Abstract
Orbital trauma is one of the most common reasons for ophthalmology specialty consultation in the emergency department setting. We survey the literature from 1990 to present to describe the role of computed tomography (CT), magnetic resonance imaging (MRI) and their associated angiography in some of the most commonly encountered orbital trauma conditions. CT orbit can often detect certain types of foreign bodies, lens dislocation, ruptured globe, choroidal or retinal detachments, or cavernous sinus thrombosis and thus complement a bedside ophthalmic exam that can sometimes be limited in the setting of trauma. CT remains the workhorse for acute orbital trauma owing to its rapidity and ability to delineate bony abnormalities; however MRI remains an important modality in special circumstances such as soft tissue assessment or with organic foreign bodies.
Keywords: Orbital wall fracture, Orbital trauma, Orbital hemorrhage, Orbital foreign body
Introduction
Despite developments and improvements in automobile safety and injury prevention, motor vehicle accidents and sports-related injuries still remain common causes of orbital trauma. Traumatic injury to the eye accounts for 3% of all visits to the emergency department (ED),1 and 40% of monocular blindness is associated with trauma.2 In many academic centers, orbital trauma can account for as much as a fifth of all emergency ophthalmology consults. From 2011 to 2012, orbital trauma comprised 23% of the consultation requests to the University of California in Irvine Medical Center (UCIMC) ED, a Level 1 trauma center. Orbital fractures were seen in 82% of all ophthalmic trauma consultations at UCIMC ED. The remainder of the injuries included ruptured globe, orbit foreign body, eyelid laceration, and hyphema.
Rapid and accurate emergency assessment of trauma to the orbit and globe of the eye is extremely important. The wide range of available imaging modalities presents a risk for delaying diagnosis if, for example, the wrong modality is utilized and/or the findings are misinterpreted.
General principles of imaging in acute orbital trauma
Common modalities for imaging the orbit and eye include radiography, ultrasound (US), MRI and CT. In general, radiography is relatively sensitive to fractures of the orbit, while having low sensitivity for soft tissue injuries. For rapid evaluation of the globe, ultrasound can be advantageous, with exception in suspicion of a ruptured globe since pressure upon the ocular surface may cause further acute eye decompensation, extravasation of intraocular contents, or both. MRI, while having superior ability to differentiate soft tissues, is usually not recommended for initial trauma evaluation, and is contraindicated in cases where suspicion exists for a metallic foreign body.
In general, CT is the primary imaging modality in orbital trauma. The sensitivity of CT for fractures is higher than that of radiography, and three-dimensional reformations after image acquisition can sometimes help to guide subsequent surgical treatment. For orbital trauma, the optimal protocol is thin-sliced CT scan with 1–2 mm cut through the orbit performed with a helical CT. The advantages of the high resolution orbital helical CT over conventional CT include (1) much shorter scanning time (<30 s compared with >5 min with traditional protocol) ,3 (2) reduced motion artifact, (3) much lower radiation exposure, (4) much more sensitive in detecting soft tissue entrapment especially in pediatric patients.4 The following section explores diagnostic imaging in the context of some of the most commonly encountered orbital trauma scenarios.
Orbital wall fractures
Orbital fractures can be classified by the bones involved, or by the direction of the fracture: blow-in vs. blow-out vs. blow-up. Blow-in fractures typically refer to superior displacement of the orbital floor. Blow-up fracture entails superior displacement of the roof into the cranial fossa without involvement of the orbital rim. Blow-out fractures are so named because of the tendency for soft tissue to herniate out of the orbit. An orbital roof blow-out fracture may warrant a neurosurgery consultation for the risks of cerebrospinal fluid leak and brain injury.
The indications for surgical repair of orbital fractures have been controversial. The size of the orbital floor fracture is often utilized as a criterion for repair. Fracture area greater than 1 cm squared or greater than 50% of the orbital floor has been described as indications for repair.5–8 One pitfall of this approach is that even large defect may not cause enophthalmos unless the suspensory ligament supporting the globe is compromised.9 As such, some large fractures treated expectantly may have good results with normal eye position and eye movement. On the other hand, some very small fractures may become problematic due to subtle effects upon the periorbita, extraocular muscles, or both. Some have observed that rounding of the typically flat inferior rectus muscle seen on coronal CT slice implies the loss of ligamentous support and hence higher likelihood of enophthalmos.10,11 Importantly, in the absence of evidence of orbital ligamentous instability, small fractures may sometimes cause orbital restriction and strabismus owing to subsequent fibrosis around the healing site.
An important feature to assess in orbital fractures is the integrity of the inferomedial orbital strut (IOS). The IOS is bony continuation from the maxillary bone to the ethmoid bone (Fig. 1). The anterior IOS gives bony support to the orbit and also serves as an attachment site for the suspensory ligaments. Involvement of the IOS creates a more challenging repair and posts greater risks for globe malposition or eye movement problems if not properly repaired.12
Figure 1.
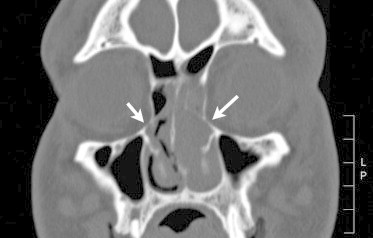
Inferomedial orbital strut. Coronal CT image of a 39-year-old male who presented to the emergency department after motor vehicle accident with left medial and floor orbital fracture. The midportion of his inferomedial orbit strut, which denotes a structural continuity from maxillary bone to ethmoidal bone, remains intact in both sides (arrows). No enophthalmos was seen at six months after the accident.
The recent literature supports one to two weeks of waiting for orbital repair until soft tissue swelling and inflammation subside, unless either muscle impingement or hemodynamically significant oculocardiac reflex is clearly evident.13–15 We recommend orbital surgery after the initial posttraumatic congestion form hemorrhage and/or edema subside since it provides safer and wider surgical views.
Entrapment of soft tissue including recti muscle, fat or connective tissue causes ischemia and can potentially lead to the development of fibrosis, necrosis, strabismus, or permanent extraocular motility.16–18 Entrapment of the inferior rectus muscle is also associated with the oculocardiac reflex especially in pediatric population. This reflex is characterized by a triad of bradycardia, nausea, and syncope. It can potentially lead to fatal arrhythmia if left untreated.19
In acute trauma, soft tissue swelling, fat stranding, intramuscular hematoma, can all render radiologic interpretation of entrapment equivocal. Thus, it is important to keep in mind that muscle entrapment is primarily a clinical rather than radiologic diagnosis. Furthermore, CT of the orbit may sometimes underestimate the size of the fracture.20 A bedside force duction test can assist in diagnosing entrapment; however we caution over-reliance on this test since it can be quite misleading owing to orbital congestion and patient resistance due to discomfort when performed awake. Usually, the diagnosis can be made with a proper eye movement assessment. If there is any suspicion for entrapment, the orbit should be explored in the operating room.
Pediatric orbital fractures
Orbital fractures account for 7–41% of all pediatric facial fractures. They may be seen less commonly than in adults owing to a lower tendency for softer pediatric orbital bones to fracture with bunt force. Children younger than 7 years are more likely to sustain roof fractures, and those older than 7 years are likely to develop orbital floor fractures.21 Such distribution is due to the differences in the craniofacial ratio and the extent of pneumatization of the frontal sinuses in children. Younger children have a larger craniofacial ratio and their sinuses are not yet pneumatized and thus are less elastic. As such, the orbital roof is more susceptible to injury, and associated neurocranial injury can be found as frequently as 36–88% of the time.21,22
Children are also more likely to develop white-eyed blow-out fractures which are so named due to the lack of clinical evidence of soft tissue trauma. The eyes and adnexa can appear deceptively nontoxic even when entrapment is present. In children, the threshold for surgical intervention is much lower than that for adults. The presence of even a small degree of diplopia and restricted eye movement with nausea, vomiting or pain with eye movement is often sufficient to call for urgent intervention.22
Surgical management within 24–48 h is indicated in patients with an extraocular muscle entrapment. Typical clinical features include: white-eyed fractures with minimal edema or ecchymosis, restriction in vertical gaze, pain with eye movement, and nausea or vomiting.16 CT typically shows a linear floor fracture with minimal displacement and little or no soft tissue herniation into the maxillary antrum (Fig. 2). Due to its greater elasticity, pediatric maxillary bone can recoil back to its normal position after fracture and in doing so can entrap soft tissue. Importantly, the CT findings can be minimal and are often misleading. Children with trapdoor fractures were found to have a shorter recovery period if the fractures were repaired 1–5 days post-trauma versus 2 weeks in adults.23
Figure 2.
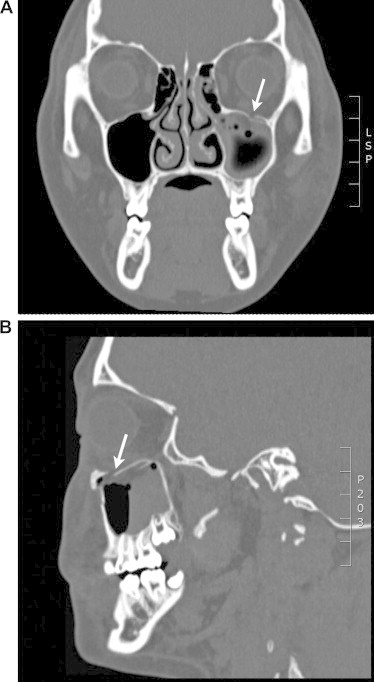
Pediatric trapdoor fracture. This 14-year-old girl presented less than 24 h status post blunt trauma to the left periaucicular area resulting in a trapdoor fracture of left orbital wall with minimal displacement and herniation of orbital contents as seen in coronal view (2A) and sagittal view (2B). Clinical exam demonstrated limited upgaze. She was operated urgently within 12 h. The infraorbital rim fracture was secured with a straight titanium plate, and a nylon foil implant was placed over the fracture site of the orbital floor.
Anterior chamber injuries
Anterior chamber (AC) injuries such as corneal laceration can be visualized on CT images. A key finding on CT of a penetrating corneal laceration is decreased anterior–posterior diameter due to a reduction in fluid volume in the AC.3 In diagnosing corneal laceration, however, subluxation of the lens anteriorly may resemble decreased anterior diameter, and must be ruled out.
Lens injuries
Injury to the lens is most commonly caused by blunt trauma to the orbit and the eye, and cause subluxation of the lens either anteriorly or posteriorly. Because the position of the iris is anterior to the lens, anterior dislocation is less common than posterior dislocation.
Diagnosis of dislocation of the lens can be made clinically, but CT can aid in the diagnosis through visualizing lens placement as well as other injuries associated with trauma. In diagnosing subluxation of the lens, non-traumatic etiologies, such as connective tissue disorders, must be ruled out.
Open globe injuries
Open globe injuries, such as ruptured globe, are a significant cause of blindness and can be diagnosed clinically if intraocular contents are visualized. If intraocular contents are unable to be visualized and rupture is suspected, CT is the imaging modality of choice24, having approximately 75% sensitivity.25 A number of CT findings that may suggest an open globe injury include: loss of globe volume, the presence of intraocular air or foreign body, discontinuity of the sclera, and aberrations in globe contour.24 Several causes of altered globe contour from non-traumatic origin indeed exist, and therefore must be ruled out. Diagnosis of injury to the globe may be complicated in the presence of previous gas bubble, silicon sponge, or scleral band treatment for retinal detachment, which mimic air or foreign bodies. Scleral buckle and silicone oil both have similar Hounsfield unit with blood on CT imaging and can be easily confused with hemorrhage. Gas in the globe can be a normal finding if a history of recent pneumatic retinopexy, recent intraocular surgeries involving air-fluid exchange or Descemet stripping endothelial keratoplasty is elicited.
Extraocular muscle injuries
Extraocular muscles can be entrapped, avulsed, or lacerated from the trauma. Medial and inferior recti muscles are most commonly involved in the setting of orbital fracture.27 The muscle injuries are often located at or near their tendon insertion.27 These events are often accompanied by intramuscular hematoma development which, due to the expansion, can exacerbate ischemia caused by an already entrapped muscle.28 To our knowledge, there are no comparative studies evaluating the sensitivity of CT versus MRI in detecting extraocular muscle injuries. However, several case reports suggest that MRI appears superior in detecting muscle contour irregularity from a lacerated muscle. These reports demonstrate muscle changes missed on the initial CT such as muscle disinsertion, that were later detected on MRI.28,29 A lacerated or avulsed muscle typically presents with weakness or no movement in the cardinal direction of the involved muscle, and this can sometimes help in distinguishing diplopia caused by an entrapped muscle. If diplopia within the central 30° or persistent diplopia especially in a minimally displaced fracture with no evidence of entrapment, diagnosis of muscle injury should be considered. While CT has greater than 70% sensitivity in detecting muscle entrapment, its sensitivity in detecting muscle laceration or intramuscular injury is much lower,29 and an MRI should be ordered for suspicion of these issues.
Foreign bodies
Intraorbital foreign bodies can be imaged well through CT, which remains the most sensitive study and should be the first imaging modality performed. MR may be used, but only after the presence of a metallic foreign body is ruled out definitively. The most common inorganic foreign bodies involved are glass and metals. The consensus in most of the ophthalmic literature is that with the exception of pure copper, lead, and possibly large iron foreign bodies lodged in the vicinity of the sclera, in the absence of signs or symptoms, metallic foreign bodies should be managed conservatively and are generally well tolerated. Indications for surgical removal include neurologic compromise, mechanical restriction of ocular movements, development of acute or chronic infection, or chronic suppurative reaction such as with copper foreign bodies. Removal of foreign bodies located close to the apex is also generally discouraged given the risk of collateral damage far outweighs the benefit.30
Detecting organic foreign bodies remains challenging. On MRI, dry wood is typically hypointense to fat on both T1- and T2-weighted studies because of its high air content and is seen as a dark cylinder, oval, or circle depending on the plane of section. On the other hand, green wood, which refers to wood recently harvested and not yet been treated or processed, is typically hypo- or isointense on T1-weighted studies depending on the amount of hydration. Pencil tip, made of graphite, can appear hyperdense on CT scan (Fig. 3). Sometimes the inflammatory and edema surrounding the organic foreign bodies can help clue to their existence. The region around the mass is often hyperintense on T2-weighted studies due to inflammation.
Figure 3.
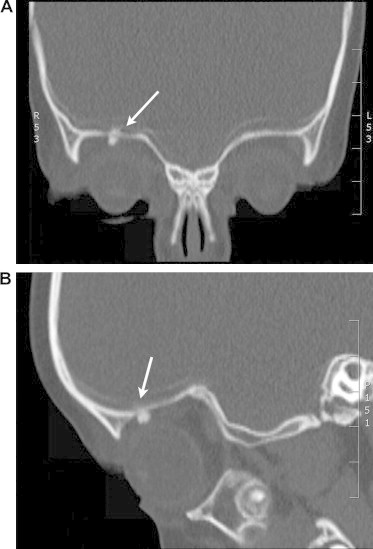
Intraorbital foreign body (crayon). Intraorbital foreign body (crayon) in a two-and-half-year-old girl who fell on a crayon in her hand. CT imaging demonstrated enhancement due to an orbital foreign body (arrow) in the superior orbit adjacent to an orbital roof fracture (3A: coronal view, 3B: sagittal view). The crayon tip was successfully removed through a superior orbitotomy.
If an intraorbital or orbito-cranial hematoma is associated with the foreign body, MRI may better delineate the hematoma and hence clue to the existence of foreign body, compared to CT. Early hemorrhage (generally less than a week old) is typically isointense to the white matter on T1 and T2 weighted images. As the hematoma becomes more organized, it turns brighter on T1 and T2 sequences.31 Eventually, the hematoma becomes fully organized and appears hypointense compared to the white matter. As a hematoma undergoes these changes, serial MRI can sometimes be helpful in detecting foreign body initially masked by an isointense early hematoma.
Neither CT nor MRI can detect all foreign bodies, and clinical suspicion should guide the decision to perform orbit exploration. If conservative management is chosen, patients should be closely monitored for development of abscess or fistula formation, both of which can help in localizing foreign bodies.
Traumatic optic neuropathy
To date, CT and MRI both still play a limited role in detecting traumatic optic neuropathy. Their role is limited to visualizing macroscopic changes in the density and signal of the optic nerve. MRI can detect macroscopic optic nerve swelling while CT detects whether there is optic canal fracture (Fig. 4), and neither is sensitive enough to pick up early post-traumatic changes that often occur at the microvascular and axonal bundle levels. As such, false negative rate remains high. Despite these pitfalls, CT may help determine the urgency of surgical intervention. If the optic nerve is partially or completely avulsed, aggressive measures are not indicated. With severe visual loss and a radiographically intact optic nerve, high-dose intravenous corticosteroids may be considered to treat a presumed traumatic optic neuropathy. However some data suggest potential overall harm with the use of mega dose steroids in this setting. Surgical exploration may be indicated to relieve nerve impingement, especially if a bone fragment or other material is visualized against the optic nerve.
Figure 4.
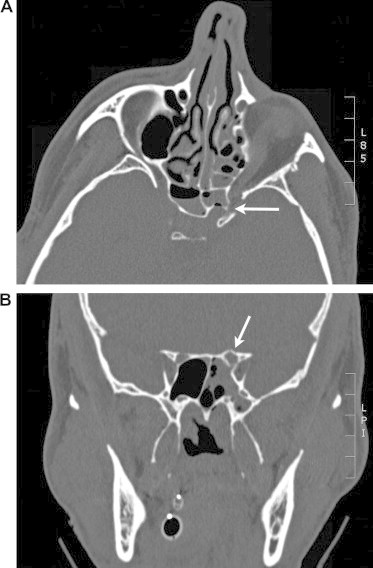
Intracanalicular fracture with traumatic optic neuropathy. A 25-year-old male fell off of a second-story balcony and hit face on a hard landing. CT scan demonstrated a left posterior optic canal fracture (arrow). (A) Axial view and (B) coronal view. Two weeks status post trauma, vision remained counting fingers corresponding to an afferent pupillary defect on flashlight exam.
Some recent case reports document a utility of diffusion-tensor MRI (DT-MRI) in detecting early changes in traumatic optic neuropathy. DT-MRI is a quantitative functional imaging that measures dynamic changes in water diffusion in nerve tissue. It is derived from taking diffusion weighted images (DWI) in at least six directions to refine the resolution of tissue microarchitecture and diffusion anisotropy. DWI is classically used to diagnose hyperacute ischemic stroke; areas of water diffusion stasis would appear hyperintense. Other causes of hyperintensity in DWI relevant to ophthalmology include orbital abscess, acute demyelinating conditions, and orbital inflammatory conditions. The apparent diffusion coefficient (ADC) is a quantitative variable derived from DWI. The ADC of the optic nerve may correlate with the post-traumatic visual acuity in patients with visual loss after injury.32
Carotid cavernous fistula
Carotid cavernous fistula may be suggested clinically through the presence of post-traumatic diplopia, proptosis, and chemosis, and these changes typically present weeks later after the initial trauma. Cavernous sinus blood flow may be reversed if the cavernous internal carotid artery is torn, forming a fistula that connects arterial and venous blood supplies. Such cases can be imaged on CT without contrast, in which the superior ophthalmic vein appears dilated. Because this finding can be a normal physiologic variant and is present in other conditions such as varix, cavernous sinus thrombosis, and Graves’ disease, diagnosis of cavernous sinus fistula must be confirmed with a CT angiogram or conventional angiography.24
Orbital compartment syndrome
Orbital compartment syndrome, like other compartment syndromes, results from a surge in pressure within a tight anatomical space leading to death of the vital tissues contained therein. Studies have shown that irreversible visual loss can occur after 60–100 min of raised orbital pressure.33 Normal intraorbital pressure has been measured at 3–6 mmHg.34 In traumatic setting, this tissue pressure can elevate above arterial pressure. If the vasa nervorum are affected, optic nerve perfusion can be affected; retinal ischemia can develop if the central retinal artery pressure is surpassed by the adnexal pressure. Since there is no lymphatic drainage to relieve intraorbital pressure, the only outflow pathway is through the major veins such as the superior ophthalmic vein, which is often compromised in the trauma too and further aggravates the compartment syndrome.
The initial management of orbital compartment syndrome is entirely clinical and unaffected by imaging data. A positive afferent pupillary defect, decreased visual acuity, tense orbit, periocular edema and hematoma, resistance to retropulsion, and a markedly raised intraocular pressure should all prompt urgent canthotomy and catholysis. Emergent imaging may be indicated when initial decompression fails to relieve the compartment syndrome, and that imaging test may reveal treatable causes such as a large sub-periosteal hematoma. CT is commonly used in this regard due to its availability. It may reveal tenting of the posterior globe. A posterior globe contour angle of less than 120° with proptosis has been shown to carry a poorer prognosis of visual recovery.35 CT can also identify the location and source of elevated orbital pressure such as retrobulbar hemorrhage, foreign bodies, or emphysema. MRI is rarely used in the acute setting when compartment syndrome is suspected. However, if used, it may provide clues regarding the age of the hematoma.
Acute hemorrhage may occur in a non-traumatic setting, resulting from venous malformations or lymphangiomas of the orbit. In these cases, magnetic resonance angiography (MRA) or magnetic resonance venography (MRV) can better guide diagnosis and treatment.36
Footnotes
References
- 1.Bord S.P., Linden J. Trauma to the globe and orbit. Emerg Med Clin North Am. 2008;26:97–123. doi: 10.1016/j.emc.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn F., Morris R., Mester V. Epidemiology and socioeconomics. Ophthalmo Clin North Am. 2002;15:145–151. doi: 10.1016/s0896-1549(02)00005-6. [DOI] [PubMed] [Google Scholar]
- 3.Zammit-Maempel I., Chadwick C.L. Radiation dose to the lens of eye and thyroid gland in paranasal siinus multisclice CT. Br J Radiol. 2003;76:418–420. doi: 10.1259/bjr/82798696. [DOI] [PubMed] [Google Scholar]
- 4.Lakets A., Prokesch R., Scholda C. Orbital helical computed tomography in the diagnosis and management of eye trauma. Ophthalmology. 1999;106:2330–2335. doi: 10.1016/S0161-6420(99)90536-5. [DOI] [PubMed] [Google Scholar]
- 5.Burnsteine M. Clinical recommendations for repair of isolated orbital floor fractures: an evidence-based analysis. Ophthalmology. 2002;109:1207–1210. doi: 10.1016/s0161-6420(02)01057-6. [DOI] [PubMed] [Google Scholar]
- 6.Cole P., Boyd V., Banerji S. Comprehensive management of orbital fractures. Plast Reconstr Surg. 2007;120:57S–63S. doi: 10.1097/01.prs.0000260752.20481.b4. [DOI] [PubMed] [Google Scholar]
- 7.Cruz A.A., Eichenberger G.C. Epidemiology and management of orbital fractures. Curr Opin Ophthalmol. 2004;15:416–421. doi: 10.1097/01.icu.0000136113.56288.87. [DOI] [PubMed] [Google Scholar]
- 8.Rinna C., Ungari C., Saltarel A. Orbital floor restoration. J Craniofac Surg. 2005;16:968–972. doi: 10.1097/01.scs.0000186308.16795.8b. [DOI] [PubMed] [Google Scholar]
- 9.Manson P.N., Clifford G.M., Su C.T. Mechanisms of globe support and posttraumatic enophthalmos: the anatomy of the ligament sling and its relation to intramuscular cone orbital fat. Plast Reconstr Surg. 1986;77:193–202. [PubMed] [Google Scholar]
- 10.Banerjee A., Moore C.C., Tse R. Rounding of the inferior rectus muscle as an indication of orbital floor fracture with periorbital disruption. J Otolaryngol. 2007;36:175–180. [PubMed] [Google Scholar]
- 11.Gilbard S.M. Management of orbital blowout fractures: the prognostic significance of computed tomography. Adv Ophthalmic Plast Reconstr Surg. 1987;6:269–280. [PubMed] [Google Scholar]
- 12.Kim J.M., Goldberg R.A., Shorr N. The inferomedial orbital strut: an anatomic and radiographic study. Ophthal Plast Recsontr Surg. 2002;18:355–362. doi: 10.1097/00002341-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Sharabi S.F., Koshy J.C., Thornton J.F. Facial fractures. Plast Reconstr Surg. 2011;127:25. doi: 10.1097/PRS.0b013e318200cb2d. [DOI] [PubMed] [Google Scholar]
- 14.Dal Canto A.J., Linberg J.V. Comparison of orbital fracture repair performed within 14 days versus 15 to 29 days after trauma. Ophthal Plast Reconstru Surg. 2008;24:437–443. doi: 10.1097/IOP.0b013e31818aac9b. [DOI] [PubMed] [Google Scholar]
- 15.Simon G.J., Syed H.M., McCann J.D. Early versus late repair of orbital blowout fractures. Ophthalmic Surg Lasers Imaging. 2009;40:141–148. doi: 10.3928/15428877-20090301-05. [DOI] [PubMed] [Google Scholar]
- 16.Jordan D.R., Allen L.H., White J. Intervention within days for some orbital floor fractures: the white-eyed blowout. Ophthal Plast Reconstr Surg. 1998;14:379–390. doi: 10.1097/00002341-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Criden M.R., Ellis F.J. Linear nondisplaced orbital fractures with muscle entrapment. J AAPOS. 2007;11:142–147. doi: 10.1016/j.jaapos.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Bansagi Z.C., Meyer D.R. Internal orbital fractures in the pediatric age group. Ophthalmology. 1998;107:829–836. doi: 10.1016/s0161-6420(00)00015-4. [DOI] [PubMed] [Google Scholar]
- 19.Sires B.S., Stanley R.B., Levine L.M. Oculocardiac reflex caused by orbital floor trapdoor fracture: an indication for urgent repair. Arch Ophthalmol. 1998;116:955–956. [PubMed] [Google Scholar]
- 20.Meyer C., Groos N., Sabateir H. Long-term outcome of surgically treated orbital floor fractures. Apropos of a series of 242 patients. Rev Stomatol Chir Maxillofac. 1998;99:149–154. [PubMed] [Google Scholar]
- 21.McGraw B.L., Randolph C.R. Pediatric maxillofacial trauma: age-related variations in injury. Arch Otolaryngol Head Neck Surg. 1990;116:41–45. doi: 10.1001/archotol.1990.01870010045014. [DOI] [PubMed] [Google Scholar]
- 22.Joshi S., Kassira W., Thaller S.R. Overview of pediatric orbital fractures. J Craniofac Surg. 2011;22:1330–1332. doi: 10.1097/SCS.0b013e31821c9365. [DOI] [PubMed] [Google Scholar]
- 23.Kwon J.H., Moon J.H., Kwon M.S. The differences of blowout fracture of the inferior orbital wall between children and adults. Arch Otolaryngol Head Neck Surg. 2005;131:723–727. doi: 10.1001/archotol.131.8.723. [DOI] [PubMed] [Google Scholar]
- 24.Kubal W.S. Imaging of orbital trauma. Radiographics. 2008;28:1729–1739. doi: 10.1148/rg.286085523. [DOI] [PubMed] [Google Scholar]
- 25.Joseph D.P., Pieramici D.J., Beauchamp N.J. Computed tomography in the diagnosis and prognosis of open globe injuries. Ophthalmology. 2000;107:1899–1906. doi: 10.1016/s0161-6420(00)00335-3. [DOI] [PubMed] [Google Scholar]
- 27.Sari A., Adiguzel U., Ismi T. An unexpected outcome of blunt ocular trauma: rupture of three muscles. Strabismus. 2009;17:95–97. doi: 10.1080/09273970903126543. [DOI] [PubMed] [Google Scholar]
- 28.Sloan B., McNab A.A. Inferior rectus rupture following blow out fracture. Aust N Z J Ophthalmol. 1998;26:171–173. doi: 10.1111/j.1442-9071.1998.tb01537.x. [DOI] [PubMed] [Google Scholar]
- 29.Myga-Porosilo J., Skrzelewski S., Sraga W. CT imaging of facial trauma. The role of different types of reconstruction. Part II – soft tissues. Pol J Radiol. 2011;76:52–58. [PMC free article] [PubMed] [Google Scholar]
- 30.Ho V.H., Wilson M.W., Fleming J.C. Retained intraorbital metallic foreign bodies. Ophthal Plast Reconstr Surg. 2009;20:232–236. doi: 10.1097/01.iop.0000129014.94384.e6. [DOI] [PubMed] [Google Scholar]
- 31.Nasr A.M., Hail B.G., Fleming J.C. Penetrating orbital injury with organic foreign bodies. Ophthalmology. 1999;106:523–532. doi: 10.1016/S0161-6420(99)90111-2. [DOI] [PubMed] [Google Scholar]
- 32.Yang Q.-T., Fan Y.-P., Zou Y. Evaluation of traumatic optic neuropathy in patients with optic canal fracture using diffusion tensor magnetic resonance imaging: a preliminary report. ORL J Otorhinolaryngol Relat Spec. 2011;73:301–307. doi: 10.1159/000330723. [DOI] [PubMed] [Google Scholar]
- 33.Hayreh S.S., Kolder W.E., Weingeist T.A. Central retinal artery occlusion and retinal tolerance time. Ophthalmology. 1980;87:75–78. doi: 10.1016/s0161-6420(80)35283-4. [DOI] [PubMed] [Google Scholar]
- 34.Kratky V., Hurwitz J.J., Avram D.R. Orbital compartment syndrome. Direct measurement of orbital tissue pressure. Can J Ophthalmol. 1990;25:293–297. [PubMed] [Google Scholar]
- 35.Dalley R.W., Robertson W.D., Rootman J. Globe tenting: a sign of increased orbital tension. AJNR. 1989;10:181–186. [PMC free article] [PubMed] [Google Scholar]
- 36.Kahana A., Lucarelli M.J., Gravey A.M. Noninvasive dynamic magnetic resonance angiography with time-resolved imaging of contrast kinetics (TRICKS) in the evaluation of orbital vascular lesions. Arch Ophthalmol. 2007;125:1635–1642. doi: 10.1001/archopht.125.12.1635. [DOI] [PubMed] [Google Scholar]



