Abstract
Transitional papilloma (inverted papilloma, Schneiderian papilloma) is a relatively common, benign epithelial neoplasm of the sinonasal tract that also occurs in the lacrimal drainage system. The name transitional papilloma is recommended because it reflects the key histological features required for pathological diagnosis, as well as the histogenesis of the tumour. The histogenesis of the tumour is reviewed, together with its natural history, which is characterized by bone remodelling and destruction, a tendency to recur and to undergo malignant transformation. Biomarkers associated with these features have been identified in the sinonasal tumours and may also be of relevance to the lacrimal sac tumours, although the necessary studies have not yet been undertaken.
Keywords: Lacrimal sac, Inverted papilloma, Schneiderian papilloma, Transitional papilloma, Transitional cell carcinoma
Introduction
Tumours of the lacrimal drainage system are not common but even small ophthalmic pathology laboratories will receive one or two each year. As in most body sites, neoplasms of the lacrimal sac may be categorized in general as benign or malignant, primary, secondary or metastatic and epithelial or non-epithelial, i.e., lymphoid, melanocytic or mesenchymal, and the variety of tumours is similar to that seen in the sinonasal tract. This review will concentrate on the commonest epithelial neoplasm of the lacrimal sac, the transitional papilloma, its characteristic histopathology and histogenesis, its malignant transformation and principles of management. The recognition and diagnosis of this tumour seem to create problems for many pathologists, in part because of the confusing array of names which has been given: inverted papilloma, inverting papilloma, squamous papilloma, Schneiderian papilloma, transitional (cell) papilloma, among others.1 To understand the natural history of this tumour, however, it is helpful to consider what is known about its much more common counterpart in the sinonasal tract.
Sinonasal papillomas
Histopathology
A papilloma is by definition a benign neoplasm of a surface epithelium. Sinonasal papillomas are conventionally classified into three morphological types:
-
•
Fungiform (exophytic) squamous papilloma
-
•
Cylindrical cell (oncocytic Schneiderian) papilloma
-
•
Inverted (transitional cell) papilloma
of which the inverted papilloma is the most common (70%).2 The adjective ‘inverted’ refers to the downward displacement of the thickened neoplastic epithelium into the underlying stroma. Although in common usage, this is an unsatisfactory term, since the papillomas may have either an endophytic or a combined endophytic/exophytic growth pattern and in small biopsies the growth pattern may be hard to recognize and the proper diagnosis may be missed. The significance of the diagnosis stems from the propensity of this benign tumour to recur and/or undergo malignant transformation. Some authors have objected to the use of the alternative name transitional cell papilloma, since there is no actual “transitional cell”.3 Nevertheless, transitional epithelium is easy to recognize by light microscopy and the term has the advantage of guiding the pathologist towards the right diagnosis if he or she receives a small biopsy with markedly thickened epithelium on its surface, even in the absence of clearly endophytic growth. The term transitional papilloma, qualified by exophytic and/or endophytic, is recommended by this author and will be used throughout this article in preference to inverted papilloma or transitional cell papilloma.
In the sinonasal tract transitional epithelium arises by metaplasia of the normal respiratory epithelium and occurs as an intermediate stage between the pseudostratified columnar ciliated epithelium and stratified squamous epithelium. Consequently, the epithelium has both glandular and squamous features:
-
•
Stratification
-
•
No surface keratin but tonofibrils identifiable on electron microscopy
-
•
No intercellular bridges
-
•
Goblet cells
-
•
Pools of mucin (microcysts), often containing neutrophils
-
•
A surface layer of columnar cells, possibly ciliated.
Histogenesis
Transitional metaplasia of the normal upper respiratory tract epithelium is a frequent accompaniment of chronic inflammation and over 20% of transitional papillomas are associated with inflammatory nasal polyps.4 Transitional metaplasia is the first step in the histogenesis of a papilloma (Fig. 1a and b) and is followed by a proliferative phase in which a hyperplastic ribbon of surface epithelium is created (Fig. 1c). To accommodate the increasing surface area, the epithelium begins to expand either outwards or inwards (Fig. 1d and e), or both. Focally, the epithelium may undergo complete squamous metaplasia with a minor amount of keratinization (Fig. 1f). During development the lining mucosa of the sinonasal tract arises from the Schneiderian membrane which is formed by the invagination of the olfactory ectoderm. It is perhaps not surprising that the mucosal epithelium should itself have an innate tendency towards endophytic growth, although what might determine this remains unclear. It is also unclear what factors drive the neoplastic transformation of the hyperplastic epithelium, although a role for inflammation has been proposed.5
Figure 1.
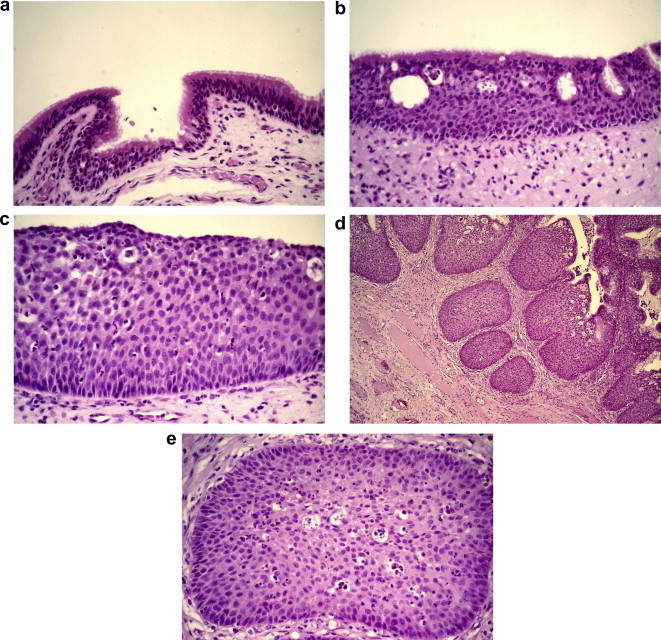
Surface epithelial changes in a recurrent transitional papilloma of the maxillary sinus. (a) Normal ciliated upper respiratory tract epithelium; (b) metaplastic transitional epithelium with surface layer of ciliated cells; (c) thickened transitional epithelium; (d) endophytic growth of surface transitional epithelium; (e) bland epithelium with microcysts and intra-epithelial neutrophils characteristic of transitional epithelium.
Schwerer reported an increased expression of p53 in sinonasal epithelium in 89% of transitional papillomas without dysplasia and, in 35% of these, the level of expression increased with transformation from respiratory-type, through transitional to squamous epithelium.6 The proliferative capacity of the epithelium, manifested as nuclear expression of Ki67 in the basal and parabasal cells, also increased from normal epithelium to papillomatous respiratory-type epithelium to transitional and squamous epithelia.7
For over two decades it has been recognized that genomic material of human papillomavirus (HPV) can be identified in transitional papillomas. Reported detection rates have shown considerable variation, possibly reflecting different methods of genotype-based testing (in situ hybridization vs. polymerase chain reaction with type-specific or consensus primers) and variable quality of archival tissue specimens. In a comprehensive review of this topic, Lawson et al. concluded that overall 16% of transitional papillomas contained evidence of HPV infection and that the ratio of low-risk (types 6/11) to high-risk (types 16/18) HPV infection was 2.8:1.8 However, in papillomas displaying high-grade dysplasia or malignant transformation the ratio of low-risk to high-risk types was reversed (1:1.1 and 1:2.4, respectively). Lawson et al. postulate that low-risk HPV types may induce the formation of transitional papillomas but the virus is subsequently lost from the cells; if primary or secondary infection occurs with high-risk types, dysplasia results with possible progression to malignancy.8
Recurrence
Reported rates of recurrence of transitional papilloma are generally between 9% and 34%, depending to some extent on the type of surgery initially performed.4,9,10 Several authors have reported on the association of HPV infection with recurrence, although others have not found this.11 Lawson et al. reported the presence of HPV in 57.9% of recurrent papillomas and Beck et al. found that the presence of HPV genomic material in a transitional papilloma had a positive predictive value of recurrence of 87%.8,12 Transitional papillomas that are destined to recur, and the recurrent tumours, show reduced expression of cytokeratin-14 (CK14) in the basal cells of the epithelium, compared with normal sinonasal epithelium and those papillomas that do not recur.13 Since this cytokeratin is associated with the integrity of hemidesmosomes, its loss may cause poor cell adhesion and a propensity for the seeding of tumour cells during the initial surgical exploration, resulting in subsequent recurrence. The Msx2 gene product, which reduces the exit of cells from the cell cycle and is localized in the basal cells of the epithelium in transitional papilloma, may play a role in the thickening of the surface epithelium; expression of the protein is increased in recurrent papillomas.14 This protein also results in increased expression of at least two bone resorption factors (TRAP, tartrate-resistant acid phosphatase and RANKL, receptor activator of nuclear factor-kappa B ligand) and these may contribute to the bone remodelling that is sometimes associated with the aggressive growth of a transitional papilloma.
Malignant transformation
Although most transitional papillomas are benign, some may undergo malignant transformation, usually to squamous cell carcinoma or mucoepidermoid carcinoma and occasionally to adenocarcinoma.1,15 Malignancy may be found within the transitional papilloma, either as carcinoma in situ or as a synchronous invasive carcinoma. Metachronous carcinomas may also occur but are less common.16 In assessing the risk of malignant transformation of a transitional papilloma, there are no well established, or universally accepted, criteria for the diagnosis of dysplasia but increased mitotic activity and nuclear atypia within the epithelium do appear to be predictive.17,18 Katori et al. demonstrated the increased expression of epidermal growth factor receptor (EGFR) and transforming growth factor-α (TGF-α), markers linked to the development of squamous cell carcinoma of the head and neck, with the transition from mild dysplasia to severe dysplasia and carcinoma in situ, although these authors do not provide criteria for the grading of dysplasia.18 Both EGFR and TGF-α expression were greater in transitional papillomas that expressed HPV (types 6/11 and 16/18), as was the Ki67 index. An inverse relation has been noted between the presence of HPV and over-expression of p53 protein in transitional papillomas that have undergone malignant transformation.15 In a series of 30 transitional papillomas with carcinoma, three cases with high-risk HPV were identified and these did not over-express p53 protein. p53 protein was over-expressed in 21 of 23 tumours lacking the evidence of HPV infection. It would appear that infection with high-risk HPV or, perhaps more importantly, a p53 mutation leading to over-expression is associated with malignant transformation, a conclusion in keeping with the studies of Finkelstein et al.19
A number of other biomarkers associated with dysplasia have been identified, including the actin cross-linking protein fascin and matrix metalloproteinases-2 and -9.20,21 With increasing severity of epithelial dysplasia within a papilloma, from a moderate degree to invasive carcinoma, the expression of these two enzymes increases significantly. Since they both have a role in the degradation of extracellular matrix, this over-expression may be linked to the early events in carcinogenesis and the aggressiveness of the tumour. Interestingly, these authors found that the expression of HPV types 6/11 and 16/18 was also increased with the degree of dysplastic/malignant change.21
Orbital invasion
Because of the proximity of the sinonasal passages to the orbit, it is not uncommon for an upper airway tumour, most frequently a squamous cell carcinoma, to invade the orbit.22 Transitional papillomas are capable of invading the orbit and Johnson et al. described four such patients with transitional papillomas of the ethmoid sinus and lateral nasal wall.22 This generally occurs because of rarefaction of the bone; papillomas are not only able to infiltrate bone but may cause a variety of radiographic changes, such as thinning, displacement and destruction.23 In a comprehensive review of sinonasal transitional papilloma, Elner et al. estimated the prevalence of orbital infiltration at 2.7%.24 These authors reviewed ten cases of their own and found focal malignant transformation into squamous cell carcinoma in six and transitional cell carcinoma in four in the portion of the tumour within the orbit. Eight of these patients required exenteration and three of them died from erosion into the brain. Orbital invasion by a transitional papilloma in the maxillary sinus may produce proptosis.25 Extension of a recurrent sinonasal transitional papilloma through the naso-lacrimal duct into the orbit has also been described.26 In that case areas of malignant transformation were identified in the excised tumour but destruction of the bony wall of the orbit was not seen.
Transitional papillomas of the lacrimal sac
Histopathology and histogenesis
Given the close connections of the lacrimal drainage system and the sinonasal tract, both embryological and anatomical, it is not surprising that the same types of tumour occur in these passages. Transitional papilloma is not only the commonest epithelial neoplasm in the lacrimal sac, it may co-exist with a sinonasal transitional papilloma. This important clinical point was recognized over 40 years ago by Harry and Ashton, who wrote:
“…we consider it advisable that all patients with lacrimal sac tumours should have their respiratory passages thoroughly examined.”27
Most of the morphological characteristics of sinonasal transitional papilloma discussed earlier are shared by the lacrimal counterpart and careful examination of the lacrimal sac tumours indicates that the same sequence of histogenetic changes can be observed in papillomas at this site.
In a series of 115 lacrimal sac neoplasms collected at the Armed Forces Institute of Pathology, Stefanyszyn et al. reported 32 cases of benign papilloma, 19 squamous in type and 13 transitional, although the authors did not indicate the criteria used to separate these two groups.28 Patients with squamous papillomas were slightly older than those with transitional papillomas (mean age of 47 vs. 40 years), which accords with the histogenetic sequence described above. In addition, there were 6 cases of papilloma with foci of malignant change in patients with a mean age of 50 years.
In their 1968 paper, in which they reported on 8 transitional neoplasms of the lacrimal sac, Harry and Ashton provided useful criteria for the diagnosis of benign and malignant tumours.27 They divided the tumours into three categories on the basis of morphology and clinical behaviour:
Type I: Transitional papilloma
-
•
A papillary tumour with a stratified transitional epithelium
-
•
Marked but smooth infolding of epithelium into the stroma
-
•
Uniform cytomorphology
Type II: Intermediate transitional tumour
-
•
A papillary tumour with a stratified epithelium
-
•
More irregular invagination of the epithelium into the stroma
-
•
Mild cellular pleomorphism
-
•
Conspicuous squamous metaplasia
-
•
Readily recognizable mitotic figures
Type III: Transitional cell carcinoma
-
•
A neoplasm with irregular, stratified epithelium
-
•
Invasive growth
-
•
Marked cellular pleomorphism
-
•
Conspicuous squamous metaplasia
-
•
Conspicuous mitotic activity.
Harry and Ashton suggested that Type I and II lesions could recur and both might give rise to frankly invasive carcinomas; however, some Type III tumours could possibly arise de novo.27 These diagnostic criteria are of use to the practising pathologist, particularly since an initial tissue sample may be a small biopsy obtained at dacryocystorhinostomy.29 A diagnosis of intermediate transitional tumour in such a biopsy should prompt consideration of a subsequent dacryocystectomy to exclude a frankly invasive carcinoma, whereas, a diagnosis of transitional papilloma might simply warrant careful follow-up of the patient.
Not surprisingly HPV DNA may be identified in some transitional papillomas.30 However, detailed molecular studies of the histogenesis have not been undertaken, in part because of the rarity of these neoplasms, and the relationship of HPV infection to dysplastic change has not been clarified.
As they do in the sinonasal tract, transitional papillomas in the lacrimal sac, whether they be exophytic or endophytic, have a propensity to recur.31,32 Although the cytomorphology may become more atypical with recurrence, in general recurrence does not seem to predispose to malignant transformation in either the sinonasal tract or the lacrimal sac.1,2,32 Extension along the naso-lacrimal duct predisposes to recurrence and the benign tumour may also extend into the nose and/or orbit by erosion of the bone of the lacrimal fossa as it grows (Fig. 2).
Figure 2.
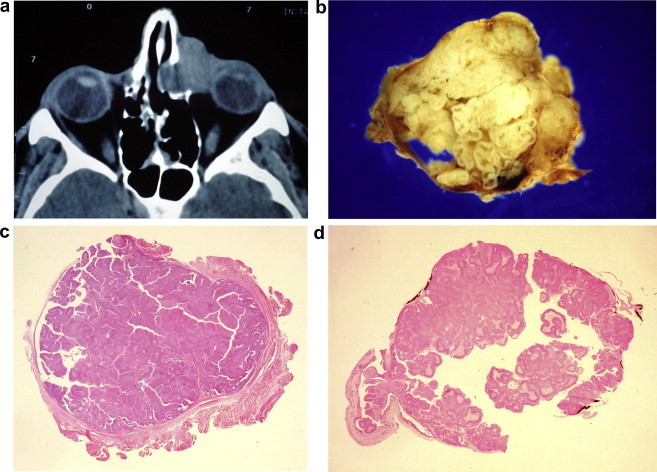
A man with a 12 month history of a lump in the left medial canthus and epiphora. (a) CT scan showing apparent invasion of orbit by mass in left lacrimal fossa, interpreted as malignant by radiologist; (b) dacryocystectomy specimen: sac lumen obliterated by papilloma with ‘ribbon’ of thickened epithelium; (c) transitional papilloma with mixed exophytic and endophytic growth pattern [haematoxylin & eosin]; (d) residual fragments of bone of lacrimal fossa on outer surface of sac stained black with von Kossa reagent.
Malignant transformation
In addition to the six carcinomas arising in papillomas of the sac, Stefanyszyn et al. identified 38 carcinomas arising de novo.28 Squamous cell carcinoma was the commonest malignancy (22/38) and transitional cell carcinoma (5/38) the second commonest. The mean age at diagnosis for squamous cell carcinoma was 63 years, considerably higher than that for transitional cell carcinoma (47 years). Ni et al. found 6 transitional cell carcinomas among 40 lacrimal sac carcinomas.33 Other reports of cases of transitional cell carcinoma have been published, arising both in transitional papilloma and de novo.34–36 Parmar and Rose described two cases of invasive transitional cell carcinoma, one with some squamous differentiation and the other with an admixed component of squamous cell carcinoma.37 Fliss et al. reported two cases of mucoepidermoid carcinoma of the lacrimal sac that had arisen in metaplastic transitional epithelium; in one of these cases the invasive carcinoma was in continuity with a predominantly exophytic transitional papilloma.38 These observations support the concept that transitional metaplasia precedes squamous differentiation and that neoplasms arising from the surface epithelium of the sac do so from transitional epithelium.
Persistent epiphora and a medial canthal mass, generally above but sometimes below the medial canthal tendon, is the classical presentation of a tumour of the lacrimal sac and the presence of pain and blood-stained tears is a strong indicator of malignancy. Transitional cell carcinoma is the second commonest carcinoma arising de novo in the lacrimal sac, after squamous cell carcinoma.28 However, as with the corresponding papillomas, the light microscopic differentiation of a transitional cell carcinoma from a non-keratinizing squamous cell carcinoma is subjective and squamous metaplasia is seen in some transitional carcinomas.27 As indicated above, the presence of atypical transitional epithelium without definite stromal invasion, should lead to a diagnosis of intermediate transitional tumour and further surgery. In the case illustrated in Fig. 3 a patient with a clinical picture suggestive of a malignant sac neoplasm was given a diagnosis of intermediate transitional tumour on biopsy but the resected specimen showed clearly invasive growth with perineural infiltration and extension along the naso-lacrimal duct. This patient was not given post-operative radiotherapy, although it has been recommended for the patients with incompletely excised tumours and for those who find radical surgery unacceptable.34,39 Firm information on the prognosis of these carcinomas is difficult to obtain because of their rarity. Metastases to regional nodes, lungs, oesophagus, liver and skeleton have been described but patients who die of tumour usually do so because of local recurrence.33,39
Figure 3.
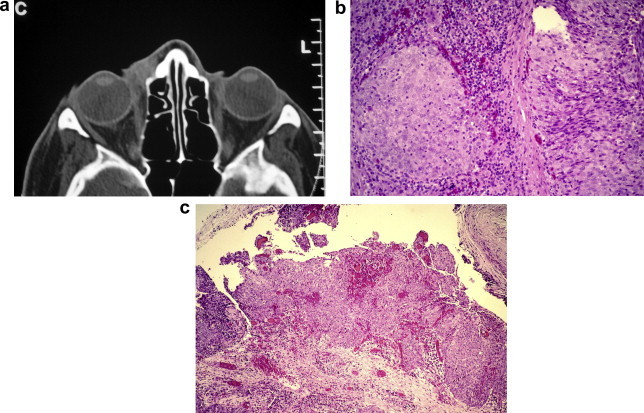
Transitional cell carcinoma of lacrimal sac. (a) CT scan showing mass in right medial canthus; (b) circumscribed nests of atypical transitional epithelium in biopsy of mass; (c) surface origin of invasive transitional cell carcinoma in dacryocystectomy specimen [haematoxylin & eosin].
Conclusion
Transitional papillomas of the sinonasal tract and lacrimal sac have identical histopathological appearances and histogenetic pathways. They also appear to have a similar natural history and the two tumours may co-exist in the same patient, although sac tumours are much less common. The pathogenetic mechanisms underlying the development and behaviour of sinonasal transitional papillomas are being elucidated but, apart from a possible role of HPV, these have not been studied in sac papillomas.
Footnotes
This review is based on a lecture given at the 25th Annual Meeting of the British Association for Ophthalmic Pathology, London, UK, April, 2006.
References
- 1.Snyder R.N., Perzin K.H. Papillomatosis of nasal cavity and paranasal sinuses (inverted papilloma, squamous papilloma). A clinicopathologic study. Cancer. 1972;30:668–690. doi: 10.1002/1097-0142(197209)30:3<668::aid-cncr2820300315>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 2.Hyams V.J. Papillomas of the nasal cavity and paranasal sinuses. A clinicopathological study of 315 cases. Ann Otol Rhinol Laryngol. 1971;80:192–206. doi: 10.1177/000348947108000205. [DOI] [PubMed] [Google Scholar]
- 3.Karcioglu Z.A., Caldwell D.R., Reed H.T. Papillomas of lacrimal drainage system: a clinicopathologic study. Ophthalmic Surg. 1984;15:670–676. [PubMed] [Google Scholar]
- 4.Yoon J.H., Kim C.H., Choi E.C. Treatment outcomes of primary and recurrent inverted papilloma: an analysis of 96 cases. J Laryngol Otol. 2002;116:699–702. doi: 10.1258/002221502760237984. [DOI] [PubMed] [Google Scholar]
- 5.Roh, Procop G.W., Batra P.S., Citardi M.J., Lanza D.C. Inflammation and pathogenesis of inverted papilloma. Am J Rhinol. 2004;18:65–74. [PubMed] [Google Scholar]
- 6.Schwerer M.J., Sailer A., Kraft K., Backzacko K., Maier H. Differentiation-related p53 protein expression in non-dysplastic sinonasal inverted papilllomas. Am J Rhinol. 2001;15:347–351. [PubMed] [Google Scholar]
- 7.Schwerer M.J., Sailer A., Kraft K., Maier H. Cell proliferation and p27Kip1 expression in endophytic scheiderian papillomas. Laryngoscope. 2002;112:852–857. doi: 10.1097/00005537-200205000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Lawson W., Schlecht N.F., Brandwein-Gensler M. The role of human papillomavirus in the pathogenesis of schneiderian inverted papillomas: an analytic over view of the evidence. Head Neck Pathol. 2008;2:49–59. doi: 10.1007/s12105-008-0048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawson W., Le Benger J., Som P., Bernard P.J., Biller H.F. Inverted papilloma: an analysis of 87 cases. Laryngoscope. 1989;99:1117–1123. doi: 10.1288/00005537-198911000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Klimek T., Atai E., Schubert M., Glanz H. Inverted papilloma of the nasal cavity and paranasal sinuses: clinical data, surgical strategy and recurrence rates. Acta Otolaryngol. 2000;120:267–272. doi: 10.1080/000164800750001071. [DOI] [PubMed] [Google Scholar]
- 11.Kraft M., Simmen D., Casas R., Pfaltz M. Significance of human papillomavirus in sinonasal papillomas. J Laryngol Otol. 2001;115:709–714. doi: 10.1258/0022215011908955. [DOI] [PubMed] [Google Scholar]
- 12.Beck J.C., McClatchey K.D., Lesperance M.M., Esclamado R.M., Carey T.E., Bradford C.R. Presence of human papillomavirus predicts recurrence of inverted papilloma. Otolaryngol Head Neck Surg. 1995;113:49–55. doi: 10.1016/s0194-5998(95)70144-3. [DOI] [PubMed] [Google Scholar]
- 13.Gunia S., Liebe D., Koch S. Loss of basal cell keratin 14 reflects increased risk of recurrence in surgically resected sinonasal inverted papilloma. J Clin Pathol. 2008;61:707–712. doi: 10.1136/jcp.2008.055954. [DOI] [PubMed] [Google Scholar]
- 14.Depondt J., Shabana E.-H., Walker F., Pibouin L., Lezot F., Berdal A. Nasal inverted papilloma expresses the muscle segment homeobox gene Msx2: possible prognostic implications. Hum Pathol. 2008;39:350–358. doi: 10.1016/j.humpath.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Buchwald C., Lindeberg H., Pedersen B.L., Franzmann M.-B. Human papilloma virus and p53 expression in carcinomas associated with sinonasal papillomas: a Danish epidemiological study 1980–1998. Laryngoscope. 2001;111:1104–1110. doi: 10.1097/00005537-200106000-00032. [DOI] [PubMed] [Google Scholar]
- 16.Von Buchwald C., Bradley P.J. Risks of malignancy in inverted papilloma of the nose and paranasal sinuses. Curr Opin Otolaryngol Head Neck Surg. 2007;15:95–98. doi: 10.1097/MOO.0b013e3280803d9b. [DOI] [PubMed] [Google Scholar]
- 17.Eggers G., Eggers H., Sander N., Koessling F., Chilla R. Histological features and malignant transformation of inverted papilloma. Eur Arch Otorhinolaryngol. 2005;262:263–268. doi: 10.1007/s00405-004-0818-9. [DOI] [PubMed] [Google Scholar]
- 18.Katori H., Nozawa A., Tsukuda M. Markers of malignant transformation of sinonasal inverted papilloma. EJSO. 2005;31:905–911. doi: 10.1016/j.ejso.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Finkelstein S.D., Tiffee J.C., Bakker A., Swalsky P., Barnes L. Malignant transformation in sinonasal papillomas is closely associated with aberrant p53 expression. Mol Diagn. 1998;3:37–41. doi: 10.154/MODI00300037. [DOI] [PubMed] [Google Scholar]
- 20.Wu H.H., Zafar S., Huan Y., Yee H., Chiriboga L., Wang B.Y. Fascin over expression is associated with dysplastic changes in sinonasal inverted papillomas: a study of 47 cases. Head Neck Pathol. 2009;3:212–216. doi: 10.1007/s12105-009-0133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katori H., Nozawa A., Tsukuda M. Increased expression of matrix metalloproteinase-2 and 9 and human papilloma virus infection are associated with malignant transformation of sinonasal inverted papilloma. J Surg Oncol. 2006;93:80–85. doi: 10.1002/jso.20386. [DOI] [PubMed] [Google Scholar]
- 22.Johnson L.N., Krohel G.B., Yeon E.B., Parnes S.M. Sinus tumors invading the orbit. Ophthalmology. 1984;91:209–217. doi: 10.1016/s0161-6420(84)34300-7. [DOI] [PubMed] [Google Scholar]
- 23.Momose K.J., Weber A.L., Goodman M., MacMillan A.S., Jr., Roberson G.H. Radiological aspects of inverted papilloma. Radiology. 1980;134:73–79. doi: 10.1148/radiology.134.1.7350638. [DOI] [PubMed] [Google Scholar]
- 24.Elner V.M., Burnstine M.A., Goodman M.L., Dortzbach R.K. Inverted papillomas that invade the orbit. Arch Ophthalmol. 1995;113:1178–1183. doi: 10.1001/archopht.1995.01100090104030. [DOI] [PubMed] [Google Scholar]
- 25.Lawton A.W., Karesh J.W., Gray W.C. Proptosis from maxillary sinus inverted papilloma with malignant transformation. Arch Ophthalmol. 1986;104:874–877. doi: 10.1001/archopht.1986.01050180108041. [DOI] [PubMed] [Google Scholar]
- 26.Chaudhry I.A., Taiba K., Al-Sadhan Y., Riley F.C. Inverted papilloma invading the orbit through the nasolacrimal duct; a case report. Orbit. 2005;24:135–139. doi: 10.1080/01676830590926530. [DOI] [PubMed] [Google Scholar]
- 27.Harry J., Ashton N. The pathology of tumours of the lacrimal sac. Trans Ophthalmol Soc UK. 1968;87:19–35. [PubMed] [Google Scholar]
- 28.Stefanyszyn M.A., Hidayat A.A., Pe’er J.J., Flanagan J.C. Lacrimal sac tumours. Ophthalmic Plast Reconstr Surg. 1994;10:169–184. doi: 10.1097/00002341-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Merkonidis C., Brewis C., Yung M., Nussbaumer M. Is routine biopsy of the lacrimal sac wall indicated at dacryocystorhinostomy? A prospective study and literature review. Br J Ophthalmol. 2005;89:1589–1591. doi: 10.1136/bjo.2005.072199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura Y., Mashima Y., Kameyama K. Human papilloma virus DNA detected in case of inverted squamous papilloma of the lacrimal sac. Br J Ophthalmol. 1995;79:392–393. doi: 10.1136/bjo.79.4.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jang J.H., Chang S.D., Choe M.S. A case of recurrent schneiderian papilloma of the lacrimal sac invading the nasal cavity. Korean J Ophthalmol. 2009;23:100–103. doi: 10.3341/kjo.2009.23.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan S.J., Font R.L. Primary epithelial neoplasms of the lacrimal sac. Am J Ophthalmol. 1973;76:73–88. doi: 10.1016/0002-9394(73)90014-7. [DOI] [PubMed] [Google Scholar]
- 33.Ni C., D’Amico D.J., Fan C.Q., Kuo P.K. Tumors of the lacrimal sac: a clinicopathological analysis of 82 cases. Int Ophthalmol Clin. 1982;22:121–140. doi: 10.1097/00004397-198202210-00010. [DOI] [PubMed] [Google Scholar]
- 34.Anderson K.K., Lessner A.M., Hood I. Invasive transitional cell carcinoma of the lacrimal sac arising in an inverted papilloma. Arch Ophthalmol. 1994;112:306–307. doi: 10.1001/archopht.1994.01090150036014. [DOI] [PubMed] [Google Scholar]
- 35.Karim R., Ghabrial R., Lin B. Transitional cell carcinoma of the nasolacrimal sac. Clin Ophthalmol. 2009;3:587–591. doi: 10.2147/opth.s7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pang C.S., Brown J.D., Ganote C.E., Youngberg G.A. A mass of the right lacrimal sac in a 53-year-old man: transitional cell carcinoma. Arch Pathol Lab Med. 2005;129:1493–1494. doi: 10.5858/2005-129-1493-AMOTRL. [DOI] [PubMed] [Google Scholar]
- 37.Parmar D.N., Rose G.E. Management of lacrimal sac tumours. Eye. 2003;17:599–606. doi: 10.1038/sj.eye.6700516. [DOI] [PubMed] [Google Scholar]
- 38.Fliss D.M., Freeman J.L., Hurwitz J.J., Heathcote J.G. Mucoepidermoid carcinoma of the lacrimal sac: a report of two cases, with observations on the histogenesis. Can J Ophthalmol. 1993;28:228–235. [PubMed] [Google Scholar]
- 39.Sagerman R.H., Fariss A.K., Chung C.T., King G.A., You H.S., Fries P.D. Radiotherapy for nasolacrimal tract epithelial cancer. Int J Radiat Oncol Biol Phys. 1994;29:177–181. doi: 10.1016/0360-3016(94)90241-0. [DOI] [PubMed] [Google Scholar]


