Abstract
Concentrations of l-tryptophan (l-Trp) and its metabolite, l-kynurenine (l-KYN), in sera of 19 normal subjects (age: 23.6 ± 3.5 y, male: 8, female: 11) were determined by high-performance liquid chromatography with mass-spectrometric detection, following their derivatization with (R)-(−)-4-(N, N-dimethylaminosulfonyl)-7-(3-isothiocyanatopyrrolidin-1-yl)-2,1,3-benzoxadiazole (DBD-PyNCS). A significant positive correlation between l-Trp and l-KYN concentrations was observed (r = 0.532, P < 0.05). Serum l-Trp concentration in male subjects (95.65 ± 4.27 μM) was significantly higher than that in female subjects (79.20 ± 3.34 μM; P < 0.05), while no significant differences in l-KYN concentration or the l-KYN:l-Trp ratio were observed between male and female subjects.
Keywords: (R)-(−)-4-(N,N-dimethylaminosulfonyl)-7-(3-isothiocyanatopyrrolidin-1-yl)-2,1,3-benzoxadiazole (DBD-PyNCS); high-performance liquid chromatography; human serum; l-kynurenine; l-tryptophan; mass spectrometry
Introduction
An essential amino acid, tryptophan (Trp), is known to be metabolized to several neuroactive substances such as serotonin, kynurenic acid, and quinolinic acid. Analyses of Trp and its metabolites in biological fluids, such as serum or plasma, are of clinical importance for diagnosis of several diseases in humans as several reports have indicated that concentrations of Trp and its metabolites are altered in various disease states.1–5 Tryptophan 2,3-dioxygenase (TDO) or indoleamine 2,3-dioxygenase (IDO) can metabolize Trp to N-formyl-l-kynurenine (KYN).6–11 KYN is also produced non-enzymatically by deformylation of N-formyl- KYN because of the instability of the latter compound. Changes in the expression and activity of IDO have been found to be associated with intractable diseases, including cancer, amyotrophic lateral sclerosis, multiple sclerosis.7,9,11
Thus far, the expression levels of IDO and TDO in cell culture or tissues of experimental rodents has been mainly investigated by western blot (WB) analysis.8,10 However, precise quantitation of the expressed enzyme by WB analysis is difficult, although the expression of TDO and IDO can be separately analyzed. Therefore, to monitor both IDO and TDO activity simultaneously requires an alternative way of measuring the concentrations of the substrate and the product, l-Trp and l-KYN, respectively. Such methodology would make it possible to assess IDO and TDO activity in vivo, because blood or urine samples can be easily obtained from human subjects or experimental animals. To date, several HPLC methods that enable determination of l-Trp and l-KYN simultaneously by using UV,12 fluorescence,13 electrochemical,14 or mass spectrometric (MS) detection,15,16 have been reported.
In this study, we developed a method employing HPLC with MS detection for simultaneous determination of l-Trp and l-KYN concentrations, by modification of our previous HPLC method17 with pre-column derivatization with a fluorescence reagent, (R)-(−)-4-(N, N-dimethylaminosulfonyl)-7- (3-isothiocyanatopyrrolidin-1-yl)-2,1,3-benzoxadiazole (DBD-PyNCS).18 Using the HPLC-MS method proposed in this study, l-Trp and l-KYN concentrations in human serum can be determined using 10 μL of serum.
Materials and Methods
Chemicals
l-Trp was obtained from Nacalai Tesque Inc. (Kyoto, Japan). (R)-(−)-DBD-PyNCS, 4-N, N-dimethylaminopyridine (DMAP), and 7-aminoheptanoic acid were purchased from Tokyo Kasei Co., Ltd. (Tokyo, Japan). Acetic acid (AcOH) of HPLC grade was obtained from Wako Pure Chemical Industries Ltd. (Osaka, Japan). l-KYN was obtained from Sigma-Aldrich (St. Louis, MO, USA). Acetonitrile (CH3CN) of HPLC grade was obtained from Kanto Kagaku Co., Ltd. (Tokyo, Japan). Millipore-purified (Nihon Millipore K.K., Tokyo, Japan) water was used in all experiments.
Serum samples from human volunteers
Healthy human volunteers (n = 19; 8 men and 11 women) participated in this study; the mean age was 23.6 ± 3.5 y (mean ± SD). Written informed consent was obtained from all subjects prior to their participation in this study, and the protocol was approved by the Ethics Committee of Toho University, Faculty of Pharmaceutical Sciences.
Five milliliters of blood was drawn from the arm vein of participants between 11:00–12:00 am, before lunch, and was collected in Venoject-II® AUTOSEP tubes (Terumo Corporation, Tokyo, Japan) for 30 min at room temperature. The tubes were then centrifuged at 1,200 × g for 15 min. The serum obtained was divided into aliquots of 100 μL each into screw-capped vials, which were stored at −80 °C.
Determination of l-Trp and l-KYN in human serum
l-Trp and l-KYN concentrations in human serum samples were simultaneously determined by an HPLC-electrospray ionization (ESI)-MS method (LC-MS), in which the detection system in our previously described HPLC method17 was changed from a fluorescence to an MS detector. Briefly, 10 μL of serum was mixed vigorously with 10 μL of CH3CN/H2O (50:50), 10 μL of a 50 μM (for l-Trp), or 2.5-μM (for l-KYN) solution of the internal standard (I.S.), 7-aminoheptanoic acid, in CH3CN/H2O (50:50), and 120 μL of CH3CN. These samples were centrifuged at 1,300 × g for 20 min at 4 °C. Ten microliters of the obtained supernatant was sampled and added to 10 μL of a 10-mM solution of (R)-(−)-DBD-Py-NCS in CH3CN and 10 μL of a 30 mM solution of DMAP in CH3CN. The resulting solution was heated at a temperature of 55 °C for 20 min, and then 70 μL of a H2O/CH3CN (80:20) mixture containing 0.1% AcOH was added to dilute the reacted solution. Thereafter, 50 μL of the final solution was injected onto the LC-MS apparatus, comprising an Agilent 1200series HPLC system (Agilent Technologies, Santa Clara, CA, U.S.A.) and a time-of-flight (TOF)-MS (JMS-T100 LP AccuTOF LC-Plus) equipped with an ESI source (JEOL Co. Ltd., Tokyo, Japan). The separation column was a TSKgel ODS-100V column (250 mm × 2.0 mm; i.d.: 5 μm; Tosoh Corporation, Tokyo, Japan), and the mobile phases A and B were H2O/CH3CN (80:20) containing 0.1% AcOH, and CH3CN/H2O (80:20) containing 0.1% AcOH, respectively. High-pressure linear gradient elution of the mobile phases A and B was carried out in the following manner: B (%: 38.0–38.0–40.0–40.0), time (min: 0–5.0–7.0–40.0). The mobile phase was constantly pumped at 0.16 mL min−1, and the column temperature was maintained at 40 °C. The conditions for ESI-MS detection were as follows: negative ion mode; needle voltage set at −2,000 V; and the ring lens and orifice 1 and 2 voltages set at −10, −35, and −7 V, respectively. Nitrogen was used as the nebulizing and desolvation gas, and pressure was maintained constant at 0.608 MPa. The desolvation chamber and orifice 1 temperatures were set to 250 °C and 120 °C, respectively. Data were obtained using Mass Center software, MS-56010MP (JEOL).
Calibration and statistical analyses
Two calibration curves were constructed using the peak area ratio of l-Trp or l-KYN to I.S. (50 or 2.5 μM), plotted against l-Trp (10–200 μM) or l-KYN (0.5–5.0 μM) concentration (n = 4). Using these calibration curves, l-Trp and l-KYN concentrations in human serum were determined. Unpaired Student’s t-tests were performed to assess the significance of differences between 2 groups. Association between 2 groups was evaluated by Pearson’s correlation test. A P-value below 0.05 was considered significant.
Results and Discussion
It is known that both IDO and TDO are key enzymes for Trp metabolism,6 and that l-Trp and l-KYN levels can be used to assess IDO and TDO activities.10 Therefore, to monitor IDO or TDO activity in vivo, determination of both l-Trp and l-KYN concentrations in bio-samples is necessary.10
Previously, we had reported the development of a method using HPLC with fluorescence detection for the determination of l-Trp and l-KYN.17 Our previous HPLC method utilized a pre-column derivatization with (R)-DBD-PyNCS, which bears a fluorescent benzofurazan moiety (2,1,3-benzoxadiazole), with relatively long excitation and emission wavelengths. Fluorescence at 565 nm that originated from (R)- DBD-PyNCS upon excitation at 440 nm was used for detection. The recovery and stability of the derivative with (R)-DBD-PyNCS were sufficient for routine analysis by HPLC.17 Under our previous HPLC conditions, a small amount of endogenous l-KYN in human serum was detected at around 49 min, while l-Trp was clearly determined at around 53 min (data not shown). Using fluorescence detection, a relatively long elution time is generally needed for separating other interfering peaks. However, marked l-KYN peak broadening occurred due to the relatively long elution time, and, for this reason, precise determination of trace l-KYN was difficult under the previous HPLC method. In contrast, selective ion monitoring (SIM) by means of MS detection can afford a single peak of analyte without complete separation.
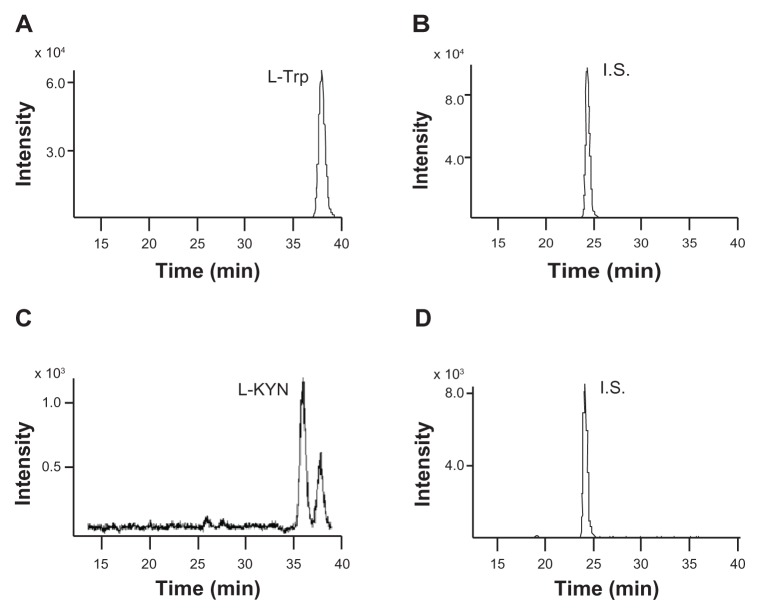
To date, several reports have described that the benzofurazan moiety mentioned above is useful not only for fluorescence, but also for MS detection.19,20 Therefore, here, we changed the detection system from a fluorescence to MS system, using a JMS-T100 LP AccuTOF LC-Plus equipped with an ESI source (JEOL Co. Ltd., Tokyo, Japan), as an alternative detection method. Optimum conditions for the ionization and detection of l-Trp and l-KYN standards derivatized with (R)-DBD-PyNCS, such as settings for needle voltage, the ring lens, and orifice 1 and 2 voltages, were carefully investigated. Moreover, we also optimized the mobile phase of the HPLC system. Consequently, the peaks of l-Trp, l-KYN, or I.S. (50 and 2.5 μM) in human serum samples could be clearly detected, as shown in Figure 1A–D. The detected ions, m/z 556.14, 560.13, and 497.16 were used for SIM of l-Trp, l-KYN, and I.S., respectively. In human serum, an unknown peak appearing after elution of the l-KYN peak was detected in SIM chromatograms at m/z 560.13 (Fig. 1C). The unknown peak may be an isotope ion peak of l-Trp derivatized with DBD-PyNCS, as the elution time of the unknown peak was consistent with that of l-Trp (Fig. 1A and C). The mass difference between l-Trp and l-KYN derivatized with DBD-PyNCS (m/z 556.14 and 560.13, respectively) is approximately 4, further supporting the likelihood that this unknown peak is an isotope ion peak of l-Trp derivatized with DBD-PyNCS.
Figure 1.
Representative chromatograms of l-Trp (A), l-KYN (B), I.S. (50 μM) (C), and I.S. (2.5 μM) (D), respectively, after derivatization with (R)-DBD-PyNCS.
The calibration curves obtained for l-Trp and l-KYN were linear (r2 = 0.9985 and 0.9978, respectively) in the range of 10–200 μM (n = 4) and 0.5–5.0 μM (n = 4), respectively. Relative standard deviation values (RSD%) for l-Trp and l-KYN were in the range of 0.24%–2.05% and 1.91%–4.44%, respectively, indicating that the proposed HPLC method was effective for the determination of l-Trp and l-KYN in human serum.
Since (R)-DBD-PyNCS, used as the derivatization reagent, has a chiral carbon in its structure, it is possible to detect both enantiomers of Trp and KYN, namely not only the l-form, but also the d-form in the chromatogram.17,21 In the present study, however, neither endogenous d-Trp, nor d-KYN, was detected in human serum. The detection and quantification limits in this study were approximately 150 nM and 0.5 μM for Trp and KYN, respectively, and, therefore, a more sensitive MS detection system would be necessary in order to detect these d-forms in human serum.
Table 1 shows serum l-Trp and l-KYN concentrations (mean ± S.E.) in male and female subjects as determined by the LC-MS method presented here. In the present study, a slight but significant gender difference was observed (P < 0.05), namely, the l-Trp concentration in the sera of male subjects was higher than that of female subjects. This result was consistent with those of a previous paper, describing that serum l-Trp concentration in males was higher than that in females.22 The ratio of l-KYN to l-Trp (l-KYN/l-Trp), which is a tryptophan breakdown index representing the sum activities of TDO and IDO,10,23 showed no gender difference.
Table 1.
Serum l-Trp and l-KYN concentrations and the ratio (mean ± S.E.) in male or female subjects determined by the proposed LC-MS method.
| Total (n = 19) | Male (n = 8) | Female (n = 11) | |
|---|---|---|---|
| l-Trp (μM) | 86.13 ± 4.12 | 95.65 ± 4.27 | 79.20 ± 3.34* |
| l-KYN (μM) | 1.84 ± 0.15 | 2.08 ± 0.17 | 1.66 ± 0.13 |
| l-KYN/l-Trp (×10−2) | 2.15 ± 0.17 | 2.12 ± 0.11 | 2.17 ± 0.21 |
Note:
P < 0.05 versus male.
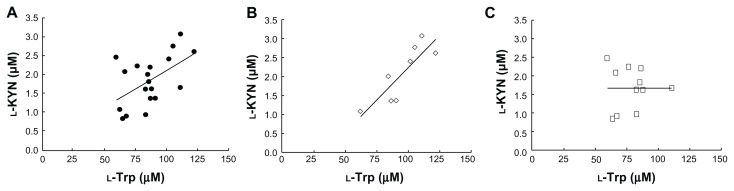
Figure 2 shows a correlation plot between l-Trp and l-KYN concentrations in human sera. As shown in Figure 2A, a significant positive correlation was observed (r = 0.532, P < 0.05), suggesting that IDO or TDO typically play a role in the decomposition of l-Trp in normal subjects. Figure 2B and C show correlation plots between l-Trp and l-KYN concentrations in sera of male and female subjects, respectively. A significant correlation was observed in male subjects (r = 0.847, P < 0.01), but not in female subjects. It is unclear why this gender difference was observed, but the menstrual cycle may affect the serum l-Trp and l-KYN concentrations in female subjects.
Figure 2.
Correlation plots of serum l-KYN concentration against l-Trp concentration in human serum (n = 19) (A), male subjects (n = 8) (B), and female subjects (n = 11) (C).
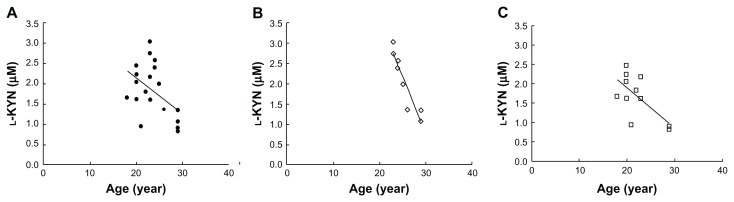
As shown in Figure 3, a slight negative correlation of l-KYN with age was observed (r = −0.466, P < 0.05), while no correlation of l-Trp with age was observed (data not shown). Figure 3B and C show correlation plots between l-KYN concentrations and age in sera of male and female subjects, respectively. In both male and female subjects, significant correlations [r = −0.928 (P < 0.01) and r = −0.651 (P < 0.05), respectively] were observed. However, in the present study, the serum samples were obtained from individuals younger than 30-years-old, and therefore, investigation of older subjects would be required in order to evaluate the correlation between age and l-KYN or l-Trp.
Figure 3.
Correlation plots of serum l-KYN concentration against age (n = 19) (A), male subjects (n = 8) (B), and female subjects (n = 11) (C).
Conclusions
l-Trp and l-KYN concentrations in human serum were determined by the proposed LC-MS method after derivatization with (R)-DBD-PyNCS. Using the proposed method, l-Trp and l-KYN concentrations in human serum can be determined using only 10 μL of the serum. This method will be helpful for the in vivo estimation of TDO and IDO activities in the field of clinical research.
Acknowledgements
The authors thank Mrs. Takahashi and Mrs. Watanabe for their kind assistance with drawing blood from volunteers, and also thank all volunteers who participated in this study.
Footnotes
Author Contributions
Conceived and designed the experiments: HO, HI, TF. Analyzed the data: HO, HI, SY, HO, MK. Contributed to the writing of the manuscript: HO, HI, TF. Jointly developed the structure and arguments for the paper: KS, HI. Made critical revisions and approved final version: HO, HI, TF. All authors reviewed and approved of the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
Funding
The present study was financially supported by grants-in- aid for Scientific Research (C) (23617027) and (22590147) from the Japan Society for the Promotion of Science (JSPS) and the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- 1.Vecsei L, Szalardy L, Fulop F, Toldi J. Kynurenines in the CNS: recent advances and new questions. Nat Rev Drug Discovery. 2013;12(1):64–82. doi: 10.1038/nrd3793. [DOI] [PubMed] [Google Scholar]
- 2.Mackay GM, Forrest CM, Stoy N, et al. Tryptophan metabolism and oxidative stress in patients with chronic brain injury. Eur J Neurol. 2006;13(1):30–42. doi: 10.1111/j.1468-1331.2006.01220.x. [DOI] [PubMed] [Google Scholar]
- 3.Huengsberg M, Winer JB, Gompels M, Round R, Ross J, Shahmanesh M. Serum kynurenine-to-tryptophan ratio increases with progressive disease in HIV-infected patients. Clin Chem. 1998;44(4):858–62. [PubMed] [Google Scholar]
- 4.Owens MJ, Nemeroff CB. Role of serotonin in the pathophysiology of depression-focus on the serotonin transporter. Clin Chem. 1994;40(2):288–95. [PubMed] [Google Scholar]
- 5.Mu S, Li Y, Tang AG, Xiao LD, Ren YP. Simultaneous determination of tyrosine, tryptophan and 5-hydroxytryptamine in serum of MDD patients by high performance liquid chromatography with fluorescence detection. Clin Chim Acta. 2012;413(11–2):973–7. doi: 10.1016/j.cca.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Peters JC. Tryptophan nutrition and metabolism: an overview. Adv Exp Med Biol. 1991;294:345–58. doi: 10.1007/978-1-4684-5952-4_32. [DOI] [PubMed] [Google Scholar]
- 7.Richard DM, Dawes MA, Mathias CW, Acheson A, Hill-Kapturczak N, Dougherty DM. l-Tryptophan: Basic metabolic functions, behavioral research and therapeutic indications. Int J Tryptophan Res. 2009;23:45–60. doi: 10.4137/ijtr.s2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funakoshi H, Kanai M, Nakamura T. Modulation of tryptophan metabolism, promotion of neurogenesis and alteration of anxiety-related behavior in tryptophan 2,3-dioxygenase-deficient mice. Int J Tryptophan Res. 2011;4:7–18. [Google Scholar]
- 9.Macchiarulo A, Camaioni E, Nuti R, Pellicciari R. Highlights at the gate of tryptophan catabolism: a review on the mechanisms of activation and regulation of indoleamine 2, 3-dioxygenase (IDO), a novel target in cancer disease. Amino Acids. 2009;37(2):219–29. doi: 10.1007/s00726-008-0137-3. [DOI] [PubMed] [Google Scholar]
- 10.Kim H, Chen L, Lim G, et al. Brain indoleamine 2,3-dioxygenase contributes to the comorbidity of pain and depression. J Clin Invest. 2012;122(8):2940–54. doi: 10.1172/JCI61884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan PH, Bharath AK. Manipulation of indoleamine 2,3 dioxygenase; a novel therapeutic target for treatment of diseases. Expert Opin Therapeutic Targets. 2009;13(8):987–1012. doi: 10.1517/14728220903018940. [DOI] [PubMed] [Google Scholar]
- 12.Zhang XQ, He Y, Ding M. Simultaneous determination of tryptophan and kynurenine in plasma samples of children patients with Kawasaki disease by high-performance liquid chromatography with programmed wavelength ultraviolet detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(16–7):1678–82. doi: 10.1016/j.jchromb.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Zhao JX, Gao PJ, Zhu DL. Optimization of Zn2+-containing mobile phase for simultaneous determination of kynurenine, kynurenic acid and tryptophan in human plasma by high performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(5–6):603–8. doi: 10.1016/j.jchromb.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Liu LH, Chen Y, Zhang YL, Wang F, Chen ZL. Determination of tryptophan and kynurenine in human plasma by liquid chromatography- electrochemical detection with multi-wall carbon nanotube-modified glassy carbon electrode. Biomed Chromatogr. 2011;25(8):938–42. doi: 10.1002/bmc.1550. [DOI] [PubMed] [Google Scholar]
- 15.Yamada K, Miyazaki T, Shibata T, Hara N, Tsuchiya M. Simultaneous measurement of tryptophan and related compounds by liquid chromatography/electrospray ionization tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;867(1):57–61. doi: 10.1016/j.jchromb.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 16.de Jong WH, Smit R, Bakker SJ, de Vries EG, Kema IP. Plasma tryptophan, kynurenine and 3-hydroxykynurenine measurement using automated online solid-phase extraction HPLC-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(7):603–9. doi: 10.1016/j.jchromb.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Iizuka H, Ishii K, Hirasa Y, Kubo K, Fukushima T. Fluorescence determination of d-and l-tryptophan concentrations in rat plasma following administration of tryptophan enantiomers using HPLC with pre-column derivatization. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(29):3208–13. doi: 10.1016/j.jchromb.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Jin DR, Nagakura K, Murofushi S, Miyahara T, Toyo’oka T. Total resolution of 17 DL-amino acids labeled with a fluorescent chiral reagent, R(−)-4-(3-isothiocyanatopyrrolidin-1-yl)-7-(N,N-dimethylaminosulfonyl)-2,1,3-benzoxadiazole, by high-performance liquid chromatography. J Chromatogr A. 1998;822(2):215–24. doi: 10.1016/s0021-9673(98)00617-7. [DOI] [PubMed] [Google Scholar]
- 19.Santa T, Fukushima T, Ichibangase T, Imai K. Recent progress in the development of derivatization reagents having a benzofurazan structure. Biomed Chromatogr. 2008;22(4):343–53. doi: 10.1002/bmc.945. [DOI] [PubMed] [Google Scholar]
- 20.Santa T, Al-Dirbashi OY, Fukushima T. Derivatization reagents in liquid chromatography/electrospray ionization tandem mass spectrometry for biomedical analysis. Drug Discov Ther. 2007;1(2):108–18. [PubMed] [Google Scholar]
- 21.Iizuka H, Hirasa Y, Kubo K, Ishii K, Toyo’oka T, Fukushima T. Enantiomeric separation of D, l-tryptophan and d, l-kynurenine by HPLC using precolumn fluorescence derivatization with R(−)-DBD-PyNCS. Biomed Chromatogr. 2011;25(7):743–7. doi: 10.1002/bmc.1525. [DOI] [PubMed] [Google Scholar]
- 22.Pitkanen HT, Oja SS, Kemppainen K, Seppa JM, Mero AA. Serum amino acid concentrations in aging men and women. Amino Acids. 2003;24(4):413–21. doi: 10.1007/s00726-002-0338-0. [DOI] [PubMed] [Google Scholar]
- 23.Kim YK, Myint AM, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Cytokine changes and tryptophan metabolites in medication-naive and medication-free schizophrenic patients. Neuropsychobiology. 2009;59(2):123–9. doi: 10.1159/000213565. [DOI] [PubMed] [Google Scholar]