Abstract
Objective
To evaluate the association between the use of medications potentially containing phthalates and urinary concentrations of specific phthalate metabolites around conception.
Methods
Women enrolled in the Environment and Reproductive Health project from 2006 to 2009 completed questionnaires about the use of medications and provided multiple urine samples before and after conception. We compared the mean urinary concentration of phthalate metabolites between users of phthalate containing medications and a matched unexposed control group.
Results
One woman used Asacol® (mesalamine), which utilizes dibutyl phthalate (DBP) as a delayed release coating material, and had a mean urinary concentration of the main DBP metabolite 200 times higher than the controls (8176 μg/L vs. 37.5 μg/L). The three users of stool softeners had a higher concentration of the main diethyl phthalate (DEP) metabolite (8636 μg/L vs. 714.2 μg/L). Neither the three additional Prilosec® (omeprazole) users nor one cyclobenzaprine user had higher urinary concentration than controls.
Conclusion
Selected medications may be important sources of DBP and DEP exposures around conception.
Keywords: Phthalate, Toxicology, Medications, Mesalamine, Male reproductive system
1. Introduction
Phthalates, diesters of 1,2-benzenedicarboxylic acid (phthalic acid), are a group of synthetic chemicals widely used in various kinds of industrial and commercial products, such as plasticizers in polyvinyl chloride plastics, food packaging, and coatings for oral medications. [1] Data from the National Health and Nutrition Examination Survey (NHANES) shows that most of the U.S. population is exposed to phthalates. [2] In experimental animals, some phthalates are developmental and reproductive toxicants. [3] Exposure of pregnant rats to dibutyl phthalate (DBP) decreased fetal testis testosterone and insulin-like factor 3 biosynthesis by Leydig cells and led to an increased prevalence of cryptorchidism and hypospadias among male offspring. [4–6] Decreased anogenital distance, a marker of feminization of the perineum, has been noted among male rats exposed prenatally, as well as among human male infants with prenatal exposure to some phthalates. [7,8] Although the evidence of potential effects of prenatal exposure to phthalates in humans is limited, there are increasing concerns regarding potential sources of exposure to phthalates in daily life, especially for women of childbearing age.
Low-molecular-weight phthalates (e.g., diethyl phthalate [DEP] and DBP) are used to make coatings for some oral medications, including those designed for timed release or release in the large bowel. [1] In a previous case report, one man who used Asacol (mesalamine with enteric coating of DBP) for ulcerative colitis was found to have a urinary concentration of monobutyl phthalate (MBP), the main DBP metabolite, more than two orders of magnitude higher than the 95th percentile reported for NHANES in 1999–2000. [9] Since then, the association between use of phthalate-containing medications and urinary concentrations of phthalate metabolites has been reported for other pharmaceuticals and other specific phthalates. [10–12] However, the role of medications as sources of phthalate exposure during fetal development has not been evaluated. In this study, we assessed the association between use of phthalate-containing medications and urinary concentrations of phthalate metabolites in the periconceptional period.
2. Materials and methods
2.1. Subjects
The Environment and Reproductive Health (EARTH) study is an ongoing study that explores the relationship of synthetic chemicals, such as phthalates, with male and female fertility and pregnancy outcomes. The EARTH study population includes adult men and women seeking evaluation and treatment for infertility at the Vincent Memorial Obstetrics and Gynecology Service, Massachusetts General Hospital (MGH), Boston, MA. The present report used data from female participants in the EARTH study that were recruited between September 2006 and May 2009. The study was approved by the institutional review boards (IRB) of MGH, Harvard School of Public Health and the U.S. Centers for Disease Control and Prevention (CDC).
2.2. Medication use assessment and urine sample collection
Study participants completed questionnaires on recent medication use and provided urine samples at recruitment and at each treatment cycle, up to twelve cycles. When the women became pregnant, they completed questionnaires and provided urine samples during follow-up visits in trimesters 1, 2 and 3. Specific brand and generic drug product names from both prescription drugs and over-the-counter (OTC) medications were recorded when possible. Standard generic names were used if the specific brand name of a product could not be identified (e.g., OTC store brand laxative). Urine samples were considered to be taken from ‘exposed’ participants when collected between the start and stop dates for the specific medication, i.e., exposure status could vary between samples within an individual. The number of hours from last ingestion of the medication to urine collection was not available.
2.3. Exposures definition
Using publicly available sources, we a priori identified medications that may contain phthalates as inactive ingredients. For the current study, we focused on the medications with formulations found to contain DEP and DBP, and their corresponding main urinary metabolites monoethyl phthalate (MEP) and MBP, respectively. Because only some specific brands and dosage forms of a medication with a given active ingredient might contain phthalates, we attempted to identify the specific brand names in the questionnaires. If this could not be achieved, we selected medications for which phthalate-containing formulation(s) were likely to account for a high proportion of use for the specific active ingredient. However, if there were many similar medications but only few brands contained phthalates (e.g., only some brands of some antihypertensives contain phthalates), we excluded them from our exposure group. From the list of 32 medications with brand names that may contain DBP or DEP, we identified 4 drugs among those reported by our study subjects: mesalamine tablets [contains DBP in Asacol (Procter & Gamble Pharmaceuticals, Cincinnati, OH)], stool softeners/laxatives [might contain DEP and DBP in some bisacodyl products], cyclobenzaprine [contains DBP in Amrix capsules (ECR Pharmaceuticals, Richmond, VA)] and omeprazole [generic capsule formulations possibly contained DEP during the study period, and other phthalates were used in Prilosec capsules (AstraZeneca, Wilmington, DE) and generics]. In addition, participants taking Canasa (rectal suppository; Axcan, Birmingham, AL) were identified as an active control for Asacol users because both commercial formulations are mesalamine products used for similar indications but Canasa does not contain DBP.
2.4. Outcome definition
Urine samples were analyzed at the CDC (Atlanta, GA, USA) for 9 phthalate metabolites: MEP, MBP, monobenzyl phthalate (MBzP), mono(3-carboxypropyl) phthalate (MCPP), monoisobutyl phthalate (MiBP), mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-oxoxyhexyl) phthalate (MEOHP), and mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP). The analytical approach involved enzymatic deconjugation of the metabolites from their glucuronidated form, solid-phase extraction, separation with high performance liquid chromatography, and detection by isotope-dilution tandem mass spectrometry. [13,14] Detection limits were in the low nanogram per milliliter range and, for each phthalate metabolite, varied slightly depending on the analytical method used. [13,14] Isotopically labeled internal standards and conjugated internal standards were used to increase precision of measurements. Along with the unknown samples, each analytical run included calibration standards, reagent blanks, and quality control (QC) materials of high and low concentration to monitor for accuracy and precision. The QC concentrations, averaged to obtain one measurement of high-concentration QC and one of low-concentration QC for each batch, were evaluated using standard statistical probability rules. [15] Analysts at the CDC were blind to all information concerning the study participants. Urine specific gravity was also measured to adjust for urinary dilution (). SG was measured at MGH, using a handheld refractometer (National Instrument Company, Inc., Baltimore, MD, USA).
2.5. Statistical analyses
We compared the urinary concentrations of each specific phthalate metabolite among users and nonusers of these medications. Each woman exposed to any of the 4 DBP or DEP containing drugs was matched on calendar time at enrollment to 2 controls who did not report using any of the 32 possibly phthalate containing medication. Subjects exposed to any medication potentially containing any phthalate were ineligible as controls to establish a truly unexposed reference group. Because each subject may have provided several urine samples during the study period, the samples were not independent and repeated measures analysis were applied for the comparison. All analyses were performed using Statistical Analysis Software for Windows, version V.9.2 (SAS Institute Inc., Cary, NC).
3. Results
From September 2006 to May 2009, 426 women between 18 and 45 years old were enrolled in the study. Of them, 317 reported using one or more medications during the study period. From this group, we identified 8 participants exposed to medications of interest and selected 15 matched controls.
As shown in Table 1 and Fig. 1, one woman who provided six urine samples over eight months reported chronic use of Asacol. The MBP concentrations adjusted by urine specific gravity were 756, 36,462, and 1363 μg/L before conception; and 3748, 2912, and 3816 μg/L after conception. The mean MBP concentration for this Asacol user was 8176 μg/L, more than 200 times higher than the mean for (i) the control group (38 μg/L), (ii) a Canasa user who provided 2 urine samples (58 μg/L), and (iii) the total NHANES population 1999–2004 (46.1 μg/L). [10] Although the mean concentration was highly influenced by the second value (36,462 μg/L), it would have been orders of magnitude higher than expected even without this sample (i.e., mean 2519). The mean concentration of MCPP, a minor metabolite of DBP [16,17], in this Asacol user (111 μg/L) was about 10 times higher compared to both the control group (14 μg/L) and the Canasa user (9 μg/L).
Table 1.
Mean urinary concentrations of select phthalate metabolites (μg/L) among pregnant women who enrolled in the EARTH study during 2006–2009.
| Metabolite | Control | Asacol | Canasa | Stool softener | Cyclobenzaprine | Omeprazole |
|---|---|---|---|---|---|---|
| No. of women | 14 | 1 | 1 | 3 | 1 | 5 |
| No. of samples | 63 | 6 | 2 | 8 | 1 | 16 |
| MBP Mean | 38 | 8176* | 58 | 44 | 29 | 35a |
| MCPP Mean | 14 | 111* | 9 | 5 | 50 | 5.9a |
| MEP Mean | 714 | 183 | 766 | 8636* | 399 | 272b |
p-value from repeated measures analysis < 0.001.
Excluding 6 urine samples from one woman with concurrent Asacol use, who had high MBP and MCPP concentrations.
Excluding 6 urine samples from one subject with stool softener use.
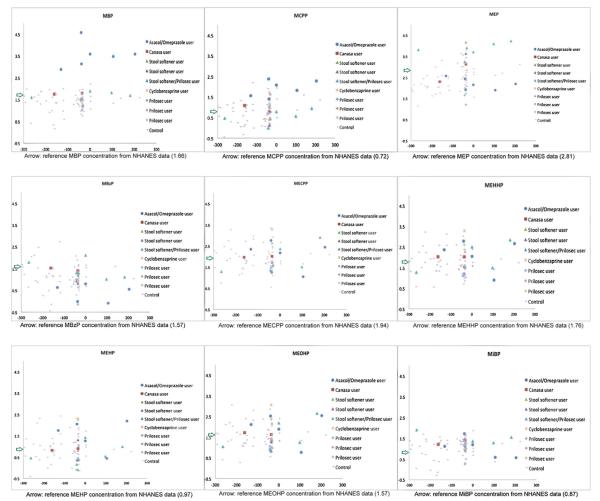
Fig. 1.
Distribution of phthalate metabolites concentrations compared to NHANES, National Health and Nutrition Examination Survey for the years 1999–2004. X-axis: estimated day to pregnancy; Y-axis: SG-adjusted phthalate concentrations (ug/L in log10 scale). In the study subjects who became pregnant during the study period, day to pregnancy was the interval between the date of urine sample and the date of pregnancy onset. For subjects who had not conceived at the time of this report (N = 17), their days to pregnancy were estimated using the average interval between the last urine sample before pregnancy and date of pregnancy in the pregnant subjects.
For those using stool softeners, 3 women provided a total of 8 urine samples. The mean SG-adjusted MBP concentration (44 μg/L) was not different from the control group (38 μg/L). However, the mean SG-adjusted MEP concentration (8636 μg/L) was 10 times higher than the corresponding means among both controls (714 μg/L) and NHANES (653 μg/L) references. [10] The SG-adjusted MEP concentrations were 1759, 7880, 6480, 5008, 12,444, 16,800, 14,533 and 4183 μg/L. Of note, 6 of these 8 samples came from a woman who was also using Prilosec (omeprazole). When the woman with concurrent omeprazole use was excluded, the mean MEP SG-adjusted concentration for the remaining 2 women was 9358 μg/L.
The user of cyclobenzaprine had a similar urinary concentration of MEP (399 μg/L) than controls (714 μg/L), based on only one urine sample obtained from this woman.
For omeprazole, 5 women who provided 16 urine samples were identified. One woman reported using omeprazole concurrently with Asacol use, three subjects specifically reported Prilosec use, and one reported concomitant Prilosec and stool softener use. The mean MEP SG-adjusted concentration (3318 μg/L) for omeprazole users was higher than the mean for the control group (714 μg/L). However, only the woman with concurrent stool softener use had MEP SG-adjusted urinary concentrations higher than the controls; when she was excluded the mean MEP concentration for the remaining 4 women was 272 μg/L, i.e., lower than the control group.
4. Discussion
Using data collected from the ongoing EARTH project, this study adds evidence to previous findings in women of childbearing age that urinary concentrations of MBP and MCPP are higher among Asacol users than non-users. [10,18] Of note, MBP urinary concentrations were higher than in the control group both in samples taken before and after conception. Because Asacol is used to treat inflammatory bowel disease, these highly elevated MBP concentrations could be related to the altered absorption of phthalates secondary to the underlying inflammatory bowel disease. However, in the current study, the woman who used Canasa for her inflammatory bowel disease had urinary concentrations of MBP similar to NHANES background levels, suggesting that the relatively high urine MBP concentrations in the Asacol users resulted from the coating on the medication and not from the underlying condition. This study is the first to provide evidence that the routine use of Asacol specifically in pregnant women could lead to much-higher than background exposures of DBP, a known experimental animal reproductive toxicant.
We had hypothesized that some stool softeners/laxatives might result in elevated MEP (stool softeners) or MBP (some bisacodyl containing laxatives) levels. We found that urinary MEP concentrations were 10 times higher among stool softeners users than among controls. The lack of association with MBP urinary concentrations might be explained by study limitations. First, there is uncertainty about which products were taken because women did not report the specific brand names. The terms stool softeners and laxatives are used interchangeably by the general public, and we therefore were concerned that subjects who reported “stool softeners” might have taken bisacodyl, a common ingredient in laxatives, some of which are coated with DBP. This concern might have been inappropriate. Second, there is uncertainty about which phthalates, if any, are contained in these products. Inactive ingredients are not well-described and sometimes may not be listed in product information. Some stool softener brands may contain DEP or DBP, other phthalates, or none, and there is uncertainty about which phthalates, if any, are contained in these products. Since stool softeners are widely used to relieve constipation, a particularly common event during pregnancy, it is important to evaluate whether they represent potential sources of exposures to phthalates.
One pregnant woman concomitantly using stool softeners and Prilosec (specific brand) had higher urinary MEP concentrations than the control group. The other three Prilosec users did not. Therefore, it is not clear whether the use of certain formulations of omeprazole increased the urinary MEP concentrations in the former subject, or whether it was the stool softener. For the latter subjects, our results suggest that either the omeprazole formulations did not contain DEP or the urine samples were collected too many hours after last dose and, because DEP has a short elimination half life (i.e., hours), urinary MEP was no longer elevated. [11]
We had a single urine sample from one cyclobenzaprine user and the urinary MEP concentration was not higher than in controls. In our list, among the cyclobenzaprine formulations, only Amrix® contains DEP in the US and the woman might have used a different brand, such as Flexeril®, Fexmid®, or a generic product. Alternatively, the interval between the time of cyclobenzaprine intake and the collection of the urine sample might have been long enough for MEP to be eliminated from the body.
Our study has some limitations. Although we matched the drug use with the urine sample on the same day, the specific time of drug intake was not available. As noted above, if the intervals between drug intake and the sample collection were long enough, the urinary concentrations of phthalates would be expected to return rapidly to background levels because of rapid elimination. [1,11,12] Therefore, we could be underestimating the levels attained shortly after medication use. On the other hand, given the small sample sizes and inability to exclude other sources, detected phthalate concentrations may not be related to drug exposure. Despite these limitations, it is of note that we observed higher urinary concentrations of MBP, MCPP and MEP among Asacol (n = 1) and stool softeners (n = 3) users than in controls, indicating these medications may be important exposure sources.
5. Conclusions
Urinary phthalate concentrations among users of medications such as Asacol can, by orders of magnitude, far exceed population levels derived from various other sources in pregnant women. Further exploration of potential toxic effects of phthalate-containing medications for the fetus is needed because of the potential for high delivered doses of phthalates during embryogenesis [19].
Acknowledgements
We thank Manori Silva, Ella Samandar, and Jim Preau for measuring the urinary concentrations of phthalate metabolites.
Funding National Institute of Environmental Health Sciences (NIEHS) (grant numbers: ES009718 and ES00002) and by the National Institute of Child Health and Human Development (grant number RO1HD059861)
Footnotes
Disclaimer The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the U.S. Centers for Disease Control and Prevention. The Pharmacoepidemiology Program at the Harvard School of Public Health is supported by training grants from Pfizer and ASISA. The Slone Epidemiology Center receives unrelated research support from various pharmaceutical manufacturers, some of which may market products containing phthalates.
References
- [1].Hauser R, Calafat AM. Phthalates and human health. Occupational and Environmental Medicine. 2005;62:806–18. doi: 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].CDC . Fourth National Report on Human Exposure to Environmental Chemicals. Centers for Disease Control and Prevention; National Center for Environmental Health; Division of Laboratory Sciences; 2010. Updated Tables JA, GA. http://www.cdc.gov/exposurereport/ [Google Scholar]
- [3].Saillenfait AM, Payan JP, Fabry JP, Beydon D, Langonne I, Gallissot F, et al. Assessment of the developmental toxicity, metabolism, and placental transfer of Di-n-butyl phthalate administered to pregnant rats. Toxicological Sciences. 1998;45:212–24. doi: 10.1006/toxs.1998.2518. [DOI] [PubMed] [Google Scholar]
- [4].Vo TT, Jung EM, Dang VH, Yoo YM, Choi KC, Yu FH, et al. Di-(2 ethylhexyl) phthalate and flutamide alter gene expression in the testis of immature male rats. Reproductive Biology and Endocrinology. 2009;7:104. doi: 10.1186/1477-7827-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gray LE, Jr, Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicological Sciences. 2000;58:350–65. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- [6].Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, et al. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicological Sciences. 2000;58:339–49. doi: 10.1093/toxsci/58.2.339. [DOI] [PubMed] [Google Scholar]
- [7].Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environmental Health Perspectives. 2005;113:1056–61. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hotchkiss AK, Parks-Saldutti LG, Ostby JS, Lambright C, Furr J, Vandenbergh JG, et al. A mixture of the “antiandrogens” linuron and butyl benzyl phthalate alters sexual differentiation of the male rat in a cumulative fashion. Biology of Reproduction. 2004;71:1852–61. doi: 10.1095/biolreprod.104.031674. [DOI] [PubMed] [Google Scholar]
- [9].Hauser R, Duty S, Godfrey-Bailey L, Calafat AM. Medications as a source of human exposure to phthalates. Environmental Health Perspectives. 2004;112:751–3. doi: 10.1289/ehp.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hernandez-Diaz S, Mitchell AA, Kelley KE, Calafat AM, Hauser R. Medications as a potential source of exposure to phthalates in the U.S. population. Environmental Health Perspectives. 2009;117:185–9. doi: 10.1289/ehp.11766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Koch H, Drexler MJ, Angerer HJ. Dibutylphthalate (DBP) in medications: are pregnant women and infants at risk? Umweltmed Forsch Prax. 2005;10:144–6. [Google Scholar]
- [12].Seckin E, Fromme H, Volkel W. Determination of total and free mono-n-butyl phthalate in human urine samples after medication of a di-n-butyl phthalate containing capsule. Toxicology Letters. 2009;188:33–7. doi: 10.1016/j.toxlet.2009.03.002. [DOI] [PubMed] [Google Scholar]
- [13].Silva MJ, Samandar E, Preau JL, Jr, Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences. 2007;860:106–12. doi: 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- [14].Kato K, Silva MJ, Needham LL, Calafat AM. Determination of 16 phthalate metabolites in urine using automated sample preparation and on-line preconcentration/high-performance liquid chromatography/tandem mass spectrometry. Analytical Chemistry. 2005;77:2985–91. doi: 10.1021/ac0481248. [DOI] [PubMed] [Google Scholar]
- [15].Caudill SP, Schleicher RL, Pirkle JL. Multi-rule quality control for the age-related eye disease study. Statistics in Medicine. 2008;27:4094–106. doi: 10.1002/sim.3222. [DOI] [PubMed] [Google Scholar]
- [16].Barr DB, Silva MJ, Kato K, Reidy JA, Malek NA, Hurtz D, et al. Assessing human exposure to phthalates using monoesters and their oxidized metabolites as biomarkers. Environmental Health Perspectives. 2003;111:1148–51. doi: 10.1289/ehp.6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Calafat AM, Silva MJ, Reidy JA, Earl Gray L, Samandar E, Preau JL, et al. Mono-(3-carboxypropyl) phthalate, a metabolite of di-n-octyl phthalate. Journal of Toxicology and Environmental Health Part A. 2006;69:215–27. doi: 10.1080/15287390500227381. [DOI] [PubMed] [Google Scholar]
- [18].Silva MJ, Samandar E, Reidy JA, Hauser R, Needham LL, Calafat AM. Metabolite profiles of di-n-butyl phthalate in humans and rats. Environmental Science and Technology. 2007;41:7576–80. doi: 10.1021/es071142x. [DOI] [PubMed] [Google Scholar]
- [19].Marsee K, Woodruff TJ, Axelrad DA, Calafat AM, Swan SH. Estimated daily phthalate exposures in a population of mothers of male infants exhibiting reduced anogenital distance. Environmental Health Perspectives. 2006;114:805–9. doi: 10.1289/ehp.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]