer and amplifier of regulatory T cell functions. Curr Med Chem. 2011;18(15):2215–21. doi: 10.2174/092986711795656027. [DOI] [PubMed] [Google Scholar]
- 41.Opitz CA, Litzenburger UM, Sahm F, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478(7368):197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 42.Qian F, Liao J, Villella J, et al. Effects of 1-methyltryptophan stereoisomers on IDO2 enzyme activity and IDO2-mediated arrest of human T cell proliferation. Cancer Immunol Immunother. 2012;61(11):2013–20. doi: 10.1007/s00262-012-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]