Abstract
Recently, primary lens extraction alone gained more acceptance as an alternative surgical approach for glaucoma management. This view was supported by the advances in phacoemulsification and intraocular lenses with greater safety and visual recovery, in addition to a substantial reduction of intraocular pressure and deepening of the anterior chamber and filtration angle. The decrease in IOP after cataract surgery in primary open-angle glaucoma (POAG) is mild, less predictable, related to baseline levels, and may return to presurgical values after an initial period of reduction. Therefore, the IOP-lowering effect of primary cataract extraction in POAG may be insufficient to achieve adequate IOP control. The IOP reduction after lens extraction is consistently greater in eyes with primary angle closure glaucoma (PACG) than in eyes with POAG. Primary lens extraction in acute PACG eliminates, or at least, reduces the risk of recurrence of acute attacks and deepens the anterior chamber and widens the angle which reduces the risk of progression of peripheral anterior synechiae and development of chronic PACG. Primary lens extraction may be more preferable to glaucoma incisional surgery in mild to moderate PACG eyes with appositional angle closure. The decision to do lens extraction as a primary treatment for glaucoma should be individualized based upon several factors other than the effect on IOP. These factors include patients’ characteristics, surgeons’ skills and preferences, status of glaucoma control, type of cataract and intraocular lens implanted, and potential harm of laser treatment for late capsular opacification and fibrosis.
Keywords: Primary lens extraction, Intraocular pressure, Open-angle glaucoma, Angle closure glaucoma, Surgical management
1. Introduction
Glaucoma management is still far from optimum. The external filtering surgery (trabeculectomy) augmented by antifibrotic agents represents the mainstay of surgical treatment. However, the high complication rate and the unpredictable postoperative course and magnitude of intraocular pressure (IOP) reduction led many to consider other treatment strategies not dependent upon external filtration. Some of these options included improvement of internal filtration (viscocanalostomy, canaloplasty, i-stent, trabectome), drainage to suprachoroidal space (golden shunt), or decreasing aqueous production (endoscopic cyclophotocoagulation) (Yalvac et al., 2004; Lewis et al., 2007; Spiegel and Kobuch, 2002; Minckler et al., 2005; Melamid et al., 2009; Uram, 1995). Another option emerged after observing a reduction of IOP after cataract surgery (Wishart and Atkinson, 1989; Greve, 1988; Gunning and Greve, 1991). The advances in the technique and technology of both phacoemulsification and intraocular lenses (IOL) made cataract surgery the most commonly performed ophthalmic procedure with highly predictable visual and refractive outcome (Resnikoff et al., 2004). These two factors (visual improvement and IOP reduction) encouraged some ophthalmologists to treat some types of glaucoma by lens extraction alone aiming for either a definite treatment or a step in the management plan that can be followed safely by another glaucoma surgical intervention when necessary (Cioffi et al., 2005).
2. Coexistence of cataract and glaucoma
Cataract and glaucoma may exist in the same eye; the most common variety of which is the primary, age-related cataract and primary types of glaucoma. Also, cataract and glaucoma coexist in the same eye when one pathology may lead to the other (resulting in secondary types of cataract or glaucoma) such as phacomorphic glaucoma, lens protein (phacolytic) glaucoma, cataract progression after glaucoma surgery, cataractogenic glaucoma medications, and glaucoma complicating cataract surgery. Additionally, both diseases may be seen in the same ocular or systemic pathology such as in pseudoexfoliation syndrome, syndromes associated with ectopia lentis and glaucoma (homocystinuria, Weill-Marchesani syndrome), congenital anomalies (Iridocorneal dysgenesis syndromes), prolonged use of topical or systemic corticosteroids, or as complications of ocular trauma and inflammation (Eid and Spaeth, 2000).
3. Treatment challenges for eyes with cataract and glaucoma
The surgical decision to treat coexisting cataract and glaucoma is challenging and needs to clarify many points such as how well glaucoma is medically controlled, how predictable is the rate of progression, how much vision the patient will gain and how much harm he can suffer, how safe and effective would be the decision to perform sequential or combined surgery for optimum management. The glaucomatous eye may have shallow anterior chamber (AC), miotic pupil, zonular instability which increase the surgical risks during cataract surgery. Also, an eye with previous glaucoma operation is more prone to increased surgical complications with subsequent cataract surgery. On the other hand, cataract extraction or subsequent YAG laser capsulotomy may compromise a successfully controlled glaucoma.
4. Treatment strategies for eyes with cataract and glaucoma
The strategies to treat coexisting cataract and glaucoma include glaucoma surgery first, cataract surgery first/alone, or combined glaucoma and cataract operation (Friedman et al., 2002). Increased glaucoma morbidity (rapidly progressive, advanced, or medically uncontrolled disease) may warrant a priori surgical intervention for glaucoma. A maximum IOP reduction can be ascertained without pressure spikes and without added intraocular or subconjunctival alterations that may limit the success of filtering operations. Additionally, filtering operations may work better in phakic than in aphakic or pseudophakic eyes. However, glaucoma surgery may be associated with increased cataract progression, early postoperative refractive and visual instability with low patient’s satisfaction, and may increase the risk of surgical complications during subsequent cataract extraction. A functioning bleb and IOP control may be compromised after cataract operation (Rebolleda and Munoz-Negrete, 2002). In fact, the benefit of better filtration in phakic eyes may be temporary and may be lost once the cataract is operated.
Combined glaucoma and cataract operation addresses the two pathologies in one setting with one admission, one procedure, and one recovery. Glaucoma operation prevents transient postoperative pressure elevation and the cataract operation will avoid the hazardous effects of subsequent surgery. Application of antifibrotic agents improves the success of filtering surgery. Additionally, many of the newer glaucoma surgeries have better outcome when combined with cataract extraction (Godfrey et al., 2009). On the other hand, combining the two techniques increases the intra- and postoperative surgical risks with longer surgery time and delayed visual rehabilitation than cataract extraction alone. In the mean time, bleb function and IOP reduction may be less than after trabeculectomy alone (Derick et al., 1998). Also, prediction of refractive outcome with combined surgery may be affected by changes in AC depth, axial length, and corneal curvature associated with the filtering operation (will be discussed later).
Cataract surgery alone (primary lens extraction) in an eye suffering from both cataract and glaucoma is gaining wide acceptance due to great advances in cataract surgery and IOLs and better medicinal control of glaucoma through a wide variety of hypotensive drops. A separate-incision phacoemulsification provides a low surgical risk procedure with short recovery time and better visual outcome in addition to a measurable decrease in IOP and deepening of the filtration angle. On the other hand, leaving the conjunctiva and sclera untouched and maintaining the intraocular anatomy decreases the risks of filtration failure of a subsequent glaucoma surgery. In this review we will explore the effectiveness of lens extraction on IOP in different types of glaucoma and the factors to be considered for glaucoma management by primary lens extraction alone.
5. Effect of lens extraction on intraocular pressure (IOP)
5.1. Nonglaucomatous eyes
There is increasing evidence supporting the IOP-lowering ability of lens extraction in nonglaucomatous eyes (Matsumura et al., 1996; Suzuki et al., 1997; Jahn, 1997; Tong and Miller, 1998; Schwenn et al., 2001; Pohjalainen et al., 2001; Shingleton et al., 2006; Kim et al., 2009) (Table 1). Poley et al. (2008) reported a reduction of IOP proportional to presurgical levels. The decrease was greatest in eyes with highest presurgical IOP and remained unchanged in eyes with lowest presurgical pressure. IOP reduction after cataract surgery in nonglaucomatous eyes is mild (1.5–2 mmHg), transient, and has no effect on diurnal variation (Kim et al., 2009). Issa et al. correlated changes in IOP with AC depth measurements before and after surgery and provided a prediction index for IOP reduction after cataract surgery calculated by dividing preoperative IOP (mmHg) by AC depth (mm). They found that eyes with higher initial IOPs and a shallower AC depth had greater IOP reductions after cataract surgery (Issa et al., 2005).
Table 1.
Magnitude of intraocular pressure reduction in nonglaucomatous eyes in different studies.
| Study (year) | Amount of IOP lowering (mmHg) | Length of follow-up |
|---|---|---|
| Matsumura et al. (1996) | 1.5 | 3 years |
| Suzuki et al. (1997) | No change from preop. level | 10 years |
| Jahn (1997) | 2 | 5 years |
| Tong and Miller (1998) | 2 | 6–8 months |
| Schwenn et al. (2001) | 0.6 (scleral tunnel), 1.5 (clear cornea) | 5 months |
| Pohjalainen et al. (2001) | 3.5 | 1–2.7 years |
| Shingleton et al. (2006) | 1.5 | 3 years |
| Kim et al. (2009) | 1.5 | 1 month |
The mechanism by which lens extraction influences IOP is not fully understood. The postulated mechanisms include: (1) increased trabecular outflow (removal of the lens causes deepening of AC and angle with backward rotation of ciliary body relieves compression on trabecular meshwork and canal of Schlemm) (Poley et al., 2008); (2) increased uveoscleral outflow due to postoperative release of prostaglandin F-2 (Mathalone et al., 2005); (3) hyposecretion of aqueous humor caused by traction on the ciliary body due to fibrosis and contraction of the posterior lens capsule after cataract surgery (Kooner et al., 1988); (4) the effect of increased AC volume after removal of a 5-mm thick crystalline lens and replacing it by a 1-mm thick artificial lens results in an increase in the volume of AC required to be filled by aqueous humor (Kim et al., 2009).
5.2. Eyes with primary open-angle glaucoma
The reduction in IOP after cataract surgery in eyes with Primary open-angle glaucoma (POAG) is modestly greater than in non glaucoma eyes. The reduction averaged 1.8–4.5 mmHg (Matsumura et al., 1996; Shingleton et al., 2006; Poley et al., 2008; Mathalone et al., 2005; Kim, 1999). Matsumura et al. (1996) reported greater IOP reduction after cataract surgery in uncontrolled (5.5 mmHg) than in controlled (2.5 mmHg) POAG. This finding may be explained by the observation by Shingleton et al. (2008) and Poley et al. (2008) that the decrease in IOP after cataract surgery may be a function of the IOP at baseline (i.e., the greater the IOP at baseline, the greater the IOP reduction after cataract extraction). In addition, Evidence has shown that IOP tends to return to baseline levels with time after initial significant reduction. Therefore, the IOP-lowering effect of primary cataract extraction in POAG may be insufficient to achieve adequate IOP control (Vizzeri and Weinreb, 2010). Friedman et al. (2002) performed a systematic literature review of 40 studies with evidence-based analysis to assess IOP control with different surgical strategies for coexisting cataract and glaucoma. They found consistent evidence that cataract extraction alone decreased IOP by 2–4 mmHg in glaucoma patients compared to a 6–8 mmHg IOP lowering after combined cataract and glaucoma operation. The authors concluded that there is strong evidence for better long-term IOP control with combined glaucoma and cataract operation compared with cataract extraction alone.
The mechanism of IOP reduction in POAG patients after phacoemulsification is poorly understood. In addition to the above mentioned mechanisms, Wang et al. (2003) hypothesized a potential IOP-lowering stress response induced in the trabecular meshwork by ultrasound energy. The procedure stimulates production of interlukin and tissue necrosis factor by the trabecular cells which in turn stimulates synthesis of matrix metalloproteinases, enzymes that catalyze tissue remodeling. This reduces extracellular matrix resistance, increasing facility of outflow.
5.3. Eyes with pseudoexfoliation
Eyes with pseudoexfoliation (PXF) were shown to have a significantly greater IOP reduction than the fellow eyes without PXF after bilateral cataract surgery (Shingleton et al., 2009). Cimetta and Cimetta (2008) demonstrated a 3.5-mmHg reduction in IOP in eyes with PXF versus a 0.48-mmHg reduction in controls at 1-year postoperative endpoint. In another study, Shingleton et al. reported a beneficial long-term response in IOP following phacoemulsification in PXF with and without glaucoma, but the mean IOP reduction in PXF eyes without glaucoma was significantly greater than in PXF eyes with glaucoma. This postoperative IOP decline correlated significantly with the preoperative IOP. They also found a small number of patients (2.7%) of PXF eyes progressed to a need for glaucoma medication and 3.7% of PXF eyes with glaucoma progressed to a need for laser and/or glaucoma surgery suggesting a protective role of phacoemulsification against the development and progression of glaucoma in PXF eyes (Shingleton et al., 2008). Despite this IOP reducing effect, PXF eyes may have greater pressure spikes in the early postoperative period after phacoemulsification especially in PXF eyes with glaucoma (Levkovitch-Verbin et al., 2008).
5.4. Eyes with angle closure glaucoma
The IOP-lowering effect of lens extraction in eyes with primary angle closure glaucoma (PACG) ranges between 2 and 6 mmHg (up to 12 mmHg in one study) (Wishart and Atkinson, 1989; Gunning and Greve, 1991, 1998; Hayashi et al., 2001; Imaizumi et al., 2006; Lai et al., 2006; Liu et al., 2006). This IOP reduction is even more manifest in eyes with acute PACG treated primarily with lens extraction (Greve, 1988; Roberts et al., 2000; Wang and Lai, 2004; Zhi et al., 2003) with superiority on laser peripheral iridotomy for definitive treatment of a post-acute attack of PACG (Lam et al., 2008). Lam et al. hypothesized that primary lens extraction in acute PACG eliminates, or at least, reduces the risk of recurrence of acute attacks and deepens the AC and widens the angle which reduces the risk of progression of peripheral anterior synechiae and development of chronic PACG (Lam et al., 2007). The IOP reduction after lens extraction was consistently greater in eyes with PACG than in eyes with POAG in many studies (Wishart and Atkinson, 1989; Hayashi et al., 2001; Mierzejewski, 2008). Progressive shallowing of the AC in predisposed eyes is mostly attributed to age-related increase in lens thickness and more anterior position of the lens (Lowe, 1970). The restoration of deeper angle configuration by removing a thickened and anteriorly positioned lens may be advantageous in eyes with PACG and may lead to a significant IOP reduction (Vizzeri and Weinreb, 2010; Pereira and Cronemberger, 2003). On the contrary, Friedman and Vedula, in a Cochrane database systematic review in 2006, concluded that despite the biological plausibility of benefit there was no evidence from good quality randomized trials or non-randomized studies to support lens extraction as treatment of chronic PACG (Friedman and Vedula, 2006). In another comprehensive literature review in 2009, Tarongoy et al. (2009) concluded that although clinical reports of lensectomy for treatment of PACG describe very favorable results, its appropriate role remains unproven. Tham et al., in two separate randomized studies (Tham et al., 2008, 2009), compared phacoemulsification alone versus combined phaco-trabeculectomy in both medically controlled and medically uncontrolled chronic PACG with coexisting cataract in terms of IOP control, number of glaucoma drops, and postoperative complications. Combined phaco-trabeculectomy controlled IOP only marginally better than phacoemulsification alone in controlled PACG. This difference was significantly better at most time points of the 24-months follow-up in uncontrolled PACG. In both studies combined phaco-trabeculectomy was associated with more complications and additional surgery in the postoperative period without a significant difference in visual acuity or disease progression between the two treatment modalities (Tham et al., 2010). The authors concluded that primary lens surgery alone is a viable surgical alternative to combined phaco-trabeculectomy in chronic PACG eyes with coexisting cataract, whether the preoperative IOP is medically controlled or not. Although combined surgery does offer additional IOP lowering and reduction of antiglaucoma drops, there are no published data that confirm such small improvement in IOP control is associated with beneficial long-term clinical outcomes and that only a small proportion of phacotreated eyes (2.9% in controlled PACG, and 14.8% in uncontrolled PACG) may eventually need a second-stage trabeculectomy (Tham et al., 2008, 2009).
In most of the above-mentioned studies, primary lens surgery entailed removal of a coexisting cataract, seeking to lower IOP as primary outcome while visual acuity improvement represented a secondary goal. When the lens is clear, primary lens extraction, although used recently on a relatively wider scale by refractive surgeons, does not receive good acceptance by many ophthalmologists. Glaucoma surgeons thrive to find a solid pathology to deal with an opaque lens for the cataract surgeons. Although many are confident that the lens position and size has a pivotal role in the mechanisms of angle closure, yet other anatomic predisposition and environmental and ethnic risk factors cannot be solely explained by the lens changes. The concept of primary clear lens surgery for treating angle closure glaucoma remains a subject of debate. In eyes with successfully aborted attacks of acute PACG a randomized trial reported superiority of primary lens extraction on laser peripheral iridotomy, the authors included only cases that had cataract. Such conclusion may not be applicable for eyes without cataract or with unaborted attacks of angle closure (Lam et al., 2008). The benefits of primary clear lens surgery in eyes with acute PACG (deepening of AC, widening of angle, IOP lowering, prevention of recurrent attacks, less number of medications, and avoiding subsequent cataract progression) should be weighed against accommodation loss, stress of surgical intervention, technical difficulties (inflamed eye, shallow AC, small atonic pupil, floppy iris, hazy cornea), intraoperative and postoperative complications (corneal endothelial decompensation, increased inflammatory response, iris atrophy, capsular contraction and phimosis, zonular instability and decentration of intraocular lenses, etc.) (Tan et al., 2006). Our mind, by instinct, avoids danger before gaining benefit (i.e., procedures with less risk may be preferred to ones with more benefits and more risks). This may be applicable, with limitations, when primary lens surgery is compared to laser iridotomy (in favor of iridotomy) and when primary lens surgery is compared to combined phacotrabeculectomy (in favor of primary lens surgery). Tham et al. (2010) demonstrated no statistically significant differences in visual acuity or glaucomatous progression between phacoemulsification and phaco-trabeculectomy for PACG, but the unwanted and unexpected events associated with higher surgical complications of combined surgery (such as more clinic visits, more operative time, inconveniences, financial costs to patients and society, and negative emotions for patients and health care professionals) should be considered when deciding the type of surgery for patients with PACG. The place of primary lens extraction in treatment of different types of PACG is demonstrated in Flow chart 1.
Flow chart 1.
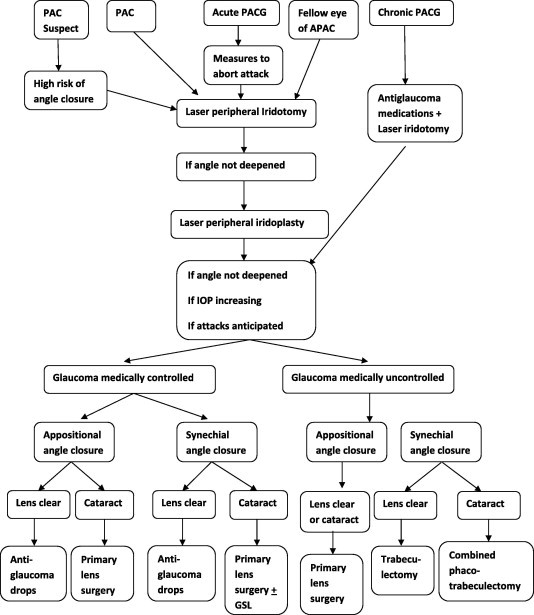
Treatment plan for eyes with primary angle closure glaucoma [PACG].
6. Special considerations when deciding primary lens surgery for management of glaucoma
6.1. Patients’ characteristics
The decision to do lens extraction as a primary treatment for glaucoma should consider several factors other than the effect on IOP. The first of these factors is related to the patient himself and include patient’s general health and associated medical problems (short versus long time procedures tolerance), associated ocular and systemic comorbidities (adverse effects of antiglaucoma medications), compliance to glaucoma treatment, visual needs and visual prognosis (age, work, activity, social life, need for rapid recovery, etc.), access for periodic long-term follow-up (disabled, short-stay travelers, remote foci), and psychological motivation of the patient.
6.2. Surgeon’s factors
Another important factor to be considered is the surgeons’ skills and preferences. Most ophthalmologists are able to do a relatively safe cataract surgery, whereas a minority of nonglaucoma specialists can cope with turbulences after glaucoma surgical intervention. This may encourage nonglaucoma surgeons to treat their glaucoma patients with primary lens extraction regardless of other considerations. Patient’s satisfaction after cataract surgery is much better than after glaucoma operation (visual gain, short recovery period, less postoperative visits, early retrieval of ordinary activity) together with the bad reputation and fear of blindness from glaucoma surgeries make both the patient and the treating ophthalmologists more in choice of less risky procedure. Some surgeons may wish to go for an easier procedure that offers certain amount of IOP lowering, short recovery, faster visual rehabilitation, less risk, less expensive and with a higher profit/effort margin. Although selected patients may benefit from clear lens surgery, other factors in glaucoma patients need to be taken into consideration (see below).
6.3. Considerations related to glaucoma status
In addition to the effect of primary lens extraction on IOP in different types of glaucoma, other factors to be considered when deciding such treatment include the type of glaucoma (primary versus secondary), glaucoma severity (mild, moderate, severe, end-stage) and rate of progression (slowly versus rapidly progressive), status of glaucoma control (medical versus laser treatment, controlled on maximum medications, treatment compliance and tolerability), target IOP (how accurate is the prediction of IOP reduction after primary lens surgery?), visual potential and comorbidity of the glaucomatous eye, and status of the fellow eye. The impact of lens extraction on various types of secondary glaucoma is different. Some secondary glaucomas may benefit directly from lens extraction such as phacomorphic and phacolytic glaucoma, pseudoexfoliation with glaucoma, nanophthalmic eyes, and eyes with malignant glaucoma (vitrectomy and lensectomy). Other types such as neovascular and uveitic glaucoma may be worsened by further violation of blood-aqueous barrier and potentiating intraocular inflammation. Traumatic glaucoma may be associated with compromise of zonular integrity with increased hazards of lens operation.
6.4. Considerations related to the crystalline lens
As mentioned previously, debate still exists regarding surgical removal of clear lenses or lenses with nonvisually significant opacities for treatment of glaucoma. Cataract extraction, even in the experienced surgeon, is not without potential complications. Visually significant cataracts can be of low or high surgical risks. Low-risk cataracts include simple primary types with uneventful operative course and greater accuracy of prediction of refractive outcome. High-risk cataracts include special types (such as posterior polar, intumescent, hypermature, pseudoexfoliative, traumatic cataract), associated ocular pathology (angle closure, neovascularization, anterior uveitis, high myopia, corneal opacity, endothelial compromise), high prediction error of refractive outcome (previous refractive surgery, very short or long eyes, hypotony after glaucoma surgery), and cataract in the demanding patient (need for rapid visual rehabilitation, supervision, spectacle independence). Primary cataract extraction for glaucoma management in such conditions may be associated with higher complication rate or higher rate of patient dissatisfaction.
6.5. Considerations related to intraocular lens (IOL) implantation
This includes implantation site, type of IOL implanted, and accurate IOL calculation and prediction error of refractive outcome in glaucoma patients. Implantation of IOL after phacoemulsification depends on intactness of capsular bag and stability of the zonules (Flow chart 2). In glaucoma eyes with severe compromise of capsular or zonular integrity, an iris-claw anterior chamber IOL may be preferred to open-loop AC lens or iris-sutured posterior chamber IOL or scleral fixation PC IOL. Regarding the type of IOL implanted, aspheric and blue light filtering lenses, through improving contrast sensitivity, are preferred in glaucoma eyes. Toric IOL implantation in glaucoma eyes carries some limitations including zonular instability and possible decentration, refractive changes after subsequent glaucoma surgery, and effect of large bleb on refraction. Implantation of multifocal IOLs in glaucoma eyes provide patients with spectacle independence for far and near vision. However, potential drawbacks include photic phenomenon, decreased contrast sensitivity which may be augmented in glaucoma eyes (Teichman and Ahmed, 2010).
Flow chart 2.
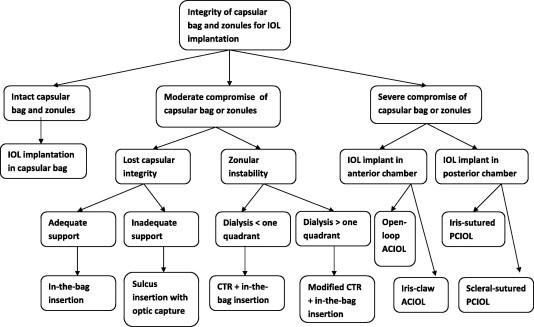
Choice of implantation site of intraocular lenses based on integrity of capsular bag and zonules.
Calculation of IOL power in glaucoma patients may be affected by changes in corneal curvature, the AC depth, and axial length which correlate positively with changes in IOP after trabeculectomy. A significant myopic shift of prediction error of postoperative refraction may be greater in glaucoma eyes with previous filtering surgery or when combined phacotrabeculectomy is performed (Eid et al., 2007; Chan et al., 2006; Muallem et al., 2009). However, changes in the above mentioned parameters after primary lens extraction in glaucoma eyes were not studied. Except for phacoemulsification in eyes with acute attack of PAC, postoperative changes in AC depth, axial length, or corneal curvature are expected to be modest and will not have a greater impact on postoperative refractive outcome. Axial length measurement without corneal indentation (the noncontact method or laser interferometer) is preferred to the contact method in glaucoma eyes especially when the IOP is low (Eid et al., 2007; Muallem et al., 2009). Caution should be taken in eyes with high preoperative IOP (risk of hyperopic surprise) and aim for slight myopia (0.5–1D) during IOL power selection is preferable (Teichman and Ahmed, 2010).
6.6. Factors related to late capsular changes after cataract surgery
Posterior capsule opacification (PCO) occurs in approximately 20–25% of eyes after cataract surgery (Schaumberg et al., 1998). The incidence of PCO is most probably similar in glaucoma eyes having cataract extraction alone or combined phaco-trabeculectomy, although mitomycin-C application may be protective against PCO (Shin et al., 2002). Capsular opacification and contraction is related to IOL material (more in PMMA, silicone, and hydrophilic lenses than hydrophobic lenses), IOL design (more with one-piece lenses and IOLs with rounded-edge design), retained cortex, chronic inflammation, and compromised zonules. Capsular contraction and phimosis may result in IOL decentration or dislocation. Treating capsular opacification with YAG laser is associated with an early transient IOP rise (>5 mmHg) in 10–40% of all individuals and in one-fifth of patients with glaucoma undergoing capsulotomy (Shani et al., 1994; Barnes et al., 2004). This rise may be related to reduction in outflow facility which is directly related to the total energy delivered (Wetzel, 1994). Use of premedications and limited capsulotomy with a low energy level can protect against these pressure spikes. Lin et al. (2008) reviewed 69 glaucoma patients who underwent YAG laser capsulotomy over a 3-year period. They found a gradual IOP elevation or a need for more aggressive therapy to be common in glaucoma patients after YAG laser posterior capsulotomy. However, it is not unclear whether this progression is related directly to the Nd:YAG laser procedure or an independent progression of the patient’s glaucoma.
7. Conclusion
Surgical decisions for management of cataract and glaucoma or considering cataract operation for glaucoma management should be individualized based upon patient and disease characteristics and surgeons’ skills and preferences.
For management of coexisting cataract and glaucoma, there are no uniform recommendations for all cases. Cataract surgery alone or glaucoma surgery alone is performed when either a visually significant cataract or advanced uncontrolled glaucoma is dominating the scene. On the other hand, there are no strict criteria for IOP level or visual acuity at which combined surgery is indicated, but a visually significant cataract and a moderate to severe medically uncontrolled glaucoma are good candidates. The advances and multiplicity of anti-glaucoma medications and glaucoma laser treatment and the less favorable visual regain with glaucoma operations may favor primary cataract extraction in eyes with mild to moderate medically controlled glaucoma.
Primary lens extraction in glaucoma eyes with nonvisually significant cataract is associated with modest IOP lowering which is less predictable, not sustained, with tendency to return to baseline, especially in POAG eyes. These patients may benefit from adding newer glaucoma surgical techniques to the phacoemulsification to augment IOP lowering. In eyes with PACG, primary lens surgery helps in restoration of anatomy of anterior chamber and filtration angle and removes an important factor in the angle closure process, beside its IOP lowering effect. These factors make primary lens extraction more preferable to glaucoma incisional surgery in mild to moderate, medically controlled PACG eyes with appositional angle closure and significant cataract. Primary clear lens extraction may have a rule in uncontrolled mild to moderate PACG eyes with non-synechial angle closure.
References
- Barnes E.A., Murdoch I.E., Subramaniam S. Neodymium:yttrium-aluminium-garnet capsulotomy and intraocular pressure in pseudophakic patients with glaucoma. Ophthalmology. 2004;111:1393–1397. doi: 10.1016/j.ophtha.2003.12.047. [DOI] [PubMed] [Google Scholar]
- Chan J.C.H., Lai J.S.M., Tham C.C.Y. Comparison of postoperative refractive outcome in phacotrabeculectomy and phacoemulsification with posterior chamber lens implantation. J. Glaucoma. 2006;15:26–29. doi: 10.1097/01.ijg.0000196620.41991.b6. [DOI] [PubMed] [Google Scholar]
- Cimetta D.J., Cimetta A.C. Intraocular pressure changes after clear cornea phacoemulsification in nonglaucomatous pseudoexfoliation syndrome. Eur. J. Ophthalmol. 2008;18:77–81. doi: 10.1177/112067210801800113. [DOI] [PubMed] [Google Scholar]
- Cioffi G.A., Friedman D.S., Pfeiffer N. Combined cataract/trabeculectomy. In: Weinreb R.N., Crowson J.G., editors. Glaucoma Surgery Open Angle Glaucoma. vol. 2. Kugler Publications; The Hague, The Netherlands: 2005. pp. 65–72. (Consensus Series. Association of International Glaucoma Societies). [Google Scholar]
- Derick R.J., Evans J., Baker N.D. Combined phacoemulsification and trabeculectomy versus trabeculectomy alone: a comparative study using mitomycin-C. Ophthalmic Surg. Lasers. 1998;29:707–713. [PubMed] [Google Scholar]
- Eid T.M., Spaeth G.L.S. The Glaucomas: Concepts and Fundamentals. Lippincott Williams & Wilkins; Philadelphia: 2000. Glaucoma associated with lens disorders. p. 160. [Google Scholar]
- Eid T.M., El-Hawary I., El-Menawy W. IOL Master for IOL power calculation in phaco-trabeculectomy: evaluation of postoperative prediction error and refractive outcome. Bull. Ophthalmol. Soc. Egypt. 2007;100:215–220. [Google Scholar]
- Friedman D.S., Vedula S.S. Cochrane Database Syst. Rev. 2006;3:CD005555. doi: 10.1002/14651858.CD005555.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D.S., Jampel H.D., Lubomski L.H. Surgical strategies for coexisting glaucoma and cataract. An evidence-based update. Ophthalmology. 2002;109:1902–1915. doi: 10.1016/s0161-6420(02)01267-8. [DOI] [PubMed] [Google Scholar]
- Godfrey D.G., Fellman R.L., Neelakantan A. Canal surgery in adult glaucoma. Curr. Opin. Ophthalmol. 2009;20:116–121. doi: 10.1097/ICU.0b013e32831eef65. [DOI] [PubMed] [Google Scholar]
- Greve E.L. Primary angle-closure glaucoma: extracapsular cataract extraction or filtering procedure. Int. Ophthalmol. 1988;12:157–162. doi: 10.1007/BF00129999. [DOI] [PubMed] [Google Scholar]
- Gunning F.P., Greve E.L. Uncontrolled primary angle-closure glaucoma: results of early intercapsular cataract extraction and posterior chamber lens implantation. Int. Ophthalmol. 1991;15:237–247. doi: 10.1007/BF00171026. [DOI] [PubMed] [Google Scholar]
- Gunning F.P., Greve E.L. Lens extraction for uncontrolled angle-closure glaucoma: long-term follow-up. J. Cataract Refract. Surg. 1998;24:1347–1356. doi: 10.1016/s0886-3350(98)80227-7. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Hayashi H., Nakao F., Hayashi F. Effect of intraocular surgery on intraocular pressure control in glaucoma patients. J. Cataract Refract. Surg. 2001;27:1779–1786. doi: 10.1016/s0886-3350(01)01036-7. [DOI] [PubMed] [Google Scholar]
- Imaizumi M., Takaki Y., Yamashita H. Phacoemulsification and intraocular lens implantation for acute angle closure glaucoma not treated or previously treated by laser iridotomy. J. Cataract Refract. Surg. 2006;32:85–90. doi: 10.1016/j.jcrs.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Issa S.A., Pacheco J., Mahmood U. A novel index for predicting intraocular pressure reduction following cataract surgery. Br. J. Ophthalmol. 2005;89:543–546. doi: 10.1136/bjo.2004.047662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn C.E. Reduced intraocular pressure after phacoemulsification and posterior chamber intraocular lens implantation. J. Cataract Refract. Surg. 1997;23:1260–1264. doi: 10.1016/s0886-3350(97)80325-2. [DOI] [PubMed] [Google Scholar]
- Kim D.D. Intraocular pressure reduction following phacoemulsification cataract extraction with posterior chamber lens implantation in glaucoma patients. Ophthalmic Surg. Lasers. 1999;30:37–40. [PubMed] [Google Scholar]
- Kim K.S., Kim J.M., Park K.H. The effect of cataract surgery on diurnal intraocular pressure fluctuation. J. Glaucoma. 2009;18:399–402. doi: 10.1097/IJG.0b013e3181879e89. [DOI] [PubMed] [Google Scholar]
- Kooner K.S., Dulaney D.O., Zimmerman T.J. Intraocular pressure following ECCE and IOL implantation in patients with glaucoma. Ophthalmic Surg. 1988;19:570–574. [PubMed] [Google Scholar]
- Lai j., Tham C., Chan J. The clinical outcome of cataract extraction by phacoemulsification in eyes with primary angle-closure glaucoma and co-existing cataract: a prospective series. J. Glaucoma. 2006;15:47–52. doi: 10.1097/01.ijg.0000196619.34368.0a. [DOI] [PubMed] [Google Scholar]
- Lam D.S.C., Tham C.C.Y., Lai J.S.M., Leung D.Y.L. Current approaches to the management of acute primary angle closure. Curr. Opin. Ophthalmol. 2007;18:146–151. doi: 10.1097/ICU.0b013e32808374c9. [DOI] [PubMed] [Google Scholar]
- Lam D.S., Leung D.Y., Tham C.C. Randomized trial of early phacoemulsification versus peripheral iridotomy to prevent intraocular pressure rise after acute primary angle closure. Ophthalmology. 2008;115:1134–1140. doi: 10.1016/j.ophtha.2007.10.033. [DOI] [PubMed] [Google Scholar]
- Levkovitch-Verbin H., Habot-Wilner Z., Burta N. Intraocular pressure elevation within the first 24 hours after cataract surgery in patients with glaucoma or pseudoexfoliation syndrome. Ophthalmology. 2008;115:104–108. doi: 10.1016/j.ophtha.2007.03.058. [DOI] [PubMed] [Google Scholar]
- Lewis R.A., Wolff K., Tets M. Canaloplasty: circumferential viscodilation and tensioning of Schlemm’s canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults. Interim clinical study analysis. J. Cataract Refract. Surg. 2007;33:1217–1226. doi: 10.1016/j.jcrs.2007.03.051. [DOI] [PubMed] [Google Scholar]
- Lin J.C., Katz L.J., Spaeth G.L., Klancnik J.M. Intraocular pressure control after Nd:YAG laser posterior capsulotomy in eyes with glaucoma. Br. J. Ophthalmol. 2008;92:337–339. doi: 10.1136/bjo.2007.125310. [DOI] [PubMed] [Google Scholar]
- Liu C., Cheng C., Wu C. Factors predicting intraocular pressure control after phacoemulsification in angle closure glaucoma. Arch. Ophthalmol. 2006;124:1390–1394. doi: 10.1001/archopht.124.10.1390. [DOI] [PubMed] [Google Scholar]
- Lowe R.F. Aetiology of the anatomical basis for primary angle closure glaucoma. Br. J. Ophthalmol. 1970;54:161–169. doi: 10.1136/bjo.54.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalone N., Hyams M., Neiman S. Long-term intraocular pressure control after clear cornea phacoemulsification in glaucoma patients. J. Cataract Refract. Surg. 2005;31:479–483. doi: 10.1016/j.jcrs.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Matsumura M., Mizogouchi T., Kuroda S. Intraocular pressure decrease after phacoemulsification-aspiration and intraocular lens implantation in primary open-angle glaucoma eyes. Nippon Ganka Gakkai Zaashi. 1996;100:885–889. (in Japanese) [PubMed] [Google Scholar]
- Melamid S., Ben Simon G.J., Goldenfeld M., Simon G. Efficacy and safety of gold micro shunt implantation to the supraciliary space in patients with glaucoma: a pilot study. Arch. Ophthalmol. 2009;127:264–269. doi: 10.1001/archophthalmol.2008.611. [DOI] [PubMed] [Google Scholar]
- Mierzejewski A. Cataract phacoemulsification and intraocular pressure in glaucoma patients. Klin Oczna. 2008;110:11–17. [PubMed] [Google Scholar]
- Minckler D.S., Baerveldt G., Alfaro M.R. Clinical results with the trabectome for treatment of open-angle glaucoma. Ophthalmology. 2005;112:962–967. doi: 10.1016/j.ophtha.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Muallem M.S., Nelson G.A., Osmanovic S. Predicted refraction versus refraction outcome in cataract surgery after trabeculectomy. J. Glaucoma. 2009;18:284–287. doi: 10.1097/IJG.0b013e318184567b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira F.A.S., Cronemberger S. Ultrasound biomicroscopic study of anterior segment changes after phacoemulsification and foldable intraocular lens implantation. Ophthalmology. 2003;110:1790–1806. doi: 10.1016/S0161-6420(03)00623-7. [DOI] [PubMed] [Google Scholar]
- Pohjalainen T., Vesti E., Usitalo R.J., Laatikainen L. Intraocular pressure after phacoemulsification and intraocular lens implantation in nonglaucoma eyes with and without exfoliation. J. Cataract Refract. Surg. 2001;27:426–431. doi: 10.1016/s0886-3350(00)00691-x. [DOI] [PubMed] [Google Scholar]
- Poley B.J., Lindstrom R.L., Samuelson T.W. Long-term effects of phacoemulsification with intraocular lens implantation in normotensive and ocular hypertensive eyes. J. Cataract Refract. Surg. 2008;34:735–742. doi: 10.1016/j.jcrs.2007.12.045. [DOI] [PubMed] [Google Scholar]
- Rebolleda G., Munoz-Negrete F.J. Phacoemulsification in eyes with functioning filtering blebs: a prospective study. Ophthalmology. 2002;109:2248–2255. doi: 10.1016/s0161-6420(02)01246-0. [DOI] [PubMed] [Google Scholar]
- Resnikoff S., Pascolini D., Etya’ale D. Global data on visual impairment on the year 2002. Bull. World Health Organ. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- Roberts T.V., Francis I.C., Lertusumitkul S. Primary phacoemulsification for uncontrolled angle-closure glaucoma. J. Cataract Refract. Surg. 2000;26:1012–1016. doi: 10.1016/s0886-3350(00)00358-8. [DOI] [PubMed] [Google Scholar]
- Schaumberg D.A., Dara M.R., Christen W.G. A systematic review of incidence of posterior capsule opacification. Ophthalmology. 1998;105:1213–1221. doi: 10.1016/S0161-6420(98)97023-3. [DOI] [PubMed] [Google Scholar]
- Schwenn O., Dick H.B., Krummenauer F. Intraocular pressure after small incision cataract surgery: temporal sclerocorneal versus clear corneal incision. J. Cataract Refract. Surg. 2001;27:421–425. doi: 10.1016/s0886-3350(00)00577-0. [DOI] [PubMed] [Google Scholar]
- Shani L., David R., Tessler Z. Intraocular pressure after neodymium:YAG laser treatments in the anterior segment. J. Cataract Refract. Surg. 1994;20:455–458. doi: 10.1016/s0886-3350(13)80184-8. [DOI] [PubMed] [Google Scholar]
- Shin D.H., Vandenbelt S.M., Kim P.H. Comparison of long-term incidence of posterior capsule opacification between phacoemulsification and phacotrabeculectomy. Am. J. Ophthalmol. 2002;133:40–47. doi: 10.1016/s0002-9394(01)01285-5. [DOI] [PubMed] [Google Scholar]
- Shingleton B.J., Pasternack J.J., Hung J.W., O’Donoghue M.W. Three and five year changes in intraocular pressure after clear cornea phacoemulsification in open-angle glaucoma patients, glaucoma suspects, and normal patients. J. Glaucoma. 2006;15:494–498. doi: 10.1097/01.ijg.0000212294.31411.92. [DOI] [PubMed] [Google Scholar]
- Shingleton B.J., Laul A., Nagao K. Effect of phacoemulsification on intraocular pressure in eyes with pseudoexfoliation. Single-surgeon series. J. Cataract Refract. Surg. 2008;34:1834–1841. doi: 10.1016/j.jcrs.2008.07.025. [DOI] [PubMed] [Google Scholar]
- Shingleton B.J., Nguyen B.C., Eagan E.F., Nagao K., O’Donoghue M.W. Outcomes of phacoemulsification in fellow eyes of patients with unilateral pseudoexfoliation. Single-surgeon series. J. Cataract Refract. Surg. 2009;34:274–279. doi: 10.1016/j.jcrs.2007.09.040. [DOI] [PubMed] [Google Scholar]
- Spiegel D., Kobuch K. Trabecular meshwork bypass tube shunt: initial case series. Br. J. Ophthalmol. 2002;86:1228–1231. doi: 10.1136/bjo.86.11.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R., Kuroki S., Fujiwara N. Ten-year follow-up of intraocular pressure after phacoemulsification and aspiration with intraocular lens implantation performed by the same surgeon. Ophthalmologica. 1997;211:79–83. doi: 10.1159/000310763. [DOI] [PubMed] [Google Scholar]
- Tan G.S.W., Hoh S-.T., Hussain R. Visual acuity after primary angle closure glaucoma and considerations for primary lens extraction. Br. J. Ophthalmol. 2006;90:14–16. doi: 10.1136/bjo.2005.077115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarongoy P., Ho C.L., Walton D.S. Angle-closure glaucoma: the role of lens in the pathogenesis, prevention, and treatment. Surv. Ophthalmol. 2009;54:211–225. doi: 10.1016/j.survophthal.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Teichman J.C., Ahmed I.K. Intraocular lens choices for patients with glaucoma. Curr. Opin. Ophthalmol. 2010;21:135–143. doi: 10.1097/ICU.0b013e3283365154. [DOI] [PubMed] [Google Scholar]
- Tham C.C.Y., Kwong Y.Y.Y., Leung D.Y.L. Phacoemulsification versus combined phaco-trabeculectomy in medically controlled chronic angle closure glaucoma with cataract. Ophthalmology. 2008;115:2167–2173. doi: 10.1016/j.ophtha.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Tham C.C.Y., Kwong Y.Y.Y., Leung D.Y.L. Phacoemulsification versus combined phaco-trabeculectomy in medically uncontrolled chronic angle closure glaucoma with cataract. Ophthalmology. 2009;116:725–731. doi: 10.1016/j.ophtha.2008.12.054. [DOI] [PubMed] [Google Scholar]
- Tham C.C.Y., Kwong Y.Y.Y., Leung D.Y.L. Phacoemulsification versus phaco-trabeculectomy in chronic angle-closure glaucoma with cataract complications. Arch. Ophthalmol. 2010;128:303–311. doi: 10.1001/archophthalmol.2010.12. [DOI] [PubMed] [Google Scholar]
- Tong J.T., Miller K.M. Intraocular pressure change after sutureless phacoemulsification and foldable posterior chamber lens implantation. J. Cataract Refract. Surg. 1998;24:256–262. doi: 10.1016/s0886-3350(98)80208-3. [DOI] [PubMed] [Google Scholar]
- Uram M. Endoscopic cyclophotocoagulation in glaucoma management. Curr. Opin. Ophthalmol. 1995;6:19–29. doi: 10.1097/00055735-199504000-00005. [DOI] [PubMed] [Google Scholar]
- Vizzeri G., Weinreb R.N. Cataract surgery and glaucoma. Curr. Opin. Ophthalmol. 2010;21:20–24. doi: 10.1097/ICU.0b013e328332f562. [DOI] [PubMed] [Google Scholar]
- Wang J.K., Lai P.C. Unusual presentation of angle-closure glaucoma treated by phacoemulsification. J. Cataract Refract. Surg. 2004;30:1371–1373. doi: 10.1016/j.jcrs.2003.10.027. [DOI] [PubMed] [Google Scholar]
- Wang N., Chintala S.K., Fini M.E., Schuman J.S. Ultrasound activates the TM ELAM-1/IL-1/NF-Kb RESPONSE: a potential mechanism for intraocular pressure reduction after phacoemulsification. Invest. Ophthalmol. Vis. Sci. 2003;44:1977–1985. doi: 10.1167/iovs.02-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel D.W. Ocular aqueous humor dynamics after photodisruptive laser surgery procedures. Ophthalmic Surg. 1994;25:298–302. [PubMed] [Google Scholar]
- Wishart P.K., Atkinson P.L. Extracapsular cataract extraction and posterior chamber lens implantation in patients chronic with primary angle-closure glaucoma: effect on intraocular pressure control. Eye. 1989;3:706–712. doi: 10.1038/eye.1989.109. [DOI] [PubMed] [Google Scholar]
- Yalvac I.S., Sahin M., Eksioglu U. Primary viscocanalostomy versus trabeculectomy for primary open-angle glaucoma; three year prospective randomized clinical trial. J. Cataract Refract. Surg. 2004;30:2050–2057. doi: 10.1016/j.jcrs.2004.02.073. [DOI] [PubMed] [Google Scholar]
- Zhi M.Z., Lim A.S., Yin Wong T. A pilot study of lens extraction in the management of acute primary angle-closure glaucoma. Am. J. Ophthalmol. 2003;135:534–536. doi: 10.1016/s0002-9394(02)02108-6. [DOI] [PubMed] [Google Scholar]


