Abstract
Angiogenesis, the process by which new blood vessels are formed, is a critical phenomenon that is activated during various stages of mammalian development. MicroRNAs (miRNAs), a class of short, single stranded, non-coding RNAs, are recognized as important regulators of angiogenesis, and the role of intracellular miRNAs in modulating angiogenesis signaling has been identified. The recent discovery of extracellular and circulating miRNAs has sparked new questions regarding their potential in modulating angiogenesis signaling not only within cells but also between cells. In this review, we discuss the characteristics of intracellular and extracellular miRNAs and decipher the potential functional roles for these molecules in regard to the angiogenic process. We summarize what is currently known about circulating miRNAs in distinct clinical populations and discuss evidence that implicates extracellular miRNAs as novel mediators of angiogenesis-associated intercellular signaling. Lastly, we offer a new perspective on the functional role of vesicle-encapsulated circulating miRNA in modulating angiogenesis signaling pathways.
Keywords: angiogenesis, exosomes, extracellular miRNA, gene expression, intracellular miRNA, microparticles, microvesicles
Introduction
Angiogenesis, the process by which new blood vessels are formed, is critical to embryonic development[1], wound healing[2], growth of tumors[3], and is triggered in response to a diverse array of stimuli, including hypoxia and tissue ischemia[4]. Endothelial cells are primary mediators of the angiogenic process through their role in remodeling of the vascular network, which involves changes in arterial shape and size, and the growth and pruning of new vasculature[1, 5]. The major promoting factors for angiogenesis have been identified[6], including vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), and basic fibroblast growth factor (bFGF), all of which are capable of activating signaling pathways to regulate endothelial cell motility, proliferation and survival[7].
Recently, microRNAs (miRNAs), a class of short, single stranded, non-coding RNAs (~22 nt), have emerged as important regulators of a variety of physiological and pathological processes[8], including angiogenesis [6, 9–12]. miRNAs are capable of regulating gene expression through the targeted degradation and translational repression of mRNA transcripts[13, 14]. Much work has focused on the role of intracellular miRNAs in angiogenesis[6]; however, the recent discovery of extracellular and circulating miRNAs has sparked new questions regarding the ability of these molecules to regulate various aspects of the angiogenic program.
In this review, we will discuss the characteristics of intracellular and extracellular miRNAs and examine evidence for their roles in mediating angiogenic signals. We will discuss recent studies that indicate extracellular or circulating miRNAs are novel mediators of cell-to-cell communication, particularly as it relates to angiogenesis. Finally, we shall offer a new perspective on the functional role of vesicle-encapsulated circulating miRNA in angiogenesis-related pathologies.
MicroRNAs: Established Roles in Normal Physiology and Disease
MiRNAs were first described in 1993 when Ambros and colleagues identified an important gene that regulated the development of Caenorhabditis elegans (C. elegans), lin-4. The product of this regulatory gene was shown to be a small, non-protein coding RNA molecule – microRNA (miRNA)[15]. Since its discovery in 1993, miRNAs have been the subject of intense study, and most have been found to be highly conserved, indicating their importance as regulatory molecules. miRNAs function by targeting specific messenger RNAs (mRNAs), which results in posttranscriptional repression of the target gene. miRNA-mediated gene repression is complex and miRNA networks have been shown to modulate many aspects of cellular homeostasis and physiology. To date, 3329 mature miRNA sequences have been cloned from a variety of species (miRBase release 18, November 2011)[16]. This number is likely to increase as miRNA research efforts continue.
Regulation of miRNA-Target Interactions
Through seed sequence-based binding, one miRNA can potentially target hundreds of mRNAs and one mRNA can be repressed by more than one miRNA. However, the relative tissue specificity of many miRNA-mRNA interactions implies an intracellular mechanism for the regulation of these interactions. Understanding the characteristics of this regulation may help to advance the development of more potent methods to regulate gene expression to either promote or inhibit cellular processes such as angiogenesis. Using bioinformatics techniques, Cui and colleagues[17] have identified several characteristics that govern miRNA-target interactions. These investigators determined that ‘adaptor’ genes, such as transcription factors and nuclear proteins, represent approximately 50% of the miRNA targets within a signal network, while receptor ligands comprise only about 9% of the miRNA targets[17]. In this context, a signal network was defined as a specific set of proteins that work together to process information generated within the extracellular or intracellular environment. Adaptor genes were defined as proteins that are capable of influencing multiple downstream signal molecules for a variety of signal networks. By preferentially targeting adaptor genes, miRNAs have the capacity to coordinate robust responses to various stimuli because adaptor genes are usually involved in multiple signal networks. This is important because miRNAs that are known to regulate angiogenesis target genes with adaptor-like characteristics[17]. Hypoxia inducible factor-1 alpha (HIF1-α) is a confirmed target of members of the pro-angiogenic miR-17~92 cluster (a group of seven miRNAs that all reside on the same gene[18]) and miR-210. HIF1-α is considered an adaptor gene because it not only promotes angiogenic signaling during hypoxia[19], but also contributes to the regulation of human metabolism[20]. In addition, PI3 kinase regulatory subunit 2, a target of pro-angiogenic miR-126, is involved in angiogenic signaling as well as apoptosis[21].
Interestingly, it was found that miRNAs are less likely to target genes that are absolutely required for basic cellular functions, such as secretion and motility[17] – two processes that have been linked to tumor angiogenesis[22]. Genes that control essential cellular processes are usually very stable and highly expressed; as a result, these genes are not preferentially targeted by miRNAs[17]. By not binding to transcripts associated with essential cellular processes, miRNAs can preferentially target those proteins which are activated or inhibited in response to specific stimuli and thereby fine tune intracellular signal transduction. The angiogenic process can be regarded as a coordinated cellular response to a series of biochemical signals – by understanding how miRNA-mRNA interactions modulate the angiogenic response, new perspectives on methods to control angiogenesis in different physiological and pathological settings can be obtained.
miRNAs as Mediators of Intracellular Angiogenic Signals
The importance of miRNAs in angiogenesis was established in studies of Dicer, a ribonuclease required for miRNA biogenesis[11, 23–25]. Dicer deficient mice had impaired blood vessel formation during embryonic development and Dicer deficiency was embryonically lethal[25]. The vascular defects in Dicer-deficient mice were associated with altered expression of vascular endothelial growth factor (VEGF), its receptors KDR (VEGFR2) and FLT1 (VEGFR1) and the angiopoietin receptor, Tie-1, suggesting that miRNAs may regulate the expression levels of crucial angiogenic factors. Also, nonlethal Dicer hypomorph females were shown to be sterile due to insufficient angiogenesis in the corpus luteum[26]. In vitro, Dicer silencing in human umbilical vein endothelial cells (HUVECs) reduced the formation of capillary-like structures and HUVEC proliferation[11, 23, 27]. Dicer knockdown also diminished cell migration and matrigel tube formation in human microvascular endothelial cells (HMECs)[28]. These studies support a role for miRNAs in regulating angiogenesis through changes in endothelial cell function. Through these studies of Dicer, and others, specific miRNAs have been implicated in the regulation of angiogenesis, including the miR-17~92 cluster, miR-15/16, miR-21[29], miR-126 and miR-210[19, 30, 31]. The role of intracellular miRNAs in angiogenesis was recently reviewed[6, 32].
Hypoxia and ischemia are potent stimuli of angiogenic signaling pathways[33]. In vitro, miR-210 is upregulated in HUVECs in response to hypoxia, and this upregulation enhances the ability of HUVECs to form capillary-like structures[34]. Hypoxia also increases miR-210 in non-endothelial cells, which likely plays a role in the cells’ ability to partake in angiogenic processes. For example, colon and breast cancer cell lines have been shown to express increased levels of miR-210 in response to hypoxia[35]. In vivo, studies of ischemia in rats demonstrated upregulation of miR-210 in brain tissue and blood[36, 37]. miR-210 was subsequently shown to modulate angiogenic pathways in endothelial cells through its confirmed mRNA targets, hypoxia inducible factor-1 alpha (HIF-1α) and ephrin-A3[19].
The process of wound healing is also known to stimulate angiogenesis[38]. Cellular redox state is a key stimulus in wound angiogenesis[27] and miRNAs can regulate the cellular redox state through alterations in the expression of p47phox (an NADPH-oxidase)[27]. Knocking down Dicer in human microvascular endothelial cells impaired their ability to form tubes in matrigel and to produce reactive oxygen species when stimulated with TNF-α and VEGF. Taken together, these data suggest that miRNAs can mediate angiogenesis via a redox-sensitive mechanism. The link between miRNA-mediated regulation of cellular redox state and wound angiogenesis was recently reviewed by Roy and Sen[38].
miRNA Dysregulation in Pathological Angiogenesis: Focus on Cancer
Activation of the angiogenic program is important during various stages of normal physiology[8, 39]; however, in cancer, angiogenesis can promote tumor growth and metastasis[40]. Various solid tumors are characterized by the abnormal expression of oncogenic miRNAs that promote tumor growth through enhancement of tumor angiogenesis. For example, the miR-17~92 cluster, known to promote angiogenesis, has been shown to be upregulated in human adenocarcinomas containing genetic mutations in MYC proto-oncogenes[41]. By downregulating anti-angiogenic factors such as thrombosbondin-1 (Tsp1) and connective tissue growth factor (CTGF), the miR-17~92 cluster is thought to enhance the tumor growth, i.e. tumorigenicity, of human adenocarcinomas[41].
The miR-17-92 cluster has been implicated in the progression of colon cancer – colon cancer cells express higher than normal levels of members of the miR-17~92 cluster[41]. In vitro, the overexpression of miR-17~92 in p53-null colon cancer cells resulted in the formation of larger and better-perfused tumors[41]. The transcripts of both thrompospondin-1 and connective tissue growth factor are predicted targets of the miR-17~92 cluster (the specific members of this cluster were not determined), and these proteins are known to be anti-angiogenic.
Whereas some miRNAs, such as the miR-17~92 cluster, are pro-angiogenic and therefore function as oncomirs, other miRNAs, such as miR-15b and miR-16, have anti-angiogenic functions and are thus regarded as tumor suppressors. Vascular endothelial growth factor (VEGF), a known mediator of tumor neovascularization in breast, endometrial, and pancreatic cancers[42], is a target of miR-15 and miR-16[43]. Hypoxia induced the downregulation of miR-15b and miR-16 in a human nasopharyngeal carcinoma cell line[43]. The transfection of this human carcinoma cell line with miR-15b and miR-16-1 led to a marked decrease in the expression of VEGF[43]. Many angiogenesis-targeting therapies have been developed to inhibit solid tumor growth[44, 45] and the described studies indicate that miRNAs could be a novel therapeutic entry point for diseases associated angiogenesis.
Hematologic malignancies are also dependent on angiogenic signaling pathways[45]. Bone marrow endothelial cells present within vascular niches secrete cytokines, including VEGF, which can interact with leukemia stem cells to promote leukemia cell proliferation and survival[46] and to stimulate angiogenesis within the bone marrow[45]. Increased bone marrow vascular density has been observed in various hematologic malignancies, including B-cell chronic lymphocytic leukemia[47], acute lymphoid leukemia[48], and acute myeloid leukemia[49]. Correspondingly, some angiogenesis-associated miRNAs have been shown to be abnormally expressed in these same hematologic cancers. Chronic lymphocytic leukemia (CLL) is characterized by a significant reduction in the expression of the anti-angiogenic miRNAs, miR-15a and miR-16[50]. Also, the microRNA signature of a common form of acute myeloid leukemia revealed higher than normal levels of the following miRNAs: let-7a-3, let-7f, and let-7c[51]. The Let-7 family of miRNAs has been shown to affect tube formation in endothelial cells and is thus considered a pro-angiogenic miRNA[19]. Interestingly many aberrantly expressed miRNAs in human cancers target angiogenesis-associated genes as well as oncogenic genes, such as the BCL2 anti-apoptotic gene (target of miR-15a/miR-16-1) and the E2F1 transcription factor gene (target of miR-17-92 cluster)[19, 52]. This observation suggests a potential link between miRNA-mediated regulation of angiogenesis and the proliferation and growth of tumor cells[45, 53, 54].
Circulating miRNAs – New Role as Intercellular Communicators
The Controlled Cellular Export of miRNAs
The discovery of distinct miRNA expression profiles in different types of body fluids[55–58] and the stability of miRNAs in the relatively harsh extracellular environment[59] indicate that extracellular export of miRNA is non-random. The regulation of miRNA biogenesis, export, and uptake was recently reviewed in depth[56, 60]. Here, we shall touch on a few recent findings that support the regulated cellular release of miRNAs.
Although the detailed mechanisms by which miRNAs are packaged and exported remains uncertain, the incorporation of miRNAs into membrane-bound vesicles and the formation of miRNA-protein/lipoprotein complexes have been shown to protect and stabilize extracellular miRNAs[56, 61–63]. The different types of miRNA-containing extracellular vesicles, including microvesicles, apoptotic bodies, and exosomes originate from distinct processes within the cell[56, 60, 61] and differ significantly in their size and their release pathway[64–70]. Apoptotic bodies are the largest of all the vesicles, ranging in size from 1 – 5 µm; apoptotic bodies are released during late apoptosis[71, 72] and originate from cellular fragments[73]. Microvesicles range in size from 100 nm – 1 µm and originate from the plasma membrane in response to various stimuli and during cellular budding or fission of the membrane[65]. Apoptotic bodies and microvesicles are jointly referred to as microparticles. A variety of cell types have been identified as being capable of producing microparticles under different stimulatory conditions, including immune cells[74], endothelial cells[75], and tumor cells[62]. Exosomes are the smallest of the extracellular vesicles, ranging in size from 10 – 50 nm. Exosomes originate from intracellular secretory organelles and are released into the extracellular space when multivesicular bodies fuse with the cell membrane[65]. The fact that all three vesicular miRNA carriers stem from three distinct pathways implies a regulated process for the sorting and subsequent export of extracellular miRNAs.
A mechanism for the sorting and export of miRNAs is also supported by in vitro data demonstrating that, within 1 h of serum deprivation, human cell lines rapidly export specific miRNAs into cell culture media via cell-derived vesicles[76], such as exosomes and microparticles. This export process was shown to be ATP-dependent, and these findings point to a potentially important miRNA trafficking system that is robust, rapid and responsive to changing extracellular conditions. Kosaka and coworkers identified an exosomal-mediated mechanism for extracellular export of miRNAs that was dependent on the ceramide pathway[56]. In cultured HEK293 and COS-7 cells, exosomal miRNA levels of miR-16 and miR-21, two miRNAs associated with angiogenic signaling[6], were proportional to their intracellular levels. This observation suggests that exosomal export of miRNAs is linked to intracellular miRNA levels.
Regulated miRNA export is further supported by the finding that disease alters the degree of miRNA association with circulating lipoproteins and miRNA enrichment in circulating microparticles. Circulating high density lipoprotein (HDL) transports miRNAs and the miRNA profile of HDL differs dramatically between individuals with familial hypercholesterolemia and healthy subjects[77]. Additionally, miRNA levels in platelet-derived microparticles isolated from the blood of patients with chronic coronary heart disease were shown to be significantly different compared to those isolated from the blood of patients with acute coronary syndrome or healthy subjects[75]. These data indicate that the cellular packaging of miRNAs into distinct extracellular transport modalities can be altered by disease[75]. Other in vivo and in vitro studies have provided further evidence that miRNA export is regulated[78, 79].
Importantly, circulating miRNAs can be taken up by recipient cells where they can subsequently suppress gene expression. Several studies have now shown that exosomes, microvesicles, apoptotic bodies, and lipoprotein particles are capable of transferring miRNA to recipient cells accompanied by a subsequent change in recipient cell function[56, 63, 65, 66, 76, 77, 80–82]. Endothelial cell-derived apoptotic bodies containing miR-126 were able to be transferred to donor cells, resulting in increased production of the CXCL 12 protein, a chemokine involved in paracrine signaling during atherosclerosis[80]. Moreover, the intravenous administration of miR-126-containing apoptotic bodies from cultured endothelial cells suppressed atherosclerotic plaque formation in mice[80]. The functional export of miRNA has also been shown for cancer cells. For example, glioblastoma-derived microvesicles containing miRNA and angiogenic proteins were taken up by endothelial cells, which subsequently resulted in the ability of these cells to form tubules[62].
While several studies have successfully demonstrated the existence of extracellular miRNAs – both in vitro and in vivo – and have provided evidence in support of the regulated release of extracellular miRNAs, there are still questions regarding the functional significance of extracellular miRNAs. Some extracellular miRNAs may be deliberately secreted by donor cells in order to be taken up by recipient target cells[83], while others may be exported by cells as a means of controlling intracellular miRNA abundance during different stimulatory conditions[76]. To decipher the potential functional roles for extracellular miRNAs, especially as it relates to a particular physiological process, namely angiogenesis, it may be useful to consider what is known about circulating miRNAs in distinct clinical populations.
Circulating Angiogenesis-Associated miRNAs in Clinical Populations
There are only a few reports directly implicating circulating or extracellular miRNAs in the regulation of angiogenesis, yet one can begin to speculate on the function of angiogenesis-associated circulating miRNAs in different clinical populations based on reported in vitro and in vivo data. For example, the expression levels of circulating miR-210 and miR-21 were shown to be higher in diffuse large B-cell lymphoma patients compared to controls[84]. Additionally, it was observed that a high serum level of miR-21 in B-cell lymphoma patients is associated with relapse-free survival[84]. This latter finding was unexpected in light of the fact that pro-angiogenic miRNAs, including miR-21, tend to promote tumor angiogenesis through their ability to modulate HIF-1α, VEGF[29] and connective tissue growth factor (CTGF) expression levels[41].
While it remains unknown why high circulating miR-21 levels correlate with better clinical prognosis in B-cell lymphoma, this observation raises several possible scenarios for the role of extracellular miRNAs in disease. Circulating/extracellular miRNAs could be released by tumor or non-tumor cells because they are part of the disease process, or because they are part of a compensatory response to the disease. For example, a potential cellular response to B-cell lymphoma could be the export of pro-angiogenic miRNAs into the circulation to lower intracellular miRNA levels. Increased vascularization indicates poor prognosis in lymphomas [85], and lymphoma growth and progression can be potentiated by autocrine stimulation of tumor cells through increased expression of VEGF and VEGF receptors[86, 87]. Moreover, high intracellular miR-21 levels (intracellular) have been observed in lymphoma tissues[88]. Since a known target of miR-21 is PTEN, a negative regulator of VEGF[29], high levels of circulating miR-21 could indicate a compensatory response by surrounding, non-tumor cells to decrease intracellular miR-21 and VEGF levels, thereby decreasing the potential for VEGF-stimulated lymphoma growth and progression. It should be noted that this discussion of the role of circulating miRNA in regard to disease-associated angiogenesis is largely conjectural.
Levels of circulating miRNAs have been identified as potential biomarkers for an array of cancers. Many of these miRNAs have been shown to have a role in regulating the angiogenesis pathway in vitro, as discussed above. Ovarian cancer patients have high circulating levels of two angiogenesis-associated miRNAs, miR-21 and miR-126[89], and gastric cancer patients have increased circulating levels of miR-17-5p and miR-21[90]. Pancreatic cancer patients are similar to gastric cancer patients in that they express high levels of a pro-angiogenic miRNA, miR-210[91]. Although the correlation between cancer prognosis and circulating miRNA levels was not reported in these studies, the finding that these pro-angiogenic miRNAs are increased in the circulation of patients with these different cancers suggests a functional role for extracellular miRNAs in the progression of both solid and hematologic malignancies. However, the mechanism responsible for changes in circulating miRNA levels in different cancers remains unknown.
Circulating/extracellular miRNAs may also be involved in the regulation of angiogenic pathways in patients with vascular disease. In a recent study of individuals with diabetes mellitus and peripheral artery disease, levels of circulating miR-126 were decreased compared to non-diabetic individuals[92]. miR-126 is an endothelial-specific miRNA[9, 10] and diabetes mellitus is a disease that is associated with an abnormal angiogenic program[93]. In order to clarify the role of circulating miRNAs in angiogenesis-related pathologies, including cancer and vascular disease, future studies will need to focus on deciphering the cellular origin of the circulating miRNAs as well as the cells that are capable of taking up miRNAs from the circulation. This information will help determine whether circulating miRNA levels are reflective of a compensatory response to disease or whether they are involved in promoting the disease process.
Current knowledge about the role of miRNAs in angiogenic signaling is based primarily on in vitro studies that describe the impact of changes in intracellular miRNA levels on endothelial cell behavior[6, 19]. In vivo, however, mounting evidence suggests that circulating/extracellular miRNAs can be taken up by a variety of cell types to modulate gene expression. Non-endothelial cell types could indirectly promote or inhibit angiogenesis signaling through their ability to regulate VEGF expression. To achieve a deeper understanding of the functional significance of circulating miRNAs in angiogenesis-related pathologies, it is necessary to consider the different cells types to which these circulating miRNAs may be targeted. The following section will examine current clinical data to discuss the mode by which miRNAs are transported in the circulation as a potential mechanism for specific targeting of recipient cells in vivo. Moreover, we will offer a new perspective on the proposed function of vesicle-encapsulated miRNAs in pathology.
Vesicular Transport of Angiogenesis-Associated miRNAs: Potential Implications in Systemic Signaling
The current understanding of circulating miRNAs is consistent with them having a role in cell-to-cell communication. It is possible that distinct miRNA transport modalities have different targeting capabilities as it pertains to angiogenesis signal transduction. For example, it was recently reported that approximately 95% of circulating miR-16 and miR-92a in the plasma of healthy subjects was non vesicle-encapsulated[63]. In contrast, miR-126, another pro-angiogenic miRNA, was found to be highly enriched in vesicles[94]. Although all three miRNAs have been shown to stimulate the angiogenic program[43, 95], the differences in their packaging could indicate distinct spatial targeting of angiogenic signals by circulating/extracellular miRNAs, i.e. localized versus systemic angiogenic signal transduction. Based on the clinical data discussed below, it is reasonable to speculate that vesicle-encapsulated miRNAs may be taken up by many different cell types and thus are part of a systemic mechanism to regulate angiogenic signaling pathway.
A role for vesicle-mediated miRNA transport in systemic signaling is supported by the molecular composition of cell-derived vesicles and the mechanisms by which these vesicles are taken up by recipient cells[96]. Several mechanisms have been offered to describe vesicle-cell interactions in vivo, the two most prominent being vesicle fusion with the cell membrane and vesicle internalization via endocytosis[96]. Because most mammalian cells are capable of endocytosis[97], a diverse array of cell types may take up circulating miRNA contained in vesicles. Proteomic studies have shown that most cell-derived vesicles contain a common set of membrane proteins[96], one that is similar to the set of proteins found on cell membranes. This common feature of cell-derived vesicles could facilitate their ability to be taken up by a variety of different cell types with minimal selectivity[98]. An example of this principle was demonstrated by Zernecke et al[80]. In vitro, endothelial cell-derived apoptotic bodies, loaded with miR-126, were shown to be successfully taken up by HUVECs and smooth muscle cells, alike, with similar effects on relative gene expression[80]. In contrast, HDL-mediated miRNA delivery was shown to be dependent on scavenger receptor class B type I, a protein that is not ubiquitously expressed by all mammalian cell types[77], suggesting a more selective/specific method for the delivery of lipoprotein-associated miRNA.
Other recent work also supports the theory that vesicle-encapsulated miRNAs are involved in the systemic regulation of angiogenesis. When miRNA enrichment in microparticles was assessed in stable CHD patients and patients with acute coronary syndrome (ACS), lower microparticle-enrichment of miR-19 and miR-21 was observed in the plasma of CHD patients compared to ACS patients. Stable CHD and ACS are both characterized by plaque formation in the coronary arteries, but ACS is the result of plaque rupture. Because increased angiogenesis is part of the process leading to plaque destabilization and rupture[95, 99], low levels of vesicle-encapsulated, pro-angiogenic miRNA in stable CHD could be a mechanism by vascular cells to decrease the level of systemic pro-angiogenic signaling. Since many aspects of circulating miRNA have yet to be elucidated, this theory is speculative.
Previous work has shown that patients with stable CHD express lower circulating levels of the pro-angiogenic miR-17, miR-92a and miR-126[58] compared to healthy subjects. It would be interesting to determine whether these differences are observed primarily in the vesicular fractions. In addition, because both healthy individuals and ACS patients appear to have relatively high circulating levels of pro-angiogenic miRNAs, it is possible that the important difference between health and disease state is the transport modality (e.g. vesicle encapsulation) of circulating miRNAs, rather than their absolute levels. Alternatively, circulating miRNAs from healthy subjects and ACS patients could differ in the way that they are taken up by recipient cells. These differences in miRNA transport or uptake could help to identify patients who are at risk for an ACS.
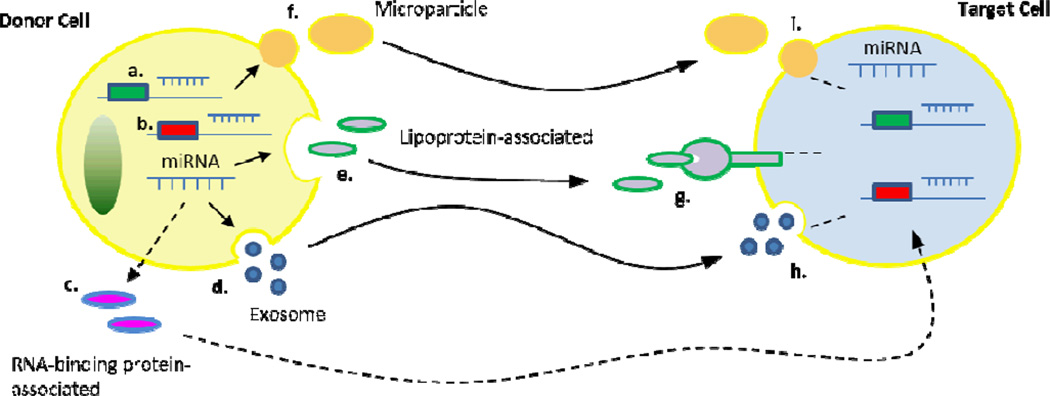
Work conducted by Zampetaki and colleagues may have provided further insight into the role of circulating miRNAs in angiogenesis. They found miR-126 to be significantly downregulated in the blood of patients with diabetes mellitus (DM)[92], a disease characterized by systemic angiogenic abnormalities[100]. Patients with DM showed a significant reduction in the level of vesicular miR-126, with little change in the level of non-vesicle-associated miR-126[92]. Decreased vesicle enrichment of miR-126 was observed prior to the manifestation of DM and was correlated with severity of DM. Moreover, in vitro experiments showed that high glucose was able to reduce the miR-126 enrichment in endothelial-cell derived apoptotic bodies. In normal health, circulating endothelial microparticles are enriched in miR-126[9, 10]. High glucose has been shown to decrease both tubule-formation of HUVECs and the expression of key angiogenesis factors, including VEGF and FGF, in human islets[101]. Taken together, these findings suggest a link between glucose levels and systemic angiogenesis signaling (both HUVECs and human islets were affected by changes in glucose abundance), one that may be mediated by changes in vesicle-encapsulated miR-126. Currently, the mechanism underlying the decreased circulating levels of pro-angiogenic miRNAs remains unknown. Future investigations will have to determine if the cellular export of these pro-angiogenic miRNAs is inhibited by disease, or whether their cellular uptake is enhanced, either of these scenarios could effectively result in decreased levels of circulating pro-angiogenic miRNA (Figure 1).
Figure 1. Schema of the proposed mechanism by which circulating miRNAs control angiogenic signaling within and between cells.
Intracellular miRNAs can bind to target mRNA sequences in the cytoplasm to posttranscriptionally regulate the expression of both a) pro-angiogenic and b) anti-angiogenic genes. Additionally, intracellular miRNAs can be packaged and exported from the cell by at least four distinct pathways: c) complexed with RNA-binding proteins; d) encapsulated in exosomes; e) bound to lipoproteins; and f) encapsulated in microvesicles and apoptotic bodies, i.e. microparticles. Angiogenic signaling can be controlled not only by the export of miRNAs but also by the uptake of extracellular miRNAs by recipient cells. Lipoprotein-associated miRNAs are taken up by recipient cells through g) a receptor-mediated mechanism, while vesicle-encapsulated miRNAs are thought to be taken up via h) the processes of endocytosis or i) fusion with the plasma membrane. The mechanism through which RNA-binding proteins transfer miRNAs to recipient cells remains unknown. By controlling intracellular levels of angiogenesis-regulatory miRNAs, via miRNA export and miRNA uptake, circulating miRNAs are likely to orchestrate both local and systemic angiogenic signaling.
While we have proposed a role for vesicle-encapsulated miRNAs in the systemic transduction of angiogenic signals, the possibility of targeted, vesicle-mediated miRNA transfer cannot be ruled out. Exosomes, for example, which are released by cells under various stimulatory conditions, express membrane proteins that vary depending on their cellular origin[96]. Endothelial-derived exosomes do not externalize phosphatidylserine (PS), a phospholipid that promotes endocytosis[98]. Rather, endothelial-derived exosomes express specific exosomal markers, including MHC class I/II[96]. Only a few specialized cells have receptors for these molecules, including T cells, and it is tempting to speculate that the incorporation of miRNAs into exosomes produced by endothelial cells is reflective of a process of specifically targeting angiogenic or other signaling pathways in specific cell types.
Concluding Remarks
A novel mechanism for intercellular communication has recently been attributed to circulating miRNAs. In this capacity, circulating miRNAs exhibit features that allow them to regulate signal transduction within and between cells. As more details regarding the regulation of miRNA release and uptake are uncovered, the roles that different extracellular miRNA transport modalities play in essential physiological processes will be identified.
The angiogenic program is involved in almost all stages of mammalian development. Despite the emergence of intracellular miRNAs as critical mediators of angiogenic signaling, the role of extracellular miRNAs in this process has yet to be elucidated. It will be important to distinguish extracellular miRNAs that have been passively released as a result of cell damage from those that have been actively secreted as part of cell-to-cell signal transduction. Moreover, the mechanism by which specific miRNAs are targeted to recipient cells remains to be clarified. Reconciliation of in vitro and animal studies with clinical data will assist in piecing together the impact of particular miRNA transport modalities on angiogenesis. In addition, because the expression levels of circulating miRNA likely represent a balance of miRNA export as well as miRNA uptake, an understanding of how both of these processes are affected by different stimulatory and disease conditions is necessary. Our understanding of signal transduction between cells will deepen as more is uncovered about the role of circulating miRNAs, and this will likely impact aspects of disease diagnostics, prognostics and treatment.
Acknowledgement
This work was supported by funding from the National Heart Lung and Blood Institute of the National Institutes of Health (HHSN268201000043C to CDS, R01 HL109559 to CDS), and a VA Merit Award (I01 BX000704 to CDS).
References
- 1.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011 May 19;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens LJ, Page-McCaw A. A Secreted MMP Is Required for Re-epithelialization during Wound Healing. Mol Biol Cell. 2012 Jan 19; [Google Scholar]
- 3.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993 Apr 29;362(6423):841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 4.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9(6):677–684. doi: 10.1038/nm0603-677. [10.1038/nm0603-677] [DOI] [PubMed] [Google Scholar]
- 5.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory Enlargement of Human Atherosclerotic Coronary Arteries. [1987/05/28];New England Journal of Medicine. 1987 316(22):1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 6.Fish JE, Srivastava D. MicroRNAs: opening a new vein in angiogenesis research. Sci Signal. 2009 Jan 6;2(52):pe1. doi: 10.1126/scisignal.252pe1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simons M. Integrative signaling in angiogenesis. Molecular and Cellular Biochemistry. 2005;264(1):99–102. doi: 10.1023/b:mcbi.0000044379.25823.03. [DOI] [PubMed] [Google Scholar]
- 8.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004 Jan 23;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S. Review] [DOI] [PubMed] [Google Scholar]
- 9.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, et al. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008 Aug;15(2):272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008 Aug;15(2):261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suarez Y, Fernandez-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007 Apr 27;100(8):1164–1173. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- 12.Poliseno L, Tuccoli A, Mariani L, Evangelista M, Citti L, Woods K, et al. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108(9):3068–3071. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- 13.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001 Oct 26;294(5543):853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 14.Lau NC, Lim LP, Weinstein EG, Bartel DP. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001 Oct 26;294(5543):858–862. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- 15.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993 Dec 3;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [Comparative Study Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S. Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 16.Griffiths-Jones S. The microRNA Registry. Nucleic Acids Research. 2004;32(suppl 1):D109–D111. doi: 10.1093/nar/gkh023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui Q, Yu Z, Purisima EO, Wang E. Principles of microRNA regulation of a human cellular signaling network. Mol Syst Biol. 2006;2 doi: 10.1038/msb4100089. [10.1038/msb4100089] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashita Y, Osada H, Tatematsu Y, Yamada H, Yanagisawa K, Tomida S, et al. A Polycistronic MicroRNA Cluster, miR-17-92, Is Overexpressed in Human Lung Cancers and Enhances Cell Proliferation. Cancer Research. 2005;65(21):9628–9632. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- 19.Wu F, Yang Z, Li G. Role of specific microRNAs for endothelial function and angiogenesis. Biochemical and Biophysical Research Communications. 2009;386(4):549–553. doi: 10.1016/j.bbrc.2009.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Formenti F, Constantin-Teodosiu D, Emmanuel Y, Cheeseman J, Dorrington KL, Edwards LM, et al. Regulation of human metabolism by hypoxia-inducible factor. Proceedings of the National Academy of Sciences. 2010;107(28):12722–12727. doi: 10.1073/pnas.1002339107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu N, Zhang D, Xie H, Zhou Z, Chen H, Hu T, et al. Endothelial-specific intron-derived miR-126 is down-regulated in human breast cancer and targets both VEGFA and PIK3R2. Molecular and Cellular Biochemistry. 2011;351(1):157–164. doi: 10.1007/s11010-011-0723-7. [DOI] [PubMed] [Google Scholar]
- 22.Funasaka T, Haga A, Raz A, Nagase H. Autocrine motility factor secreted by tumor cells upregulates vascular endothelial growth factor receptor (Flt-1) expression in endothelial cells. Int J Cancer. 2002 Sep 20;101(3):217–223. doi: 10.1002/ijc.10617. [DOI] [PubMed] [Google Scholar]
- 23.Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007 Jul 6;101(1):59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- 24.Suarez Y, Fernandez-Hernando C, Yu J, Gerber SA, Harrison KD, Pober JS, et al. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc Natl Acad Sci U S A. 2008 Sep 16;105(37):14082–14087. doi: 10.1073/pnas.0804597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang WJ, Yang DD, Na S, Sandusky GE, Zhang Q, Zhao G. Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem. 2005 Mar 11;280(10):9330–9335. doi: 10.1074/jbc.M413394200. [DOI] [PubMed] [Google Scholar]
- 26.Otsuka M, Zheng M, Hayashi M, Lee J-D, Yoshino O, Lin S, et al. Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. The Journal of Clinical Investigation. 2008;118(5):1944–1954. doi: 10.1172/JCI33680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shilo S, Roy S, Khanna S, Sen CK. Evidence for the Involvement of miRNA in Redox Regulated Angiogenic Response of Human Microvascular Endothelial Cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(3):471–477. doi: 10.1161/ATVBAHA.107.160655. [DOI] [PubMed] [Google Scholar]
- 28.Shilo S, Roy S, Khanna S, Sen CK. Evidence for the involvement of miRNA in redox regulated angiogenic response of human microvascular endothelial cells. Arterioscler Thromb Vasc Biol. 2008 Mar;28(3):471–477. doi: 10.1161/ATVBAHA.107.160655. [DOI] [PubMed] [Google Scholar]
- 29.Liu L-Z, Li C, Chen Q, Jing Y, Carpenter R, Jiang Y, et al. MiR-21 Induced Angiogenesis through AKT and ERK Activation and HIF-1α Expression. PLoS One. 2011;6(4):e19139. doi: 10.1371/journal.pone.0019139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doebele C, Bonauer A, Fischer A, Scholz A, Reiss Y, Urbich C, et al. Members of the microRNA-17-92 cluster exhibit a cell-intrinsic antiangiogenic function in endothelial cells. Blood. 2010 Jun 10;115(23):4944–4950. doi: 10.1182/blood-2010-01-264812. [DOI] [PubMed] [Google Scholar]
- 31.O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005 Jun 9;435(7043):839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 32.Biyashev D, Qin G. E2F and microRNA regulation of angiogenesis. Am J Cardiovasc Dis. 2011;1(2):110–118. [PMC free article] [PubMed] [Google Scholar]
- 33.Rocha S. Gene regulation under low oxygen: holding your breath for transcription. Trends Biochem Sci. 2007 Aug;32(8):389–397. doi: 10.1016/j.tibs.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Fasanaro P, D'Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, et al. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008 Jun 6;283(23):15878–15883. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, et al. A microRNA signature of hypoxia. Mol Cell Biol. 2007 Mar;27(5):1859–1867. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke. 2008 Mar;39(3):959–966. doi: 10.1161/STROKEAHA.107.500736. [DOI] [PubMed] [Google Scholar]
- 37.Pulkkinen K, Malm T, Turunen M, Koistinaho J, Yla-Herttuala S. Hypoxia induces microRNA miR-210 in vitro and in vivo ephrin-A3 and neuronal pentraxin 1 are potentially regulated by miR-210. FEBS Lett. 2008 Jul 9;582(16):2397–2401. doi: 10.1016/j.febslet.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 38.Roy S, Sen CK. miRNA in wound inflammation and angiogenesis. Microcirculation. 2011 doi: 10.1111/j.1549-8719.2011.00156.x. [10.1111/j.1549-8719.2011.00156.x] no-no. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ambros V. The functions of animal microRNAs. Nature. 2004 Sep 16;431(7006):350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 40.Chung AS, Ferrara N. Developmental and Pathological Angiogenesis. [2011/11/10];Annual Review of Cell and Developmental Biology. 2011 27(1):563–584. doi: 10.1146/annurev-cellbio-092910-154002. [DOI] [PubMed] [Google Scholar]
- 41.Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E, et al. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet. 2006 Sep;38(9):1060–1065. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hicklin DJ, Ellis LM. Role of the Vascular Endothelial Growth Factor Pathway in Tumor Growth and Angiogenesis. Journal of Clinical Oncology. 2005;23(5):1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 43.Hua Z, Lv Q, Ye W, Wong CK, Cai G, Gu D, et al. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS One. 2006;1:e116. doi: 10.1371/journal.pone.0000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000 Jan 7;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt T, Carmeliet P. Angiogenesis: a target in solid tumors, also in leukemia? Hematology Am Soc Hematol Educ Program. 2011;2011:1–8. doi: 10.1182/asheducation-2011.1.1. [DOI] [PubMed] [Google Scholar]
- 46.Hatfield K, Ryningen A, Corbascio M, Bruserud O. Microvascular endothelial cells increase proliferation and inhibit apoptosis of native human acute myelogenous leukemia blasts. Int J Cancer. 2006 Nov 15;119(10):2313–2321. doi: 10.1002/ijc.22180. [DOI] [PubMed] [Google Scholar]
- 47.Molica S, Vacca A, Ribatti D, Cuneo A, Cavazzini F, Levato D, et al. Prognostic value of enhanced bone marrow angiogenesis in early B-cell chronic lymphocytic leukemia. Blood. 2002 Nov 1;100(9):3344–3351. doi: 10.1182/blood-2002-01-0084. [DOI] [PubMed] [Google Scholar]
- 48.Perez-Atayde AR, Sallan SE, Tedrow U, Connors S, Allred E, Folkman J. Spectrum of tumor angiogenesis in the bone marrow of children with acute lymphoblastic leukemia. Am J Pathol. 1997 Mar;150(3):815–821. [PMC free article] [PubMed] [Google Scholar]
- 49.Hussong JW, Rodgers GM, Shami PJ. Evidence of increased angiogenesis in patients with acute myeloid leukemia. Blood. 2000 Jan 1;95(1):309–313. [PubMed] [Google Scholar]
- 50.Hanlon K, Rudin CE, Harries LW. Investigating the targets of MIR-15a and MIR-16-1 in patients with chronic lymphocytic leukemia (CLL) PLoS One. 2009;4(9):e7169. doi: 10.1371/journal.pone.0007169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garzon R, Garofalo M, Martelli MP, Briesewitz R, Wang L, Fernandez-Cymering C, et al. Distinctive microRNA signature of acute myeloid leukemia bearing cytoplasmic mutated nucleophosmin. Proc Natl Acad Sci U S A. 2008 Mar 11;105(10):3945–3950. doi: 10.1073/pnas.0800135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ha TY. MicroRNAs in Human Diseases: From Cancer to Cardiovascular Disease. Immune Netw. 2011 Jun;11(3):135–154. doi: 10.4110/in.2011.11.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suarez Y, Fernandez-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circulation research. 2007 Apr 27;100(8):1164–1173. doi: 10.1161/01.RES.0000265065.26744.17. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 54.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002 Nov 26;99(24):15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laterza OF, Lim L, Garrett-Engele PW, Vlasakova K, Muniappa N, Tanaka WK, et al. Plasma MicroRNAs as sensitive and specific biomarkers of tissue injury. Clin Chem. 2009 Nov;55(11):1977–1983. doi: 10.1373/clinchem.2009.131797. [DOI] [PubMed] [Google Scholar]
- 56.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010 Jun 4;285(23):17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dimmeler S, Zeiher AM. Circulating microRNAs: novel biomarkers for cardiovascular diseases? Eur Heart J. 2010 Nov;31(22):2705–2707. doi: 10.1093/eurheartj/ehq221. [DOI] [PubMed] [Google Scholar]
- 58.Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010 Sep 3;107(5):677–684. doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 59.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008 Jul 29;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu H, Fan GC. Extracellular/circulating microRNAs and their potential role in cardiovascular disease. Am J Cardiovasc Dis. 2011 Jul 30;1(2):138–149. [PMC free article] [PubMed] [Google Scholar]
- 61.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007 Jun;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 62.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Curry WT, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [10.1038/ncb1800] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011 Mar 22;108(12):5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anderson HC, Mulhall D, Garimella R. Role of extracellular membrane vesicles in the pathogenesis of various diseases, including cancer, renal diseases, atherosclerosis, and arthritis. Lab Invest. 2010 Nov;90(11):1549–1557. doi: 10.1038/labinvest.2010.152. [DOI] [PubMed] [Google Scholar]
- 65.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010 Nov;78(9):838–848. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 66.Jy W, Horstman LL, Ahn YS. Microparticle size and its relation to composition, functional activity, and clinical significance. Semin Thromb Hemost. 2010 Nov;36(8):876–880. doi: 10.1055/s-0030-1267041. [DOI] [PubMed] [Google Scholar]
- 67.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010 Sep 10;73(10):1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 68.Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol. 2011 May 15;81(10):1171–1182. doi: 10.1016/j.bcp.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 69.Silverman JM, Reiner NE. Exosomes and other microvesicles in infection biology: organelles with unanticipated phenotypes. Cell Microbiol. 2011 Jan;13(1):1–9. doi: 10.1111/j.1462-5822.2010.01537.x. [DOI] [PubMed] [Google Scholar]
- 70.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009 Aug;9(8):581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 71.Dini L, Lentini A, Diez GD, Rocha M, Falasca L, Serafino L, et al. Phagocytosis of apoptotic bodies by liver endothelial cells. J Cell Sci. 1995 Mar;108(Pt 3):967–973. doi: 10.1242/jcs.108.3.967. [DOI] [PubMed] [Google Scholar]
- 72.Savill J, Fadok V, Henson P, Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993 Mar;14(3):131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- 73.Zhang J, Reedy MC, Hannun YA, Obeid LM. Inhibition of Caspases Inhibits the Release of Apoptotic Bodies: Bcl-2 Inhibits the Initiation of Formation of Apoptotic Bodies in Chemotherapeutic Agent-induced Apoptosis. The Journal of Cell Biology. 1999;145(1):99–108. doi: 10.1083/jcb.145.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Köppler B, Cohen C, Schlöndorff D, Mack M. Differential mechanisms of microparticle transfer toB cells and monocytes: anti-inflammatory propertiesof microparticles. European Journal of Immunology. 2006;36(3):648–660. doi: 10.1002/eji.200535435. [10.1002/eji.200535435] [DOI] [PubMed] [Google Scholar]
- 75.Diehl P, Fricke A, Sander L, Stamm J, Bassler N, Htun N, et al. Microparticles: major transport vehicles for distinct miRNAs in circulation. Cardiovascular research. 2012 Jan 18; doi: 10.1093/cvr/cvs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010 Nov;38(20):7248–7259. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011 Apr;13(4):423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ohshima K, Inoue K, Fujiwara A, Hatakeyama K, Kanto K, Watanabe Y, et al. Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS One. 2010;5(10):e13247. doi: 10.1371/journal.pone.0013247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pigati L, Yaddanapudi SC, Iyengar R, Kim DJ, Hearn SA, Danforth D, et al. Selective release of microRNA species from normal and malignant mammary epithelial cells. PLoS One. 2010;5(10):e13515. doi: 10.1371/journal.pone.0013515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009 Dec 8;2(100):ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 81.Eldh M, Ekstrom K, Valadi H, Sjostrand M, Olsson B, Jernas M, et al. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One. 2010;5(12):e15353. doi: 10.1371/journal.pone.0015353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hristov M, Erl W, Linder S, Weber PC. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood. 2004 Nov 1;104(9):2761–2766. doi: 10.1182/blood-2003-10-3614. [DOI] [PubMed] [Google Scholar]
- 83.Chen X, Liang H, Zhang J, Zen K, Zhang C-Y. Secreted microRNAs: a new form of intercellular communication. Trends in Cell Biology. (0) doi: 10.1016/j.tcb.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 84.Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K, et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol. 2008 May;141(5):672–675. doi: 10.1111/j.1365-2141.2008.07077.x. [DOI] [PubMed] [Google Scholar]
- 85.Farinha P, Kyle AH, Minchinton AI, Connors JM, Karsan A, Gascoyne RD. Vascularization predicts overall survival and risk of transformation in follicular lymphoma. Haematologica. 2010 Dec;95(12):2157–2160. doi: 10.3324/haematol.2009.021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ruan J, Hajjar K, Rafii S, Leonard JP. Angiogenesis and antiangiogenic therapy in non-Hodgkin's lymphoma. Ann Oncol. 2009 Mar;20(3):413–424. doi: 10.1093/annonc/mdn666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Medinger M, Fischer N, Tzankov A. Vascular endothelial growth factor-related pathways in hemato-lymphoid malignancies. J Oncol. 2010;2010:729725. doi: 10.1155/2010/729725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lawrie CH, Soneji S, Marafioti T, Cooper CD, Palazzo S, Paterson JC, et al. MicroRNA expression distinguishes between germinal center B cell-like and activated B cell-like subtypes of diffuse large B cell lymphoma. Int J Cancer. 2007 Sep 1;121(5):1156–1161. doi: 10.1002/ijc.22800. [DOI] [PubMed] [Google Scholar]
- 89.Resnick KE, Alder H, Hagan JP, Richardson DL, Croce CM, Cohn DE. The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol Oncol. 2009 Jan;112(1):55–59. doi: 10.1016/j.ygyno.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 90.Tsujiura M, Ichikawa D, Komatsu S, Shiozaki A, Takeshita H, Kosuga T, et al. Circulating microRNAs in plasma of patients with gastric cancers. Br J Cancer. 2010;102(7):1174–1179. doi: 10.1038/sj.bjc.6605608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ho AS, Huang X, Cao H, Christman-Skieller C, Bennewith K, Le QT, et al. Circulating miR-210 as a Novel Hypoxia Marker in Pancreatic Cancer. Transl Oncol. 2010 Apr;3(2):109–113. doi: 10.1593/tlo.09256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010 Sep 17;107(6):810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- 93.Abaci A, Kahraman S, Eryol NK, Arinç H, Ergin A. Effect of Diabetes Mellitus on Formation of Coronary Collateral Vessels. Circulation. 1999 1999 May 4;99(17):2239–2242. doi: 10.1161/01.cir.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 94.Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One. 2008;3(11):e3694. doi: 10.1371/journal.pone.0003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haver VG, Slart RHJA, Zeebregts CJ, Peppelenbosch MP, Tio RA. Rupture of Vulnerable Atherosclerotic Plaques: MicroRNAs Conducting the Orchestra? Trends in Cardiovascular Medicine. 2010;20(2):65–71. doi: 10.1016/j.tcm.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 96.Simpson RJ, Jensen SS, Lim JWE. Proteomic profiling of exosomes: Current perspectives. PROTEOMICS. 2008;8(19):4083–4099. doi: 10.1002/pmic.200800109. [10.1002/pmic.200800109] [DOI] [PubMed] [Google Scholar]
- 97.Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiological Reviews. 1997;77(3):759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 98.Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol. 2011 Jan;31(1):27–33. doi: 10.1161/ATVBAHA.110.218123. [DOI] [PubMed] [Google Scholar]
- 99.Scalbert E, Bril A. Implication of microRNAs in the cardiovascular system. Current Opinion in Pharmacology. 2008;8(2):181–188. doi: 10.1016/j.coph.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 100.Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Medicinal Research Reviews. 2003;23(2):117–145. doi: 10.1002/med.10024. [10.1002/med.10024] [DOI] [PubMed] [Google Scholar]
- 101.Dubois S, Madec AM, Mesnier A, Armanet M, CENRhikh K, Berney T, et al. Glucose inhibits angiogenesis of isolated human pancreatic islets. J Mol Endocrinol. 2010 Aug;45(2):99–105. doi: 10.1677/JME-10-0020. [DOI] [PubMed] [Google Scholar]