Summary
The hallmark of acquired immunodeficiency syndrome (AIDS) pathogenesis is a progressive depletion of CD4+ T-cell populations in close association with progressive impairment of cellular immunity and increasing susceptibility to opportunistic infections (OI). Disease progression in untreated human immunodeficiency virus (HIV) infection can take many years, and it was originally hypothesized to be a consequence of slow, viral-mediated CD4+ T-cell destruction. However, massive CD4+ memory T-cell destruction is now known to occur quite early in infection, almost always without overt immunodeficiency. In most individuals, this initial destruction is countered by CD4+ memory T-cell regeneration that preserves CD4+ T-cell numbers and functions above the threshold associated with overt immunodeficiency. This regeneration, which occurs in the setting of chronic immune activation and immune dysregulation does not, however, restore all functionally important CD4+ T-cell populations and is not stable over the long term. Ultimately, CD4+ memory T-cell homeostasis fails and critical effector populations decline below the level necessary to prevent OI. Thus, the onset of overt immune deficiency appears to be intimately linked with CD4+ memory T-cell dynamics and reflects the complex interplay of direct viral cytopathogenicity and the indirect effects of persistent immune activation on CD4+ memory T-cell proliferation, differentiation, and survival.
Keywords: AIDS, CD4+ T lymphocytes, lymphocyte depletion, central memory, rhesus macaque
Introduction
It has been 30 years since the discovery and identification of a family of deadly human lymphotropic retroviruses now known as human immunodeficiency viruses (HIV)(1–3). This new virus type was isolated from patients presenting with the then recently described acquired immune deficiency syndrome (AIDS), a syndrome characterized by the development of previously rare opportunistic infections (OIs) and/or malignancies in previously healthy young homosexual men and/or intravenous drug users (4). The earliest reported AIDS cases included subjects with Pneumocystis carinii pneumonia and/or Kaposi's sarcoma (5, 6), but subsequently, other AIDS-associated OIs were identified, including Mycobacterium tuberculosis, M. avium intracellulare, Cryptococcus neoformans, Toxoplasma gondii, cytomegalovirus, adenovirus, herpes simplex virus, and Candida albicans (7, 8). A common thread of impaired cellular immunity linked these OIs. In keeping with this observation, early laboratory studies documented that subjects with AIDS manifested marked lymphopenia, low lymphocyte proliferative responses in vitro after stimulation with antigens or mitogens, anergy to cutaneous skin tests, and an inversion in the ratio of T-helper cells to cytotoxic T cells (5–7). Subsequent studies confirmed that HIV selectively infected and killed CD4+ T cells in vitro and that the numbers of circulating CD4+ T cells in HIV+ subjects predicted the onset of overt immunodeficiency (9, 10). Later still, it was found that suppressing HIV replication with antiretroviral therapy (ART) rapidly increased peripheral blood CD4+ T-cell counts and reversed immunodeficiency (11, 12).
Overall, these observations provide strong evidence that a profoundly impaired cellular immune response due to depletion of CD4+ T cells and loss of CD4+ T-cell function was the underlying cause of immunodeficiency present in these patients. Further evidence for this conclusion came from analysis of experimental infections of nonhuman primates (NHPs) with certain strains of chimeric simian/human immunodeficiency viruses (SHIV). In these infections, systemic, acute, pan-CD4+ T-cell depletion led to rapid development of an AIDS-like syndrome and death early after infection (13, 14). Taken together, these observations suggested a model of HIV pathogenesis in which viral-mediated destruction of CD4+ helper T cells results in impaired immunity to pathogenic agents typically restricted by T-cell-mediated immunity, and ultimately, the emergence of one or more fatal OIs.
The loss in CD4+ T cells was initially thought to be a gradual process as the timing to overt immunodeficiency and AIDS in untreated patients was typically within 10–12 years from primary infection (15–17). However, the concept that HIV disease progression results from slow, viral-mediated CD4+ T-cell destruction was brought into question by a number of observations. First, HIV replication was shown to be continuous and high throughout the course of infection, despite the slow progression to end-stage disease (12, 18). Second, because of the use of CCR5 as a viral co-receptor (CCR5 tropism), infecting strains preferentially infect memory CD4+ T cells (particularly the more differentiated effector memory subset) and these preferentially targeted cells, which compromise the majority of CD4+ T cells in extra-lymphoid effector sites such as the intestinal mucosa, are rapidly and profoundly depleted during acute HIV infection, long before the onset of AIDS (19–22). Third, the level of immune activation in HIV-infected subjects predicts disease progression as well or better than the levels of virus replication (23–26). Taken together, these observations suggested that AIDS pathogenesis was not well explained by the direct viral killing hypothesis and must involve a more complex interplay between the host immune system and both direct and indirect effects of active viral replication. Indeed, the discovery that the simian immunodeficiency virus (SIV) infections of African NHPs (the larger viral family from which HIV originated) are largely nonpathogenic vividly illustrates this conclusion. The SIVs that infect these natural hosts are just as cytopathic to NHP CD4+ T cells as HIV is to human CD4+ T cells, but in the vast majority of these animals, CD4+ T-cell depletion is not progressive and AIDS does not ensue (27–31). These hosts have obviously adapted to viral replication and CD4+ T-cell destruction, whereas pathogenic infections (HIV infections of humans and SIV infections of Asian NHPs) reflect cross-species transmission of infection to non-adapted hosts, a situation in which genetically determined differences in host response result in vastly different outcomes from otherwise similar viral infections.
Over the past decade, a more nuanced and dynamic model of AIDS pathogenesis has emerged. In this model, viral replication drives pathogenesis but does not directly cause immunodeficiency. Instead, immunodeficiency results from dysregulation and ultimately failure of host homeostatic mechanisms and cellular immune networks. Here, we review the evolution of this new model, with the hope of providing a better explanation for why a rapidly replicating, cytotoxic virus causes such a slowly progressing disease.
Cellular dynamics of HIV/SIV infection
One of the more critical discoveries in the investigation of AIDS pathogenesis was the demonstration that HIV entry (and therefore, the targeting of the infection) is dependent not only on expression of a primary receptor, CD4, but also on expression of co-receptors. These co-receptors, which were shown to be chemokine receptors, typically CCR5 or CXCR4, are differentially expressed on CD4+ T cells (21, 32). CXCR4 is highly expressed on the vast majority of peripheral T cells and can indeed mediate a generalized infection and destruction of all CD4+ T cells in infections with viruses using CXCR4 as co-receptor, as demonstrated by the profound generalized CD4+ T-cell destruction and acute onset of AIDS in NHPs infected with a CXCR4-using SHIV (13, 14). However, this co-receptor is not used by transmitted strains of HIV, and ′CXCR4-tropism′ typically develops only in a proportion of infected individuals very late in infection, a switch in co-receptor usage which, in keeping with CXCR4 distribution, is often observed in association with profound pan-CD4+ T-cell depletion and end-stage disease (33–36). As implied by this late switch, transmitted strains of HIV use CCR5 as co-receptor, which confers ′CCR5-tropism′. Importantly, CCR5 is not expressed by all CD4+ T cells, but rather, in keeping with its role in direction of the migration of effector-differentiated T cells to extra-lymphoid sites of inflammation or host defense, its expression is upregulated as part of the late differentiation of effector and effector memory T cells. It is therefore predominantly expressed by CD4+ T cells in effector sites like the lamina propria of the intestinal mucosa (37, 38). CCR5-tropism is not accidental. It represents a fundamental adaption of the virus that allows infection of a subset of CD4+ T cells (effector-differentiated cells in extra-lymphoid effector sites and their immediate precursors in secondary lymphoid tissues) that can be rapidly regenerated from less differentiated, CCR5− precursors (20, 39–41). This adaptation provides the virus with a large initial target population (CD4+ T cells in effector sites), and once these are depleted, the host courteously provides a continuous stream of new targets that maintain infection over the long-term and increase the likelihood of viral transmission.
The importance of this selective targeting in AIDS pathogenesis was first demonstrated in the SIV model of pathogenesis in rhesus macaques (RMs), where it was shown that highly pathogenic, CCR5-tropic SIV infection was associated with widespread infection and massive destruction of CD4+ T cells in intestinal lamina propria and other extra-lymphoid effector sites during the first 2–3 weeks of infection (22, 42–44). The wholesale destruction of CD4+ T cells in these sites has been attributed to both direct infection and Fas/Fas ligand-mediated apoptosis (42). Studies in HIV-infected patients have since shown a similar pattern of preferential and profound depletion of CD4+ T cells within the gastrointestinal tract during acute infection (20, 45, 46). These findings demonstrated that depletion of HIV/SIV viral targets (CD4+CCR5+ memory T cells) is not slow, as originally hypothesized, but rather early and profound.
CCR5-expressing CD4+ T cells in blood and secondary lymphoid tissues are also infected and destroyed in acute infection, but these cells comprise only a subset of CD4+ T cells in these sites, and both the CD4+ naive (TN) and central memory (TCM) T-cell compartments in these tissues are relatively spared from destruction in acute infection. Indeed, within a few days of peak effector memory (TEM) CD4+ destruction in acute SIV infection of RMs, proliferating CD4+ TCM and early transitional effector memory (TTrM) T cells are observed, and these cells migrate to extra-lymphoid effector sites and either stabilize or partially regenerate CD4+ effector memory cells in these sites (39). Indeed, among RMs infected with CCR5-tropic SIV, early collapse of the CD4+ memory T-cell regenerative response is strongly associated with rapid disease progression (onset of AIDS in the first 6 months of infection), whereas robust CD4+ memory T-cell regeneration is associated with survival into the chronic phase of infection (22). Our recent observation that regeneration is preserved in RMs with experimental ablation of the CD4+ TN compartment prior to SIV infections indicates that the CD4+ TCM population is the critical population in this regenerative response (47).
These observations together indicate that the use of CCR5 as the major HIV/SIV co-receptor results in the ability of the virus to target the terminally differentiated CD4+ TEM population, while leaving relatively intact a precursor CD4+ TCM population capable of continuous, high level production of new TEM (Fig. 1). As a result, the virus is assured of a continuous supply of new targets, most of which are expendable to the host, as long as the production of new TEM cells is sufficient to maintain effective immunity (39–41). This would suggest that the profound depletion of CCR5+CD4+ memory T cells (viral targets) during acute infection would not lead to AIDS progression if the regenerative potential of the CCR5−CD4+ T-cell compartments (TCM and TN) could be effectively maintained over the long term. Indeed, this seems to be the case with non-pathogenic SIV infections of African monkeys (′natural hosts′). Via a variety of adaptations that both allow for more efficient sparing of CD4+ TCM populations (48, 49) and provide for effective long-term CD4+ memory T-cell regeneration, these NHPs are able to regenerate and maintain nearly normal CD4+ T-memory compartments after SIV infection, allowing them to tolerate levels of cytopathic SIV replication that are as high or higher than in pathogenic infections of humans and RMs (27, 29, 50–52).
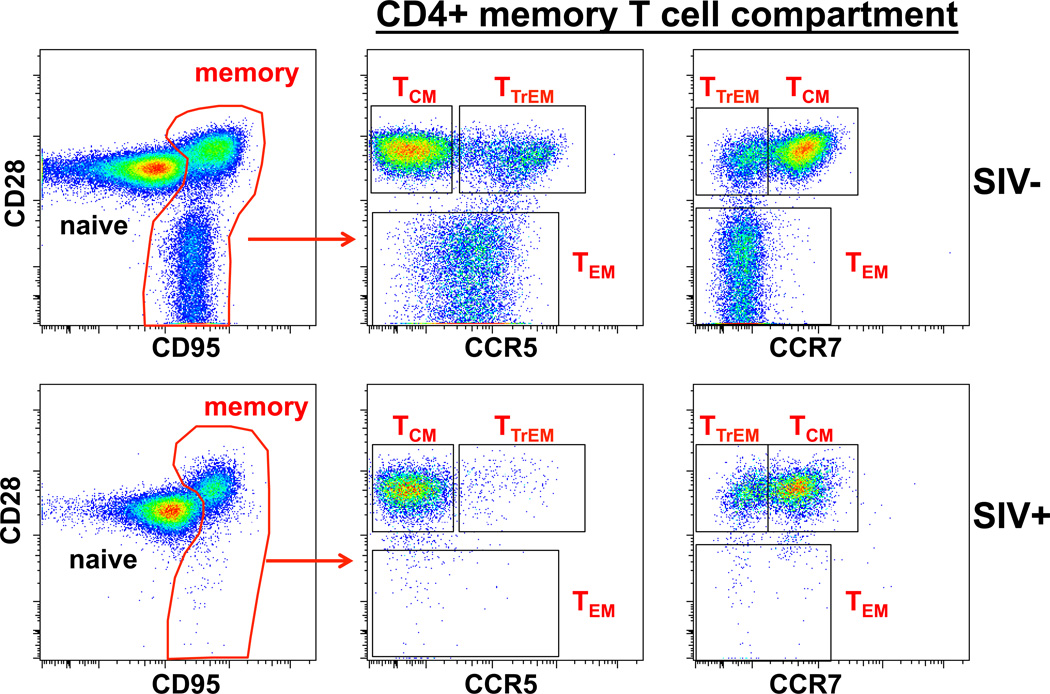
Fig. 1. CCR5+CD4+ memory T cells are primary targets for HIV/SIV.
The figure shows CD28 versus CCR5 or CCR7 on gated CD4+ memory (CD95+) T cells from the peripheral blood of a healthy normal RM (SIV−) and the same animal during chronic infection with the CCR5-tropic SIVmac239. CCR7 downregulation and CCR5 upregulation are key phenotypic markers of TTrM and TEM differentiation and therefore constitute prime HIV/SIV targets. In contrast, CD4+ TN and TCM are CCR7+ and CCR5−, making them inefficient targets for CCR5-tropic HIV and SIV. Regeneration of depleted CD4+ TEM compartments is dependent on continuous recruitment of new cells from their CD4+ TCM precursors.
Connecting CD4+ T-cell depletion with chronic disease progression
These observations suggest that CD4+ T-cell depletion in HIV/SIV infection might be considered a three-stage process: (i) initial profound destruction of optimal CCR5+ viral targets (effector-differentiated CD4+ memory T cells), (ii) a regeneration of the CD4+ TEM compartment from CD4+ TCM precursors, and (iii) progressive CD4+ memory T-cell homeostatic failure in which declining CD4+ TCM populations ultimately lead to CD4+ T-effector cell insufficiency and overt AIDS. As described above, the massive depletion of CD4+ effector-differentiated T cells in infections with CCR5-tropic SIVs (which typically result in AIDS after 1–2 years of infection) is closely followed by a dramatic increase in CD4+ TCM proliferation and CD4+ TEM production that partially regenerates depleted mucosal compartments and prevents early onset of overt immune deficiency. However, this regenerative process does not completely restore immunity and is not stable over time. Unlike T-cell homeostasis in healthy uninfected RMs, in which proliferating cells are predominantly long-lived, the regeneration in SIV infection is a high-turnover process driven by immune activation that produces short-lived cells (53). Extraordinarily high levels of proliferation are necessary to maintain effector memory compartments even at subnormal levels, and not all functionally important CD4+ subsets are regenerated to the same degree (see below). It is now appreciated that apparently healthy monkeys in early chronic phase SIV infection manifest major changes in their intestinal ′virome′, including multiple subclinical invasive viral infections, consistent with covert immune deficiency (54).
The quasi-stable equilibrium between viral- and immune activation-mediated CD4+ T-cell destruction and host efforts to regenerate these populations appears to degrade over time as the substrate population, lymphoid tissue-based CD4+ TCM cells, decline, resulting in progressively reduced regenerative capacity. Indeed, the progressive failure of CD4+ TCM homeostasis and the consequent inability to maintain CD4+ TEM cell production appears to play a major role in setting the tempo of disease progression and the timing of progression to overt immunodeficiency in pathogenic SIV infection (39) (Fig. 2). The mechanisms responsible for the instability of the CD4+ TCM compartment in progressive CCR5-tropic HIV/SIV infection are not yet completely defined. The immune activation-induced high turnover state does not, by itself, explain CD4+ TCM homeostatic failure as CD8+ TCM turnover is similarly increased, but CD8+ memory T-cell homeostasis remains stable over the course of progressive infection (39). While CD8+ and CD4+ TCM populations obviously differ in the fact that cells in the latter population, even with low CCR5 expression, can be killed by direct viral infection, this mechanism, by itself, would only be able to account for progressive CD4+ TCM cell decline if the rate of CD4+ TCM cell infection and direct destruction increased over time (otherwise homeostatic regeneration would maintain a stable equilibrium). In this regard, CD4+ TCM cell decline has been observed in animals with stable viral replication dynamics and stable or even declining levels of CD4+ TCM cell infection (39).
Fig. 2. CD4+ TCM homeostasis is dysregulated in progressive HIV/SIV infection.
In normal (healthy uninfected) individuals, proliferating cells are predominantly long-lived, while effector site CD4+ TEM populations are stable and maintained at levels required for effective immune function. In progressive HIV/SIV infection, immune activation drives a high turnover process that produces short-lived cells. As the regenerative CD4+ TCM cell population slowly diminishes over time, it precipitates the decline in production and influx of new CD4+ TEM cells below a crucial threshold required to keep OIs at bay. Thus, progressive failure of CD4+ TCM homeostasis and its consequent inability to produce new TEM cells plays a major role in setting the tempo of disease progression and the timing to overt immunodeficiency and AIDS.
The slow nature of CD4+ TCM cell homeostatic failure in progressive SIV infection suggests that either CD4+ TCM cells have intrinsic or extrinsic limitations in their ability to self-renew or that the balance between their self-renewal and their production of effector-differentiated progeny is dysregulated, ultimately leaving the CD4+ TCM cell population too small to maintain the level of continuous effector cell production necessary to maintain minimal levels of immune competence. With respect to the former, it has been shown that the secondary lymphoid tissue microenvironments that support TCM cell homeostasis are damaged by the ongoing inflammation associated with progressive infection, with the highly organized reticulin structure of the sites replaced by disorganized scarring (55, 56). Such microenvironmental destruction would limit TCM cell access to IL-7, resulting in a reduction in the size of the total body TCM cell population that can be maintained (39). It has also been suggested that over time, TCM cells undergo proliferative senescence due to telomere shortening, resulting in an inability to maintain the cell division necessary to keep the population from declining. It remains controversial whether telomere shortening actually occurs in the CD4+ TCM cells responsible for maintaining CD4+ memory T-cell homeostasis or whether induction of telomerase maintains the proliferative potential of this population (57–59).
If microenvironment destruction and/or proliferative senescence were solely responsible for CD4+ TCM cell homeostatic failure, the expectation would be that as HIV or SIV infections progress to AIDS, there would be an absolute limitation in CD4+ memory T-cell regeneration, even if viral replication were suppressed. This does not seem to be the case, as anti-retroviral therapy of end-stage infections can result in a rapid burst of regeneration (60–63)(Fig. 3A,B). Thus, dysregulation of homeostatic mechanisms almost certainly plays a major role in disease progression. Negative regulators such as the programmed death-1 receptor (PD-1) is highly expressed on CD4+ and CD8+ memory T cells in chronic HIV and SIV infection as a consequence of persistent viral replication and hyperimmune activation (64–67). While it is generally appreciated that engagement of PD-1 and other negative regulators with their ligands dampens T-cell receptor-mediated signaling and activation, downmodulating pathogen-specific T-cell responses (64, 68), it is possible that these negative regulators have a more generic effect on the homeostasis of the memory compartment. Indeed, antibody-mediated blockade of the PD-1 pathway in chronically SIV-infected RMs induces robust CD4+ TCM cell proliferative responses and an increase in CD4+ memory T-cell numbers, indicating that these pathways strongly inhibit CD4+ TCM homeostasis (Okoye, et al., manuscript in preparation). Induction of negative regulator expression by CD4+ TCM cells is not the only mechanism by which chronic immune activation might dysregulate CD4+ TCM cell homeostasis. The proinflammatory cytokines associated with this immune activation drive effector differentiation (69–72), and by this mechanism lead to an imbalance between CD4+ TCM cell renewal and TCM to TEM cell differentiation, ultimately leading to smaller CD4+ TCM populations and an overall reduction in effector cell production.
Fig. 3. CD4+ memory T-cell regenerative capacity can be restored with ART even at end-stage disease.
At end-stage disease, the total CD4+ memory T-cell proliferative response declines below a crucial threshold that typically signals the failure of the CD4+ TCM regenerative potential and subsequent effector site CD4+ TEM population collapse. (A) Administration of ART leads to a robust proliferative burst in CD4+ TCM cells in the peripheral blood of an RM at end-stage disease. (B) This proliferative response is also observed in secondary lymphoid tissues (spleen, axillary and mesenteric lymph nodes) and results in the influx of new CD4+ TEM cells into extra-lymphoid effector sites (such as the BAL). (C) Administration of partially suppressive ART to chronically SIVmac239-infected RMs induces proliferative responses in both CD4+ TCM and CD8+ TCM cells in the peripheral blood.
Dysregulation of CD4+ T-cell functional heterogeneity
Up to this point, we have discussed mechanisms leading to depletion of the overall CD4+ T-cell memory compartment. Of course, the CD4+ memory T-cell population is not functionally homogenous but rather is comprised of a number of distinct subsets, Th1, Th2, Th17, T-follicular helper (Tfh), and T-regulatory (Treg), among others, with highly specialized immunologic functions and differing requirements for homeostasis and regeneration. These subsets differentially contribute to host defense against a wide variety of invading pathogens as well as prevention of autoimmunity by regulating tolerance to self-antigens, and a functional deficiency in any one of them could potentially lead to immune insufficiency or other immunopathology. Thus, to understand the mechanisms responsible for progression to AIDS, it is necessary to understand the impact of viral replication and immune activation not only on the overall CD4+ memory T-cell compartment but also on the dynamics and homeostasis of each of these functional CD4+ memory T-cell subsets, with particular attention to the effect of infection on the specific factors that mediate development and maintenance of these subsets.
CD4+ Th17 cells are important mediators in the hosts′ defense against extracellular pathogens such as bacteria and fungi, and in the intestinal mucosa, these cells are associated with maintaining the integrity of the gut epithelial barriers. They are characterized by expression of the transcription factor retinoic acid-related orphan receptor-γt (RORγt) and secretion of the IL-17 family of cytokines. Th17 cells promote neutrophil recruitment and induce the production of antibacterial defensins. They also promote tissue repair by inducing the proliferation and survival of epithelial cells (73). Th17 cells appear to play a central role in HIV pathogenesis (74). They are susceptible to direct viral infection in vitro and in vivo and the massive loss of CD4+ memory T cells from the gut early after infection results in significant alterations in CD4+ Th17 homeostasis. CD4+ Th17 cells are depleted in HIV-infected patients, resulting in a skewing in the fraction of CD4+ memory T-cell subsets in the gut from a Th17 to more of a Th1 phenotype (75). Studies in NHPs have shown similar results. In healthy animals, the fractions of Th17 cells are higher in mucosal tissues (lung, colon, jejunum) than in the blood or peripheral lymph nodes. However, after SIV infection, the fraction of Th17 cells in the gut decrease significantly and are never restored. As the infection progresses, CD4+ Th1 cells become the dominant population in the gut mucosa (75, 76). These observations suggest that the conditions of CD4+ memory T-cell regeneration in HIV/SIV infection are not conducive to Th17 regeneration, leaving infected individuals with persistent defects in the functions mediated by this subset. Besides increasing susceptibility to bacterial and fungal infections, loss of this subset may compromise intestinal mucosal integrity, increasing permeability to microbial products and contributing to the chronic immune activation that drives pathogenesis (51, 77, 78).
Another CD4+ helper T lineage subset of primary importance to HIV pathogenesis are Tfh cells. Tfh cells interact with antigen-specific B cells within specialized structures known as germinal centers (GCs) of secondary lymphoid tissues. This interaction enhances antibody affinity maturation and the differentiation of follicular B cells into long-lived memory B cells and/or plasma cells. Crucial to Tfh cell development is the expression of the transcription factor Bcl-6. They are further characterized by expression of the chemokine receptor CXCR5, PD-1, ICOS, and IL-21 secretion. Tfh cells are predominantly located within secondary lymphoid tissues (e.g. lymph nodes, spleen, tonsils, Peyer′s patches) (79, 80). A number of groups have recently shown significant alterations in Tfh cell dynamics in HIV and SIV infection. Like Th17 cells, Tfh are susceptible to infection in vivo and can support active viral replication and production in vitro (81, 82). Localization within secondary lymphoid tissues exposes them to high concentrations of HIV particles that are trapped within GC resident follicular dendritic cells (FDCs) in the form of immune complexes (with antibody and/or complement) (83). FDCs also play a role in recruiting and retaining antigen-specific Tfh cells within the GC (84). Our group has recently shown that the persistence of live attenuated SIVs (LAV) was associated with their ability to selectively replicate in CD4+ Tfh cells in lymph nodes. At necropsy, secondary lymphoid tissues contained significantly higher levels of cell-associated viral RNA and DNA than bone marrow or tissues from extra-lymphoid effector sites (jejunum, liver, and lung), suggesting lymph node-resident CD4+ Tfh cells serve as a sanctuary for LAV persistence (85). In chronic HIV infection, Tfh cells are enriched for HIV-specific cells and contain a high proportion of HIV DNA+ cells (81, 82). Interestingly, the fraction of Tfh cells in peripheral lymph nodes is significantly increased in HIV and pathogenic SIV infection (81, 86). It is not clear the extent to which this relative expansion of Tfh in infected individuals is due to the preservation of these cells from viral-mediated destruction or the selective expansion of these cells after infection, although both likely have a role. Increases in plasma IL-6, a proinflammatory cytokine that regulates Tfh differentiation, suggests IL-6-mediated signaling could play a role in driving Tfh differentiation and expansion in HIV/SIV infection (70, 86). Overall, these observations suggest that the ratio of Tfh to other CD4+ memory T-cell subsets within secondary lymphoid tissues is significantly skewed resulting in higher frequencies of Tfh with progressive infection (81, 82, 86, 87). In both HIV infection of humans and pathogenic SIV infection of RMs, expansion of Tfh cells was associated with increased gammaglobulinemia and a high frequency of GC B cells. Expansion of Tfh cells, which parallels the follicular hyperplasia and hypergammaglobulinemia observed early in infection, might predispose infected individuals to autoimmunity commonly associated with AIDS progression. Indeed, it has been shown that the generation of autoreactive antibodies targeting platelet glycoproteins, phospholipids, and T-cell antigens in SIV-infected macaques was associated with severe pathologic outcomes including the depletion of CD4+ T cells (88).
HIV/SIV infection also affects the dynamics of CD4+ Tregs. Tregs represent a regulatory subset within the CD4+ T-helper lineage involved in suppressing the activation, proliferation, and cytokine production of effector T cells. They are important in the maintenance of immune tolerance and the control of immune activation. Their differentiation is regulated by the transcription factor forkhead box protein 3 (Foxp3) and is characterized by expression of the α chain of the IL-2 receptor CD25, Foxp3, and low expression of the IL-7 receptor CD127. Tregs are susceptible to HIV infection in vitro and are rapidly depleted from the gut during acute infection resulting in a reduction in Treg suppressor activity (89). In chronic SIV infection, there is a progressive decline in absolute Treg cell counts in the blood. Animals with high plasma viral loads manifested low numbers of circulating Tregs. This depletion was not preferential but reflected the overall loss of CD4+ memory T cells (90). In HIV-infected patients, a higher frequency of Tregs was observed in the blood, despite an overall decline in CD4+ T-cell counts. These frequencies were mostly normalized and returned to baseline levels in patients on ART or individuals with elite control of viral replication (91, 92). These observations would suggest progressive HIV/SIV infection alters the dynamics of peripheral CD4+ T-cell subset distribution resulting in higher frequencies of circulating Tregs at the expense of other CD4+ helper T-lineage differentiation and subsequent production of their effector-differentiated progeny. In SIV-infected pigtail macaques, the onset of systemic immune activation and disease progression was associated with a loss in the balance between Th17 and Tregs during acute infection. This loss in the normal ratio of Th17/Treg was not observed in nonpathogenic SIV infection of African green monkeys (AGM) (93). HIV disease progression is also associated with a loss in Th17/Treg balance, which was linked with the induction of indoleamine 2,3-dioxygenase 1 (IDO1) (94). IDO1 is a rate-limiting enzyme for tryptophan catabolism that plays a role in maintaining immune tolerance. IDO1 is induced during inflammation by interferons (and other agents), and its activity has been linked with the induction of Foxp3 expression and Treg differentiation while subsequently suppressing Th17 development (95). Thus, as Th17 cells are progressively depleted during the course of HIV/SIV infection, increased IDO1 activity does not support their regeneration but instead favors a suppressor Treg phenotype. Apart from the negative effects associated with Th17 deficiency (as discussed earlier), higher frequencies of Tregs could lead to delayed or diminished virus-specific immune responses, potentially impairing the ability of the host to limit HIV or SIV replication, as well as effectively respond to developing OIs.
Dysregulation of CD4+ memory T-cell homeostasis and immune reconstitution after anti-retroviral therapy
The advent of ART has dramatically improved the life span of HIV-infected individuals. These drugs are able to suppress viral replication to undetectable levels and significantly restore CD4+ memory T cells in the blood, secondary lymphoid tissues, and extra-lymphoid effector sites. As viral replication is suppressed and chronic immune activation is resolved, at least in part, it is still unclear whether the homeostatic mechanisms associated with regulating CD4+ memory T-cell homeostasis are fully normalized in patients on ART. As previously discussed, end-stage disease is often characterized by the severe depletion of optimal target cells (CCR5+CD4+ memory T cells) and a decline in the CD4+ TCM proliferative response to below a crucial threshold required to maintain CD4+ TEM in extra lymphoid effector sites (22). However, even at this stage of disease, ART induces a burst in CD4+ memory T-cell regeneration with the production of new tissue homing CCR5+CD4+ TEM cells. This regenerative response is not solely due to suppression of direct virus-mediated cytopathicity, as CD8+ memory T cells, which are resistant to infection and depletion, can also respond to ART (47) (Fig. 3C). In a large fraction of HIV-infected individuals, however, ART fails to effectively reconstitute CD4+ T cells to pre-infection levels despite fully suppressed viral replication (96, 97). Although the mechanisms for this are unclear, recent evidence suggests that initiation of therapy during primary infection rather than later in chronic infection significantly improves the prospects for enhanced immune recovery. Patients treated within the first 4 months of infection significantly correlated with higher restoration of CD4+ T-cell counts (>900cells/ml) in the blood (98). Interestingly, initiation of ART within this early (<4 month) time period coincided with the spontaneous restoration of CD4+ T-cell counts observed in HIV-1-infected patients who were not receiving therapy. However, in patients who were not on ART, CD4+ T-cell counts initially rebounded but then progressively declined. Patients who initiated ART in chronic infection were less likely to have enhanced CD4+ T-cell recovery. In a different study, patients treated for 48 weeks with ART in primary infection were less likely to progress to disease (or have CD4+ T-cell counts drop below 350 cells/ml) than patients treated for just 12 weeks or no treatment at all (99). Overall, these observations suggest that CD4+ T-cell regenerative potential declines over time in untreated infection, likely due to the destructive effects of viral replication and chronic immune activation on cell substrates, homeostatic microenvironments, and the balance of regulatory mechanisms regulating CD4+ memory T-cell regeneration. This concept would argue that early intervention aimed at preserving CD4+ TCM cell regenerative capacity should lead to improved CD4+ T-cell recovery and better long-term immune function in ART-treated subjects. This idea is supported by data showing that low CD4+ T-cell nadir (baseline CD4+ T-cell counts before ART) has been implicated with incomplete immune recovery after ART (100). In addition, Lederman et al. (101) showed that patients with immunologic failure (CD4+ T-cell counts < 350 cells/ml) had diminished CD4+ TCM and TEM cell populations, with high turnover rates and increased levels of immune activation despite suppressive ART. It is important to note that other factors such as age, ongoing viral replication (possibly due to viral sanctuaries), and duration of therapy have also been linked to immune reconstitution in HIV-infected patients (97). Dysregulation in CD4+ T cell homeostasis appears to be a key contributor to this process.
Viral persistence during optimally suppressive ART has also been linked with CD4+ T-cell homeostasis. The major barrier to eradication of HIV is thought to be the pool of latently infected, long-lived, resting CD4+ memory T cells, including both CD4+ TCM and TTrM cells, that constitute the major cellular reservoirs for HIV persistence in patients on ART (102). Thus, the maintenance of the viral reservoir is intimately linked with the regulation of CD4+ TCM and TTrM homeostasis. Homeostatic proliferation of these latently infected cells might lead to the regeneration and maintenance of the viral reservoir without cell death. Interestingly, it has been shown that patients with low CD4+ T-cell nadir manifest the highest levels of cell-associated HIV-1 DNA (103). This observation suggests that the level of CD4+ T-cell depletion prior to initiating therapy plays a role in determining the size of the viral reservoir. In addition, virally suppressed patients on long-term ART with low CD4+ T-cell counts (<350 cells/ml) had significantly higher levels of cell-associated HIV RNA and proviral DNA than patients with high CD4+ T-cell counts after therapy. Interestingly, patients with low CD4+ T-cell counts had higher levels of PD-1 expression, predominantly in the CD4+ TCM compartment (104). These observations suggest that the homeostatic mechanisms associated with CD4+ T-cell regeneration have a significant effect not only on the ability to reconstitute immunity after ART but also on the HIV reservoir that maintains infection and hinders therapies aimed at HIV cure.
Concluding remarks
The development of AIDS in untreated HIV and pathogenic SIV infection has the paradoxical quality of being a slowly progressive pathogenetic process involving rapid, highly dynamic mechanisms, a continuously ′negotiated′ balance between direct and indirect effects on HIV/SIV replication that compromise CD4+ memory T-cell survival or function and the ability of the host to replace these lost cells and maintain CD4+ memory T-cell populations by cell proliferation and differentiation. This process leaves its imprint on all clinical aspects of HIV/SIV infection, including the degree to which immunity does or does not normalize after viral suppression with ART and the dynamics of the HIV reservoir in ART-treated subjects. A better understanding of these mechanisms and the development of approaches for their therapeutic manipulation will be critical for management of subjects for whom viral replication cannot be controlled, as well as the development of cure strategies for individuals whose infections are controlled by ART.
Acknowledgements
This work was funded by grants from NIH (R37-AIO54292 and P51-OD011092). We thank Lori Boshears for proofreading the manuscript. The authors declare no competing financial interests.
References
- 1.Barre-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 2.Gallo RC, et al. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984;224:500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- 3.Levy JA, Hoffman AD, Kramer SM, Landis JA, Shimabukuro JM, Oshiro LS. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984;225:840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- 4.Quagliarello V. The Acquired Immunodeficiency Syndrome: current status. Yale J Biol Med. 1982;55:443–452. [PMC free article] [PubMed] [Google Scholar]
- 5.Gottlieb MS, et al. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med. 1981;305:1425–1431. doi: 10.1056/NEJM198112103052401. [DOI] [PubMed] [Google Scholar]
- 6.Masur H, et al. An outbreak of community-acquired Pneumocystis carinii pneumonia: initial manifestation of cellular immune dysfunction. N Engl J Med. 1981;305:1431–1438. doi: 10.1056/NEJM198112103052402. [DOI] [PubMed] [Google Scholar]
- 7.Small CB, Klein RS, Friedland GH, Moll B, Emeson EE, Spigland I. Community-acquired opportunistic infections and defective cellular immunity in heterosexual drug abusers and homosexual men. Am J Med. 1983;74:433–441. doi: 10.1016/0002-9343(83)90970-1. [DOI] [PubMed] [Google Scholar]
- 8.Vieira J, Frank E, Spira TJ, Landesman SH. Acquired immune deficiency in Haitians: opportunistic infections in previously healthy Haitian immigrants. N Engl J Med. 1983;308:125–129. doi: 10.1056/NEJM198301203080303. [DOI] [PubMed] [Google Scholar]
- 9.Klatzmann D, et al. Selective tropism of lymphadenopathy associated virus (LAV) for helper-inducer T lymphocytes. Science. 1984;225:59–63. doi: 10.1126/science.6328660. [DOI] [PubMed] [Google Scholar]
- 10.Masur H, et al. CD4 counts as predictors of opportunistic pneumonias in human immunodeficiency virus (HIV) infection. Ann Intern Med. 1989;111:223–231. doi: 10.7326/0003-4819-111-3-223. [DOI] [PubMed] [Google Scholar]
- 11.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 12.Wei X, et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 13.Reimann KA, et al. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishimura Y, et al. Resting naive CD4+ T cells are massively infected and eliminated by X4-tropic simian-human immunodeficiency viruses in macaques. Proc Natl Acad Sci USA. 2005;102:8000–8005. doi: 10.1073/pnas.0503233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munoz A, et al. Acquired immunodeficiency syndrome (AIDS)-free time after human immunodeficiency virus type 1 (HIV-1) seroconversion in homosexual men Multicenter AIDS Cohort Study Group. Am J Epidemiol. 1989;130:530–539. doi: 10.1093/oxfordjournals.aje.a115367. [DOI] [PubMed] [Google Scholar]
- 16.Lackner AA, Lederman MM, Rodriguez B. HIV pathogenesis: the host. Cold Spring Harbor Persp Med. 2012;2 doi: 10.1101/cshperspect.a007005. a007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendriks JC, Satten GA, van Ameijden EJ, van Druten HA, Coutinho RA, van Griensven GJ. The incubation period to AIDS in injecting drug users estimated from prevalent cohort data, accounting for death prior to an AIDS diagnosis. AIDS. 1998;12:1537–1544. doi: 10.1097/00002030-199812000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Pantaleo G, et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 19.Chun TW, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 20.Brenchley JM, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grivel JC, et al. Human immunodeficiency virus type 1 coreceptor preferences determine target T-cell depletion and cellular tropism in human lymphoid tissue. J Virol. 2000;74:5347–5351. doi: 10.1128/jvi.74.11.5347-5351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picker LJ, et al. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J Exp Med. 2004;200:1299–1314. doi: 10.1084/jem.20041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deeks SG, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 24.Hazenberg MD, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 25.Giorgi JV, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez B, et al. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA. 2006;296:1498–1506. doi: 10.1001/jama.296.12.1498. [DOI] [PubMed] [Google Scholar]
- 27.Silvestri G, et al. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18:441–452. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 28.Gordon SN, et al. Short-lived infected cells support virus replication in sooty mangabeys naturally infected with simian immunodeficiency virus: implications for AIDS pathogenesis. J Virol. 2008;82:3725–3735. doi: 10.1128/JVI.02408-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandrea I, et al. Simian immunodeficiency virus SIVagm dynamics in African green monkeys. J Virol. 2008;82:3713–3724. doi: 10.1128/JVI.02402-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstein S, et al. Comparison of simian immunodeficiency virus SIVagmVer replication and CD4+ T-cell dynamics in vervet and sabaeus African green monkeys. J Virol. 2006;80:4868–4877. doi: 10.1128/JVI.80.10.4868-4877.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chahroudi A, Bosinger SE, Vanderford TH, Paiardini M, Silvestri G. Natural SIV hosts: showing AIDS the door. Science. 2012;335:1188–1193. doi: 10.1126/science.1217550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doms RW. Chemokine receptors and HIV entry. AIDS. 2001;15(Suppl):S34–S35. doi: 10.1097/00002030-200102001-00051. [DOI] [PubMed] [Google Scholar]
- 33.Scarlatti G, et al. In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression. Nat Med. 1997;3:1259–1265. doi: 10.1038/nm1197-1259. [DOI] [PubMed] [Google Scholar]
- 34.Harouse JM, Gettie A, Tan RC, Blanchard J, Cheng-Mayer C. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science. 1999;284:816–819. doi: 10.1126/science.284.5415.816. [DOI] [PubMed] [Google Scholar]
- 35.Connor RI, Sheridan KE, Ceradini D, Choe S, Landau NR. Change in coreceptor use correlates with disease progression in HIV-1--infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koot M, et al. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann Intern Med. 1993;118:681–688. doi: 10.7326/0003-4819-118-9-199305010-00004. [DOI] [PubMed] [Google Scholar]
- 37.Poles MA, Elliott J, Taing P, Anton PA, Chen IS. A preponderance of CCR5(+) CXCR4(+) mononuclear cells enhances gastrointestinal mucosal susceptibility to human immunodeficiency virus type 1 infection. J Virol. 2001;75:8390–8399. doi: 10.1128/JVI.75.18.8390-8399.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veazey RS, et al. Dynamics of CCR5 expression by CD4(+) T cells in lymphoid tissues during simian immunodeficiency virus infection. J Virol. 2000;74:11001–11007. doi: 10.1128/jvi.74.23.11001-11007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okoye A, et al. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J Exp Med. 2007;204:2171–2185. doi: 10.1084/jem.20070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Picker LJ. Immunopathogenesis of acute AIDS virus infection. Curr Opin Immunol. 2006;18:399–405. doi: 10.1016/j.coi.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Picker LJ, Watkins DI. HIV pathogenesis: the first cut is the deepest. Nat Immunol. 2005;6:430–432. doi: 10.1038/ni0505-430. [DOI] [PubMed] [Google Scholar]
- 42.Li Q, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 43.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, et al. Massive infection and loss of CD4+ T cells occurs in the intestinal tract of neonatal rhesus macaques in acute SIV infection. Blood. 2007;109:1174–1181. doi: 10.1182/blood-2006-04-015172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guadalupe M, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehandru S, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okoye AA, et al. Naive T cells are dispensable for memory CD4+ T cell homeostasis in progressive simian immunodeficiency virus infection. J Exp Med. 2012;209:641–651. doi: 10.1084/jem.20112071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paiardini M, et al. Bone marrow-based homeostatic proliferation of mature T cells in nonhuman primates: implications for AIDS pathogenesis. Blood. 2009;113:612–621. doi: 10.1182/blood-2008-06-159442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paiardini M, et al. Low levels of SIV infection in sooty mangabey central memory CD(4)(+) T cells are associated with limited CCR5 expression. Nat Med. 2011;17:830–836. doi: 10.1038/nm.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beaumier CM, et al. CD4 downregulation by memory CD4+ T cells in vivo renders African green monkeys resistant to progressive SIVagm infection. Nat Med. 2009;15:879–885. doi: 10.1038/nm.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 52.Brenchley JM, et al. Differential infection patterns of CD4+ T cells and lymphoid tissue viral burden distinguish progressive and nonprogressive lentiviral infections. Blood. 2012;120:4172–4181. doi: 10.1182/blood-2012-06-437608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat Med. 2006;12:289–295. doi: 10.1038/nm1380. [DOI] [PubMed] [Google Scholar]
- 54.Handley SA, et al. Pathogenic simian immunodeficiency virus infection is associated with expansion of the enteric virome. Cell. 2012;151:253–266. doi: 10.1016/j.cell.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeng M, Haase AT, Schacker TW. Lymphoid tissue structure and HIV-1 infection: life or death for T cells. Trends Immunol. 2012;33:306–314. doi: 10.1016/j.it.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 56.Zeng M, et al. Critical role of CD4 T cells in maintaining lymphoid tissue structure for immune cell homeostasis and reconstitution. Blood. 2012;120:1856–1867. doi: 10.1182/blood-2012-03-418624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Franzese O, et al. Telomerase activity, hTERT expression, and phosphorylation are downregulated in CD4(+) T lymphocytes infected with human immunodeficiency virus type 1 (HIV-1) J Med Virol. 2007;79:639–646. doi: 10.1002/jmv.20855. [DOI] [PubMed] [Google Scholar]
- 58.Palmer LD, Weng N, Levine BL, June CH, Lane HC, Hodes RJ. Telomere length, telomerase activity, and replicative potential in HIV infection: analysis of CD4+ and CD8+ T cells from HIV-discordant monozygotic twins. J Exp Med. 1997;185:1381–1386. doi: 10.1084/jem.185.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dagarag M, Ng H, Lubong R, Effros RB, Yang OO. Differential impairment of lytic and cytokine functions in senescent human immunodeficiency virus type 1-specific cytotoxic T lymphocytes. J Virol. 2003;77:3077–3083. doi: 10.1128/JVI.77.5.3077-3083.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tortajada C, et al. Comparison of T-cell subsets' reconstitution after 12 months of highly active antiretroviral therapy initiated during early versus advanced states of HIV disease. J Acquir Immune Defic Syndr. 2000;25:296–305. doi: 10.1097/00042560-200012010-00002. [DOI] [PubMed] [Google Scholar]
- 61.Li TS, Tubiana R, Katlama C, Calvez V, Ait Mohand H, Autran B. Long-lasting recovery in CD4 T-cell function and viral-load reduction after highly active antiretroviral therapy in advanced HIV-1 disease. Lancet. 1998;351:1682–1686. doi: 10.1016/s0140-6736(97)10291-4. [DOI] [PubMed] [Google Scholar]
- 62.Hammer SM, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 63.D'Amico R, et al. Lower CD4+ T lymphocyte nadirs may indicate limited immune reconstitution in HIV-1 infected individuals on potent antiretroviral therapy: analysis of immunophenotypic marker results of AACTG 5067. J Clin Immunol. 2005;25:106–115. doi: 10.1007/s10875-005-2816-0. [DOI] [PubMed] [Google Scholar]
- 64.Porichis F, Kaufmann DE. Role of PD-1 in HIV pathogenesis and as target for therapy. Curr HIV/AIDS Rep. 2012;9:81–90. doi: 10.1007/s11904-011-0106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trautmann L, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 66.Rosignoli G, Lim CH, Bower M, Gotch F, Imami N. Programmed death (PD)-1 molecule and its ligand PD-L1 distribution among memory CD4 and CD8 T cell subsets in human immunodeficiency virus-1-infected individuals. Clin Exp Immunol. 2009;157:90–97. doi: 10.1111/j.1365-2249.2009.03960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Day CL, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 68.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahmad R, Sindhu ST, Toma E, Morisset R, Ahmad A. Elevated levels of circulating interleukin-18 in human immunodeficiency virus-infected individuals: role of peripheral blood mononuclear cells and implications for AIDS pathogenesis. J Virol. 2002;76:12448–12456. doi: 10.1128/JVI.76.24.12448-12456.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Breen EC, et al. Infection with HIV is associated with elevated IL-6 levels and production. J Immunol. 1990;144:480–484. [PubMed] [Google Scholar]
- 71.Emilie D, et al. Production of interleukins in human immunodeficiency virus-1-replicating lymph nodes. J Clin Invest. 1990;86:148–159. doi: 10.1172/JCI114678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Molina JM, Scadden DT, Byrn R, Dinarello CA, Groopman JE. Production of tumor necrosis factor alpha and interleukin 1 beta by monocytic cells infected with human immunodeficiency virus. J Clin Invest. 1989;84:733–737. doi: 10.1172/JCI114230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huber S, Gagliani N, Flavell RA. Life, death, and miracles: Th17 cells in the intestine. Eur J Immunol. 2012;42:2238–2245. doi: 10.1002/eji.201242619. [DOI] [PubMed] [Google Scholar]
- 74.Elhed A, Unutmaz D. Th17 cells and HIV infection. Curr Opin HIV AIDS. 2010;5:146–150. doi: 10.1097/COH.0b013e32833647a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brenchley JM, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cecchinato V, et al. Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques. Mucosal Immunol. 2008;1:279–288. doi: 10.1038/mi.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brenchley JM, Douek DC. Microbial translocation across the GI tract. Annu Rev Immunol. 2012;30:149–73. doi: 10.1146/annurev-immunol-020711-075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 2013;21:6–13. doi: 10.1016/j.tim.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ma CS, Deenick EK, Batten M, Tangye SG. The origins, function, and regulation of T follicular helper cells. J Exp Med. 2012;209:1241–1253. doi: 10.1084/jem.20120994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nutt SL, Tarlinton DM. Germinal center B and follicular helper T cells: siblings, cousins or just good friends? Nat Immunol. 2011;12:472–477. doi: 10.1038/ni.2019. [DOI] [PubMed] [Google Scholar]
- 81.Lindqvist M, et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest. 2012;122:3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perreau M, et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med. 2013;210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smith-Franklin BA, et al. Follicular dendritic cells and the persistence of HIV infectivity: the role of antibodies and Fcgamma receptors. J Immunol. 2002;168:2408–2414. doi: 10.4049/jimmunol.168.5.2408. [DOI] [PubMed] [Google Scholar]
- 84.Estes JD, et al. Follicular dendritic cell regulation of CXCR4-mediated germinal center CD4 T cell migration. J Immunol. 2004;173:6169–6178. doi: 10.4049/jimmunol.173.10.6169. [DOI] [PubMed] [Google Scholar]
- 85.Fukazawa Y, et al. Lymph node T cell responses predict the efficacy of live attenuated SIV vaccines. Nat Med. 2012;18:1673–1681. doi: 10.1038/nm.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Petrovas C, et al. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest. 2012;122:3281–3294. doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hong JJ, Amancha PK, Rogers K, Ansari AA, Villinger F. Spatial alterations between CD4(+) T follicular helper, B, and CD8(+) T cells during simian immunodeficiency virus infection: T/B cell homeostasis, activation, and potential mechanism for viral escape. J Immunol. 2012;188:3247–3256. doi: 10.4049/jimmunol.1103138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kuwata T, et al. Association of progressive CD4(+) T cell decline in SIV infection with the induction of autoreactive antibodies. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000372. e1000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chase AJ, et al. Severe depletion of CD4+ CD25+ regulatory T cells from the intestinal lamina propria but not peripheral blood or lymph nodes during acute simian immunodeficiency virus infection. J Virol. 2007;81:12748–12757. doi: 10.1128/JVI.00841-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pereira LE, et al. Simian immunodeficiency virus (SIV) infection influences the level and function of regulatory T cells in SIV-infected rhesus macaques but not SIV-infected sooty mangabeys. J Virol. 2007;81:4445–4456. doi: 10.1128/JVI.00026-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Angin M, et al. Preserved function of regulatory T cells in chronic HIV-1 infection despite decreased numbers in blood and tissue. J Infect Dis. 2012;205:1495–1500. doi: 10.1093/infdis/jis236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Presicce P, Orsborn K, King E, Pratt J, Fichtenbaum CJ, Chougnet CA. Frequency of circulating regulatory T cells increases during chronic HIV infection and is largely controlled by highly active antiretroviral therapy. PLoS ONE. 2011;6:e28118. doi: 10.1371/journal.pone.0028118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Favre D, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000295. e1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Favre D, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2:32ra6. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kanwar B, Favre D, McCune JM. Th17 and regulatory T cells: implications for AIDS pathogenesis. Curr Opin HIV AIDS. 2010;5:151–157. doi: 10.1097/COH.0b013e328335c0c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Autran B, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 97.Guihot A, Bourgarit A, Carcelain G, Autran B. Immune reconstitution after a decade of combined antiretroviral therapies for human immunodeficiency virus. Trends Immunol. 2011;32:131–137. doi: 10.1016/j.it.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 98.Le T, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med. 2013;368:218–230. doi: 10.1056/NEJMoa1110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fidler S, et al. Short-course antiretroviral therapy in primary HIV infection. N Engl J Med. 2013;368:207–217. doi: 10.1056/NEJMoa1110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Negredo E, et al. Nadir CD4 T cell count as predictor and high CD4 T cell intrinsic apoptosis as final mechanism of poor CD4 T cell recovery in virologically suppressed HIV-infected patients: clinical implications. Clin Infect Dis. 2010;50:1300–1308. doi: 10.1086/651689. [DOI] [PubMed] [Google Scholar]
- 101.Lederman MM, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis. 2011;204:1217–1226. doi: 10.1093/infdis/jir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chomont N, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boulassel MR, Chomont N, Pai NP, Gilmore N, Sekaly RP, Routy JP. CD4 T cell nadir independently predicts the magnitude of the HIV reservoir after prolonged suppressive antiretroviral therapy. J Clin Virol. 2012;53:29–32. doi: 10.1016/j.jcv.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 104.Hatano H, et al. Cell-based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD-1)-expressing CD4+ T cells. J Infect Dis. 2012 doi: 10.1093/infdis/jis630. [DOI] [PMC free article] [PubMed] [Google Scholar]