Abstract
Objectives
The purpose of this study was to investigate the efficacy and safety of 0.1% tacrolimus powder in Oraguard-B for the treatment of patients with symptomatic oral lichen planus (OLP).
Methods
This was a nonrandomized, nonblinded study conducted in the outpatient department. The 20 patients with symptomatic OLP oral lichen planus who were asked to participate in the study were provided with 20-g containers of the study medication. Patients were asked to use the medication over the symptomatic areas three times a day until resolution of the lesion. Patients were recalled to assess the drug response every 15 days.
Results
The duration of treatment ranged from 30 to 183 days, with a mean of 81.8 ± 44.4 days; all 20 patients reported a favourable response to the topical tacrolimus therapy. Eleven patients had complete resolution of their lesions. In 16 of 20 patients, there was marked resolution in symptoms as recorded by visual analogue scale. Out of 10 patients followed up for a period of 3 months, 5 had recurrence of their lesions but with less intensity, and the patients were symptomless. No serious side effects were associated with the study medication.
Conclusion
Topical tacrolimus 0.1% in Oraguard-B was effective and safe in treating patients with OLP. However, there is still a need to undertake more detailed and objective clinical studies to determine the exact benefit of tacrolimus compared with conventional therapies and examine the influence of different dose regimes and formulations and assess the incidence of recurrence.
Keywords: Oral lichen planus, Topical tacrolimus, Oraguard-B
1. Introduction
Oral lichen planus (OLP) is a chronic inflammatory mucocutaneous disorder affecting the stratified squamous epithelium, with a prevalence of 0.02–1.2% among the various populations (Eisen et al., 2005). Among the prevalent group, 15% of patients with predominantly OLP develop cutaneous lesions (Eisen, 2003).
OLP most commonly affects individuals in the 5th and 6th decade of life with a female preponderance. Intraoral presentation of lesions is divided into three forms, namely, reticular, atrophic (erythematous), and erosive (ulcerative and bullous), with the posterior buccal mucosa the most frequently affected site (Eisen, 2003; DeRossi and Ciarrocca, 2005; Sugerman and Savage, 2002). The risk of malignant transformation of OLP is very low but cannot be overlooked (Rajentheran et al., 1999).
Aetiological factors include hepatitis C virus; psychological stress (high levels of anxiety or depression); contact hypersensitivity to dental materials, especially to amalgam; herpes viruses (HSV1, EBV, and HHV6); HIV; HPV; and hepatitis B virus (Pilli et al., 2002; Thornhill et al., 2003). Although the pathogenesis of OLP is chiefly unknown, a large body of evidence supports the role of immune dysregulation. Numerous topical and systemic treatments for OLP have been reported to be effective, including corticosteroids (both topical and systemic), retinoids, ultraviolet phototherapy, steroid sparing agents (hydroxychloroquine, azathioprine, mycophenolate mofetil), and pimecrolimus.
Although the above-mentioned drugs have shown positive results in the treatment of OLP, resistance to treatment and a high risk of toxicities limit their use (Al-Hashimi et al., 2007). Tacrolimus is a newer immunosuppressant that has recently been shown to be effective and safe in the treatment of symptomatic OLP (Kaliakatsou et al., 2002). Tacrolimus is 10–100 times as potent as cyclosporine in its ability to inhibit IL-2 mRNA synthesis, and it inhibits mediator release from basophils and mast cells. It inhibits enzyme calcineurin phosphatase activity, resulting in decreased IL-2 synthesis and secretion, hence inhibiting T cell multiplication (Letko et al., 1999).
Topical tacrolimus has been extensively used in dermatology for the treatment of atopic dermatitis, plaque psoriasis, pyoderma gangrenosum, and generalized erythroderma with good results (Leonardi et al., 2006). With such an insight, this clinical trial was done to assess the safety and efficacy of 0.1% tacrolimus powder in Oraguard-B when treating the lesions of patients with symptomatic OLP.
2. Materials and methods
Approval to conduct the study was given by the Ethics Committee of the Kothiwal Dental College and Research Centre, Moradabad, India, in compliance with the principles of the Declaration of Helsinki. The tacrolimus powder for the study was sponsored by Ranbaxy Pharmaceuticals (Gurgaon, Haryana, India) and the base Oraguard-B was purchased from Colgate Palmolive (New York, NY, USA).
The tacrolimus ointment was made using tacrolimus powder with Oraguard-B as a base. Five hundred milligrams of tacrolimus powder was mixed with 300 g Oraguard-B on a clean glass slab using a stainless steel mixing spatula under aseptic conditions. This preparation was then packed into plastic containers so that each container contained at least 20 g of the medication.
The subjects for the study were selected among the patients attending the outpatient department who fulfilled the inclusion and exclusion criteria. A written consent was obtained from every patient before initiating treatment. The following inclusion and exclusion criteria were designed for the study.
2.1. Inclusion criteria
-
(a)
Clinical evidence of the lesion.
-
(b)
Histopathological confirmation of the disease.
-
(c)
Patients recalcitrant to treatment with other medications or having recurrent lesions (see Table 1).
Table 1.
Past history of treatment.
| Serial no. | Patient’s age/sex | Past history of treatment |
|---|---|---|
| 1 | 45/F | Tab Betnesol 5 mg thrice daily (swish and swallow), Kenacort topical ointment and Tablet Vermisol |
| 2 | 45/F | No past history of treatment |
| 3 | 38/F | Tab Vermisol, Topical Kenacort ointment |
| 4 | 55/F | No past drug history |
| 5 | 49/M | History of antioxidant intake for 15–20 days |
| 6 | 30/F | No past history of treatment |
| 7 | 58/M | Topical Kenacort ointment, Tab Vermisole, Tab Betnesol, Tab Prednisolone |
| 8 | 45/F | Topical Kenacort and Prednisolone |
| 9 | 40/F | Tab Vermisole, Orasep-OT mouth paint. Betnisol 5 mg, Kenacort topical ointment |
| 10 | 22/M | No past history of treatment |
| 11 | 30/M | Topical Kenacort ointment, Tab Vermisol, Tab Prednisolone |
| 12 | 27/M | Topical Kenacort, Mucopain ointment |
| 13 | 42/F | No past history of treatment |
| 14 | 55/F | Orasep-OT mouth paint |
| 15 | 40/M | No past history of treatment |
| 16 | 32/F | Topical Kenacort, Betnesol (swish and swallow) |
| 17 | 44/M | Tab Vermisol, Tab Prednisolone, and topical Kenacort |
| 18 | 30/F | Topical Kenacort ointment |
| 19 | 18/F | No past history of treatment |
| 20 | 24/M | No past history of treatment |
2.2. Exclusion criteria
Patients on medication for other systemic diseases.
2.3. Study intervention
The study preparation was administered for 3–6 months or till the lesions had completely healed, then the patients were instructed to use the medication three times daily after meals.
Detailed information regarding the study protocol and tacrolimus therapy was explained verbally to each subject. Subsequently, informed consent was obtained. A minimum data set (patient age, gender, medical history, and habits) was documented by means of case history proforma. All topical and systemic medication previously prescribed for OLP was stopped at least 2 weeks before the initiation of tacrolimus therapy. Use of routine analgesics was allowed during the course of the study. Subjective assessment was done by means of a visual analogue scale (VAS) for symptoms of pain and burning. The extent of eroded or ulcerated areas was recorded by using a scoring system based on the variation criteria (Table 2). Before the commencement of therapy, baseline subjective and objective assessments were recorded together with blood pressure, complete blood count, liver biochemistry, blood urea, and random blood glucose levels. The patients were instructed to sparingly apply 0.1% tacrolimus powder in Oraguard-B topically over the affected areas after meals and not to eat or rinse for at least 45 min after applying the study preparation.
Table 2.
Clinical scoring.
| Score | Clinical status |
|---|---|
| 0 | No Lesion |
| 1 | White striae only |
| 2 | White striae and erosion <1 cm2 |
| 3 | White striae and erosion >1 cm2 |
| 4 | White striae and ulceration <1 cm2 |
| 5 | White striae and ulceration >1 cm2 |
Adopted from J. Am. Acad. Dermatol. 2002;46:35–41.
Recall assessments were done every 15 days to assess the treatment response.
2.4. Data analysis
As the sample size was small, the distributions were checked for normality using the Kolmogorov–Smirnov test and were found to be asymmetric and abnormal, hence a nonparametric analysis plan was adopted. Changes from pretreatment values for VAS and clinical scoring were compared using the Wilcoxon signed rank test. The confidence level of the study was kept at 95%, hence a P value less than 0.05 indicated a statistically significant change.
3. Results
Twenty patients (13 women and 7 men) were enrolled in the study. Their mean age was 38.25 ± 11.19 (range, 18–58), and the duration of treatment ranged from 30 to 183 days, with a mean of 81.8 ± 44.4 days. Demographic and clinical characteristics of the patients are shown in Table 3. None of the patients withdrew from the study. All 20 patients enrolled for the study were responsive to the topical tacrolimus therapy; 11 patients had complete resolution of the lesions including the reticular component, while 14 had complete healing of the erosive component. Desquamative gingivitis was present in 6 patients, of which 3 showed complete healing. Clinical improvement in the various forms of symptomatic lichen planus has been observed in the study (Figs. 1–4). Relief in pain symptoms recorded by the visual analogue scale showed complete relief of pain (Graph 1).
Table 3.
Demographic and clinical characteristics of the patients.
| Serial no. | Characteristic | Statistic |
|---|---|---|
| 1 | Mean age ± SD (range) in years | 38.25 ± 11.19 (18–58) |
| 2 | Male:female | 7 (35%):13 (65%) |
| 3 | No. of patients with involvement of right buccal mucosa (%) | 17 (85%) |
| 4 | No. of patients with involvement of left buccal mucosa (%) | 17 (85%) |
| 5 | No. of patients with desquamative gingivitis (%) | 6 (30%) |
| 6 | Mean duration of treatment ± SD (range) in days | 81.8 ± 44.4 (30–183) |
Figure 1.
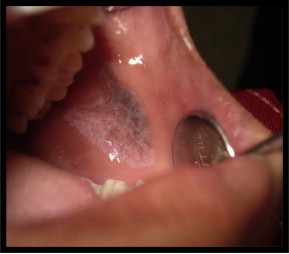
(Pre treatment) papular lichen planus involving left buccal mucosa.
Figure 2.
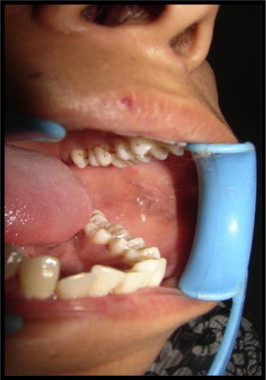
(Post treatment) healed lesions on the left buccal mucosa.
Figure 3.
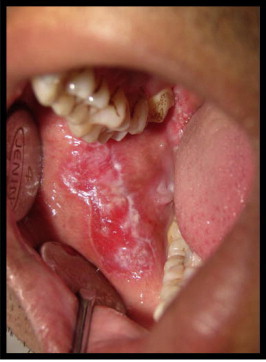
(Pre treatment) erosive lesions over the right buccal mucosa.
Figure 4.
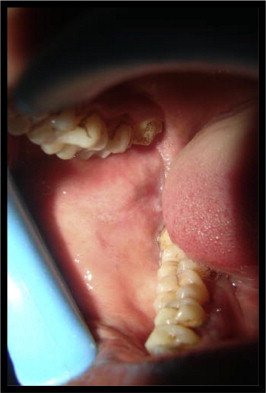
(Post treatment) healed mucosal lesion.
Graph 1.
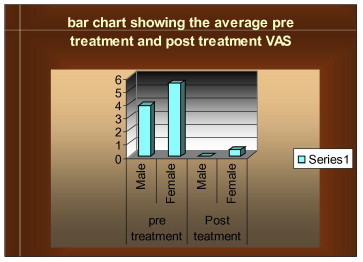
The above bar graph shows the pre-treatment and post-treatment visual analogue scale results in males and females on topical tacrolimus therapy. A significant difference is observed on the bar graph in pre and post treatment results.
No serious adverse effects were reported by any of the patients under study. The only adverse effect reported with the use of tacrolimus therapy was a burning sensation on the first 3–4 applications, which was not so marked as to discontinue use. Moreover, the burning sensation diminished spontaneously on subsequent applications, and it was present in only 5 patients. No patient developed a candidal infection during the tacrolimus therapy. Values of the visual analogue scale, both pre- and posttreatment, for male and female patients in terms of mean ±, standard deviation, and paired t test to test the significant differences in pre- and postobservational scores are listed in Table 4. Statistically, a significant decrease in mean VAS and clinical scores was observed (P < 0.05). The results of this pilot study are encouraging; however, in order to substantiate it further, it can be done in a more detailed and objective manner to determine the exact benefit of tacrolimus compared with conventional therapies and to examine the influence of different dosage regimes and formulations.
Table 4.
VAS and Clinical score in pre and post-treatment patients.
| Serial no. | Parameter | Pre-treatment |
Post-treatment |
Change in values |
Significance of change (Wilcoxon signed rank test) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | z | p | ||
| 1 | VAS score (n = 20) | 4.95 | 2.23 | 0.50 | 1.24 | 4.45 | 2.11 | 3.956 | <0.001 |
| 2 | Clinical score (right buccal mucosa) (n = 17) | 2.71 | 0.89 | 0.59 | 0.94 | 2.12 | 0.99 | 3.564 | <0.001 |
| 3 | Clinical score (left buccal mucosa) (n = 17) | 2.82 | 0.53 | 0.41 | 0.71 | 2.41 | 0.94 | 3.656 | <0.001 |
| 4 | Clinical score (desquamative gingivitis) (n = 7) | 2.71 | 0.49 | 0.43 | 0.79 | 2.29 | 0.84 | 2.414 | 0.016 |
4. Discussion
Topical tacrolimus was found to be safe and effective in all the 20 patients who participated in the study. There was a decrease in overall representation of the lesion, which included the surface area and visual analogue scores. The demographic characteristics of our patients were similar to those patients previously reported (Lozada-Nur and Sroussi, 2006). Moreover, in most of the previously reported studies, the tacrolimus therapy was administered only for erosive or ulcerative OLP (Olivier et al., 2002; Sanchez et al., 2004; Rabnal et al., 2007; Donovan et al., 2005; Rozycki et al., 2002; Morrison et al., 2002).
Few studies have shown the role of tacrolimus therapy in the treatment of symptomatic reticular OLP (Olivier et al., 2002). In our study, 61.1% (13 of 20 patients) complete resolution of erosive, ulcerated, and even the reticular form of OLP was observed. A marked improvement in the symptoms of burning and pain occurred in 80% (16 of 20 patients). Eight of our patients used the study preparation for the first time, and the results were very promising. In 2 of our patients, healing took place with pigmentation. Ten patients were followed up for a period of 3 months. Of these, 5 patients had recurrence of their lesions but the intensity of recurrence (as observed clinically) was mild, and the patients were symptomless. Adverse effects associated with the therapy were mild and transient; they were limited only to a burning sensation. Because of the established safe systemic blood levels of tacrolimus, the tests to measure the blood concentration of tacrolimus were not performed. The reason for fewer adverse effects may be attributed to the fact that compounds having a mass unit greater than approximately 500 Da scarcely penetrate the epidermis or epithelium of normal skin mucosa. Inflamed mucosa, due to increased permeability, allows penetration of molecules of higher molecular weight such as tacrolimus 823 Da. Once the inflammation (and permeability) decreases due to the anti-inflammatory activity of topical tacrolimus, the compound will penetrate the epithelium less when the lesions have improved, thereby limiting the potential side effects of this particular regime (Kaliakatsou et al., 2002). The treatment was not discontinued on any patient for any reason. In our studies, Oraguard-B has been a promising base, showing good mucoadhesive properties through patients’ responses. Since the study was nonblinded and noncomparative, whether topical tacrolimus can be used as the first line of treatment or used in other concentrations need to be assessed.
5. Conclusion
The encouraging results of our pilot prospective study lead us to conclude that topical tacrolimus powder 0.1% in Oraguard-B is a safe and effective treatment of symptomatic OLP. As per our finding, topical 0.1% tacrolimus can be used effectively in patients whose ere lesions are recalcitrant to treatment with topical or systemic medications, especially corticosteroids. However, there is a need of more detailed, randomized, double-blinded clinical study with 1 or 2 other traditional medications to substantiate the findings of this pilot study.
Conflict of interest
Nil.
Footnotes
Peer review under responsibility of King Saud University.
References
- Rabnal Alejandro, Bral Michael, Goldstein Gray. Management of a patient with severe erosive lichen planus in need of an immediate complete denture: a clinical report. J. Prosthet. Dent. 2007;97(5):252–255. doi: 10.1016/j.prosdent.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Al-Hashimi I., Schifter M., Lockhart P.B., Wray D., Brennan M., Migliorati C.A. Oral lichen planus and oral lichenoid lesions: diagnostic and therapeutic considerations. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007;103:S25.e1–S25.e12. doi: 10.1016/j.tripleo.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Sanchez A.R., Sheridan Phillip J., Rogers Roy S. Successful treatment of oral lichen planus-like chronic graft versus host disease with topical tacrolimus: a case report. J. Periodontol. 2004;5(4):613–619. doi: 10.1902/jop.2004.75.4.613. [DOI] [PubMed] [Google Scholar]
- Donovan J.C., Hayes R.C., Burgess K., Leong I.T., Rosen C.F. Refractory erosive oral lichen planus associated with hepatitis C: response to topical tacrolimus ointment. J. Cutan. Med. Surg. 2005;9(2):43–46. doi: 10.1007/s10227-005-0038-y. [DOI] [PubMed] [Google Scholar]
- Eisen D. The clinical manifestations and treatment of oral lichen planus. Dermatol. Clin. 2003;21(1):79–89. doi: 10.1016/s0733-8635(02)00067-0. [DOI] [PubMed] [Google Scholar]
- Eisen D., Carrozzo M., Bagan Sebastian J.V., Thongprasom K. Oral lichen planus: clinical features and management. J. Oral Dis. 2005;11(6):338–349. doi: 10.1111/j.1601-0825.2005.01142.x. [DOI] [PubMed] [Google Scholar]
- Kaliakatsou F., Hodgson T.A., Lewsey J.D., Hegarty A.M., Murphy A.G., Porter S.R. Management of recalcitrant ulcerative oral lichen planus with topical tacrolimus. J. Am. Acad. Dermatol. 2002;46(1):35–41. doi: 10.1067/mjd.2002.120535. [DOI] [PubMed] [Google Scholar]
- Leonardi S., Rotolo N., Marchese G., La Rosa M. Efficacy and safety of tacrolimus ointment 0.03% treatment in a 1-month-old “red baby”: a case report. Allergy Asthma Proc. 2006;27(6):523–526. doi: 10.2500/aap.2006.27.2897. [DOI] [PubMed] [Google Scholar]
- Letko E., Bhol K., Pinar V., Foster C.S., Ahmed A.R. Tacrolimus (FK 506) Ann. Allergy Asthma Immunol. 1999;83(3):179–189. doi: 10.1016/S1081-1206(10)62636-1. [DOI] [PubMed] [Google Scholar]
- Lozada-Nur F.I., Sroussi H.Y. Tacrolimus powder in Orabase 0.1% for the treatment of oral lichen planus and oral lichenoid lesions: an open clinical trial. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006;102(6):744–749. doi: 10.1016/j.tripleo.2006.02.033. [DOI] [PubMed] [Google Scholar]
- Morrison L., Kratochvil F.J., 3rd, Gorman A. An open trial of topical tacrolimus for erosive oral lichen planus. J. Am. Acad. Dermatol. 2002;47(4):617–620. doi: 10.1067/mjd.2002.126275. [DOI] [PubMed] [Google Scholar]
- Olivier V., Lacour J.P., Mousnier A., Garraffo R., Monteil R.A., Ortonne J.P. Treatment of chronic erosive oral lichen planus with low concentrations of topical tacrolimus: an open prospective study. Arch. Dermatol. 2002;138(10):1335–1338. doi: 10.1001/archderm.138.10.1335. [DOI] [PubMed] [Google Scholar]
- Pilli M., Penna A., Zerbini A., Vescovi P., Manfredi M., Negro F., Carrozzo M., Mori C., Giuberti T., Ferrari C., Missale G. Oral lichen planus pathogenesis: a role for the HCV-specific cellular immune response. Hepatology. 2002;36(6):1446–1452. doi: 10.1053/jhep.2002.37199. [DOI] [PubMed] [Google Scholar]
- Rajentheran R., Mc Lean N.R., Kelly C.G., Reed M.F., Nolan A. Malignant transformation of oral lichen planus. Eur. J. Surg. Oncol. 1999;25(5):520–523. doi: 10.1053/ejso.1999.0689. [DOI] [PubMed] [Google Scholar]
- Rozycki T.W., Rogers R.S., Pittelkow M.R., McEvoy M.T., El-Azhary R.A., Bruce A.J., Fiore J.P., Davis M.D. Topical tacrolimus in the treatment of symptomatic oral lichen planus: a series of 13 patients. J. Am. Acad. Dermatol. 2002;46(1):27–34. doi: 10.1067/mjd.2002.119648. [DOI] [PubMed] [Google Scholar]
- DeRossi Scott S., Ciarrocca Katharine N. Lichen planus, lichenoid drug reactions, and lichenoid mucositis. Dent. Clin. N. Am. 2005;49:77–89. doi: 10.1016/j.cden.2004.08.004. e vii. [DOI] [PubMed] [Google Scholar]
- Sugerman P.B., Savage N.W. Oral lichen planus: causes, diagnosis and management. Aust. Dent. J. 2002;47(4):290–297. doi: 10.1111/j.1834-7819.2002.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Thornhill M.H., Pemberton M.N., Simmons R.K. Amalgam-contact hypersensitivity lesions and oral lichen planus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003 Mar;95(3):291–299. doi: 10.1067/moe.2003.115. [DOI] [PubMed] [Google Scholar]
Further reading
- Byrd J.A., Davis M.D., Bruce A.J., Drage L.A., Rogers R.S. Response of oral lichen planus to topical tacrolimus in 37 patients. Arch. Dermatol. 2004;140(12):1508–1512. doi: 10.1001/archderm.140.12.1508. [DOI] [PubMed] [Google Scholar]
- Luger T., Paul C. Potential new indications of topical calcineurin inhibitors. Dermatology. 2007;215(Suppl. 1):45–54. doi: 10.1159/000102119. [DOI] [PubMed] [Google Scholar]



