Abstract
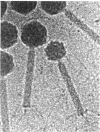
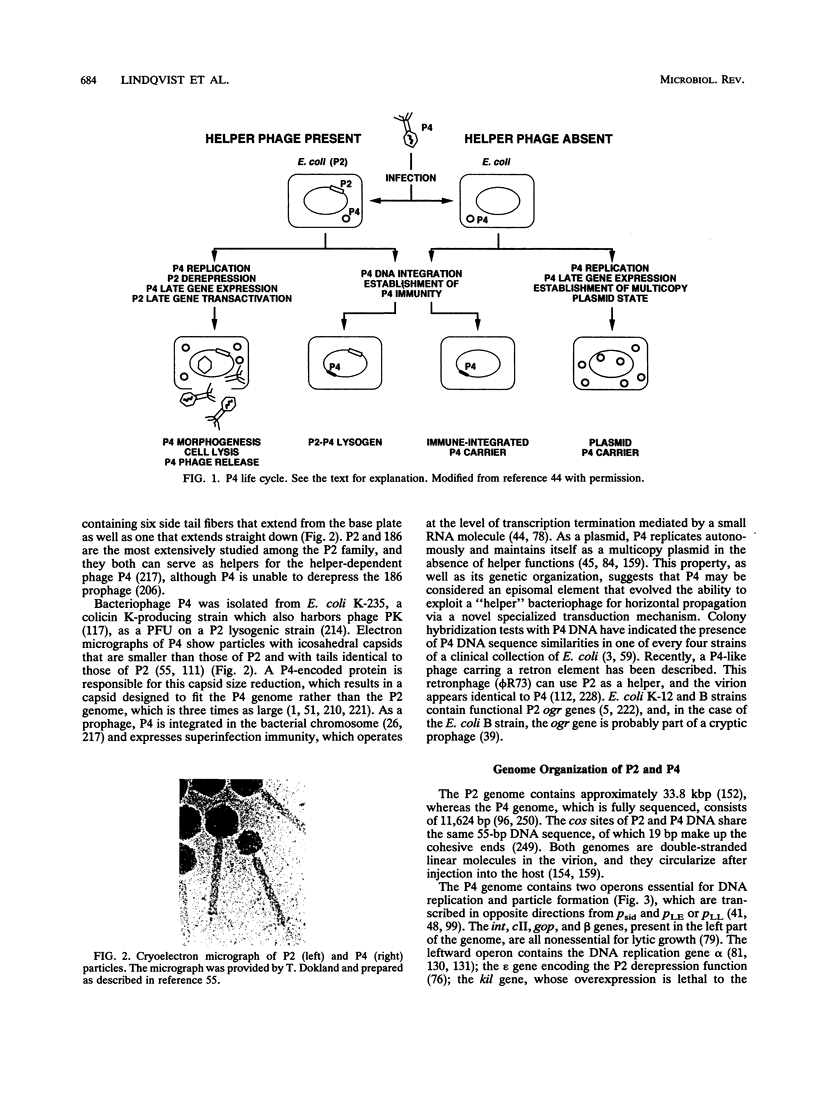
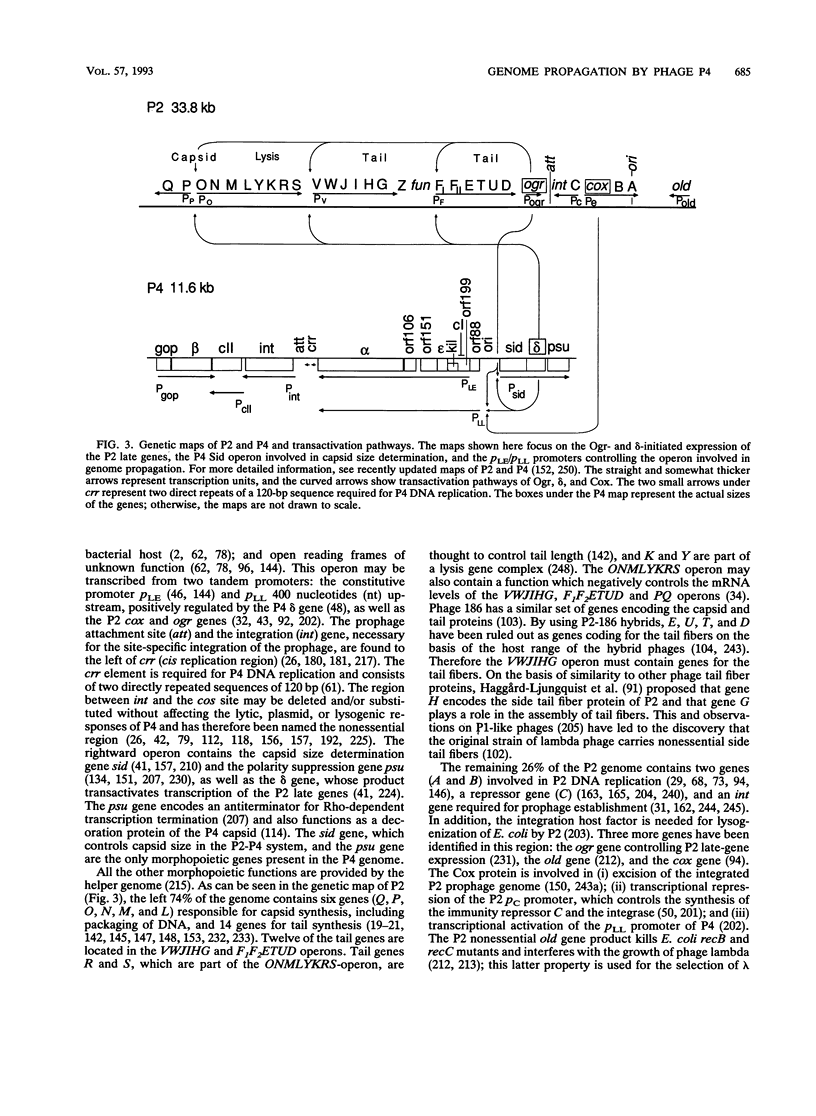
Temperate coliphage P2 and satellite phage P4 have icosahedral capsids and contractile tails with side tail fibers. Because P4 requires all the capsid, tail, and lysis genes (late genes) of P2, the genomes of these phages are in constant communication during P4 development. The P4 genome (11,624 bp) and the P2 genome (33.8 kb) share homologous cos sites of 55 bp which are essential for generating 19-bp cohesive ends but are otherwise dissimilar. P4 turns on the expression of helper phage late genes by two mechanisms: derepression of P2 prophage and transactivation of P2 late-gene promoters. P4 also exploits the morphopoietic pathway of P2 by controlling the capsid size to fit its smaller genome. The P4 sid gene product is responsible for capsid size determination, and the P2 capsid gene product, gpN, is used to build both sizes. The P2 capsid contains 420 capsid protein subunits, and P4 contains 240 subunits. The size reduction appears to involve a major change of the whole hexamer complex. The P4 particles are less stable to heat inactivation, unless their capsids are coated with a P4-encoded decoration protein (the psu gene product). P4 uses a small RNA molecule as its immunity factor. Expression of P4 replication functions is prevented by premature transcription termination effected by this small RNA molecule, which contains a sequence that is complementary to a sequence in the transcript that it terminates.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal M., Arthur M., Arbeit R. D., Goldstein R. Regulation of icosahedral virion capsid size by the in vivo activity of a cloned gene product. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2428–2432. doi: 10.1073/pnas.87.7.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alano P., Dehò G., Sironi G., Zangrossi S. Regulation of the plasmid state of the genetic element P4. Mol Gen Genet. 1986 Jun;203(3):445–450. doi: 10.1007/BF00422069. [DOI] [PubMed] [Google Scholar]
- Argos P., Landy A., Abremski K., Egan J. B., Haggard-Ljungquist E., Hoess R. H., Kahn M. L., Kalionis B., Narayana S. V., Pierson L. S., 3rd The integrase family of site-specific recombinases: regional similarities and global diversity. EMBO J. 1986 Feb;5(2):433–440. doi: 10.1002/j.1460-2075.1986.tb04229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro V., Haggård-Ljungquist E. Attachment sites for bacteriophage P2 on the Escherichia coli chromosome: DNA sequences, localization on the physical map, and detection of a P2-like remnant in E. coli K-12 derivatives. J Bacteriol. 1992 Jun;174(12):4086–4093. doi: 10.1128/jb.174.12.4086-4093.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett K. J., Blinkova A., Arnold G. The bacteriophage P4 alpha gene is the structural gene for bacteriophage P4-induced RNA polymerase. J Virol. 1983 Oct;48(1):157–169. doi: 10.1128/jvi.48.1.157-169.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett K. J., Gibbs W., Calendar R. A transcribing activity induced by satellite phage P4. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2986–2990. doi: 10.1073/pnas.69.10.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett K. J., Marsh M. L., Calendar R. Interactions between a satellite bacteriophage and its helper. J Mol Biol. 1976 Sep 25;106(3):683–707. doi: 10.1016/0022-2836(76)90259-x. [DOI] [PubMed] [Google Scholar]
- Bazinet C., King J. Initiation of P22 procapsid assembly in vivo. J Mol Biol. 1988 Jul 5;202(1):77–86. doi: 10.1016/0022-2836(88)90520-7. [DOI] [PubMed] [Google Scholar]
- Bertani L. E. Abortive induction of bacteriophage P2. Virology. 1968 Sep;36(1):87–103. doi: 10.1016/0042-6822(68)90119-0. [DOI] [PubMed] [Google Scholar]
- Biere A. L., Citron M., Schuster H. Transcriptional control via translational repression by c4 antisense RNA of bacteriophages P1 and P7. Genes Dev. 1992 Dec;6(12A):2409–2416. doi: 10.1101/gad.6.12a.2409. [DOI] [PubMed] [Google Scholar]
- Birkeland N. K., Christie G. E., Lindqvist B. H. Directed mutagenesis of the bacteriophage P2 ogr gene defines an essential function. Gene. 1988 Dec 20;73(2):327–335. doi: 10.1016/0378-1119(88)90497-0. [DOI] [PubMed] [Google Scholar]
- Birkeland N. K., Lindquist B. H. Coliphage P2 late control gene ogr. DNA sequence and product identification. J Mol Biol. 1986 Apr 5;188(3):487–490. doi: 10.1016/0022-2836(86)90170-1. [DOI] [PubMed] [Google Scholar]
- Birkeland N. K., Lindqvist B. H., Christie G. E. Control of bacteriophage P2 gene expression: analysis of transcription of the ogr gene. J Bacteriol. 1991 Nov;173(21):6927–6934. doi: 10.1128/jb.173.21.6927-6934.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden D. W., Calendar R. Maturation of bacteriophage P2 DNA in vitro: A complex, site-specific system for DNA cleavage. J Mol Biol. 1979 Mar 25;129(1):1–18. doi: 10.1016/0022-2836(79)90055-x. [DOI] [PubMed] [Google Scholar]
- Bowden D. W., Modrich P. In vitro maturation of circular bacteriophage P2 DNA. Purification of ter components and characterization of the reaction. J Biol Chem. 1985 Jun 10;260(11):6999–7007. [PubMed] [Google Scholar]
- Bowden D. W., Modrich P. In vitro studies on the bacteriophage P2 terminase system. Prog Clin Biol Res. 1981;64:223–230. [PubMed] [Google Scholar]
- Bowden D. W., Twersky R. S., Calendar R. Escherichia coli deoxyribonucleic acid synthesis mutants: their effect upon bacteriophage P2 and satellite bacteriophage P4 deoxyribonucleic acid synthesis. J Bacteriol. 1975 Oct;124(1):167–175. doi: 10.1128/jb.124.1.167-175.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley C., Ling O. P., Egan J. B. Isolation of phage P2-186 intervarietal hybrids and 186 insertion mutants. Mol Gen Genet. 1975 Sep 29;140(2):123–135. doi: 10.1007/BF00329780. [DOI] [PubMed] [Google Scholar]
- Bullas L. R., Mostaghimi A. R., Arensdorf J. J., Rajadas P. T., Zuccarelli A. J. Salmonella phage PSP3, another member of the P2-like phage group. Virology. 1991 Dec;185(2):918–921. doi: 10.1016/0042-6822(91)90573-t. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- COHEN D. A variant of phage P2 originating in Escherichia coli, strain B. Virology. 1959 Jan;7(1):112–126. doi: 10.1016/0042-6822(59)90180-1. [DOI] [PubMed] [Google Scholar]
- Calendar R., Lindqvist B., Sironi G., Clark A. J. Characterization of REP- mutants and their interaction with P2 phage. Virology. 1970 Jan;40(1):72–83. doi: 10.1016/0042-6822(70)90380-6. [DOI] [PubMed] [Google Scholar]
- Calendar R., Ljungquist E., Deho G., Usher D. C., Goldstein R., Youderian P., Sironi G., Six E. W. Lysogenization by satellite phage P4. Virology. 1981 Aug;113(1):20–38. doi: 10.1016/0042-6822(81)90133-1. [DOI] [PubMed] [Google Scholar]
- Chattoraj D. K., Inman R. B. Location of DNA ends in P2, 186, P4 and lambda bacteriophage heads. J Mol Biol. 1974 Jul 25;87(1):11–22. doi: 10.1016/0022-2836(74)90556-7. [DOI] [PubMed] [Google Scholar]
- Chattoraj D. K. Strand-specific break near the origin of bacteriophage P2 DNA replication. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1685–1689. doi: 10.1073/pnas.75.4.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie G. E., Calendar R. Bacteriophage P2 late promoters. II. Comparison of the four late promoter sequences. J Mol Biol. 1985 Feb 5;181(3):373–382. doi: 10.1016/0022-2836(85)90226-8. [DOI] [PubMed] [Google Scholar]
- Christie G. E., Calendar R. Bacteriophage P2 late promoters. Transcription initiation sites for two late mRNAs. J Mol Biol. 1983 Jul 15;167(4):773–790. doi: 10.1016/s0022-2836(83)80110-7. [DOI] [PubMed] [Google Scholar]
- Christie G. E., Calendar R. Interactions between satellite bacteriophage P4 and its helpers. Annu Rev Genet. 1990;24:465–490. doi: 10.1146/annurev.ge.24.120190.002341. [DOI] [PubMed] [Google Scholar]
- Christie G. E., Haggård-Ljungquist E., Feiwell R., Calendar R. Regulation of bacteriophage P2 late-gene expression: the ogr gene. Proc Natl Acad Sci U S A. 1986 May;83(10):3238–3242. doi: 10.1073/pnas.83.10.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron M., Schuster H. The c4 repressor of bacteriophage P1 is a processed 77 base antisense RNA. Nucleic Acids Res. 1992 Jun 25;20(12):3085–3090. doi: 10.1093/nar/20.12.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron M., Schuster H. The c4 repressors of bacteriophages P1 and P7 are antisense RNAs. Cell. 1990 Aug 10;62(3):591–598. doi: 10.1016/0092-8674(90)90023-8. [DOI] [PubMed] [Google Scholar]
- Coleman J. E. Zinc proteins: enzymes, storage proteins, transcription factors, and replication proteins. Annu Rev Biochem. 1992;61:897–946. doi: 10.1146/annurev.bi.61.070192.004341. [DOI] [PubMed] [Google Scholar]
- Cores de Vries G., Wu X. S., Haggård-Ljungquist E. Genetic analysis of the DNA recognition sequence of the P2 Cox protein. J Virol. 1991 Sep;65(9):4665–4669. doi: 10.1128/jvi.65.9.4665-4669.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale E. C., Christie G. E., Calendar R. Organization and expression of the satellite bacteriophage P4 late gene cluster. J Mol Biol. 1986 Dec 20;192(4):793–803. doi: 10.1016/0022-2836(86)90029-x. [DOI] [PubMed] [Google Scholar]
- Dehò G. Circular genetic map of satellite bacteriophage P4. Virology. 1983 Apr 15;126(1):267–278. doi: 10.1016/0042-6822(83)90478-6. [DOI] [PubMed] [Google Scholar]
- Dehò G., Ghisotti D., Alano P., Zangrossi S., Borrello M. G., Sironi G. Plasmid mode of propagation of the genetic element P4. J Mol Biol. 1984 Sep 15;178(2):191–207. doi: 10.1016/0022-2836(84)90139-6. [DOI] [PubMed] [Google Scholar]
- Dehó G., Zangrossi S., Ghisotti D., Sironi G. Alternative promoters in the development of bacteriophage plasmid P4. J Virol. 1988 May;62(5):1697–1704. doi: 10.1128/jvi.62.5.1697-1704.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana C., Dehò G., Geisselsoder J., Tinelli L., Goldstein R. Viral interference at the level of capsid size determination by satellite phage P4. J Mol Biol. 1978 Dec 15;126(3):433–445. doi: 10.1016/0022-2836(78)90050-5. [DOI] [PubMed] [Google Scholar]
- Dibbens J. A., Egan J. B. Control of gene expression in the temperate coliphage 186. IX. B is the sole phage function needed to activate transcription of the phage late genes. Mol Microbiol. 1992 Sep;6(18):2629–2642. doi: 10.1111/j.1365-2958.1992.tb01440.x. [DOI] [PubMed] [Google Scholar]
- Dibbens J. A., Gregory S. L., Egan J. B. Control of gene expression in the temperate coliphage 186. X. The cI repressor directly represses transcription of the late control gene B. Mol Microbiol. 1992 Sep;6(18):2643–2650. doi: 10.1111/j.1365-2958.1992.tb01441.x. [DOI] [PubMed] [Google Scholar]
- Dokland T., Isaksen M. L., Fuller S. D., Lindqvist B. H. Capsid localization of the bacteriophage P4 Psu protein. Virology. 1993 Jun;194(2):682–687. doi: 10.1006/viro.1993.1308. [DOI] [PubMed] [Google Scholar]
- Dokland T., Lindqvist B. H., Fuller S. D. Image reconstruction from cryo-electron micrographs reveals the morphopoietic mechanism in the P2-P4 bacteriophage system. EMBO J. 1992 Mar;11(3):839–846. doi: 10.1002/j.1460-2075.1992.tb05121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi Y., Itoh T., Tomizawa J. Antisense RNA. Annu Rev Biochem. 1991;60:631–652. doi: 10.1146/annurev.bi.60.070191.003215. [DOI] [PubMed] [Google Scholar]
- Finkel S., Halling C., Calendar R. Selection of lambda Spi- transducing phages using the P2 old gene cloned onto a plasmid. Gene. 1986;46(1):65–69. doi: 10.1016/0378-1119(86)90167-8. [DOI] [PubMed] [Google Scholar]
- Flensburg J., Calendar R. Bacteriophage P4 DNA replication. Nucleotide sequence of the P4 replication gene and the cis replication region. J Mol Biol. 1987 May 20;195(2):439–445. doi: 10.1016/0022-2836(87)90664-4. [DOI] [PubMed] [Google Scholar]
- Frankel A. D., Bredt D. S., Pabo C. O. Tat protein from human immunodeficiency virus forms a metal-linked dimer. Science. 1988 Apr 1;240(4848):70–73. doi: 10.1126/science.2832944. [DOI] [PubMed] [Google Scholar]
- Fujiki H., Palm P., Zillig W., Calendar R., Sunshine M. Identification of a mutation within the structural gene for the a subunit of DNA-dependent RNA polymerase of E. coli. Mol Gen Genet. 1976 Apr 23;145(1):19–22. doi: 10.1007/BF00331552. [DOI] [PubMed] [Google Scholar]
- Funnell B. E., Inman R. B. Bacteriophage P2 DNA replication. Characterization of the requirement of the gene B protein in vivo. J Mol Biol. 1983 Jun 25;167(2):311–334. doi: 10.1016/s0022-2836(83)80338-6. [DOI] [PubMed] [Google Scholar]
- Funnell B. E., Inman R. B. Physical evidence for early transcription in intracellular bacteriophage P2 DNA. J Mol Biol. 1982 Jan 5;154(1):85–101. doi: 10.1016/0022-2836(82)90419-3. [DOI] [PubMed] [Google Scholar]
- Garrett S., Silhavy T. J. Isolation of mutations in the alpha operon of Escherichia coli that suppress the transcriptional defect conferred by a mutation in the porin regulatory gene envZ. J Bacteriol. 1987 Apr;169(4):1379–1385. doi: 10.1128/jb.169.4.1379-1385.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisselsoder J., Mandel M., Calendar R., Chattoraj D. K. In vivo transcription patterns of temperate coliphage P2. J Mol Biol. 1973 Jul 5;77(3):405–415. doi: 10.1016/0022-2836(73)90447-6. [DOI] [PubMed] [Google Scholar]
- Geisselsoder J., Sedivy J. M., Walsh R. B., Goldstein R. Capsid structure of satellite phage P4 and its P2 helper. J Ultrastruct Res. 1982 May;79(2):165–173. doi: 10.1016/s0022-5320(82)90028-4. [DOI] [PubMed] [Google Scholar]
- Geisselsoder J. Strand-specific discontinuity in republicating P2 DNA. J Mol Biol. 1976 Jan 5;100(1):13–22. doi: 10.1016/s0022-2836(76)80030-7. [DOI] [PubMed] [Google Scholar]
- Geisselsoder J., Youdarian P., Dehò G., Chidambaram M., Goldstein R., Ljungquist E. Mutants of satellite virus P4 that cannot derepress their bacteriophage P2 helper. J Mol Biol. 1981 May 5;148(1):1–19. doi: 10.1016/0022-2836(81)90232-1. [DOI] [PubMed] [Google Scholar]
- Ghisotti D., Chiaramonte R., Forti F., Zangrossi S., Sironi G., Dehò G. Genetic analysis of the immunity region of phage-plasmid P4. Mol Microbiol. 1992 Nov;6(22):3405–3413. doi: 10.1111/j.1365-2958.1992.tb02208.x. [DOI] [PubMed] [Google Scholar]
- Ghisotti D., Finkel S., Halling C., Dehò G., Sironi G., Calendar R. Nonessential region of bacteriophage P4: DNA sequence, transcription, gene products, and functions. J Virol. 1990 Jan;64(1):24–36. doi: 10.1128/jvi.64.1.24-36.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs W., Eisen H., Calendar R. In vitro activation of bacteriophage P2 late gene expression by extracts from phage P4-infected cells. J Virol. 1983 Sep;47(3):392–398. doi: 10.1128/jvi.47.3.392-398.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs W., Goldstein R. N., Wiener R., Lindqvist B., Calendar R. Satellite bacteriophage P4: characterization of mutants in two essential genes. Virology. 1973 May;53(1):24–39. doi: 10.1016/0042-6822(73)90462-5. [DOI] [PubMed] [Google Scholar]
- Giffard P. M., Booth I. R. The rpoA341 allele of Escherichia coli specifically impairs the transcription of a group of positively-regulated operons. Mol Gen Genet. 1988 Sep;214(1):148–152. doi: 10.1007/BF00340193. [DOI] [PubMed] [Google Scholar]
- Goldstein R., Lengyel J., Pruss G., Barrett K., Calendar R., Six E. Head size determination and the morphogenesis of satellite phage P4. Curr Top Microbiol Immunol. 1974;(68):59–75. doi: 10.1007/978-3-642-66044-3_3. [DOI] [PubMed] [Google Scholar]
- Goldstein R., Sedivy J., Ljungquist E. Propagation of satellite phage P4 as a plasmid. Proc Natl Acad Sci U S A. 1982 Jan;79(2):515–519. doi: 10.1073/pnas.79.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V. Viral proteins containing the purine NTP-binding sequence pattern. Nucleic Acids Res. 1989 Nov 11;17(21):8413–8440. doi: 10.1093/nar/17.21.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V., Wolf Y. I. A new superfamily of putative NTP-binding domains encoded by genomes of small DNA and RNA viruses. FEBS Lett. 1990 Mar 12;262(1):145–148. doi: 10.1016/0014-5793(90)80175-i. [DOI] [PubMed] [Google Scholar]
- Grambow N. J., Birkeland N. K., Anders D. L., Christie G. E. Deletion analysis of a bacteriophage P2 late promoter. Gene. 1990 Oct 30;95(1):9–15. doi: 10.1016/0378-1119(90)90407-i. [DOI] [PubMed] [Google Scholar]
- Guo P. X., Erickson S., Xu W., Olson N., Baker T. S., Anderson D. Regulation of the phage phi 29 prohead shape and size by the portal vertex. Virology. 1991 Jul;183(1):366–373. doi: 10.1016/0042-6822(91)90149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann L., Agarwal M., Arthur M., Campanelli C., Goldstein R. A phasmid shuttle vector for the cloning of complex operons in Salmonella. Plasmid. 1990 Jan;23(1):42–58. doi: 10.1016/0147-619x(90)90043-c. [DOI] [PubMed] [Google Scholar]
- Haggård-Ljungquist E., Barreiro V., Calendar R., Kurnit D. M., Cheng H. The P2 phage old gene: sequence, transcription and translational control. Gene. 1989 Dec 21;85(1):25–33. doi: 10.1016/0378-1119(89)90460-5. [DOI] [PubMed] [Google Scholar]
- Haggård-Ljungquist E., Halling C., Calendar R. DNA sequences of the tail fiber genes of bacteriophage P2: evidence for horizontal transfer of tail fiber genes among unrelated bacteriophages. J Bacteriol. 1992 Mar;174(5):1462–1477. doi: 10.1128/jb.174.5.1462-1477.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggård-Ljungquist E., Kockum K., Bertani L. E. DNA sequences of bacteriophage P2 early genes cox and B and their regulatory sites. Mol Gen Genet. 1987 Jun;208(1-2):52–56. doi: 10.1007/BF00330421. [DOI] [PubMed] [Google Scholar]
- Halling C., Calendar R. Bacteriophage P2 ogr and P4 delta genes act independently and are essential for P4 multiplication. J Bacteriol. 1990 Jul;172(7):3549–3558. doi: 10.1128/jb.172.7.3549-3558.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halling C., Sunshine M. G., Lane K. B., Six E. W., Calendar R. A mutation of the transactivation gene of satellite bacteriophage P4 that suppresses the rpoA109 mutation of Escherichia coli. J Bacteriol. 1990 Jul;172(7):3541–3548. doi: 10.1128/jb.172.7.3541-3548.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. B. Structure and regulation of the lytic replicon of phage P1. J Mol Biol. 1989 May 5;207(1):135–149. doi: 10.1016/0022-2836(89)90445-2. [DOI] [PubMed] [Google Scholar]
- Harris J. D., Calendar R. Transcription map of satellite coliphage P4. Virology. 1978 Apr;85(2):343–358. doi: 10.1016/0042-6822(78)90443-9. [DOI] [PubMed] [Google Scholar]
- Hauser M. A., Scocca J. J. Location of the host attachment site for phage HPl within a cluster of Haemophilus influenzae tRNA genes. Nucleic Acids Res. 1990 Sep 11;18(17):5305–5305. doi: 10.1093/nar/18.17.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser M. A., Scocca J. J. Site-specific integration of the Haemophilus influenzae bacteriophage HP1. Identification of the points of recombinational strand exchange and the limits of the host attachment site. J Biol Chem. 1992 Apr 5;267(10):6859–6864. [PubMed] [Google Scholar]
- Heisig A., Riedel H. D., Dobrinski B., Lurz R., Schuster H. Organization of the immunity region immI of bacteriophage P1 and synthesis of the P1 antirepressor. J Mol Biol. 1989 Oct 20;209(4):525–538. doi: 10.1016/0022-2836(89)90591-3. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W., Casjens S. R. Assembly of bacteriophage lambda heads: protein processing and its genetic control in petit lambda assembly. J Mol Biol. 1975 Jan 15;91(2):187–199. doi: 10.1016/0022-2836(75)90159-x. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W., Duda R. L. Bacteriophage lambda PaPa: not the mother of all lambda phages. Science. 1992 Nov 13;258(5085):1145–1148. doi: 10.1126/science.1439823. [DOI] [PubMed] [Google Scholar]
- Hocking S. M., Egan J. B. Genetic characterization of twelve P2-186 hybrid bacteriophages. Mol Gen Genet. 1982;187(1):174–176. doi: 10.1007/BF00384403. [DOI] [PubMed] [Google Scholar]
- Hocking S. M., Egan J. B. Genetic studies of coliphage 186. I. Genes associated with phage morphogenesis. J Virol. 1982 Dec;44(3):1056–1067. doi: 10.1128/jvi.44.3.1056-1067.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwich A. L., Neupert W., Hartl F. U. Protein-catalysed protein folding. Trends Biotechnol. 1990 May;8(5):126–131. doi: 10.1016/0167-7799(90)90153-o. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Ishihama A. Bipartite functional map of the E. coli RNA polymerase alpha subunit: involvement of the C-terminal region in transcription activation by cAMP-CRP. Cell. 1991 Jun 14;65(6):1015–1022. doi: 10.1016/0092-8674(91)90553-b. [DOI] [PubMed] [Google Scholar]
- Ilyina T. V., Gorbalenya A. E., Koonin E. V. Organization and evolution of bacterial and bacteriophage primase-helicase systems. J Mol Evol. 1992 Apr;34(4):351–357. doi: 10.1007/BF00160243. [DOI] [PubMed] [Google Scholar]
- Inman R. B., Schnös M., Simon L. D., Six E. W., Walker D. H., Jr Some morphological properties of P4 bacteriophage and P4 DNA. Virology. 1971 Apr;44(1):67–72. doi: 10.1016/0042-6822(71)90153-x. [DOI] [PubMed] [Google Scholar]
- Inouye S., Sunshine M. G., Six E. W., Inouye M. Retronphage phi R73: an E. coli phage that contains a retroelement and integrates into a tRNA gene. Science. 1991 May 17;252(5008):969–971. doi: 10.1126/science.1709758. [DOI] [PubMed] [Google Scholar]
- Isaksen M. L., Dokland T., Lindqvist B. H. Characterization of the capsid associating activity of bacteriophage P4's Psu protein. Virology. 1993 Jun;194(2):674–681. doi: 10.1006/viro.1993.1307. [DOI] [PubMed] [Google Scholar]
- Isaksen M. L., Rishovd S. T., Calendar R., Lindqvist B. H. The polarity suppression factor of bacteriophage P4 is also a decoration protein of the P4 capsid. Virology. 1992 Jun;188(2):831–839. doi: 10.1016/0042-6822(92)90538-z. [DOI] [PubMed] [Google Scholar]
- Ishii T., Yamaguchi Y., Yanagida M. Binding of the structural protein soc to the head shell of bacteriophage T4. J Mol Biol. 1978 Apr 25;120(4):533–544. doi: 10.1016/0022-2836(78)90352-2. [DOI] [PubMed] [Google Scholar]
- Ishii T., Yanagida M. The two dispensable structural proteins (soc and hoc) of the T4 phage capsid; their purification and properties, isolation and characterization of the defective mutants, and their binding with the defective heads in vitro. J Mol Biol. 1977 Feb 5;109(4):487–514. doi: 10.1016/s0022-2836(77)80088-0. [DOI] [PubMed] [Google Scholar]
- JESAITIS M. A., HUTTON J. J. Properties of a bacteriophage derived from Escherichia coli K235. J Exp Med. 1963 Feb 1;117:285–302. doi: 10.1084/jem.117.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M. L., Timblin C. R. Gene fusion vehicles for the analysis of gene expression in Rhizobium meliloti. J Bacteriol. 1984 Jun;158(3):1070–1077. doi: 10.1128/jb.158.3.1070-1077.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M. L., Ziermann R., Dehò G., Ow D. W., Sunshine M. G., Calendar R. Bacteriophage P2 and P4. Methods Enzymol. 1991;204:264–280. doi: 10.1016/0076-6879(91)04013-e. [DOI] [PubMed] [Google Scholar]
- Kahn M., Ow D., Sauer B., Rabinowitz A., Calendar R. Genetic analysis of bacteriophage P4 using P4-plasmid ColE1 hybrids. Mol Gen Genet. 1980 Feb;177(3):399–412. doi: 10.1007/BF00271478. [DOI] [PubMed] [Google Scholar]
- Kainuma-Kuroda R., Okazaki R. Mechanism of DNA chain growth. XII. Asymmetry of replication of P2 phage DNA. J Mol Biol. 1975 May 15;94(2):213–228. doi: 10.1016/0022-2836(75)90079-0. [DOI] [PubMed] [Google Scholar]
- Kalionis B., Dodd I. B., Egan J. B. Control of gene expression in the P2-related template coliphages. III. DNA sequence of the major control region of phage 186. J Mol Biol. 1986 Sep 20;191(2):199–209. doi: 10.1016/0022-2836(86)90257-3. [DOI] [PubMed] [Google Scholar]
- Kalionis B., Pritchard M., Egan J. B. Control of gene expression in the P2-related temperate coliphages. IV. Concerning the late control gene and control of its transcription. J Mol Biol. 1986 Sep 20;191(2):211–220. doi: 10.1016/0022-2836(86)90258-5. [DOI] [PubMed] [Google Scholar]
- Kanda T., Watanabe S., Zanma S., Sato H., Furuno A., Yoshiike K. Human papillomavirus type 16 E6 proteins with glycine substitution for cysteine in the metal-binding motif. Virology. 1991 Dec;185(2):536–543. doi: 10.1016/0042-6822(91)90523-e. [DOI] [PubMed] [Google Scholar]
- Katsura I. Structure and inherent properties of the bacteriophage lambda head shell. IV. Small-head mutants. J Mol Biol. 1983 Dec 15;171(3):297–317. doi: 10.1016/0022-2836(83)90095-5. [DOI] [PubMed] [Google Scholar]
- Keener J., Dale E. C., Kustu S., Calendar R. In vitro transcription from the late promoter of bacteriophage P4. J Bacteriol. 1988 Aug;170(8):3543–3546. doi: 10.1128/jb.170.8.3543-3546.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. A., Anders D. L., Christie G. E. Site-directed mutagenesis of an amino acid residue in the bacteriophage P2 ogr protein implicated in interaction with Escherichia coli RNA polymerase. Mol Microbiol. 1992 Nov;6(22):3313–3320. doi: 10.1111/j.1365-2958.1992.tb02199.x. [DOI] [PubMed] [Google Scholar]
- Koonin E. V., Gorbalenya A. E. The superfamily of UvrA-related ATPases includes three more subunits of putative ATP-dependent nucleases. Protein Seq Data Anal. 1992;5(1):43–45. [PubMed] [Google Scholar]
- Krevolin M. D., Calendar R. The replication of bacteriophage P4 DNA in vitro. Partial purification of the P4 alpha gene product. J Mol Biol. 1985 Apr 20;182(4):509–517. doi: 10.1016/0022-2836(85)90237-2. [DOI] [PubMed] [Google Scholar]
- Krevolin M. D., Inman R. B., Roof D., Kahn M., Calendar R. Bacteriophage P4 DNA replication. Location of the P4 origin. J Mol Biol. 1985 Apr 20;182(4):519–527. doi: 10.1016/0022-2836(85)90238-4. [DOI] [PubMed] [Google Scholar]
- Kurosawa Y., Okazaki R. Mechanism of DNA chain growth. XIII. Evidence for discontinuous replication of both strands of P2 phage DNA. J Mol Biol. 1975 May 15;94(2):229–241. doi: 10.1016/0022-2836(75)90080-7. [DOI] [PubMed] [Google Scholar]
- Lagos R., Goldstein R. Phasmid P4: manipulation of plasmid copy number and induction from the integrated state. J Bacteriol. 1984 Apr;158(1):208–215. doi: 10.1128/jb.158.1.208-215.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos R., Jiang R. Z., Kim S., Goldstein R. Rho-dependent transcription termination of a bacterial operon is antagonized by an extrachromosomal gene product. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9561–9565. doi: 10.1073/pnas.83.24.9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont I., Brumby A. M., Egan J. B. UV induction of coliphage 186: prophage induction as an SOS function. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5492–5496. doi: 10.1073/pnas.86.14.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont I., Kalionis B., Egan J. B. Control of gene expression in the P2-related temperate coliphages. V. The use of sequence analysis of 186 Vir mutants to indicate presumptive repressor binding sites. J Mol Biol. 1988 Jan 20;199(2):379–382. doi: 10.1016/0022-2836(88)90321-x. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Christie G. E. Purification and properties of the bacteriophage P2 ogr gene product. A prokaryotic zinc-binding transcriptional activator. J Biol Chem. 1990 May 5;265(13):7472–7477. [PubMed] [Google Scholar]
- Lengyel J. A., Calendar R. Control of bacteriophage P2 protein and DNA synthesis. Virology. 1974 Feb;57(2):305–313. doi: 10.1016/0042-6822(74)90170-6. [DOI] [PubMed] [Google Scholar]
- Lengyel J. A., Goldstein R. N., Marsh M., Calendar R. Structure of the bacteriophage P2 tail. Virology. 1974 Nov;62(1):161–174. doi: 10.1016/0042-6822(74)90312-2. [DOI] [PubMed] [Google Scholar]
- Lengyel J. A., Goldstein R. N., Marsh M., Sunshine M. G., Calendar R. Bacteriophage P2 head morphogenesis: cleavage of the major capsid protein. Virology. 1973 May;53(1):1–23. doi: 10.1016/0042-6822(73)90461-3. [DOI] [PubMed] [Google Scholar]
- Lin C. S. Nucleotide sequence of the essential region of bacteriophage P4. Nucleic Acids Res. 1984 Nov 26;12(22):8667–8684. doi: 10.1093/nar/12.22.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl G. Bacteriophage P2: replication of the chromosome requires a protein which acts only on the genome that coded for it. Virology. 1970 Oct;42(2):522–533. doi: 10.1016/0042-6822(70)90295-3. [DOI] [PubMed] [Google Scholar]
- Lindahl G. Characterization of conditional lethal mutants of bacteriophage P2. Mol Gen Genet. 1974 Feb 6;128(3):249–260. doi: 10.1007/BF00267114. [DOI] [PubMed] [Google Scholar]
- Lindahl G. Genetic map of bacteriophage P2. Virology. 1969 Dec;39(4):839–860. doi: 10.1016/0042-6822(69)90021-x. [DOI] [PubMed] [Google Scholar]
- Lindahl G. On the control of transcription in bacteriophage P2. Virology. 1971 Dec;46(3):620–633. doi: 10.1016/0042-6822(71)90065-1. [DOI] [PubMed] [Google Scholar]
- Lindahl G., Sironi G., Bialy H., Calendar R. Bacteriophage lambda; abortive infection of bacteria lysogenic for phage P2. Proc Natl Acad Sci U S A. 1970 Jul;66(3):587–594. doi: 10.1073/pnas.66.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl G., Sunshine M. Excision-deficient mutants of bacteriophage P2. Virology. 1972 Jul;49(1):180–187. doi: 10.1016/s0042-6822(72)80019-9. [DOI] [PubMed] [Google Scholar]
- Linderoth N. A., Calendar R. L. The Psu protein of bacteriophage P4 is an antitermination factor for rho-dependent transcription termination. J Bacteriol. 1991 Nov;173(21):6722–6731. doi: 10.1128/jb.173.21.6722-6731.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linderoth N. A., Ziermann R., Haggård-Ljungquist E., Christie G. E., Calendar R. Nucleotide sequence of the DNA packaging and capsid synthesis genes of bacteriophage P2. Nucleic Acids Res. 1991 Dec;19(25):7207–7214. doi: 10.1093/nar/19.25.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist B. H., Bovre K. Asymmetric transcription of the coliphage P2 genome during infection. Virology. 1972 Sep;49(3):690–699. doi: 10.1016/0042-6822(72)90526-0. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H. Expression of phage transcription in P2 lysogens infected with helper-dependent coliphage P4. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2752–2755. doi: 10.1073/pnas.71.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist B. H. Recombination between satellite phage P4 and its helper P2. I. In vivo and in vitro construction of P4: :P2 hybrid satellite phage. Gene. 1981 Sep;14(4):231–241. doi: 10.1016/0378-1119(81)90156-6. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H. Recombination between satellite phage P4 and its helper P2. II. In vitro construction of a helper-independent P4: :P2 hybrid phage. Gene. 1981 Sep;14(4):243–250. doi: 10.1016/0378-1119(81)90157-8. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H., Six E. W. Replication of bacteriophage P4 DNA in a nonlysogenic host. Virology. 1971 Jan;43(1):1–7. doi: 10.1016/0042-6822(71)90218-2. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H. Vegetative DNA of temperate coliphage P2. Mol Gen Genet. 1971;110(2):178–196. doi: 10.1007/BF00332647. [DOI] [PubMed] [Google Scholar]
- Lindsey D. F., Mullin D. A., Walker J. R. Characterization of the cryptic lambdoid prophage DLP12 of Escherichia coli and overlap of the DLP12 integrase gene with the tRNA gene argU. J Bacteriol. 1989 Nov;171(11):6197–6205. doi: 10.1128/jb.171.11.6197-6205.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungquist E., Bertani L. E. Properties and products of the cloned int gene of bacteriophage P2. Mol Gen Genet. 1983;192(1-2):87–94. doi: 10.1007/BF00327651. [DOI] [PubMed] [Google Scholar]
- Ljungquist E., Kockum K., Bertani L. E. DNA sequences of the repressor gene and operator region of bacteriophage P2. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3988–3992. doi: 10.1073/pnas.81.13.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo M. J., Bagga D., Miller C. G. Mutations in rpoA affect expression of anaerobically regulated genes in Salmonella typhimurium. J Bacteriol. 1991 Dec;173(23):7511–7518. doi: 10.1128/jb.173.23.7511-7518.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist B., Bertani G. Immunity repressor of bacteriophage P2. Identification and DNA-binding activity. J Mol Biol. 1984 Sep 25;178(3):629–651. doi: 10.1016/0022-2836(84)90242-0. [DOI] [PubMed] [Google Scholar]
- Marschalek R., Brechner T., Amon-Böhm E., Dingermann T. Transfer RNA genes: landmarks for integration of mobile genetic elements in Dictyostelium discoideum. Science. 1989 Jun 23;244(4911):1493–1496. doi: 10.1126/science.2567533. [DOI] [PubMed] [Google Scholar]
- Matsuyama S., Mizushima S. Novel rpoA mutation that interferes with the function of OmpR and EnvZ, positive regulators of the ompF and ompC genes that code for outer-membrane proteins in Escherichia coli K12. J Mol Biol. 1987 Jun 20;195(4):847–853. doi: 10.1016/0022-2836(87)90489-x. [DOI] [PubMed] [Google Scholar]
- Mazodier P., Thompson C., Boccard F. The chromosomal integration site of the Streptomyces element pSAM2 overlaps a putative tRNA gene conserved among actinomycetes. Mol Gen Genet. 1990 Jul;222(2-3):431–434. doi: 10.1007/BF00633850. [DOI] [PubMed] [Google Scholar]
- Monaco H. L., Crawford J. L., Lipscomb W. N. Three-dimensional structures of aspartate carbamoyltransferase from Escherichia coli and of its complex with cytidine triphosphate. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5276–5280. doi: 10.1073/pnas.75.11.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaratnam S., Myles G. M., Strange R. W., Sancar A. Evidence from extended X-ray absorption fine structure and site-specific mutagenesis for zinc fingers in UvrA protein of Escherichia coli. J Biol Chem. 1989 Sep 25;264(27):16067–16071. [PubMed] [Google Scholar]
- Nehlin J. O., Carlberg M., Ronne H. Control of yeast GAL genes by MIG1 repressor: a transcriptional cascade in the glucose response. EMBO J. 1991 Nov;10(11):3373–3377. doi: 10.1002/j.1460-2075.1991.tb04901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Iordanescu S., Projan S. J., Kornblum J., Edelman I. pT181 plasmid replication is regulated by a countertranscript-driven transcriptional attenuator. Cell. 1989 Oct 20;59(2):395–404. doi: 10.1016/0092-8674(89)90300-0. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Freundlich M. Mechanism for the autogenous control of the crp operon: transcriptional inhibition by a divergent RNA transcript. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5000–5004. doi: 10.1073/pnas.83.14.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ow D. W., Ausubel F. M. Conditionally replicating plasmid vectors that can integrate into the Klebsiella pneumoniae chromosome via bacteriophage P4 site-specific recombination. J Bacteriol. 1983 Aug;155(2):704–713. doi: 10.1128/jb.155.2.704-713.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ow D. W., Ausubel F. M. Recombinant P4 bacteriophages propagate as viable lytic phages or as autonomous plasmids in Klebsiella pneumoniae. Mol Gen Genet. 1980;180(1):165–175. doi: 10.1007/BF00267366. [DOI] [PubMed] [Google Scholar]
- Pansegrau W., Lanka E. A common sequence motif among prokaryotic DNA primases. Nucleic Acids Res. 1992 Sep 25;20(18):4931–4931. doi: 10.1093/nar/20.18.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson L. S., 3rd, Kahn M. L. Cloning of the integration and attachment regions of bacteriophage P4. Mol Gen Genet. 1984;195(1-2):44–51. doi: 10.1007/BF00332722. [DOI] [PubMed] [Google Scholar]
- Pierson L. S., 3rd, Kahn M. L. Integration of satellite bacteriophage P4 in Escherichia coli. DNA sequences of the phage and host regions involved in site-specific recombination. J Mol Biol. 1987 Aug 5;196(3):487–496. doi: 10.1016/0022-2836(87)90026-x. [DOI] [PubMed] [Google Scholar]
- Polissi A., Bertoni G., Acquati F., Dehò G. Cloning and transposon vectors derived from satellite bacteriophage P4 for genetic manipulation of Pseudomonas and other gram-negative bacteria. Plasmid. 1992 Sep;28(2):101–114. doi: 10.1016/0147-619x(92)90041-8. [DOI] [PubMed] [Google Scholar]
- Prevelige P. E., Jr, Thomas D., King J. Scaffolding protein regulates the polymerization of P22 coat subunits into icosahedral shells in vitro. J Mol Biol. 1988 Aug 20;202(4):743–757. doi: 10.1016/0022-2836(88)90555-4. [DOI] [PubMed] [Google Scholar]
- Pritchard M., Egan J. B. Control of gene expression in P2-related coliphages: the in vitro transcription pattern of coliphage 186. EMBO J. 1985 Dec 16;4(13A):3599–3604. doi: 10.1002/j.1460-2075.1985.tb04123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss G. J., Calendar R. Maturation of bacteriophage P2 DNA. Virology. 1978 May 15;86(2):454–467. doi: 10.1016/0042-6822(78)90085-5. [DOI] [PubMed] [Google Scholar]
- Pruss G. J., Wang J. C., Calendar R. In vitro packaging of covalently closed circular monomers of bacteriophage DNA. J Mol Biol. 1975 Nov 5;98(3):465–478. doi: 10.1016/s0022-2836(75)80080-5. [DOI] [PubMed] [Google Scholar]
- Pruss G., Barrett K., Lengyel J., Goldstein R., Calendar R. Phage head size determination and head protein cleavage in vitro. J Supramol Struct. 1974;2(2-4):337–348. doi: 10.1002/jss.400020223. [DOI] [PubMed] [Google Scholar]
- Pruss G., Goldstein R. N., Calendar R. In vitro packaging of satellite phage P4 DNA. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2367–2371. doi: 10.1073/pnas.71.6.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi A., Donghi R., Montaguti A., Pessina A., Dehò G. Analysis of spontaneous deletion mutants of satellite bacteriophage P4. J Virol. 1985 Apr;54(1):233–235. doi: 10.1128/jvi.54.1.233-235.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter W. D., Palm P., Yeats S. Transfer RNA genes frequently serve as integration sites for prokaryotic genetic elements. Nucleic Acids Res. 1989 Mar 11;17(5):1907–1914. doi: 10.1093/nar/17.5.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards K. E., Williams R. C., Calendar R. Mode of DNA packing within bacteriophage heads. J Mol Biol. 1973 Aug 5;78(2):255–259. doi: 10.1016/0022-2836(73)90114-9. [DOI] [PubMed] [Google Scholar]
- Richardson H., Puspurs A., Egan J. B. Control of gene expression in the P2-related temperate coliphage 186. VI. Sequence analysis of the early lytic region. J Mol Biol. 1989 Mar 5;206(1):251–255. doi: 10.1016/0022-2836(89)90539-1. [DOI] [PubMed] [Google Scholar]
- Rishovd S., Lindqvist B. Bacteriophage P2 and P4 morphogenesis: protein processing and capsid size determination. Virology. 1992 Apr;187(2):548–554. doi: 10.1016/0042-6822(92)90457-z. [DOI] [PubMed] [Google Scholar]
- Rowland G. C., Giffard P. M., Booth I. R. phs Locus of Escherichia coli, a mutation causing pleiotropic lesions in metabolism, is an rpoA allele. J Bacteriol. 1985 Nov;164(2):972–975. doi: 10.1128/jb.164.2.972-975.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnels J. M., Soltis D., Hey T., Snyder L. Genetic and physiological studies of the role of the RNA ligase of bacteriophage T4. J Mol Biol. 1982 Jan 15;154(2):273–286. doi: 10.1016/0022-2836(82)90064-x. [DOI] [PubMed] [Google Scholar]
- Saha S., Haggård-Ljungquist E., Nordström K. Activation of prophage P4 by the P2 Cox protein and the sites of action of the Cox protein on the two phage genomes. Proc Natl Acad Sci U S A. 1989 Jun;86(11):3973–3977. doi: 10.1073/pnas.86.11.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S., Haggård-Ljungquist E., Nordström K. Integration host factor is necessary for lysogenization of Escherichia coli by bacteriophage P2. Mol Microbiol. 1990 Jan;4(1):3–11. doi: 10.1111/j.1365-2958.1990.tb02009.x. [DOI] [PubMed] [Google Scholar]
- Saha S., Haggård-Ljungquist E., Nordström K. The cox protein of bacteriophage P2 inhibits the formation of the repressor protein and autoregulates the early operon. EMBO J. 1987 Oct;6(10):3191–3199. doi: 10.1002/j.1460-2075.1987.tb02631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S., Lundqvist B., Haggård-Ljungquist E. Autoregulation of bacteriophage P2 repressor. EMBO J. 1987 Mar;6(3):809–814. doi: 10.1002/j.1460-2075.1987.tb04823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmeier H., Iida S., Arber W. DNA inversion regions Min of plasmid p15B and Cin of bacteriophage P1: evolution of bacteriophage tail fiber genes. J Bacteriol. 1992 Jun;174(12):3936–3944. doi: 10.1128/jb.174.12.3936-3944.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B., Calendar R., Ljungquist E., Six E., Sunshine M. G. Interaction of satellite phage P4 with phage 186 helper. Virology. 1982 Jan 30;116(2):523–534. doi: 10.1016/0042-6822(82)90145-3. [DOI] [PubMed] [Google Scholar]
- Sauer B., Ow D., Ling L., Calendar R. Mutants of satellite bacteriophage P4 that are defective in the suppression of transcriptional polarity. J Mol Biol. 1981 Jan 5;145(1):29–46. doi: 10.1016/0022-2836(81)90333-8. [DOI] [PubMed] [Google Scholar]
- Schachman H. K., Pauza C. D., Navre M., Karels M. J., Wu L., Yang Y. R. Location of amino acid alterations in mutants of aspartate transcarbamoylase: Structural aspects of interallelic complementation. Proc Natl Acad Sci U S A. 1984 Jan;81(1):115–119. doi: 10.1073/pnas.81.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnös M., Inman R. B. Starting point and direction of replication in P2 DNA. J Mol Biol. 1971 Jan 14;55(1):31–38. doi: 10.1016/0022-2836(71)90278-6. [DOI] [PubMed] [Google Scholar]
- Shore D., Dehò G., Tsipis J., Goldstein R. Determination of capsid size by satellite bacteriophage P4. Proc Natl Acad Sci U S A. 1978 Jan;75(1):400–404. doi: 10.1073/pnas.75.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Kleckner N. Biological regulation by antisense RNA in prokaryotes. Annu Rev Genet. 1988;22:567–600. doi: 10.1146/annurev.ge.22.120188.003031. [DOI] [PubMed] [Google Scholar]
- Sironi G., Bialy H., Lozeron H. A., Calendar R. Bacteriophage P2: interaction with phage lambda and with recombination-deficient bacteria. Virology. 1971 Nov;46(2):387–396. doi: 10.1016/0042-6822(71)90040-7. [DOI] [PubMed] [Google Scholar]
- Sironi G. Mutants of Escherichia coli unable to be lysogenized by the temperate bacteriophage P2. Virology. 1969 Feb;37(2):163–176. doi: 10.1016/0042-6822(69)90196-2. [DOI] [PubMed] [Google Scholar]
- Six E. W., Klug C. A. Bacteriophage P4: a satellite virus depending on a helper such as prophage P2. Virology. 1973 Feb;51(2):327–344. doi: 10.1016/0042-6822(73)90432-7. [DOI] [PubMed] [Google Scholar]
- Six E. W., Lindqvist B. H. Multiplication of bacteriophage P4 in the absence of replication of the DNA of its helper. Virology. 1971 Jan;43(1):8–15. doi: 10.1016/0042-6822(71)90219-4. [DOI] [PubMed] [Google Scholar]
- Six E. W., Lindqvist B. H. Mutual derepression in the P2-P4 bacteriophage system. Virology. 1978 Jun 15;87(2):217–230. doi: 10.1016/0042-6822(78)90127-7. [DOI] [PubMed] [Google Scholar]
- Six E. W., Sunshine M. G., Williams J., Haggård-Ljungquist E., Lindqvist B. H. Morphopoietic switch mutations of bacteriophage P2. Virology. 1991 May;182(1):34–46. doi: 10.1016/0042-6822(91)90645-r. [DOI] [PubMed] [Google Scholar]
- Six E. W. The helper dependence of satellite bacteriophage P4: which gene functions of bacteriophage P2 are needed by P4? Virology. 1975 Sep;67(1):249–263. doi: 10.1016/0042-6822(75)90422-5. [DOI] [PubMed] [Google Scholar]
- Slettan A., Gebhardt K., Kristiansen E., Birkeland N. K., Lindqvist B. H. Escherichia coli K-12 and B contain functional bacteriophage P2 ogr genes. J Bacteriol. 1992 Jun;174(12):4094–4100. doi: 10.1128/jb.174.12.4094-4100.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snopek T. J., Wood W. B., Conley M. P., Chen P., Cozzarelli N. R. Bacteriophage T4 RNA ligase is gene 63 product, the protein that promotes tail fiber attachment to the baseplate. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3355–3359. doi: 10.1073/pnas.74.8.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza L., Calendar R., Six E. W., Lindqvist B. H. A transactivation mutant of satellite phage P4. Virology. 1977 Aug;81(1):81–90. doi: 10.1016/0042-6822(77)90060-5. [DOI] [PubMed] [Google Scholar]
- Souza L., Geisselsoder J., Hopkins A., Calender R. Physical mapping of the satellite phage P4 genome. Virology. 1978 Apr;85(2):335–342. doi: 10.1016/0042-6822(78)90442-7. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Weisberg R. Packaging of coliphage lambda DNA. II. The role of the gene D protein. J Mol Biol. 1977 Dec 15;117(3):733–759. doi: 10.1016/0022-2836(77)90067-5. [DOI] [PubMed] [Google Scholar]
- Strack B., Lessl M., Calendar R., Lanka E. A common sequence motif, -E-G-Y-A-T-A-, identified within the primase domains of plasmid-encoded I- and P-type DNA primases and the alpha protein of the Escherichia coli satellite phage P4. J Biol Chem. 1992 Jun 25;267(18):13062–13072. [PubMed] [Google Scholar]
- Sun J., Inouye M., Inouye S. Association of a retroelement with a P4-like cryptic prophage (retronphage phi R73) integrated into the selenocystyl tRNA gene of Escherichia coli. J Bacteriol. 1991 Jul;173(13):4171–4181. doi: 10.1128/jb.173.13.4171-4181.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunshine M. G., Sauer B. A bacterial mutation blocking P2 phage late gene expression. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2770–2774. doi: 10.1073/pnas.72.7.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunshine M. G., Thorn M., Gibbs W., Calendar R., Kelly B. P2 phage amber mutants: characterization by use of a polarity suppressor. Virology. 1971 Dec;46(3):691–702. doi: 10.1016/0042-6822(71)90071-7. [DOI] [PubMed] [Google Scholar]
- Sunshine M., Six E. Relief of P2 bacteriophage amber mutant polarity by the satellite bacteriophage P4. J Mol Biol. 1976 Sep 25;106(3):673–682. doi: 10.1016/0022-2836(76)90258-8. [DOI] [PubMed] [Google Scholar]
- Sunshine M., Six E. sub, a host mutation that specifically allows growth of replication-deficient gene B mutants of coliphage P2. Mol Gen Genet. 1986 Aug;204(2):359–361. doi: 10.1007/BF00425523. [DOI] [PubMed] [Google Scholar]
- Temple L. M., Forsburg S. L., Calendar R., Christie G. E. Nucleotide sequence of the genes encoding the major tail sheath and tail tube proteins of bacteriophage P2. Virology. 1991 Mar;181(1):353–358. doi: 10.1016/0042-6822(91)90502-3. [DOI] [PubMed] [Google Scholar]
- Van Bokkelen G. B., Dale E. C., Halling C., Calendar R. Mutational analysis of a bacteriophage P4 late promoter. J Bacteriol. 1991 Jan;173(1):37–45. doi: 10.1128/jb.173.1.37-45.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. H., Jr, Anderson T. F. Morphological variants of coliphage P1. J Virol. 1970 Jun;5(6):765–782. doi: 10.1128/jvi.5.6.765-782.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C., Martin K. V., Calendar R. On the sequence similarity of the cohesive ends of coliphage P4, P2, and 186 deoxyribonucleic acid. Biochemistry. 1973 May 22;12(11):2119–2123. doi: 10.1021/bi00735a016. [DOI] [PubMed] [Google Scholar]
- Westö A., Ljungquist E. Cloning of the immunity repressor determinant of bacteriophage P2 in the pBR322 plasmid. Mol Gen Genet. 1980 Apr;178(1):101–109. doi: 10.1007/BF00267218. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Inokuchi H., Ozeki H. Identification of transfer RNA suppressors in Escherichia coli. IV. Amber suppressor Su+6 a double mutant of a new species of leucine tRNA. J Mol Biol. 1984 Aug 25;177(4):627–644. doi: 10.1016/0022-2836(84)90041-x. [DOI] [PubMed] [Google Scholar]
- Yu A., Bertani L. E., Haggård-Ljungquist E. Control of prophage integration and excision in bacteriophage P2: nucleotide sequences of the int gene and att sites. Gene. 1989 Aug 1;80(1):1–11. doi: 10.1016/0378-1119(89)90244-8. [DOI] [PubMed] [Google Scholar]
- Yu A., Haggård-Ljungquist E. Characterization of the binding sites of two proteins involved in the bacteriophage P2 site-specific recombination system. J Bacteriol. 1993 Mar;175(5):1239–1249. doi: 10.1128/jb.175.5.1239-1249.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziermann R., Calendar R. Characterization of the cos sites of bacteriophages P2 and P4. Gene. 1990 Nov 30;96(1):9–15. doi: 10.1016/0378-1119(90)90334-n. [DOI] [PubMed] [Google Scholar]
- Zou C., Fujita N., Igarashi K., Ishihama A. Mapping the cAMP receptor protein contact site on the alpha subunit of Escherichia coli RNA polymerase. Mol Microbiol. 1992 Sep;6(18):2599–2605. doi: 10.1111/j.1365-2958.1992.tb01437.x. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig M., Cummings D. J. Cleavage of head and tail proteins during bacteriophage T5 assembly: selective host involvement in the cleavage of a tail protein. J Mol Biol. 1973 Nov 5;80(3):505–518. doi: 10.1016/0022-2836(73)90418-x. [DOI] [PubMed] [Google Scholar]