Abstract
Purpose
To study the histopathological findings of the early cases of failed DSAEK grafts and to analyze the causes of graft failure.
Methods
Retrospective study of 13 failed DSAEK grafts (four grafts submitted alone with no host cornea) of 12 patients. The histopathologic features are correlated with the clinical and operative findings.
Results
Significant attenuation of the endothelial cells found in 10/13 cases (77%), retained recipient Descemet’s membrane in 7/13 (54%), variability of graft thickness in 5/13 (38%) and two of these had stromal irregularity. Retrocorneal fibrous membrane along the donor’s Descemet’s membrane was found in 4/13 (31%) resulting in endothelial detachment in one case. Eight of the nine host cornea–graft specimens were found to have: total graft-cornea detachment (in one), subtotal in four and partial (⩽50% of graft length) in three. The detached flaps showed infection at the interface of the graft–host cornea in two, epithelial ingrowth and fibrous proliferation along the anterior stromal surface of the graft (one case each). An additional histopathological finding was secondary amyloid deposition within the host stroma (in one).
Conclusion
Irregular or thick graft, graft–host interface fibrous/epithelial ingrowth, and infection all predispose to DSAEK failures related to graft detachment. Endothelial cells attenuation and retrocorneal fibrous membrane are major causes for primary graft failure.
Keywords: Endothelial keratoplasty, Corneal graft failure, Descemet’s stripping
Introduction
Descemet’s stripping with automated endothelial keratoplasty (DSAEK) is rapidly gaining popularity as a primary treatment option for patients with corneal endothelial cell dysfunction such as Fuch’s endothelial dystrophy and pseudophakic bullous keratopathy.1,2 It offers several potential advantages over full-thickness penetrating keratoplasty (PKP) including more rapid visual rehabilitation, more predictable refractive outcomes, decreased risk of rejection, and retention of corneal structural integrity.2–6 However, DSAEK involves more donor tissue manipulation, which increases the likelihood of endothelial cell loss compared with PKP. Furthermore, the learning curve and challenges in patients with aphakia, trabeculectomies and tube shunts introduce additional factors that can affect the success.7
Materials and methods
A retrospective review of all cases of failed DSAEK grafts that were removed either as a posterior failed donor lenticule during repeated DSAEK or as a full cornea/flap during penetrating keratoplasty at King Khaled Eye Specialist Hospital (KKESH) over 2 years (2008 and 2009). The corneal tissue is submitted in formalin for histopathologic examination. Routine gross examination of the corneal tissue is performed and half of the buttons are submitted for routine tissue processing and staining with Hematoxylin–eosin and periodic acid–Schiff stains. Special stains for infectious etiology are performed whenever applicable. Thirteen cases in 12 patients are included for review of the histopathologic findings with correlation to their clinical and operative findings obtained. The charts are reviewed to gather demographic, clinical and surgical information using a predesigned data sheet. Donor corneal tissue is obtained from USA. The donor DSAEK flap is prepared Hanna anterior chamber maintainer and trephine. Pachymetry is performed after scraping of donor epithelium and Moria microkeratome is used to separate the flap which is trephined. The endothelial surface is protected by viscoelastic. The DSAEK flaps are implanted against the posterior stroma of the host cornea either using a Busin spatula, glide or folding forceps depending on the surgeon’s preference. The histologic slides are reviewed by a single pathologist and the relevant findings are documented. This study has been approved by the institution Research Department and HEC/IRB (Project #0935-R).
Results
Twelve patients were included. One patient had a repeated DSAEK in the same eye because of the failed initial procedure. The patients’ age ranged from 28 years to 72 years with the median of 65 years. Seven females and five males were included. Other associated ophthalmic problems were present in five patients (42%) and included controlled glaucoma in four and history of retinal detachment repair in one.
The indications for surgery included pseudophakic bullous keratopathy (PBK) in nine corneas (69%), failed PKP in two corneas (15%), failed DSAEK in one (8%) and corneal edema with cataract in one (8%). Descemet’s membrane of the last patient removed at initial DSAEK showed possible Non-guttata endothelial dystrophy.
Clinically identified causes for failure included: persistent detachment of the graft – first noted in the immediate post-operative period – as the commonest clinically identified cause in 6/13 despite reattachment surgical intervention in 3/13 cases, primary failure – inspite of well positioned and attached DSAEK graft – in 4/13, suspected herpetic keratouveitis in 2/13, vitreous endothelial touch in one cornea and graft rejection because of poor compliance with the use of post-operative medications in one patient. Infection was clinically identified in one cornea with partial response to antifungal therapy. The clinical causes for the DSAEK failure are summarized in Table 1.
Table 1.
The clinically identified causes of failed DSAEK in 13 cases.
| Cause | No. | Comment |
|---|---|---|
| Persistent detachment of the graft | 6 | Failed reattachment procedure in 2 Dislocation of flap in 1 |
| Primary graft failure | 4 | Endothelial attenuation by histopathology in 3 Intraoperative excessive manipulation in 1 |
| Herpetic keratouveitis | 2 | Clinical diagnosis Endothelial cells pigment deposition by histopathology |
| Graft rejection | 1 | Successful reattachment of the graft |
| Infection (fungal) | 1 | Subtotal detachment by histopathology |
| Vitreous-endothelial touch | 1 | Managed by PK/anterior vitrectomy |
Operative notes were reviewed for all cases to identify any specific intraoperative event that might contribute to subsequent graft dislocation or failure. All the cases had a smooth uneventful procedure except for one (case 8). In that case, intraoperative difficulty in introducing the graft and excessive manipulation due to air escaping posteriorly were noted. Post-operative complications were documented following nine procedures (69%). A summary of all the cases is presented in Table 2. The commonest complication was initial detachment of the DSAEK graft in 6/9. The graft showed significant inferior dislocation in one of these detached grafts and reattachment was tried for three grafts. The other four noted complications included hypotony in a patient who had a combined procedure where cyclophotocoagulation (CPC) was also performed, recurrent epithelial defect in one patient, stromal infiltrate at the donor–host interface in one and finally persistent edema of the DSAEK graft with no evidence of detachment in one.
Table 2.
Clinical and operative data of 13 failed DSAEK grafts with relevant histopathologic findings.
| Specimen no. | Age (years) | Preoperative diagnosis | Procedure | Complication or identified clinical risk factor | Second procedure | Main histopathologic findings |
|---|---|---|---|---|---|---|
| 1 | 65 | Failed PKP Glaucoma |
DSAEK | Graft detachment Fungal infection |
Penetrating keratoplasty | Graft detachment (subtotal) Fungal stromal keratitis Retrocorneal fibrous membrane Endothelial cell loss |
| 2 | 67 | PBK Pseudoexfoliation glaucoma |
DSAEK + CPC | Graft detachment Hypotony |
Penetrating keratoplasty | Graft detachment (subtotal) Gram positive cocci Endothelial cell loss |
| 3 | 87 | PBK Glaucoma High myopia |
DSAEK | Graft detachment (persistent)a | Penetrating keratoplasty | Graft detachment (subtotal) Interface fibrous membrane Endothelial attenuation |
| 4b | 72 | Corneal edema Cataract |
DSAEK + cataract extraction | Graft detachment (persistent)a Dislocation of the graft Herpetic keratouveitis |
DSAEK | Graft detachment (total) Endothelial cells pigment deposition |
| 5b | 72 | Failed DSAEK | DSAEK | Herpetic keratouveitis | Penetrating keratoplasty | Graft detachment (subtotal) Endothelial cell loss Endothelial cells pigment deposition Amyloid stromal deposits |
| 6 | 61 | PBK | DSAEK | Graft detachment | Penetrating keratoplasty | Graft detachment (partial) Endothelial cell loss |
| 7 | 68 | Failed PKP | DSAEK | Graft detachment | DSAEK | Interface epithelial growth |
| 8 | 65 | PBK | DSAEK | Intra-operative manipulation Primary graft failure |
Penetrating keratoplasty | Graft detachment (partial) Retrocorneal fibrous membrane Endothelial detachment |
| 9 | 71 | PBK | DSAEK | Vitreous in AC touching the graft | Penetrating keratoplasty Anterior vitrectomy |
Graft detachment (partial) Retrocorneal fibrous membrane Endothelial cell loss |
| 10 | 60 | PBK | DSAEK | Primary graft failure | Penetrating keratoplasty | Endothelial cell loss |
| 11 | 58 | PBK Glaucoma |
DSAEK | Primary graft failure | DSAEK | Endothelial cell loss |
| 12 | 65 | PBK | DSAEK | Primary graft failure | DSAEK | Endothelial cell loss |
| 13 | 28 | PBK S/P RD repair |
DSAEK | Graft detachment (initial) Graft rejection |
DSAEK | Retrocorneal fibrous membrane Endothelial attenuation |
Persistent detachment following reattachment surgery.
Specimens from the same patient with repeated DSAEK, both of which have failed.
Histopathologically 10 cases showed significant attenuation (moderate to severe) of the endothelial cells equally along Descemet’s membrane of the graft (77%) resulting in primary graft failure (Figs. 1a and 1b of specimen 11).
Figure 1a.
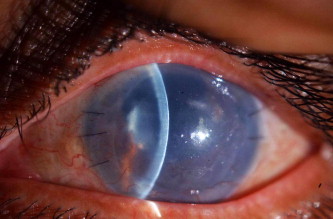
An example of primary graft failure in the left eye.
Figure 1b.
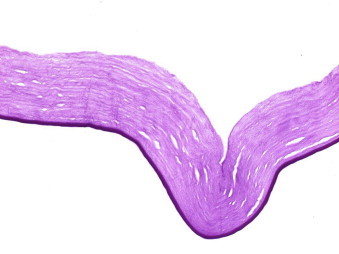
Corresponding histopathological appearance of his DSAEK graft (specimen 11) showing endothelial attenuation (periodic acid–Schiff, original magnification ×200).
Retained recipient Descemet’s membrane was found in seven cases (54%).
The flap was variably increased in thickness in five cases (38%) with irregularity of the anterior stromal surface in two cases out of these (Figs. 2a and 2b).
Figure 2a.
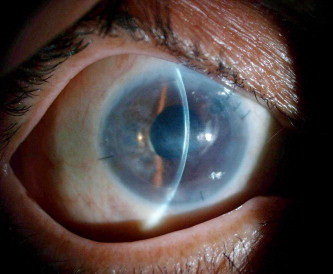
Clinical photo of the right eye in another failed DSAEK case.
Figure 2b.
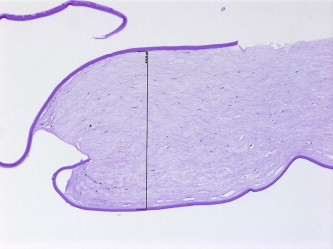
Histopathologic appearance of the same case (specimen 12) with thick DSAEK flap and retained host DM (periodic acid–Schiff, original magnification ×200).
Retrocorneal fibrous membrane along the donor’s Descemet’s membrane was observed in four cases (31%).
In regard to the graft detachment, the histopathological documented cases of total detachment or incomplete attachment of the flap in full thickness cornea + flap specimens were 8/9, excluding the four cases where only the DSAEK flap is received which accounts for 89%. The main histopathologic findings along the graft–host interface in these cases included fibrous proliferation (Figs. 3a and 3b for specimen 3), epithelial ingrowth (Figs. 4a and 4b for specimen 7) and infection with documentation of organisms in two specimens (one bacterial-specimen 2 and one fungal-specimen 1 as shown in Figs. 5a and 5b).
Figure 3a.
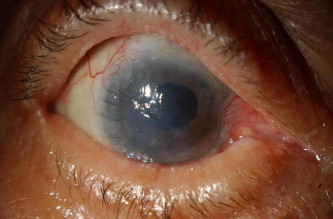
Clinical photo of failed reattachment of the DSAEK flap in the right eye.
Figure 3b.
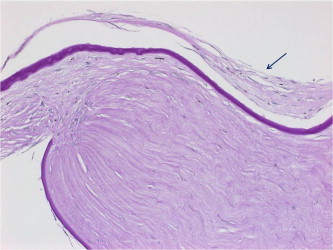
Fibrous ingrowth along host–graft interface in the same case (specimen 3) indicated by the black arrow (periodic acid–Schiff, original magnification ×400).
Figure 4a.
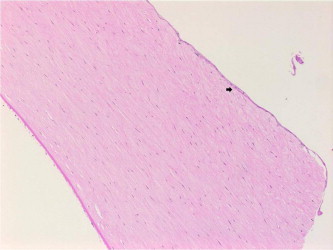
Epithelial ingrowth (arrow) at the graft–host interface of specimen 7 (hematoxylin–eosin, original magnification ×200).
Figure 4b.
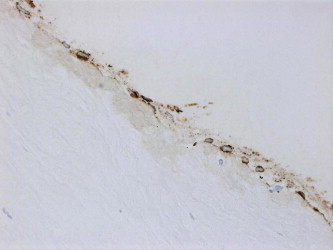
Cytokeratin positive epithelial ingrowth (cytokeratin, original magnification ×400).
Figure 5a.
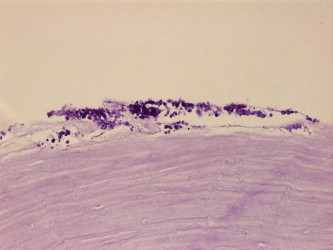
Fungal stromal keratitis of the flap in specimen 2 (periodic acid–Schiff, original magnification ×400).
Figure 5b.
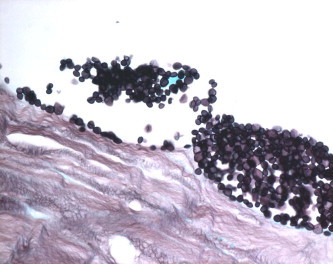
Yeast within the graft stroma (Grocott methenamine silver, original magnification ×1000 oil immersion).
Other histopathologic findings included secondary Amyloid deposition within the host stroma of the cornea as a sequela of chronic viral keratitis (Fig. 6 for specimen 5∗).
Figure 6.
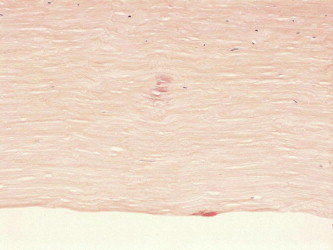
Stromal amyloid deposits within the stroma (Congo red, original magnification ×200).
The 12 initially failed DSAEK corneas were eventually managed by penetrating keratoplasty in eight and repeated DSAEK procedure in four corneas. All the surgical procedures were performed more than 80 days of the initial DSAEK. The patient who had a repeated DSAEK within our project period developed edema and reactivation of herpetic keratouveitis in that eye with subsequent failure of his DSAEK and eventual treatment by penetrating keratoplasty (specimens 4∗ and 5∗).
Discussion
Descemet’s stripping with endothelial keratoplasty DSEK is a rapidly advancing procedure used to treat patients with corneal endothelial cell dysfunction. In DSEK the recipient Descemet’s membrane and endothelium are stripped and a posterior lamellar graft, or DSEK graft, then is inserted and allowed to unfold with subsequent recipient-to-donor stromal adherence.
Adhesion of the DSEK graft allows for eventual detumescence of the recipient cornea as the donor endothelial cells begin their pump action. Preparation of the posterior lamellar graft, containing the donor posterior stroma, Descemet’s membrane, and endothelium, has been simplified by use of a microkeratome on a corneoscleral button. This variant in procedure has been termed Descemet’s stripping automated endothelial keratoplasty (DSAEK), but most use the terms DSEK and DSAEK interchangeably because almost all now use the microkeratome for button preparation.
The most common post-operative complication in DSAEK cases is graft detachment with a reported rate of 6% in DSAEK cases for experienced surgeons ,3,8–10 and 88% of histopathologically studied primary graft failure cases following DSAEK by Oster et al.11 Detached grafts can be reattached with repositioning of the graft termed “repositioning” and injection of an air bubble termed “rebubbling”.3 Proposed causes of graft detachment include patient eye rubbing and poor donor tissue dissection.3,9 Our results have shown the frequent occurence of this complication in 89% of failed cases where the full thickness host and donor tissue were histologically examined which is quite similar to the rate reported above. This detachment was persistent in six cases despite the reattachment procedures in two patients. The remaining cases had other contributing factors to their failure, identified as: graft rejection in one case following successful reattachment of the graft (specimen 13) and infection which was partially responding to antifungal therapy (specimen 1). Graft detachment can be attributed to irregular thickness of the graft in five cases, fibrous proliferation along the graft–host interface in one case and finally epithelial ingrowth along the interface in another.
Suh et al. have concluded that the presence of interface material such as Descemet’s membrane, fibrous proliferation or epithelium is the potential cause of dislocation.12
On the other hand, Romaniv et al. in their case report described tight adherence of an endothelial keratoplasty (EK) donor button to a prior failed PKP with a retained Descemet’s membrane and endothelium. The failure of their case was attributed to folding and partial detachment of Descemet’s membrane from EK donor button.13
The thickness of the flap is of major concern in regard to the stable attachment of the graft and its functional survival. Our five cases with histopathologically irregular thick donor flaps demonstrated clinically proven initial detachment (in one case) and persistent detachment (in four cases). The use of femtosecond laser might be useful in creating a deeper and more consistent cutting depth resulting in a better donor tissue lenticule than can be produced with the microtome.14
Infection at the host–graft interface is a new finding in our study which has not been described before and has occurred in association with subtotal graft detachment in two cases. The first showed infiltration by yeast with associated mild stromal keratitis (specimen 1). The other showed collection of gram positive cocci at the stromal interface, with no associated inflammation (specimen 2) similar to what is seen in cases of infectious crystalline keratopathy, following PKP.
Another potential post-operative complication described is the graft rejection, although is found to be lower with EK than PKP.14,15 In our cases we had a single graft rejection which was actually related to poor compliance with the use of post-operative steroids (specimen 13).
Persistent edema despite successful primary apposition of the graft, termed “primary graft failure” is another cause explained by minimal endothelial function that is inadequate for graft clarity. We had four cases of primary failure, all of whom have shown endothelial attenuation by histopathologic examination. One of these cases has shown formation of a retrocorneal fibrous membrane along the donor Descemet’s membrane with evidence of endothelial detachment (specimen 8). This particular pseudophakic case was subjected to excessive manipulation during surgery due to posterior air escape as documented in the patient chart. Mehta reported two cases of primary graft failure with complete loss of endothelial cells.16 Lee et al. in a study of eight cases concluded the common finding of marked endothelial loss with an interesting pattern of greater loss at the periphery and relative preservation of central cells.17 In our cases, endothelial attenuation was the most common histopathologic finding in 77%, however, no specific pattern to the endothelial loss was identified. This was similar to the findings of Oster et al. who detected atrophic endothelium in 75% of their 16 cases and concluded that it is a prominent feature in primary graft failure.11 Suh et al. had a higher rate of endothelial absence accounting for 84% of their 19 cases and Table 3 compares the results of both studies with additional findings in our 13 cases.
Table 3.
Comparison of histopathologic findings in failed DSAEK grafts.
| Cause | Alkatan et al. (13 cases) | Suh et al. (19 cases) |
|---|---|---|
| Endothelial attenuation | 10 (77%) | 16 (84%) |
| Retained host Descemet’s membrane | 7 (54%) | 5 (26%) |
| Variable graft thickness | 5 (38%) | Not mentioned |
| Retrocorneal fibrous membrane | 4 (31%) | Not reported |
| Growing organisms at the interface | 2 (15%) | Not reported |
| Fibrocellular membrane at the interface | 1 (8%) | (58%) |
| Epithelial ingrowth at the interface | 1 (8%) | 4 (21%) |
| Decentered graft | Not detected | 4 (21%) |
Patient selection for this procedure is also important. Our patient who had repeated DSAEK procedure, had a clinically proven persistent detachment of his first flap with no additional features to explain its failure. However, when the full-thickness specimen was studied at his second failed DSAEK procedure (specimen 5∗), the host cornea showed subtotal absence of Bowman’s layer, alteration of the normal stromal lamellar architecture and secondary Amyloid deposits as sequela of herpetic keratitis. This diseased host stromal tissue could have added to the procedure failure. Rose et al. clarified that not all patients are ideal candidates for DSAEK. They related the difficulty of the procedure in aphakic and vitrectomized eyes to the migration of the supporting air bubble into the posterior segment.14 This was experienced in one of our cases with pseudophakic bullous keratopathy.
Conclusions
In conclusion DSAEK is an advantageous procedure for the management of endothelial dysfunction. Improved surgical techniques and developing skills are needed to reduce the risk of graft detachment and endothelial cell loss.
In regard to the detachment, the irregularity of the DSAEK flap thickness seems to affect the stable attachment of the graft. Epithelial and fibrous ingrowth may interfere with the adherence of the flap. Infection is not well understood and has not been previously reported. We believe, however, that persistent detachment of the flap is the real initial step in DSAEK failure as it allows the development of epithelial and/or fibrous ingrowth in the stromal interface as well as the chance for organisms to grow causing infection-related failures.
Other than graft detachment, endothelial attenuation remains a major cause of primary graft failure. Retrocorneal fibrous membrane is a new finding also which is expected to adversely affect the graft survival, in a similar way to penetrating keratoplasty cases.
Better understanding of the mechanism of DSAEK failure partially aided by the histopathologic findings can guide us in refining our surgical skills and technique, to improve the outcome of this procedure and reduce its failure rate.
References
- 1.Melles G.R., Eggink F.A., Lander F. A surgical technique for posterior lamellar keratoplasty. Cornea. 1998;17:618–626. doi: 10.1097/00003226-199811000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Price F.W., Jr, Price M.O. Descemet’s stripping with endothelial keratoplasty in 50 eyes: a refractive neutral corneal transplant. J Refract Surg. 2005;21:339–345. doi: 10.3928/1081-597X-20050701-07. [DOI] [PubMed] [Google Scholar]
- 3.Price F.W., Jr, Price M.O. Descemet’s stripping with endothelial keratoplasty in 200 eyes: early challenges and techniques to enhance donor adherence. J Cataract Refract Surg. 2006;32:411–418. doi: 10.1016/j.jcrs.2005.12.078. [DOI] [PubMed] [Google Scholar]
- 4.Mearza A.A., Qureshi M.A., Rostron C.K. Experience and 12-month results of Descemet-stripping endothelial keratoplasty (DSEK) with a small-incision technique. Cornea. 2007;26:279–283. doi: 10.1097/ICO.0b013e31802cd8c2. [DOI] [PubMed] [Google Scholar]
- 5.Koenig S.B., Covert D.J. Early results of small-incision Descemet’s stripping and automated endothelial keratoplasty. Ophthalmology. 2007;114:221–226. doi: 10.1016/j.ophtha.2006.07.056. [DOI] [PubMed] [Google Scholar]
- 6.Terry M.A., Shamie N., Chen E.S. Endothelial keratoplasty: a simplified technique to minimize graft dislocation, iatrogenic graft failure, and pupillary block. Ophthalmology. 2008;115:1179–1186. doi: 10.1016/j.ophtha.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Takeshi Ide. Subconjunctival leakage after descemet stripping automated endothelial keratoplasty (DSAEK) in a post trabeculectomy eye. Open ophthalmol J. 2009;3:1–2. doi: 10.2174/1874364100903010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terry M.A., Hoar K.L., Wall J., Ousley P. History of dislocations in endothelial keratoplasty (DSEK and DLEK: a laboratory based, surgical solution to dislocation in 100 consecutive DSAEK eyes. Cornea. 2006;25:926–932. doi: 10.1097/01.ico.0000243958.07027.f2. [DOI] [PubMed] [Google Scholar]
- 9.Gorovoy M.S. Descemet’s stripping automated endothelial keratoplasty. Cornea. 2006;25:886–889. doi: 10.1097/01.ico.0000214224.90743.01. [DOI] [PubMed] [Google Scholar]
- 10.Suh L.H., Yoo S.H., Deobhakta A. Complications of Descemet’s stripping with automated endothelial keratoplasty; survey of 118 eyes at one institute. Ophthalmology. 2008;116:1517–1524. doi: 10.1016/j.ophtha.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Oster S.F., Ebrahimi K.B., Eberhart C.G., Schein O.D., Stark W.J., Jun A.S. A clinicopathologic series of primary graft failure after Descemet’s stripping and automated endothelial keratoplasty. Ophthalmology. 2009;116:609–614. doi: 10.1016/j.ophtha.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 12.Suh L.H., Dawson D.G., Mutapcic L., Rosenfeld S.I., Cultbertson W.W., Yoo S.H., O’brien T.P., Dubovy S.R., Leejee H., Suh M.D. Histopathological examination of failed grafts in descemet stripping with automated endothelial keratoplasty. Ophthalmology. 2009;116:603–608. doi: 10.1016/j.ophtha.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Romaniv N., Price M.O., Price F.W., Mamalis N. Donor descemet membrane detachment after endothelial keratoplasty. Cornea. 2006;25(8):943–947. doi: 10.1097/01.ico.0000243950.99472.f5. [DOI] [PubMed] [Google Scholar]
- 14.Rose L., Kelliher C., Jun A.S. Endothelial keratoplasty: historical perspectives, current techniques, future directions. Can J Ophthalmol. 2009;44(4):401–405. doi: 10.3129/i09-090. [DOI] [PubMed] [Google Scholar]
- 15.Allan B.D., Terry M.A., Price F.W., Jr, Price M.O., Griffin M.B., Claesson M. Corneal transplant rejection rate and severity after endothelial keratoplasty. Cornea. 2007;26:1039–1042. doi: 10.1097/ICO.0b013e31812f66e5. [DOI] [PubMed] [Google Scholar]
- 16.Mehta J.S., Chua J., Poh R., Beuerman R.W., Tan D. Primary graft failure after Descemet-stripping automated endothelial keratoplasty: clinico-pathological study. Cornea. 2008;27(6):722–726. doi: 10.1097/QAI.0b013e31815e92ac. [DOI] [PubMed] [Google Scholar]
- 17.Lee J.A., Djalilian A.R., Riaz K.M., Sugar J., Tu E.Y., Wadia H., Edward D.P. Clinical and histopathologic features of failed Descemet-stripping automated endothelial keratoplasty grafts. Cornea. 2009;28(5):530–535. doi: 10.1097/ICO.0b013e31818d3b1c. [DOI] [PubMed] [Google Scholar]


