Abstract
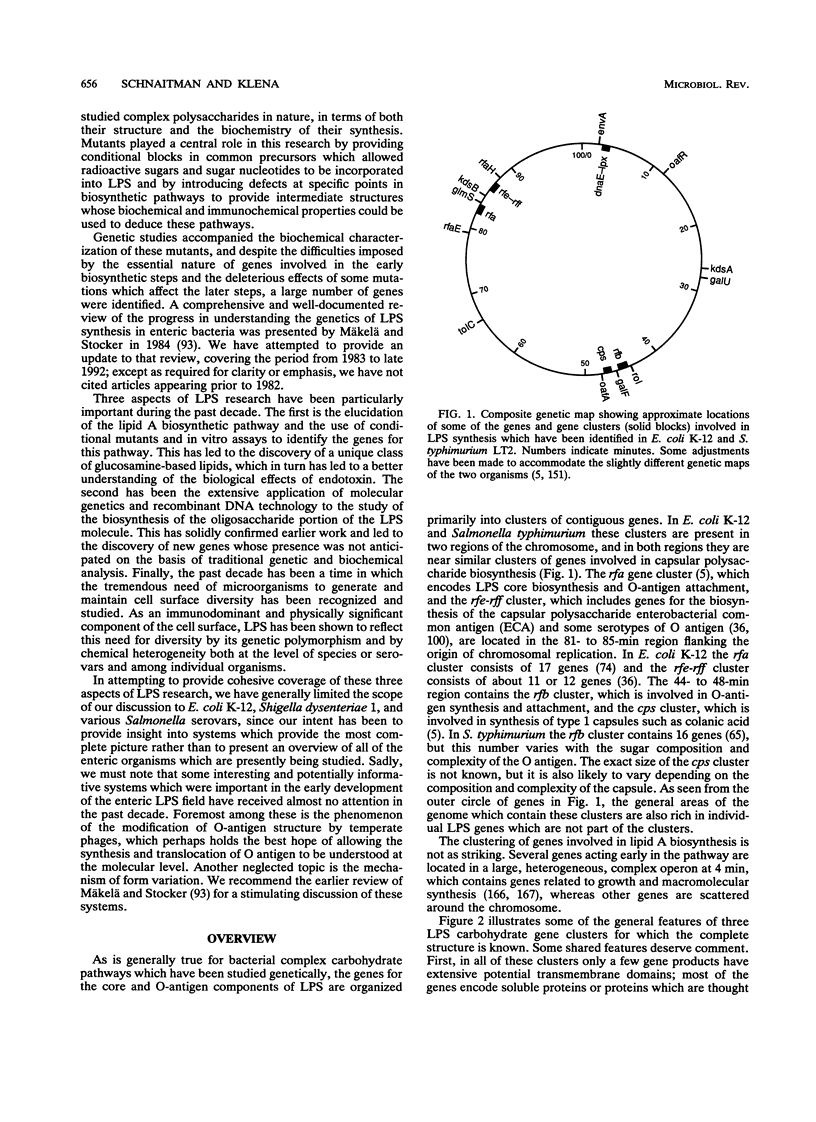
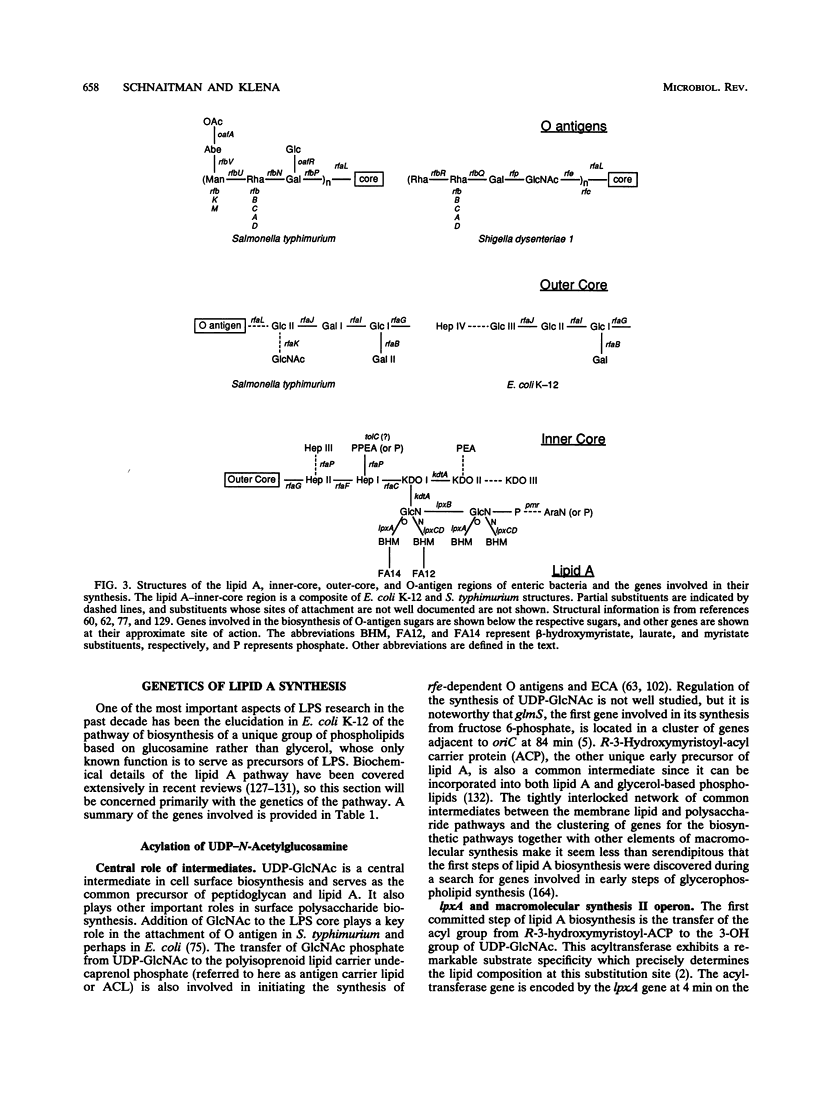
From a historical perspective, the study of both the biochemistry and the genetics of lipopolysaccharide (LPS) synthesis began with the enteric bacteria. These organisms have again come to the forefront as the blocks of genes involved in LPS synthesis have been sequenced and analyzed. A number of new and unanticipated genes were found in these clusters, indicating a complexity of the biochemical pathways which was not predicted from the older studies. One of the most dramatic areas of LPS research has been the elucidation of the lipid A biosynthetic pathway. Four of the genes in this pathway have now been identified and sequenced, and three of them are located in a complex operon which also contains genes involved in DNA and phospholipid synthesis. The rfa gene cluster, which contains many of the genes for LPS core synthesis, includes at least 17 genes. One of the remarkable findings in this cluster is a group of several genes which appear to be involved in the synthesis of alternate rough core species which are modified so that they cannot be acceptors for O-specific polysaccharides. The rfb gene clusters which encode O-antigen synthesis have been sequenced from a number of serotypes and exhibit the genetic polymorphism anticipated on the basis of the chemical complexity of the O antigens. These clusters appear to have originated by the exchange of blocks of genes among ancestral organisms. Among the large number of LPS genes which have now been sequenced from these rfa and rfb clusters, there are none which encode proteins that appear to be secreted across the cytoplasmic membrane and surprisingly few which encode integral membrane proteins or proteins with extensive hydrophobic domains. These data, together with sequence comparison and complementation experiments across strain and species lines, suggest that the LPS biosynthetic enzymes may be organized into clusters on the inner surface of the cytoplasmic membrane which are organized around a few key membrane proteins.
Full text
PDF

























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aasland R., Coleman J., Holck A. L., Smith C. L., Raetz C. R., Kleppe K. Identity of the 17-kilodalton protein, a DNA-binding protein from Escherichia coli, and the firA gene product. J Bacteriol. 1988 Dec;170(12):5916–5918. doi: 10.1128/jb.170.12.5916-5918.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. S., Raetz C. R. Biosynthesis of lipid A precursors in Escherichia coli. A cytoplasmic acyltransferase that converts UDP-N-acetylglucosamine to UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine. J Biol Chem. 1987 Apr 15;262(11):5159–5169. [PubMed] [Google Scholar]
- Austin E. A., Graves J. F., Hite L. A., Parker C. T., Schnaitman C. A. Genetic analysis of lipopolysaccharide core biosynthesis by Escherichia coli K-12: insertion mutagenesis of the rfa locus. J Bacteriol. 1990 Sep;172(9):5312–5325. doi: 10.1128/jb.172.9.5312-5325.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M. J., Koronakis V., Schmoll T., Hughes C. Escherichia coli HlyT protein, a transcriptional activator of haemolysin synthesis and secretion, is encoded by the rfaH (sfrB) locus required for expression of sex factor and lipopolysaccharide genes. Mol Microbiol. 1992 Apr;6(8):1003–1012. doi: 10.1111/j.1365-2958.1992.tb02166.x. [DOI] [PubMed] [Google Scholar]
- Bastin D. A., Romana L. K., Reeves P. R. Molecular cloning and expression in Escherichia coli K-12 of the rfb gene cluster determining the O antigen of an E. coli O111 strain. Mol Microbiol. 1991 Sep;5(9):2223–2231. doi: 10.1111/j.1365-2958.1991.tb02152.x. [DOI] [PubMed] [Google Scholar]
- Bastin D. A., Stevenson G., Brown P. K., Haase A., Reeves P. R. Repeat unit polysaccharides of bacteria: a model for polymerization resembling that of ribosomes and fatty acid synthetase, with a novel mechanism for determining chain length. Mol Microbiol. 1993 Mar;7(5):725–734. doi: 10.1111/j.1365-2958.1993.tb01163.x. [DOI] [PubMed] [Google Scholar]
- Batchelor R. A., Alifano P., Biffali E., Hull S. I., Hull R. A. Nucleotide sequences of the genes regulating O-polysaccharide antigen chain length (rol) from Escherichia coli and Salmonella typhimurium: protein homology and functional complementation. J Bacteriol. 1992 Aug;174(16):5228–5236. doi: 10.1128/jb.174.16.5228-5236.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor R. A., Haraguchi G. E., Hull R. A., Hull S. I. Regulation by a novel protein of the bimodal distribution of lipopolysaccharide in the outer membrane of Escherichia coli. J Bacteriol. 1991 Sep;173(18):5699–5704. doi: 10.1128/jb.173.18.5699-5704.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belunis C. J., Mdluli K. E., Raetz C. R., Nano F. E. A novel 3-deoxy-D-manno-octulosonic acid transferase from Chlamydia trachomatis required for expression of the genus-specific epitope. J Biol Chem. 1992 Sep 15;267(26):18702–18707. [PubMed] [Google Scholar]
- Belunis C. J., Raetz C. R. Biosynthesis of endotoxins. Purification and catalytic properties of 3-deoxy-D-manno-octulosonic acid transferase from Escherichia coli. J Biol Chem. 1992 May 15;267(14):9988–9997. [PubMed] [Google Scholar]
- Beutin L., Manning P. A., Achtman M., Willetts N. sfrA and sfrB products of Escherichia coli K-12 are transcriptional control factors. J Bacteriol. 1981 Feb;145(2):840–844. doi: 10.1128/jb.145.2.840-844.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulnois G. J., Jann K. Bacterial polysaccharide capsule synthesis, export and evolution of structural diversity. Mol Microbiol. 1989 Dec;3(12):1819–1823. doi: 10.1111/j.1365-2958.1989.tb00168.x. [DOI] [PubMed] [Google Scholar]
- Boulnois G. J., Roberts I. S., Hodge R., Hardy K. R., Jann K. B., Timmis K. N. Analysis of the K1 capsule biosynthesis genes of Escherichia coli: definition of three functional regions for capsule production. Mol Gen Genet. 1987 Jun;208(1-2):242–246. doi: 10.1007/BF00330449. [DOI] [PubMed] [Google Scholar]
- Brade H., Brade L., Nano F. E. Chemical and serological investigations on the genus-specific lipopolysaccharide epitope of Chlamydia. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2508–2512. doi: 10.1073/pnas.84.8.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmbhatt H. N., Lindberg A. A., Timmis K. N. Shigella lipopolysaccharide: structure, genetics, and vaccine development. Curr Top Microbiol Immunol. 1992;180:45–64. doi: 10.1007/978-3-642-77238-2_3. [DOI] [PubMed] [Google Scholar]
- Brahmbhatt H. N., Wyk P., Quigley N. B., Reeves P. R. Complete physical map of the rfb gene cluster encoding biosynthetic enzymes for the O antigen of Salmonella typhimurium LT2. J Bacteriol. 1988 Jan;170(1):98–102. doi: 10.1128/jb.170.1.98-102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazas R., Davie E., Farewell A., Rothfield L. I. Transcriptional organization of the rfaGBIJ locus of Salmonella typhimurium. J Bacteriol. 1991 Oct;173(19):6168–6173. doi: 10.1128/jb.173.19.6168-6173.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. K., Romana L. K., Reeves P. R. Cloning of the rfb gene cluster of a group C2 Salmonella strain: comparison with the rfb regions of groups B and D. Mol Microbiol. 1991 Aug;5(8):1873–1881. doi: 10.1111/j.1365-2958.1991.tb00811.x. [DOI] [PubMed] [Google Scholar]
- Brown P. K., Romana L. K., Reeves P. R. Molecular analysis of the rfb gene cluster of Salmonella serovar muenchen (strain M67): the genetic basis of the polymorphism between groups C2 and B. Mol Microbiol. 1992 May;6(10):1385–1394. doi: 10.1111/j.1365-2958.1992.tb00859.x. [DOI] [PubMed] [Google Scholar]
- Brozek K. A., Hosaka K., Robertson A. D., Raetz C. R. Biosynthesis of lipopolysaccharide in Escherichia coli. Cytoplasmic enzymes that attach 3-deoxy-D-manno-octulosonic acid to lipid A. J Biol Chem. 1989 Apr 25;264(12):6956–6966. [PubMed] [Google Scholar]
- Brozek K. A., Raetz C. R. Biosynthesis of lipid A in Escherichia coli. Acyl carrier protein-dependent incorporation of laurate and myristate. J Biol Chem. 1990 Sep 15;265(26):15410–15417. [PubMed] [Google Scholar]
- Carstenius P., Flock J. I., Lindberg A. Nucleotide sequence of rfaI and rfaJ genes encoding lipopolysaccharide glycosyl transferases from Salmonella typhimurium. Nucleic Acids Res. 1990 Oct 25;18(20):6128–6128. doi: 10.1093/nar/18.20.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C. A., Beltrame J., Manning P. A. The oac gene encoding a lipopolysaccharide O-antigen acetylase maps adjacent to the integrase-encoding gene on the genome of Shigella flexneri bacteriophage Sf6. Gene. 1991 Oct 30;107(1):43–52. doi: 10.1016/0378-1119(91)90295-m. [DOI] [PubMed] [Google Scholar]
- Clementz T., Raetz C. R. A gene coding for 3-deoxy-D-manno-octulosonic-acid transferase in Escherichia coli. Identification, mapping, cloning, and sequencing. J Biol Chem. 1991 May 25;266(15):9687–9696. [PubMed] [Google Scholar]
- Clementz T. The gene coding for 3-deoxy-manno-octulosonic acid transferase and the rfaQ gene are transcribed from divergently arranged promoters in Escherichia coli. J Bacteriol. 1992 Dec;174(23):7750–7756. doi: 10.1128/jb.174.23.7750-7756.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J., Raetz C. R. First committed step of lipid A biosynthesis in Escherichia coli: sequence of the lpxA gene. J Bacteriol. 1988 Mar;170(3):1268–1274. doi: 10.1128/jb.170.3.1268-1274.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman W. G., Jr The rfaD gene codes for ADP-L-glycero-D-mannoheptose-6-epimerase. An enzyme required for lipopolysaccharide core biosynthesis. J Biol Chem. 1983 Feb 10;258(3):1985–1990. [PubMed] [Google Scholar]
- Collins L. V., Hackett J. Molecular cloning, characterization, and nucleotide sequence of the rfc gene, which encodes an O-antigen polymerase of Salmonella typhimurium. J Bacteriol. 1991 Apr;173(8):2521–2529. doi: 10.1128/jb.173.8.2521-2529.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeger E. S., Chen J. F., Rothfield L. I. Cloning of genes for bacterial glycosyltransferases. II. Selection of a hybrid plasmid carrying the rfah gene. J Biol Chem. 1979 Feb 10;254(3):811–815. [PubMed] [Google Scholar]
- Creeger E. S., Rothfield L. I. Cloning of genes for bacterial glycosyltransferases. I. Selection of hybrid plasmids carrying genes for two glucosyltransferases. J Biol Chem. 1979 Feb 10;254(3):804–810. [PubMed] [Google Scholar]
- Creeger E. S., Schulte T., Rothfield L. I. Regulation of membrane glycosyltransferases by the sfrB and rfaH genes of Escherichia coli and Salmonella typhimurium. J Biol Chem. 1984 Mar 10;259(5):3064–3069. [PubMed] [Google Scholar]
- Crowell D. N., Reznikoff W. S., Raetz C. R. Nucleotide sequence of the Escherichia coli gene for lipid A disaccharide synthase. J Bacteriol. 1987 Dec;169(12):5727–5734. doi: 10.1128/jb.169.12.5727-5734.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChavigny A., Heacock P. N., Dowhan W. Sequence and inactivation of the pss gene of Escherichia coli. Phosphatidylethanolamine may not be essential for cell viability. J Biol Chem. 1991 Mar 15;266(8):5323–5332. [PubMed] [Google Scholar]
- Dicker I. B., Seetharam S. Cloning and nucleotide sequence of the firA gene and the firA200(Ts) allele from Escherichia coli. J Bacteriol. 1991 Jan;173(1):334–344. doi: 10.1128/jb.173.1.334-344.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker I. B., Seetharam S. What is known about the structure and function of the Escherichia coli protein FirA? Mol Microbiol. 1992 Apr;6(7):817–823. doi: 10.1111/j.1365-2958.1992.tb01532.x. [DOI] [PubMed] [Google Scholar]
- Dmitriev B. A., Knirel Y. A., Kochetkov N. K. Somatic antigens of shigella. Structural investigation on the O-specific polysaccharide chain of Shigella dysenteriae type 1 lipopolysaccharide. Eur J Biochem. 1976 Jul 15;66(3):559–566. doi: 10.1111/j.1432-1033.1976.tb10582.x. [DOI] [PubMed] [Google Scholar]
- Drickamer K. Clearing up glycoprotein hormones. Cell. 1991 Dec 20;67(6):1029–1032. doi: 10.1016/0092-8674(91)90278-7. [DOI] [PubMed] [Google Scholar]
- Farewell A., Brazas R., Davie E., Mason J., Rothfield L. I. Suppression of the abnormal phenotype of Salmonella typhimurium rfaH mutants by mutations in the gene for transcription termination factor Rho. J Bacteriol. 1991 Aug;173(16):5188–5193. doi: 10.1128/jb.173.16.5188-5193.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G. Progress in unraveling pathways of Golgi traffic. Annu Rev Cell Biol. 1985;1:447–488. doi: 10.1146/annurev.cb.01.110185.002311. [DOI] [PubMed] [Google Scholar]
- Fält I. C., Schweda E. K., Weintraub A., Sturm S., Timmis K. N., Lindberg A. A. Expression of the Shigella dysenteriae type-1 lipopolysaccharide repeating unit in Escherichia coli K12/Shigella dysenteriae type-1 hybrids. Eur J Biochem. 1993 Apr 1;213(1):573–581. doi: 10.1111/j.1432-1033.1993.tb17796.x. [DOI] [PubMed] [Google Scholar]
- Galloway S. M., Raetz C. R. A mutant of Escherichia coli defective in the first step of endotoxin biosynthesis. J Biol Chem. 1990 Apr 15;265(11):6394–6402. [PubMed] [Google Scholar]
- Gibson B. W., Webb J. W., Yamasaki R., Fisher S. J., Burlingame A. L., Mandrell R. E., Schneider H., Griffiss J. M. Structure and heterogeneity of the oligosaccharides from the lipopolysaccharides of a pyocin-resistant Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1989 Jan;86(1):17–21. doi: 10.1073/pnas.86.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Bolling T. J., Kohlbrenner W. E., Kim Y., Fox J. L. Primary structure of CTP:CMP-3-deoxy-D-manno-octulosonate cytidylyltransferase (CMP-KDO synthetase) from Escherichia coli. J Biol Chem. 1986 Dec 5;261(34):15831–15835. [PubMed] [Google Scholar]
- Goldman R. C., Hunt F. Mechanism of O-antigen distribution in lipopolysaccharide. J Bacteriol. 1990 Sep;172(9):5352–5359. doi: 10.1128/jb.172.9.5352-5359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golenbock D. T., Hampton R. Y., Qureshi N., Takayama K., Raetz C. R. Lipid A-like molecules that antagonize the effects of endotoxins on human monocytes. J Biol Chem. 1991 Oct 15;266(29):19490–19498. [PubMed] [Google Scholar]
- Grossman N., Schmetz M. A., Foulds J., Klima E. N., Jimenez-Lucho V. E., Leive L. L., Joiner K. A., Jiminez V. Lipopolysaccharide size and distribution determine serum resistance in Salmonella montevideo. J Bacteriol. 1987 Feb;169(2):856–863. doi: 10.1128/jb.169.2.856-863.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Guerry P., Seid R. C., Jr, Kapfer C., Wingfield M. E., Reaves C. B., Baron L. S., Formal S. B. Expression of lipopolysaccharide O antigen in Escherichia coli K-12 hybrids containing plasmid and chromosomal genes from Shigella dysenteriae 1. Infect Immun. 1984 Nov;46(2):470–475. doi: 10.1128/iai.46.2.470-475.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi G. E., Hull R. A., Krallmann-Wenzel U., Hull S. I. Molecular cloning and expression of the O4 polysaccharide gene cluster from Escherichia coli. Microb Pathog. 1989 Feb;6(2):123–132. doi: 10.1016/0882-4010(89)90015-6. [DOI] [PubMed] [Google Scholar]
- Haraguchi G. E., Zähringer U., Jann B., Jann K., Hull R. A., Hull S. I. Genetic characterization of the O4 polysaccharide gene cluster from Escherichia coli. Microb Pathog. 1991 May;10(5):351–361. doi: 10.1016/0882-4010(91)90080-t. [DOI] [PubMed] [Google Scholar]
- Hasin M., Kennedy E. P. Role of phosphatidylethanolamine in the biosynthesis of pyrophosphoethanolamine residues in the lipopolysaccharide of Escherichia coli. J Biol Chem. 1982 Nov 10;257(21):12475–12477. [PubMed] [Google Scholar]
- Helander I. M., Moran A. P., Mäkelä P. H. Separation of two lipopolysaccharide populations with different contents of O-antigen factor 122 in Salmonella enterica serovar typhimurium. Mol Microbiol. 1992 Oct;6(19):2857–2862. doi: 10.1111/j.1365-2958.1992.tb01465.x. [DOI] [PubMed] [Google Scholar]
- Helander I. M., Vaara M., Sukupolvi S., Rhen M., Saarela S., Zähringer U., Mäkelä P. H. rfaP mutants of Salmonella typhimurium. Eur J Biochem. 1989 Nov 20;185(3):541–546. doi: 10.1111/j.1432-1033.1989.tb15147.x. [DOI] [PubMed] [Google Scholar]
- Heuzenroeder M. W., Beger D. W., Thomas C. J., Manning P. A. Molecular cloning and expression in Escherichia coli K-12 of the O101 rfb region from E. coli B41 (O101:K99/F41) and the genetic relationship to other O101 rfb loci. Mol Microbiol. 1989 Mar;3(3):295–302. doi: 10.1111/j.1365-2958.1989.tb00174.x. [DOI] [PubMed] [Google Scholar]
- Hirvas L., Koski P., Vaara M. The ompH gene of Yersinia enterocolitica: cloning, sequencing, expression, and comparison with known enterobacterial ompH sequences. J Bacteriol. 1991 Feb;173(3):1223–1229. doi: 10.1128/jb.173.3.1223-1229.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson A. L., Bird P., Nisbet I. T. Cloning, nucleotide sequence, and expression in Escherichia coli of the phospholipase D gene from Corynebacterium pseudotuberculosis. J Bacteriol. 1990 Mar;172(3):1256–1261. doi: 10.1128/jb.172.3.1256-1261.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst O., Brade H. Isolation and identification of 3-deoxy-5-O-alpha-L-rhamnopyranosyl-D-manno-2-octulopyranosonate from the inner core region of the lipopolysaccharide of Escherichia coli K-12. Carbohydr Res. 1990 Oct 25;207(2):327–331. doi: 10.1016/0008-6215(90)84060-8. [DOI] [PubMed] [Google Scholar]
- Holst O., Röhrscheidt-Andrzejewski E., Brade H. Isolation and characterisation of 3-deoxy-D-manno-2-octulopyranosonate 7-(2-aminoethyl phosphate) from the inner core region of Escherichia coli K-12 and Salmonella minnesota lipopolysaccharides. Carbohydr Res. 1990 Sep 5;204:93–102. doi: 10.1016/0008-6215(90)84024-o. [DOI] [PubMed] [Google Scholar]
- Holst O., Zähringer U., Brade H., Zamojski A. Structural analysis of the heptose/hexose region of the lipopolysaccharide from Escherichia coli K-12 strain W3100. Carbohydr Res. 1991 Aug 20;215(2):323–335. doi: 10.1016/0008-6215(91)84031-9. [DOI] [PubMed] [Google Scholar]
- Jansson P. E., Lindberg A. A., Lindberg B., Wollin R. Structural studies on the hexose region of the core in lipopolysaccharides from Enterobacteriaceae. Eur J Biochem. 1981 Apr;115(3):571–577. doi: 10.1111/j.1432-1033.1981.tb06241.x. [DOI] [PubMed] [Google Scholar]
- Jiang X. M., Neal B., Santiago F., Lee S. J., Romana L. K., Reeves P. R. Structure and sequence of the rfb (O antigen) gene cluster of Salmonella serovar typhimurium (strain LT2). Mol Microbiol. 1991 Mar;5(3):695–713. doi: 10.1111/j.1365-2958.1991.tb00741.x. [DOI] [PubMed] [Google Scholar]
- John C. M., Griffiss J. M., Apicella M. A., Mandrell R. E., Gibson B. W. The structural basis for pyocin resistance in Neisseria gonorrhoeae lipooligosaccharides. J Biol Chem. 1991 Oct 15;266(29):19303–19311. [PubMed] [Google Scholar]
- Karow M., Fayet O., Cegielska A., Ziegelhoffer T., Georgopoulos C. Isolation and characterization of the Escherichia coli htrB gene, whose product is essential for bacterial viability above 33 degrees C in rich media. J Bacteriol. 1991 Jan;173(2):741–750. doi: 10.1128/jb.173.2.741-750.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karow M., Fayet O., Georgopoulos C. The lethal phenotype caused by null mutations in the Escherichia coli htrB gene is suppressed by mutations in the accBC operon, encoding two subunits of acetyl coenzyme A carboxylase. J Bacteriol. 1992 Nov;174(22):7407–7418. doi: 10.1128/jb.174.22.7407-7418.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karow M., Georgopoulos C. Isolation and characterization of the Escherichia coli msbB gene, a multicopy suppressor of null mutations in the high-temperature requirement gene htrB. J Bacteriol. 1992 Feb;174(3):702–710. doi: 10.1128/jb.174.3.702-710.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karow M., Georgopoulos C. Sequencing, mutational analysis, and transcriptional regulation of the Escherichia coli htrB gene. Mol Microbiol. 1991 Sep;5(9):2285–2292. doi: 10.1111/j.1365-2958.1991.tb02159.x. [DOI] [PubMed] [Google Scholar]
- Kastowsky M., Gutberlet T., Bradaczek H. Molecular modelling of the three-dimensional structure and conformational flexibility of bacterial lipopolysaccharide. J Bacteriol. 1992 Jul;174(14):4798–4806. doi: 10.1128/jb.174.14.4798-4806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido N., Ohta M., Iida K., Hasegawa T., Ito H., Arakawa Y., Komatsu T., Kato N. Partial deletion of the cloned rfb gene of Escherichia coli O9 results in synthesis of a new O-antigenic lipopolysaccharide. J Bacteriol. 1989 Jul;171(7):3629–3633. doi: 10.1128/jb.171.7.3629-3633.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klena J. D., Ashford R. S., 2nd, Schnaitman C. A. Role of Escherichia coli K-12 rfa genes and the rfp gene of Shigella dysenteriae 1 in generation of lipopolysaccharide core heterogeneity and attachment of O antigen. J Bacteriol. 1992 Nov;174(22):7297–7307. doi: 10.1128/jb.174.22.7297-7307.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klena J. D., Pradel E., Schnaitman C. A. Comparison of lipopolysaccharide biosynthesis genes rfaK, rfaL, rfaY, and rfaZ of Escherichia coli K-12 and Salmonella typhimurium. J Bacteriol. 1992 Jul;174(14):4746–4752. doi: 10.1128/jb.174.14.4746-4752.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klena J. D., Pradel E., Schnaitman C. A. The rfaS gene, which is involved in production of a rough form of lipopolysaccharide core in Escherichia coli K-12, is not present in the rfa cluster of Salmonella typhimurium LT2. J Bacteriol. 1993 Mar;175(5):1524–1527. doi: 10.1128/jb.175.5.1524-1527.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeda Y., Icho T., Iino T. Effects of galU mutation on flagellar formation in Escherichia coli. J Bacteriol. 1977 Feb;129(2):908–915. doi: 10.1128/jb.129.2.908-915.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski P., Rhen M., Kantele J., Vaara M. Isolation, cloning, and primary structure of a cationic 16-kDa outer membrane protein of Salmonella typhimurium. J Biol Chem. 1989 Nov 15;264(32):18973–18980. [PubMed] [Google Scholar]
- Kröncke K. D., Boulnois G., Roberts I., Bitter-Suermann D., Golecki J. R., Jann B., Jann K. Expression of the Escherichia coli K5 capsular antigen: immunoelectron microscopic and biochemical studies with recombinant E. coli. J Bacteriol. 1990 Feb;172(2):1085–1091. doi: 10.1128/jb.172.2.1085-1091.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. J., Romana L. K., Reeves P. R. Cloning and structure of group C1 O antigen (rfb gene cluster) from Salmonella enterica serovar montevideo. J Gen Microbiol. 1992 Feb;138(2):305–312. doi: 10.1099/00221287-138-2-305. [DOI] [PubMed] [Google Scholar]
- Lee S. J., Romana L. K., Reeves P. R. Sequence and structural analysis of the rfb (O antigen) gene cluster from a group C1 Salmonella enterica strain. J Gen Microbiol. 1992 Sep;138(9):1843–1855. doi: 10.1099/00221287-138-9-1843. [DOI] [PubMed] [Google Scholar]
- Lehmann V., Lüderitz O., Westphal O. The linkage of pyrophosphorylethanolamine to heptose in the core of Salmonella minnesota lipopolysaccharides. Eur J Biochem. 1971 Aug 16;21(3):339–347. doi: 10.1111/j.1432-1033.1971.tb01474.x. [DOI] [PubMed] [Google Scholar]
- Lesse A. J., Campagnari A. A., Bittner W. E., Apicella M. A. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Immunol Methods. 1990 Jan 24;126(1):109–117. doi: 10.1016/0022-1759(90)90018-q. [DOI] [PubMed] [Google Scholar]
- Levy S. B., Leive L. An equilibrium between two fractions of lipopolysaccharide in Escherichia coli. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1435–1439. doi: 10.1073/pnas.61.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew H. C., Mäkelä P. H., Kuhn H. M., Mayer H., Nikaido H. Biosynthesis of enterobacterial common antigen requires dTDPglucose pyrophosphorylase determined by a Salmonella typhimurium rfb gene and a Salmonella montevideo rfe gene. J Bacteriol. 1986 Nov;168(2):715–721. doi: 10.1128/jb.168.2.715-721.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A. A., Holme T. Influence of O side chains on the attachment of the Felix O-1 bacteriophage to Salmonella bacteria. J Bacteriol. 1969 Aug;99(2):513–519. doi: 10.1128/jb.99.2.513-519.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Verma N. K., Romana L. K., Reeves P. R. Relationships among the rfb regions of Salmonella serovars A, B, and D. J Bacteriol. 1991 Aug;173(15):4814–4819. doi: 10.1128/jb.173.15.4814-4819.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLachlan P. R., Kadam S. K., Sanderson K. E. Cloning, characterization, and DNA sequence of the rfaLK region for lipopolysaccharide synthesis in Salmonella typhimurium LT2. J Bacteriol. 1991 Nov;173(22):7151–7163. doi: 10.1128/jb.173.22.7151-7163.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marolda C. L., Valvano M. A. Identification, expression, and DNA sequence of the GDP-mannose biosynthesis genes encoded by the O7 rfb gene cluster of strain VW187 (Escherichia coli O7:K1). J Bacteriol. 1993 Jan;175(1):148–158. doi: 10.1128/jb.175.1.148-158.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marolda C. L., Welsh J., Dafoe L., Valvano M. A. Genetic analysis of the O7-polysaccharide biosynthesis region from the Escherichia coli O7:K1 strain VW187. J Bacteriol. 1990 Jul;172(7):3590–3599. doi: 10.1128/jb.172.7.3590-3599.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marumo K., Lindqvist L., Verma N., Weintraub A., Reeves P. R., Lindberg A. A. Enzymatic synthesis and isolation of thymidine diphosphate-6-deoxy-D-xylo-4-hexulose and thymidine diphosphate-L-rhamnose. Production using cloned gene products and separation by HPLC. Eur J Biochem. 1992 Mar 1;204(2):539–545. doi: 10.1111/j.1432-1033.1992.tb16665.x. [DOI] [PubMed] [Google Scholar]
- McGrath B. C., Osborn M. J. Localization of the terminal steps of O-antigen synthesis in Salmonella typhimurium. J Bacteriol. 1991 Jan;173(2):649–654. doi: 10.1128/jb.173.2.649-654.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Dieter U., Barr K., Starman R., Hatch L., Rick P. D. Nucleotide sequence of the Escherichia coli rfe gene involved in the synthesis of enterobacterial common antigen. Molecular cloning of the rfe-rff gene cluster. J Biol Chem. 1992 Jan 15;267(2):746–753. [PubMed] [Google Scholar]
- Meier-Dieter U., Starman R., Barr K., Mayer H., Rick P. D. Biosynthesis of enterobacterial common antigen in Escherichia coli. Biochemical characterization of Tn10 insertion mutants defective in enterobacterial common antigen synthesis. J Biol Chem. 1990 Aug 15;265(23):13490–13497. [PubMed] [Google Scholar]
- Meier U., Mayer H. Genetic location of genes encoding enterobacterial common antigen. J Bacteriol. 1985 Aug;163(2):756–762. doi: 10.1128/jb.163.2.756-762.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. D., Dykhuizen D. E., Hartl D. L. Fitness effects of a deletion mutation increasing transcription of the 6-phosphogluconate dehydrogenase gene in Escherichia coli. Mol Biol Evol. 1988 Nov;5(6):691–703. doi: 10.1093/oxfordjournals.molbev.a040522. [DOI] [PubMed] [Google Scholar]
- Misra R., Reeves P. R. Role of micF in the tolC-mediated regulation of OmpF, a major outer membrane protein of Escherichia coli K-12. J Bacteriol. 1987 Oct;169(10):4722–4730. doi: 10.1128/jb.169.10.4722-4730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morona R., Manning P. A., Reeves P. Identification and characterization of the TolC protein, an outer membrane protein from Escherichia coli. J Bacteriol. 1983 Feb;153(2):693–699. doi: 10.1128/jb.153.2.693-699.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulford C. A., Osborn M. J. An intermediate step in translocation of lipopolysaccharide to the outer membrane of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1159–1163. doi: 10.1073/pnas.80.5.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlradt P. Biosynthesis of Salmonella lipopolysaccharide. The in vitro transfer of phosphate to the heptose moiety of the core. Eur J Biochem. 1969 Dec;11(2):241–248. doi: 10.1111/j.1432-1033.1969.tb00766.x. [DOI] [PubMed] [Google Scholar]
- Nakae T., Nikaido H. Multiple molecular forms of uridine diphosphate glucose pyrophosphorylase from Salmonella typhimurium. II. Genetic determination of multiple forms. J Biol Chem. 1971 Jul 25;246(14):4397–4403. [PubMed] [Google Scholar]
- Neal B. L., Tsiolis G. C., Heuzenroeder M. W., Manning P. A., Reeves P. R. Molecular cloning and expression in Escherichia coli K-12 of chromosomal genes determining the O antigen of an E. coli O2: K1 strain. FEMS Microbiol Lett. 1991 Aug 15;66(3):345–351. doi: 10.1016/0378-1097(91)90286-j. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Nikaido K., Rapin A. M. Biosynthesis of thymidine diphosphate L-rhamnose in Escherichia coli K-12. Biochim Biophys Acta. 1965 Dec 16;111(2):548–551. doi: 10.1016/0304-4165(65)90068-1. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Structure of cell wall lipopolysaccharide from Salmonella typhimurium. Further studies on the linkage between O side chains and R core. Eur J Biochem. 1970 Jul;15(1):57–62. doi: 10.1111/j.1432-1033.1970.tb00975.x. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Boman H. G., Matsson E. Mutant of Escherichia coli with anomalous cell division and ability to decrease episomally and chromosomally mediated resistance to ampicillin and several other antibiotics. J Bacteriol. 1969 Mar;97(3):1334–1342. doi: 10.1128/jb.97.3.1334-1342.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Rick P. D., Rasmussen N. S. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Translocation and integration of an incomplete mutant lipid A into the outer membrane. J Biol Chem. 1980 May 10;255(9):4246–4251. [PubMed] [Google Scholar]
- Parker C. T., Kloser A. W., Schnaitman C. A., Stein M. A., Gottesman S., Gibson B. W. Role of the rfaG and rfaP genes in determining the lipopolysaccharide core structure and cell surface properties of Escherichia coli K-12. J Bacteriol. 1992 Apr;174(8):2525–2538. doi: 10.1128/jb.174.8.2525-2538.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. T., Pradel E., Schnaitman C. A. Identification and sequences of the lipopolysaccharide core biosynthetic genes rfaQ, rfaP, and rfaG of Escherichia coli K-12. J Bacteriol. 1992 Feb;174(3):930–934. doi: 10.1128/jb.174.3.930-934.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegues J. C., Chen L. S., Gordon A. W., Ding L., Coleman W. G., Jr Cloning, expression, and characterization of the Escherichia coli K-12 rfaD gene. J Bacteriol. 1990 Aug;172(8):4652–4660. doi: 10.1128/jb.172.8.4652-4660.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips N. J., John C. M., Reinders L. G., Gibson B. W., Apicella M. A., Griffiss J. M. Structural models for the cell surface lipooligosaccharides of Neisseria gonorrhoeae and Haemophilus influenzae. Biomed Environ Mass Spectrom. 1990 Nov;19(11):731–745. doi: 10.1002/bms.1200191112. [DOI] [PubMed] [Google Scholar]
- Pradel E., Parker C. T., Schnaitman C. A. Structures of the rfaB, rfaI, rfaJ, and rfaS genes of Escherichia coli K-12 and their roles in assembly of the lipopolysaccharide core. J Bacteriol. 1992 Jul;174(14):4736–4745. doi: 10.1128/jb.174.14.4736-4745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradel E., Schnaitman C. A. Effect of rfaH (sfrB) and temperature on expression of rfa genes of Escherichia coli K-12. J Bacteriol. 1991 Oct;173(20):6428–6431. doi: 10.1128/jb.173.20.6428-6431.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- Raetz C. R., Brozek K. A., Clementz T., Coleman J. D., Galloway S. M., Golenbock D. T., Hampton R. Y. Gram-negative endotoxin: a biologically active lipid. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):973–982. doi: 10.1101/sqb.1988.053.01.112. [DOI] [PubMed] [Google Scholar]
- Raetz C. R., Dowhan W. Biosynthesis and function of phospholipids in Escherichia coli. J Biol Chem. 1990 Jan 25;265(3):1235–1238. [PubMed] [Google Scholar]
- Raina S., Georgopoulos C. The htrM gene, whose product is essential for Escherichia coli viability only at elevated temperatures, is identical to the rfaD gene. Nucleic Acids Res. 1991 Jul 25;19(14):3811–3819. doi: 10.1093/nar/19.14.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralling G., Linn T. Evidence that Rho and NusA are involved in termination in the rplL-rpoB intercistronic region. J Bacteriol. 1987 May;169(5):2277–2280. doi: 10.1128/jb.169.5.2277-2280.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B. L., Painter G., Raetz C. R. The biosynthesis of gram-negative endotoxin. Formation of lipid A disaccharides from monosaccharide precursors in extracts of Escherichia coli. J Biol Chem. 1984 Apr 25;259(8):4852–4859. [PubMed] [Google Scholar]
- Reeves P. Evolution of Salmonella O antigen variation by interspecific gene transfer on a large scale. Trends Genet. 1993 Jan;9(1):17–22. doi: 10.1016/0168-9525(93)90067-R. [DOI] [PubMed] [Google Scholar]
- Rehemtulla A., Kadam S. K., Sanderson K. E. Cloning and analysis of the sfrB (sex factor repression) gene of Escherichia coli K-12. J Bacteriol. 1986 May;166(2):651–657. doi: 10.1128/jb.166.2.651-657.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick P. D., Osborn M. J. Lipid A mutants of Salmonella typhimurium. Characterization of a conditional lethal mutant in 3-deoxy-D-mannooctulosonate-8-phosphate synthetase. J Biol Chem. 1977 Jul 25;252(14):4895–4903. [PubMed] [Google Scholar]
- Rick P. D., Young D. A. Isolation and characterization of a temperature-sensitive lethal mutant of Salmonella typhimurium that is conditionally defective in 3-deoxy-D-manno-octulosonate-8-phosphate synthesis. J Bacteriol. 1982 May;150(2):447–455. doi: 10.1128/jb.150.2.447-455.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche P., Debellé F., Maillet F., Lerouge P., Faucher C., Truchet G., Dénarié J., Promé J. C. Molecular basis of symbiotic host specificity in Rhizobium meliloti: nodH and nodPQ genes encode the sulfation of lipo-oligosaccharide signals. Cell. 1991 Dec 20;67(6):1131–1143. doi: 10.1016/0092-8674(91)90290-f. [DOI] [PubMed] [Google Scholar]
- Romana L. K., Santiago F. S., Reeves P. R. High level expression and purification of dthymidine diphospho-D-glucose 4,6-dehydratase (rfbB) from Salmonella serovar typhimurium LT2. Biochem Biophys Res Commun. 1991 Jan 31;174(2):846–852. doi: 10.1016/0006-291x(91)91495-x. [DOI] [PubMed] [Google Scholar]
- Roncero C., Casadaban M. J. Genetic analysis of the genes involved in synthesis of the lipopolysaccharide core in Escherichia coli K-12: three operons in the rfa locus. J Bacteriol. 1992 May;174(10):3250–3260. doi: 10.1128/jb.174.10.3250-3260.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncero C., Sanderson K. E., Casadaban M. J. Analysis of the host ranges of transposon bacteriophages Mu, MuhP1, and D108 by use of lipopolysaccharide mutants of Salmonella typhimurium LT2. J Bacteriol. 1991 Aug;173(16):5230–5233. doi: 10.1128/jb.173.16.5230-5233.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfield L., Pearlman-Kothencz M. Synthesis and assembly of bacterial membrane components. A lipopolysaccharide-phospholipid-protein complex excreted by living bacteria. J Mol Biol. 1969 Sep 28;44(3):477–492. doi: 10.1016/0022-2836(69)90374-x. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, edition VII. Microbiol Rev. 1988 Dec;52(4):485–532. doi: 10.1128/mr.52.4.485-532.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A., Parker C. T., Klena J. D., Pradel E. L., Pearson N. B., Sanderson K. E., MacClachlan P. R. Physical maps of the rfa loci of Escherichia coli K-12 and Salmonella typhimurium. J Bacteriol. 1991 Dec;173(23):7410–7411. doi: 10.1128/jb.173.23.7410-7411.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. A., Romanowska E. Structure and biology of Shigella flexneri O antigens. J Med Microbiol. 1987 Jun;23(4):289–302. doi: 10.1099/00222615-23-4-289. [DOI] [PubMed] [Google Scholar]
- Sirisena D. M., Brozek K. A., MacLachlan P. R., Sanderson K. E., Raetz C. R. The rfaC gene of Salmonella typhimurium. Cloning, sequencing, and enzymatic function in heptose transfer to lipopolysaccharide. J Biol Chem. 1992 Sep 15;267(26):18874–18884. [PubMed] [Google Scholar]
- Stevenson G., Lee S. J., Romana L. K., Reeves P. R. The cps gene cluster of Salmonella strain LT2 includes a second mannose pathway: sequence of two genes and relationship to genes in the rfb gene cluster. Mol Gen Genet. 1991 Jun;227(2):173–180. doi: 10.1007/BF00259668. [DOI] [PubMed] [Google Scholar]
- Strain S. M., Armitage I. M., Anderson L., Takayama K., Qureshi N., Raetz C. R. Location of polar substituents and fatty acyl chains on lipid A precursors from a 3-deoxy-D-manno-octulosonic acid-deficient mutant of Salmonella typhimurium. Studies by 1H, 13C, and 31P nuclear magnetic resonance. J Biol Chem. 1985 Dec 25;260(30):16089–16098. [PubMed] [Google Scholar]
- Sturm S., Jann B., Jann K., Fortnagel P., Timmis K. N. Genetic and biochemical analysis of Shigella dysenteriae 1 O antigen polysaccharide biosynthesis in Escherichia coli K-12: 9 kb plasmid of S. dysenteriae 1 determines addition of a galactose residue to the lipopolysaccharide core. Microb Pathog. 1986 Jun;1(3):299–306. doi: 10.1016/0882-4010(86)90055-0. [DOI] [PubMed] [Google Scholar]
- Sturm S., Jann B., Jann K., Fortnagel P., Timmis K. N. Genetic and biochemical analysis of Shigella dysenteriae 1 O antigen polysaccharide biosynthesis in Escherichia coli K-12: structure and functions of the rfb gene cluster. Microb Pathog. 1986 Jun;1(3):307–324. doi: 10.1016/0882-4010(86)90056-2. [DOI] [PubMed] [Google Scholar]
- Sturm S., Timmis K. N. Cloning of the rfb gene region of Shigella dysenteriae 1 and construction of an rfb-rfp gene cassette for the development of lipopolysaccharide-based live anti-dysentery vaccines. Microb Pathog. 1986 Jun;1(3):289–297. doi: 10.1016/0882-4010(86)90054-9. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Kido N., Komatsu T., Ohta M., Kato N. Expression of the cloned Escherichia coli O9 rfb gene in various mutant strains of Salmonella typhimurium. J Bacteriol. 1991 Jan;173(1):55–58. doi: 10.1128/jb.173.1.55-58.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Mascagni P., Nashed M. A., Anderson L., Raetz C. R. Fatty acyl derivatives of glucosamine 1-phosphate in Escherichia coli and their relation to lipid A. Complete structure of A diacyl GlcN-1-P found in a phosphatidylglycerol-deficient mutant. J Biol Chem. 1983 Jun 25;258(12):7379–7385. [PubMed] [Google Scholar]
- Thome B. M., Hoffschulte H. K., Schiltz E., Müller M. A protein with sequence identity to Skp (FirA) supports protein translocation into plasma membrane vesicles of Escherichia coli. FEBS Lett. 1990 Aug 20;269(1):113–116. doi: 10.1016/0014-5793(90)81132-8. [DOI] [PubMed] [Google Scholar]
- Tomasiewicz H. G., McHenry C. S. Sequence analysis of the Escherichia coli dnaE gene. J Bacteriol. 1987 Dec;169(12):5735–5744. doi: 10.1128/jb.169.12.5735-5744.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda M., Ohtsubo E. Mapping of insertion element IS5 in the Escherichia coli K-12 chromosome. Chromosomal rearrangements mediated by IS5. J Mol Biol. 1990 May 20;213(2):229–237. doi: 10.1016/S0022-2836(05)80186-X. [DOI] [PubMed] [Google Scholar]
- Valvano M. A., Crosa J. H. Molecular cloning and expression in Escherichia coli K-12 of chromosomal genes determining the O7 lipopolysaccharide antigen of a human invasive strain of E. coli O7:K1. Infect Immun. 1989 Mar;57(3):937–943. doi: 10.1128/iai.57.3.937-943.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma N. K., Brandt J. M., Verma D. J., Lindberg A. A. Molecular characterization of the O-acetyl transferase gene of converting bacteriophage SF6 that adds group antigen 6 to Shigella flexneri. Mol Microbiol. 1991 Jan;5(1):71–75. doi: 10.1111/j.1365-2958.1991.tb01827.x. [DOI] [PubMed] [Google Scholar]
- Vuorio R., Vaara M. Mutants carrying conditionally lethal mutations in outer membrane genes omsA and firA (ssc) are phenotypically similar, and omsA is allelic to firA. J Bacteriol. 1992 Nov;174(22):7090–7097. doi: 10.1128/jb.174.22.7090-7097.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walenga R. W., Osborn M. J. Biosynthesis of lipid A. In vivo formation of an intermediate containing 3-deoxy-D-mannoctulosonate in a mutant of Salmonella typhimurium. J Biol Chem. 1980 May 10;255(9):4252–4256. [PubMed] [Google Scholar]
- Wandersman C., Delepelaire P. TolC, an Escherichia coli outer membrane protein required for hemolysin secretion. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4776–4780. doi: 10.1073/pnas.87.12.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C., Létoffé S. Involvement of lipopolysaccharide in the secretion of Escherichia coli alpha-haemolysin and Erwinia chrysanthemi proteases. Mol Microbiol. 1993 Jan;7(1):141–150. doi: 10.1111/j.1365-2958.1993.tb01105.x. [DOI] [PubMed] [Google Scholar]
- Wang L., Romana L. K., Reeves P. R. Molecular analysis of a Salmonella enterica group E1 rfb gene cluster: O antigen and the genetic basis of the major polymorphism. Genetics. 1992 Mar;130(3):429–443. doi: 10.1093/genetics/130.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Nakamura A., Timmis K. N. Small virulence plasmid of Shigella dysenteriae 1 strain W30864 encodes a 41,000-dalton protein involved in formation of specific lipopolysaccharide side chains of serotype 1 isolates. Infect Immun. 1984 Oct;46(1):55–63. doi: 10.1128/iai.46.1.55-63.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Timmis K. N. A small plasmid in Shigella dysenteriae 1 specifies one or more functions essential for O antigen production and bacterial virulence. Infect Immun. 1984 Jan;43(1):391–396. doi: 10.1128/iai.43.1.391-396.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R. G., Gemski P., Jr, Stocker B. A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]
- Woisetschläger M., Hödl-Neuhofer A., Högenauer G. Localization of the kdsA gene with the aid of the physical map of the Escherichia coli chromosome. J Bacteriol. 1988 Nov;170(11):5382–5384. doi: 10.1128/jb.170.11.5382-5384.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woisetschläger M., Högenauer G. The kdsA gene coding for 3-deoxy-D-manno-octulosonic acid 8-phosphate synthetase is part of an operon in Escherichia coli. Mol Gen Genet. 1987 May;207(2-3):369–373. doi: 10.1007/BF00331603. [DOI] [PubMed] [Google Scholar]
- Wollin R., Creeger E. S., Rothfield L. I., Stocker B. A., Lindberg A. A. Salmonella typhimurium mutants defective in UDP-D-galactose:lipopolysaccharide alpha 1,6-D-galactosyltransferase. Structural, immunochemical, and enzymologic studies of rfaB mutants. J Biol Chem. 1983 Mar 25;258(6):3769–3774. [PubMed] [Google Scholar]
- Yao Z., Liu H., Valvano M. A. Acetylation of O-specific lipopolysaccharides from Shigella flexneri 3a and 2a occurs in Escherichia coli K-12 carrying cloned S. flexneri 3a and 2a rfb genes. J Bacteriol. 1992 Dec;174(23):7500–7508. doi: 10.1128/jb.174.23.7500-7508.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]



