Abstract
Purpose
Anterior chamber depth (ACD) is an important preoperative parameter in anterior segment surgery. Several factors are known to influence ACD, including race and geography. Our purpose was to sample data from various countries to characterize differences in ACD worldwide and, if any, assess their level of clinical significance.
Setting
International, multicenter.
Methods
Cross-sectional study. Using the Pentacam Eye Scanner (OCULUS GmbH, Wetzlar, Germany), we analyzed ACD measurements from 1077 eyes of 568 normal adults from nine countries spanning six continents. Differences between countries were assessed by comparison of 95% confidence intervals and by ANOVA. Normative thresholds were constructed at three standard deviations (SD) above and below the mean.
Results
Mean ACD was 3.11 mm overall, ranging from 2.91 mm (New Zealand) to 3.24 mm (United States). The ACD among New Zealanders was significantly shallower (P < .0001) than that among Chinese, Egyptians, Germans, Indians, and Americans. The maximum difference in the mean ACDs was 0.33 mm, between New Zealand and the United States. The shallowest 0.15% of normal ACD values occurred below 2.04 mm overall, ranging from 1.69 mm (New Zealand) to 2.42 mm (United States). The deepest 0.15% of normal ACD values occurred above 4.18 mm overall, ranging from 4.03 mm (Saudi Arabia) to 4.35 mm (Brazil).
Conclusions
ACD did not vary significantly in the countries studied, with the notable exception of New Zealand. Surgeons should anticipate a greater likelihood of a shallow ACD when evaluating patients from New Zealand. Clinical examination and direct measurement of ACD are recommended. Finally, deep ACD has limited clinical utility in screening for keratoconus.
Keywords: Anterior chamber depth, Scheimpflug, Pentacam, International
1. Introduction
Anterior chamber depth (ACD) is an established anterior segment biometric parameter. Anatomically, it represents the distance between the corneal endothelium and the anterior capsule of the crystalline lens. Clinically, ACD carries preoperative importance for intraocular surgery. For example, cataract surgeons rely on biometric intraocular lens (IOL) power formulas, the latest generations of which increasingly respect the role of preoperative ACD measurement (Lee et al., 2008). Forty-two percent of refractive error after IOL implantation may be attributed to inaccuracy in ACD measurement, more than from axial length (36%) or corneal power (22%; Olsen, 2007). When ACD is shallow, surgeons must also anticipate an increased risk of corneal endothelial injury during routine cataract extraction. Similarly, safe implantation of phakic intraocular lenses requires adequate ACD.
The construction of normal reference ranges for ACD is challenging due to the contributions of multiple variables. ACD is influenced by gender and negatively correlated with age (Casson, 2008; Edmonds et al., 2009; Rabsilber et al., 2006), while the effect of refractive error has been less consistent (Ucakhan et al., 2008; Utine et al., 2009). ACD also is an inheritable trait affected by race (Casson, 2008; Leung et al., 2010). Using geography as a proxy for race, one can appreciate variations in ACD across many regional studies worldwide (Alonso et al., 2010; Buehl et al., 2006; Dinc et al., 2010; Doors et al., 2009; Edmonds et al., 2009; Elbaz et al., 2007; Emre et al., 2007; Fontes et al., 2010a; Fontes et al., 2010b; Fu et al., 2010; Huang et al., 2011; Kovacs et al., 2010; Lackner et al., 2005; Nemeth et al., 2006; Rabsilber et al., 2006; Reuland et al., 2007; Salouti et al., 2010; Savant et al., 2008; Su et al., 2008; Ucakhan et al., 2008; Utine et al., 2009; Woodmass and Rocha, 2009; Yazici et al., 2010; Yi et al., 2008). However, a systematic study to examine normal ACD values and variation across multiple races and countries has not, to our knowledge, been reported. Furthermore, the United States Food and Drug Administration (FDA) encourages the collection of race-specific data to determine the effectiveness of medical devices (Food and Drug Administration, 2005). Therefore, we sampled normative data from various countries to test for significant differences in ACD as measured by a rotating Scheimpflug camera.
2. Materials and methods
This was a cross-sectional study conducted at multiple international centers. After receiving local Institutional Review Board exemption at the primary site, de-identified data were received from each center. Data consisted of Pentacam Eye Scanner (OCULUS GmbH, Wetzlar, Germany) examinations of one or both eyes of adult subjects from Brazil, China, Egypt, Germany, India, Japan, New Zealand, Saudi Arabia, and the United States. Recruited subjects were between the ages of 25–65, representative of their geographic area, with normal ocular health by local standard criteria, and with simple myopia, myopic astigmatism, or emmetropia. Exclusion criteria included foreign birth, mixed astigmatism, hyperopia, prior eye surgery, or personal or family history of corneal ectatic disease.
We analyzed ACD data measured at the corneal apex in all eyes. Preliminary analysis revealed these data were statistically similar and highly correlated between the paired right and left eyes of applicable subjects. Data were normally distributed whether examining right eyes only, left eyes only, or all eyes. Subsequently, one-way ANOVA with pairwise Bonferroni post-tests were used to assess for differences between countries. Statistical significance was set at P < .05. Confidence intervals (CI) were calculated at the standard 95% confidence level. Finally, normative gates were constructed from these sample data at three standard deviations (SD) to encompass 99.7% of normal values.
3. Results
We analyzed 1077 eyes of 568 normal adult subjects representing nine countries spanning six continents Table 1. ACD did not differ significantly between right eyes, left eyes, and all eyes in any country (P > .05, two-tailed unpaired t-test). Mean ACD (95% CI) for all eyes ranged from a low in New Zealand of 2.91 (2.83–2.98) mm to a high in the United States of 3.24 (3.17–3.31) mm, with a collective mean of 3.11 (3.09–3.14) mm (all countries; Fig. 1). While Saudi Arabia was not shown because a small sample size precluded the calculation of a meaningful 95% CI, Saudi data were included in the collective analysis.
Table 1.
Sample sizes by country.
| Country | n | Right eyes | Left eyes | All eyes |
|---|---|---|---|---|
| Brazil | 68 | 62 | 65 | 127 |
| China | 100 | 90 | 89 | 179 |
| Egypt | 75 | 75 | 74 | 149 |
| Germany | 66 | 66 | 66 | 132 |
| India | 104 | 104 | 101 | 205 |
| Japan | 61 | 46 | 54 | 100 |
| New Zealand | 56 | 56 | 56 | 112 |
| Saudi Arabia | 8 | 6 | 7 | 13 |
| United States | 30 | 30 | 30 | 60 |
| All | 568 | 535 | 542 | 1077 |
Figure 1.
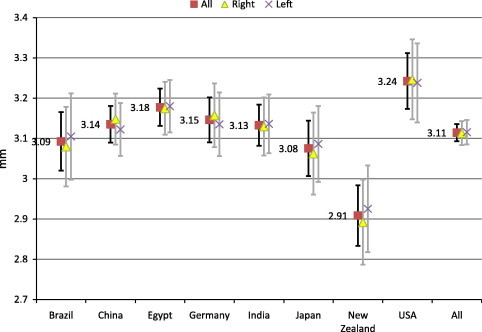
Estimated population means for anterior chamber depth by country. Sample means for ACD in countries with n > 25. Error bars indicate 95% confidence intervals for the estimation of each country’s true population mean.
The only 95% CI without considerable overlap with the others belonged to New Zealand. Statistical analysis confirmed that New Zealand was significantly different (P < .0001, ANOVA), with shallower ACD than China, Egypt, Germany, India, and the United States (P < .05 each, pairwise post-tests). The maximum difference in mean ACDs was 0.33 mm, between New Zealand and the United States. Differences between New Zealand and Brazil or Japan did not achieve significance. These relationships persisted when examining right eyes only (P < .0001, ANOVA) and left eyes only (P = .0019, ANOVA).
To assess the normal spread of ACD in each country, we calculated 3 SD gates above and below each mean (Fig. 2). One could expect 99.7% of normal values to fall between these upper and lower thresholds. The lower threshold, below which only 0.15% of normal ACD values occur, ranged from a low in New Zealand of 1.69 mm to a high in the United States of 2.42 mm, with a collective value of 2.04 mm. The upper threshold, above which only 0.15% of normal ACD values occur, ranged from a low in Saudi Arabia of 4.03 mm to a high in Brazil of 4.35 mm, with a collective value of 4.18 mm.
Figure 2.
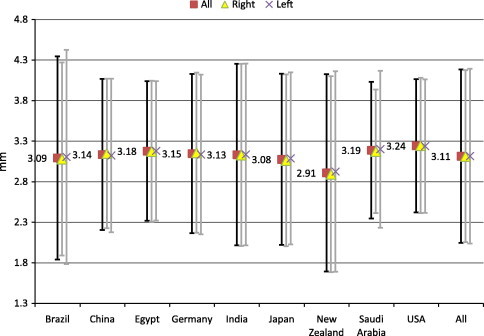
Distribution of normal anterior chamber depth by country. Sample means for ACD by country. Error bars indicate ± 3 SD, encompassing 99.7% of normal subjects.
4. Discussion
Review of the literature revealed published normal ACD values from the Pentacam system for Brazil (Alonso et al., 2010; Fontes et al., 2010a; Fontes et al., 2010b), China (Fu et al., 2010; Huang et al., 2011), Germany (Rabsilber et al., 2006; Reuland et al., 2007), and the United States (Edmonds et al., 2009). Our data agreed with those published values. Mean ACD (± SD) for Brazil was 3.09 ± 0.42 mm compared to 3.07 ± 0.42 mm; for China, 3.14 ± 0.31 mm compared to 3.17 ± 0.27; for Germany, 3.15 ± 0.33 compared to 3.10 ± 0.36; and for the United States, 3.24 ± 0.27 compared to 3.18 ± 0.28. These similarities appear to confirm the normative validity of these data in these countries.
Unfortunately, historical controls were not available for Egypt, India, Japan, New Zealand, and Saudi Arabia. Conversely, we found published Pentacam-based normal values from countries not examined in this study, such as Turkey (Dinc et al., 2010; Emre et al., 2007; Ucakhan et al., 2008; Utine et al., 2009; Yazici et al., 2010), Iran (Salouti et al., 2010), and Israel (Elbaz et al., 2007) in the Middle East; Hungary (Kovacs et al., 2010; Nemeth et al., 2006), Austria (Buehl et al., 2006; Lackner et al., 2005), Holland (Doors et al., 2009), and England (Savant et al., 2008) in Europe; South Korea (Yi et al., 2008) and Taiwan (Su et al., 2008) in East Asia; and Canada in North America (Woodmass and Rocha, 2009). These may suggest future areas for further investigation.
Our data were notable for statistically shallower mean ACD in New Zealand, the magnitude of which measured up to 0.33 mm and appeared clinically significant (Fig. 1). This result was surprising, as we expected the shallowest ACD data to come from East Asia. This result may represent greater heterogeneity in the local population of New Zealand, whether due to heterogeneity of race or age distribution, for example. Consistent with those possibilities, New Zealand data were among the most widely spread, as evidenced by a large SD. ACD is known to decrease with increasing age and (commonly age-related) cataract size.
Indeed, a weakness of this study is the absence of associated age, gender, and cataract data. To create a normative database, the study was designed to leave age distribution up to the local discretion of the recruiting co-investigator. The definition of a normal subject was left deliberately ambiguous with regards to non-ocular parameters, such as age, race, and gender. Co-investigators at each site were given complete autonomy to determine their local definition of normal. Nevertheless, supplementation of our ACD data with demographic data would allow adjustment for age, gender, and presence of cataract, and would yield more rigorous analysis and conclusions, especially when comparing a country with younger demographics to another with an older population.
We limited our study to myopes and emmetropes as our database was an extension of prior work which excluded hyperopes (Khachikian and Belin, 2009). This study might, therefore, overestimate mean ACD in regions where hyperopes constitute a significant proportion of the normal population, given that hyperopes are known to have shallower anterior chambers. However, the proportion of hyperopes in various geographic areas is unknown. We also acknowledge that ACD variation may occur within countries. While our data are in agreement with available historical controls, sampling error remains possible without multiple study sites within each country. Finally, our findings may be limited to ACD as measured by Scheimpflug imaging. ACD can also be measured by ultrasound (A-scan, ultrasound biomicroscopy, very high frequency digital ultrasound), partial coherence interferometry, anterior segment optical coherence tomography, and slit-scanning videokeratography. Differences have been reported in ACD measurements between these modalities, some by as much as 0.17 mm (Lee et al., 2008), which is greater than the mean differences between most countries examined in this study.
Several Pentacam-based studies have noted significantly deeper mean ACD in eyes with keratoconus than normal controls. Specifically, mean ACD in eyes with keratoconus ranged from 3.29 to 3.34 mm (Edmonds et al., 2009; Kovacs et al., 2010). In a third study, mean ACD ranged from 3.3 mm in moderate keratoconus to 3.7 mm in severe disease (Emre et al., 2007). Our data suggest that these differences, while real, have limited clinical utility in screening for keratoconus. Collective ACD data from all nine countries yielded a 3 SD upper threshold of 4.18 mm (Fig. 2). The corresponding 2 SD and 1 SD upper thresholds were 3.83 and 3.47 mm, respectively. Therefore, the mean ACD for eyes with keratoconus lies well within 1 SD, and one could expect more 16% of normal eyes to exceed 3.29–3.34 mm. Furthermore, more than 2.5% of normal eyes would exceed 3.7 mm without having severe keratoconus. Deeper ACD correlated significantly with increased posterior corneal elevation (Kovacs et al., 2010), and we recommend continued use of the latter for keratoconus screening (Khachikian and Belin, 2009).
5. Conclusions
In summary, it is preferable to establish racial/geographic-specific normative values where possible. ACD did not vary significantly in the countries studied, with the notable exception of New Zealand. Surgeons planning to operate on patients from New Zealand should anticipate a greater likelihood of a shallow ACD. Clinical examination and direct measurement of ACD are recommended. Future studies should examine a hyperopic population as the dimensions and requirement for hyperopic phakic IOL’s differ from those for myopia. Finally, deep ACD has poor clinical utility in screening for keratoconus.
References
- Alonso R.S., Ambrósio R., Jr., Paranhos A., Jr., Sakata L.M., Ventura M.P. Glaucoma anterior chamber morphometry based on optical Scheimpflug images. Arq. Bras. Oftalmol. 2010;73:497–500. doi: 10.1590/s0004-27492010000600005. [DOI] [PubMed] [Google Scholar]
- Buehl W., Stojanac D., Sacu S., Drexler W., Findl O. Comparison of three methods of measuring corneal thickness and anterior chamber depth. Am. J. Ophthalmol. 2006;141:7–12. doi: 10.1016/j.ajo.2005.08.048. [DOI] [PubMed] [Google Scholar]
- Casson R.J. Anterior chamber depth and primary angle-closure glaucoma: an evolutionary perspective. Clin. Exp. Ophthalmol. 2008;36:3–4. doi: 10.1111/j.1442-9071.2008.01672.x. [DOI] [PubMed] [Google Scholar]
- Dinc U.A., Gorgun E., Oncel B., Yenerel M.N., Alimgil L. Assessment of anterior chamber depth using Visante optical coherence tomography, slitlamp optical coherence tomography, IOL Master, Pentacam and Orbscan IIz. Ophthalmologica. 2010;224:341–346. doi: 10.1159/000313815. [DOI] [PubMed] [Google Scholar]
- Doors M., Cruysberg L.P., Berendschot T.T., de Brabander J., Verbakel F., Webers C.A., Nuijts R.M. Comparison of central corneal thickness and anterior chamber depth measurements using three imaging technologies in normal eyes and after phakic intraocular lens implantation. Graefes Arch. Clin. Exp. Ophthalmol. 2009;247:1139–1146. doi: 10.1007/s00417-009-1086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds C.R., Wung S.F., Pemberton B., Surrett S. Comparison of anterior chamber depth of normal and keratoconus eyes using Scheimpflug photography. Eye Contact Lens. 2009;35:120–122. doi: 10.1097/ICL.0b013e31819cf5a6. [DOI] [PubMed] [Google Scholar]
- Elbaz U., Barkana Y., Gerber Y., Avni I., Zadok D. Comparison of different techniques of anterior chamber depth and keratometric measurements. Am. J. Ophthalmol. 2007;143:48–53. doi: 10.1016/j.ajo.2006.08.031. [DOI] [PubMed] [Google Scholar]
- Emre S., Doganay S., Yologlu S. Evaluation of anterior segment parameters in keratoconic eyes measured with the Pentacam system. J. Cataract Refract. Surg. 2007;33:1708–1712. doi: 10.1016/j.jcrs.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Fontes B.M., Ambrósio R., Jr., Jardim D., Velarde G.C., Nosé W. Ability of corneal biomechanical metrics and anterior segment data in the differentiation of keratoconus and healthy corneas. Arq. Bras. Oftalmol. 2010;73:333–337. doi: 10.1590/s0004-27492010000400006. [DOI] [PubMed] [Google Scholar]
- Fontes B.M., Ambrósio R., Jr, Jardim D., Velarde G.C., Nosé W. Corneal biomechanical metrics and anterior segment parameters in mild keratoconus. Ophthalmology. 2010;117:673–679. doi: 10.1016/j.ophtha.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration (FDA), 2005. Guidance for industry: Collection of race and ethnicity data in clinical trials. US Department of Health and Human Services (DHHS). <http://www.fda.gov/downloads/RegulatoryInformation/Guidances/ucm126396.pdf> (accessed 10/22/10).
- Fu J., Wang X., Li S., Wu G., Wang N. Comparative study of anterior segment measurement with Pentacam and anterior segment optical coherence tomography. Can. J. Ophthalmol. 2010;45:627–631. doi: 10.3129/i10-068. [DOI] [PubMed] [Google Scholar]
- Huang J., Pesudovs K., Wen D., Chen S., Wright T., Wang X., Li Y., Wang Q. Comparison of anterior segment measurements with rotating Scheimpflug photography and partial coherence reflectometry. J. Cataract Refract. Surg. 2011;37:341–348. doi: 10.1016/j.jcrs.2010.08.044. [DOI] [PubMed] [Google Scholar]
- Khachikian S.S., Belin M.W. Posterior elevation in keratoconus. Ophthalmology. 2009;116:816. doi: 10.1016/j.ophtha.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Kovacs I., Mihaltz K., Nemeth J., Nagy Z.Z. Anterior chamber characteristics of keratoconus assessed by rotating Scheimpflug imaging. J. Cataract Refract. Surg. 2010;36:1101–1106. doi: 10.1016/j.jcrs.2009.12.046. [DOI] [PubMed] [Google Scholar]
- Lackner B., Schmidinger G., Skorpik C. Validity and repeatability of anterior chamber depth measurements with Pentacam and Orbscan. Optom. Vis. Sci. 2005;82:858–861. doi: 10.1097/01.opx.0000177804.53192.15. [DOI] [PubMed] [Google Scholar]
- Lee A.C., Qazi M.A., Pepose J.S. Biometry and intraocular lens power calculation. Curr. Opin. Ophthalmol. 2008;19:13–17. doi: 10.1097/ICU.0b013e3282f1c5ad. [DOI] [PubMed] [Google Scholar]
- Leung C.K., Palmiero P.M., Weinreb R.N., Li H., Sbeity Z., Dorairaj S., Leung D., Liu S., Liebmann J.M., Congdon N., Lam D.S., Ritch R. Comparisons of anterior segment biometry between Chinese and Caucasians using anterior segment optical coherence tomography. Br. J. Ophthalmol. 2010;94:1184–1189. doi: 10.1136/bjo.2009.167296. [DOI] [PubMed] [Google Scholar]
- Nemeth G., Vajas A., Kolozsvari B., Berta A., Modis L., Jr Anterior chamber depth measurements in phakic and pseudophakic eyes: Pentacam versus ultrasound device. J. Cataract Refract. Surg. 2006;32:1331–1335. doi: 10.1016/j.jcrs.2006.02.057. [DOI] [PubMed] [Google Scholar]
- Olsen T. Calculation of intraocular lens power: a review. Acta Ophthalmol. Scand. 2007;85:472–485. doi: 10.1111/j.1600-0420.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- Rabsilber T.M., Khoramnia R., Auffarth G.U. Anterior chamber measurements using Pentacam rotating Scheimpflug camera. J. Cataract Refract. Surg. 2006;32:456–459. doi: 10.1016/j.jcrs.2005.12.103. [DOI] [PubMed] [Google Scholar]
- Reuland M.S., Reuland A.J., Nishi Y., Auffarth G.U. Corneal radii and anterior chamber depth measurements using the IOLmaster versus the Pentacam. J. Refract. Surg. 2007;23:368–373. doi: 10.3928/1081-597X-20070401-09. [DOI] [PubMed] [Google Scholar]
- Salouti R., Nowroozzadeh M.H., Zamani M., Ghoreyshi M., Salouti R. Comparison of anterior chamber depth measurements using Galilei, HR Pentacam, and Orbscan II. Optometry. 2010;81:35–39. doi: 10.1016/j.optm.2009.04.100. [DOI] [PubMed] [Google Scholar]
- Savant V., Chavan R., Pushpoth S., Ilango B. Comparability and intra-/interobserver reliability of anterior chamber depth measurements with the Pentacam and IOLMaster. J. Refract. Surg. 2008;24:615–618. doi: 10.3928/1081597X-20080601-11. [DOI] [PubMed] [Google Scholar]
- Su P.F., Lo A.Y., Hu C.Y., Chang S.W. Anterior chamber depth measurement in phakic and pseudophakic eyes. Optom. Vis. Sci. 2008;85:1193–1200. doi: 10.1097/OPX.0b013e31818e8ceb. [DOI] [PubMed] [Google Scholar]
- Ucakhan O.O., Gesoglu P., Ozkan M., Kanpolat A. Corneal elevation and thickness in relation to the refractive status measured with the Pentacam Scheimpflug system. J. Cataract Refract. Surg. 2008;34:1900–1905. doi: 10.1016/j.jcrs.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Utine C.A., Altin F., Cakir H., Perente I. Comparison of anterior chamber depth measurements taken with the Pentacam, Orbscan IIz and IOLMaster in myopic and emmetropic eyes. Acta Ophthalmol. 2009;87:386–391. doi: 10.1111/j.1755-3768.2008.01278.x. [DOI] [PubMed] [Google Scholar]
- Woodmass J., Rocha G. A comparison of Scheimpflug imaging simulated and Holladay equivalent keratometry values with partial coherence interferometry keratometry measurements in phakic eyes. Can. J. Ophthalmol. 2009;44:700–704. doi: 10.3129/i09-172. [DOI] [PubMed] [Google Scholar]
- Yazici A.T., Bozkurt E., Alagoz C., Alagoz N., Pekel G., Kaya V., Yilmaz O.F. Central corneal thickness, anterior chamber depth, and pupil diameter measurements using Visante OCT, Orbscan, and Pentacam. J. Refract. Surg. 2010;26:127–133. doi: 10.3928/1081597X-20100121-08. [DOI] [PubMed] [Google Scholar]
- Yi J.H., Hong S., Seong G.J., Kang S.Y., Ma K.T., Kim C.Y. Anterior chamber measurements by pentacam and AS-OCT in eyes with normal open angles. Korean J. Ophthalmol. 2008;22:242–245. doi: 10.3341/kjo.2008.22.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]


