Abstract
Optical disector counting is currently applied most often to cryosections, followed in frequency by resin-embedded tissues, paraffin, and vibratome sections. The preservation quality of these embedding options differs considerably; yet, the effect of tissue morphology on numerical estimates is unknown. We tested whether different embedding media significantly influence numerical estimates in optical disector counting, using the previously calibrated trochlear motor nucleus of hatchling chickens. Animals were perfusion-fixed with paraformaldehyde (PFA) only or in addition with glutaraldehyde (GA), or by Methacarn immersion fixation. Brains were prepared for paraffin, cryo-, vibratome- or celloidin sectioning. Complete penetration of the thionin stain was verified by z-axis analysis. Neuronal nuclei were counted using an unbiased counting rule, numbers were averaged for each group and compared by ANOVA. In paraffin sections, 906 ± 12 (SEM) neurons were counted, similar to previous calibrated data series, and results obtained from fixation with Methacarn or PFA were statistically indistinguishable. In celloidin sections, 912 ± 28 neurons were counted—not statistically different from paraffin. In cryosections, 812 ± 12 neurons were counted (underestimate of 10.4%) when fixed with PFA only, but 867 ± 17 neurons were counted when fixed with PFA and GA. Vibratome sections had the most serious aberration with 729 ± 31 neurons—a deficit of 20%. Thus, our analysis shows that PFA-fixed cryosections and vibratome sections result in a substantial numerical deficit. The addition of GA to the PFA fixative significantly improved counts in cryosections. These results may explain, in part, the significant numerical differences reported from different labs and should help investigators select optimal conditions for quantitative morphological studies.
Keywords: stereology, paraffin, celloidin, morphology, histology, particle counting, bias
Introduction
Quantitative morphology is important in developmental and clinical biology as well as in the biology of aging. To quantify neurodegeneration, or to evaluate the effects of mechanical or pharmacological manipulations in animals, it is important to measure the extent of cell survival. To determine absolute numbers or changes in particle number in tissues, such tissues generally have to be sectioned and examined under the microscope.
Previously used quantification techniques such as “profile counting” (2D counting, counting of profiles of each particle in equally spaced thin sections, Abercrombie, 1946; Clarke and Oppenheim, 1995; Konigsmark, 1970) are now increasingly replaced by techniques that cut relatively thick sections, count samples in 3D space, and then apply densities from representative samples to the total reference space (Gundersen et al., 1988; Howard and Reed, 1998; Williams and Rakic, 1988). This approach uniquely identifies each particle and abolishes the necessity of counting profiles and using correction formulae to adjust for over-counts of multiple profiles. It is most efficient to use “optical sections” that define a counting box within thick tissue sections (hence the name “optical disector”). The optical disector has been applied to all major types of tissue sections, including paraffin, resin, vibratome, and cryosections (Gardella et al., 2003). Cryosections are currently the type of tissue sections used most often in optical disector counting (Baryshnikova et al., 2006).
Although numerical estimates obtained by optical disector counting were initially thought to be—theoretically—unbiased, major numerical discrepancies between optical disector studies have been reported (Guillery and Herrup, 1997; Mouton, 2002; Myers et al., 2004; Schmitz et al., 1999; Tandrup, 2004). Several sources of bias appear to contribute to these discrepancies (Geuna, 2005; Guillery, 2002; Guillery and August, 2002; Mouton, 2002; Schmitz and Hof, 2005). Some biases may be explained by z-axis compression and loss of particles during tissue section processing (Baryshnikova et al., 2006; Gardella et al., 2003; Hatton and von Bartheld, 1999), but this source of bias alone cannot explain the entire extent of discrepancies between studies.
It is known that different tissue embedding and processing methods result in varying preservation of tissue qualities and such quality differences are clinically significant (Medeiros et al., 2005; Pinto et al., 2001; Prieto et al., 2003; Taylor, 2001; Wootipoom et al., 2006). For example, Methacarn (acid) fixation and subsequent paraffin embedding and sectioning produce superior resolution of particles when compared with vibratome or cryosections (Aldana Marcos et al., 1996; Durham et al., 1981). Disadvantages of frozen sections include poor preservation of morphology, poor resolution at higher magnifications, and difficulty in cutting over paraffin sections. Vibratome sectioning is slow and difficult with soft and poorly fixed tissues, and chatter marks or vibratome lines are often apparent in the sections. While preservation of tissue morphology is difficult to define and quantify, differences in tissue preservation between tissue processing conditions are commonly agreed upon among investigators and these include staining properties, the degree of resolution, preservation of membranes, and three-dimensional resolution when focusing through densely stacked particles in the z-axis of tissue sections.
To date, the four most commonly used types of tissue sections have not been examined for their possible influence on numerical estimates derived from optical disector counting. Here, we provide a detailed analysis of different tissue processing conditions including paraffin sections fixed with Methacarn or 4% paraformaldehyde (PFA), cryosections fixed with 4% PFA or with PFA and 0.1% glutaraldehyde (GA), vibratome sections fixed with 4% PFA or with PFA and 0.1% GA, and celloidin plastic sections of tissue that was fixed with 4% PFA. Neuron counts of the chicken trochlear nucleus were compared with neuron numbers previously established and calibrated by complete 3D reconstruction of serial sections (Hatton and von Bartheld, 1999) and with previously established nerve fiber counts in the trochlear nerve of the posthatch chicken (Croes et al., 2007; Sohal et al., 1985). Our study reveals a substan tial (10–20%) underestimate of actual neuronal num bers when optical disector counting rules are applied to cryo- or vibratome sections that were prepared by conventional tissue processing protocols. Preliminary data of this work have been presented in abstract form (Ward et al., 2007).
Materials and Methods
Sources of Tissue
Fertilized White Leghorn chicken eggs were ob tained from local sources and incubated at 37.58C until hatching. All procedures were conducted in accordance with the Policies on the Use of Animals and Humans in Neuroscience Research (1995), and animal protocols were approved by the local animal care committees. Hatchling chicks were killed at 12–36 h post hatch (P0.5–P1.5). For Methacarn fixation, hatchlings were decapitated and heads were fixed in three changes of Methacarn (methanol, chloroform, and acetic acid at volume ratios of 6:3:1). For PFA fixation, hatchlings were deeply anesthetized with sodium pentobarbital (Nembutal, 50 mg/kg body weight) and perfused through the left cardiac ventricle with cold 4% PFA in phosphate-buffered saline (PBS, pH 7.5) or with cold 4% PFA and 0.1% GA (EM-grade, Ted Pella) (PFA + GA in PBS, pH 7.5). PFA-fixed brains were removed from the cranium and stored in the same fixative over night at room temperature. For numbers of nuclei per condition see Table 1. The following protocols were designed to produce tissue sections with a final thick ness of about 20–30 lm. For all conditions, serial sec tions were generated in the same (transverse) plane.
Table 1. Quantitative data of samples, statistics, and ranges of numerical data.
| Conditions | Trochlear nuclei (number) | Average number of sections (range) | Nominal section thickness (μm) | Average section thickness (μm) | Average number of neurons (range) | Difference of neuron numbers vs. combined paraffin: 906 (P-values) |
|---|---|---|---|---|---|---|
| Paraffin | ||||||
| Methacarn | 6 | 14 (13–14) | 35 | 31.7 (29.8–33.9) | 904 (856–942) | −0.2% (0.937) |
| PFA | 3 | 10 (10–11) | 35 | 24.4 (14–35.5) | 909 (876–960) | +0.3% (0.906) |
| Cryosection | ||||||
| PFA | 8 | 9 (9–10) | 60 | 21.7 (15.1–30.6) | 812 (762–861) | −10.4% (<0.001) |
| PFA + GA | 5 | 9 (8–9) | 60 | 30 (22.8–39.3) | 867 (818–917) | −4.3% (0.088) |
| Vibratome | ||||||
| PFA | 6 | 8 (7–9) | 70 | 17.3 (11–26.9) | 747 (614–836) | −17.5% (<0.001) |
| PFA + GA | 3 | 8 (8–8) | 70 | 14.9 (12.1–17.4) | 672 (670–674) | −25.8% (<0.001) |
| Celloidin | ||||||
| PFA | 4 | 13 (11–13) | 30 | 26.2 (22.4–32.5) | 912 (853–981) | +0.7% (0.815) |
PFA, 4% paraformaldehyde; GA, 0.1% glutaraldehyde.
Paraffin Sections
The PFA-fixed brains were stored overnight in 70% ethanol, dehydrated in 70, 95, and 100% ethanol, and cleared in two changes of 100% methylsalicylate (Sigma). Methacarn-fixed brains were stored in Methacarn fixative overnight and dehydrated in two changes of methanol and cleared in two changes of 100% xylene. All tissues were then placed in two changes of 100% paraffin at 64–68°C (Paraplast Plus, Oxford Labware, St. Louis, MO; melting point, 56°C) and embedded in fresh paraffin. Blocks were allowed to cool down at room temperature before storage at 4–8°C. Blocks were warmed to room temperature and sectioned at 35 μm on a rotary microtome (AO Spencer 820) using dispos able metal blades (Accu-Edge®, Sakura Finetek, Torrance, CA; low profile microtome blades). Sections were floated for 20–40 s in a distilled water bath at 43°C, and serial sections were collected on silane-coated glass slides. Sections were dried (20–60 min) at room temperature and baked at 40–45°C overnight. They were deparaffinized in xylene (20 min), hydrated in a graded ethanol series (1–3 min each), and stained for 20– 25 min with 0.03% thionin (Sigma, dissolved in water). Sections were then dehydrated in a graded ethanol series and cleared in two changes of 100% xylene for 5 min each. Sections were coverslipped with Corning (11/2, 170 lm thick) cover glasses in di-n-butyl-phthalate-xylene (DPX) mounting medium with a refractive index of 1.52 (Electron Microscopy Sciences, Fort Washington, PA).
Cryosections
Brains fixed with 4% PFA (or 4% PFA and 0.1% GA) were sunk overnight in 30% sucrose. Brains were embedded in Tissue-Tek® OCT compound (Sakura Finetek, Torrance, CA), frozen on dry ice, and cut at 60 μm (Leica CM 3500) using disposable metal blades (Accu-Edge). Serial sections were cut at a temperature of −20°C, thawed on gelatin-subbed slides, and dried at room temperature. Sections were stained for 20– 25 min with 0.03% thionin (Sigma, dissolved in water), dehydrated in a graded ethanol series, and cleared in two changes of 100% xylene for 5 min each. Sections were then coverslipped with Corning (11/2, 170 μm thick) cover glasses in DPX mounting medium.
Vibratome Sections
PFA-fixed brains (or PFA + GA-fixed brains) were glued to a metal chuck with super glue plus™ (Kwik Fix). The right ventral midbrain was stabbed with a needle to generate a needle track that aided in the identification of the right and left sides in case of section flipping during subsequent processing steps. The chuck was mounted on a vibratome (Series 1000, The Vibratome Company, St. Louis, MO), the chamber was filled with PBS, and serial sections were cut with Personna (American Safety Razor Company) platinum chrome blades. The settings on the vibratome were for a speed of 4.5 (maximal range 1–10), an amplitude of 5.0 (maximal range 1–10), and a nominal section thickness of 70 μm. Floating sections were collected on gelatin-subbed slides and dried overnight. Sections were stained for 20–25 min with 0.03% thionin (Sigma, dissolved in water), dehydrated in a graded ethanol series and cleared in two changes of 100% xylene for 5 min each. Sections were then coverslipped with Corning (11/2, 170 μm thick) cover glasses in DPX mounting medium.
Celloidin Sections
Brains were embedded in celloidin as described in detail elsewhere (Williams et al., 2003). In brief, PFA-fixed midbrains were postfixed for several weeks before embedding. Following washes in distilled water and dehydration with graded ethanols, they were immersed in a 1:1 solution of 100% ethanol and ether before embedding for 1 week in a 3% solution of celloidin (Fisher Scientific) and a 1:1 solution of 100% ethanol and ethyl ether, followed by 12% celloidin for 3 days. One corner of the celloidin block was nicked for orientation to prevent right–left reversal of sections. After hardening, the blocked brains were sectioned on a sliding microtome (Leica Microsystems) at 30 μm. Serial sections were stored in 80% ethanol for 1–2 weeks and stained free-floating with 0.5% thionin for 3 min. After dehydration in graded alcohols, the sections were stored in terpineol for 1–5 days. Sections were cleared in xylene and mounted in order on Fisher-Brand (Fisher Scientific, Chicago, IL) slides (50 × 75 mm2) and coverslipped with Permount (Fisher Scientific, refractive index [dry] = 1.529).
z-Axis Analysis
A z-axis distribution analysis was performed on each series of sections to verify complete stain penetration (Gardella et al., 2003). In short, the position of 200–300 particles was measured relative to the section thickness within the view field. Measurements within the z-axis were converted into percentiles (because of slight differences in section thickness) and graphed in 10 or 20% z-axis bins. Any cases with substantial and progressive loss of counts in the bins adjacent to the glass slide (indicative of incomplete stain penetration) were excluded from further evaluation. For counting par ticles, centers of nuclei (rather than tops or bottoms of particles) were scored (Heller et al., 2001), to avoid any over- or undercount in the bins adjacent to the surface at the top or bottom of the tissue section.
Neuron Counts and Analysis in Serial Sections
Serial sections were collected from the midbrain at the level of the trochlear motor nuclei. Only complete serial sections through the entire trochlear nucleus with verified complete stain penetration were quantified. Incomplete stain penetration was rare (4 of 39 cases). Sections were viewed using a 100× immersion oil objective (NA 1.25) on a Nikon Optiphot microscope equipped with a microcator (MFC-1 focus controller and DRV-1-OPTI drive) producing a practical resolution of 0.4 μm. A modified optical disector method described by Hatton and von Bartheld (1999) was used to count the nucleus of every motor neuron in the trochlear nucleus that met the following inclusion criteria. Every nucleus with a diameter larger than 6 μm falling within the counting frame and not intersecting the exclusion line was counted using an unbiased counting rule (Gundersen et al., 1988; Howard and Reed, 1998). The same investigator (T.S.W.) analyzed all serial sections to eliminate interobserver variability. Parameters measured for statistical analyses included the mean, SEM, and differences between groups by ANOVA, degrees of freedom (F-values), and measurement of statistical significance with P values of <0.05, using Sigma Stat software.
Results
Different processing and sectioning methods yield morphological quality differences of tissue sections. To determine whether fixation, embedding, and sectioning choices affect total neuron estimates obtained by optical disector counting, we made total neuron counts within the trochlear nucleus of 1-day-old (P1) hatchling chicks that were processed and sectioned using six different protocols. The trochlear nucleus of the hatchling chick is a compact and well-defined nucleus located dorsomedially in the midbrain tegmentum. This nucleus contains relatively large neurons with coarse Nissl bodies (Figs. 1A–1F). For all cases, z-axis analyses of sections were performed to provide an objective measure of stain penetration, since incomplete stain penetration could compromise recognition and thus accurate estimation of numbers of trochlear neurons. Representative graphs of z-axis distributions for all major conditions are summarized in Figures 2A–2F. These graphs demonstrate adequate stain penetration and verify previous reports of symmetric z-axis distributions (Baryshnikova et al., 2006; Gardella et al., 2003; Hatton and von Bartheld, 1999), and thus indicate retention of particles at section surfaces (Baryshnikova et al., 2006).
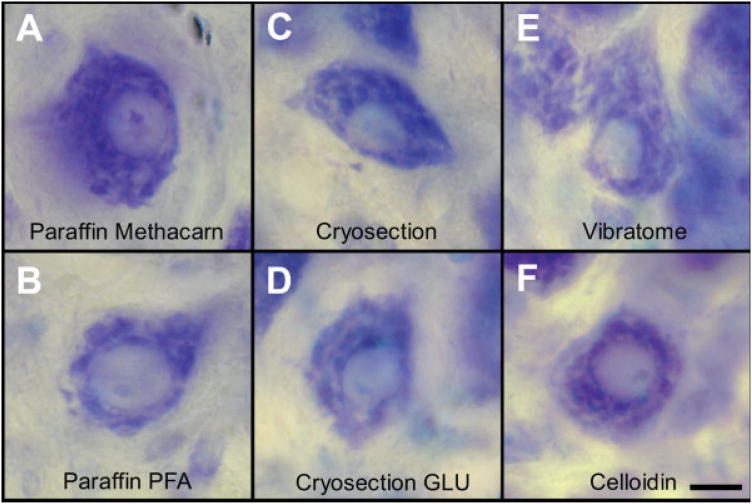
Fig. 1.
(A–F) Representative photomicrographs show tissue sections of trochlear motoneurons from hatchling chickens for six different tissue processing (fixation, embedding, and sectioning) protocols. All sections were stained with thionin and images were obtained with a Nikon coolpix camera. (A) Paraffin section after fixation with Methacarn. (B) Paraffin section after fixation with 4% paraformaldehyde. (C) Cryosection after fixation with paraformaldehyde. (D) Cryosection after fixation with paraformaldehyde and glutaraldehyde (GLU). (E) Vibratome section after fixation with paraformaldehyde. (F) Celloidin section after fixation with paraformaldehyde. The bar (10 μm) in panel F applies to all panels. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
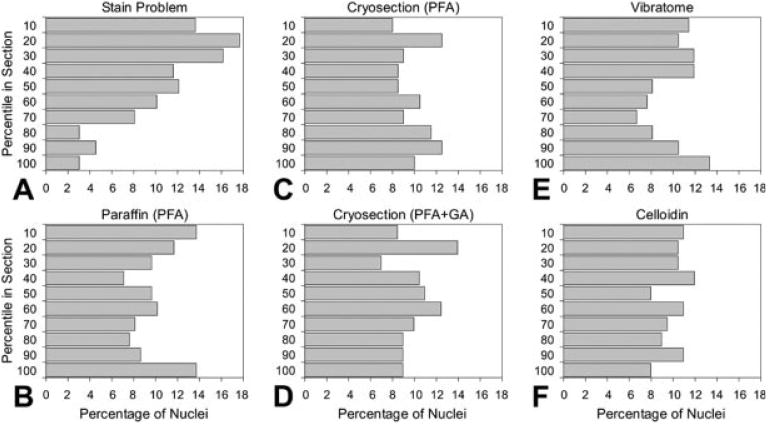
Fig. 2.
(A–F) Examples of z-axis distributions to verify complete stain penetration and lack of “lost caps.” The distribution of a total of 200–300 particles (centers of neuronal nuclei) was plotted. (A) Incomplete stain penetration (example of a rare case that was excluded from analysis). (B) Paraffin section with paraformaldehyde (PFA) fixation. Paraffin sections with Methacarn fixation had the same z-axis distribution pattern, but are not shown here, since these were previously published (Hatton and von Bartheld, 1999; Gardella et al., 2003). (C) Cryosection with paraformaldehyde fixation. (D) Cryosection with additional glutaraldehyde (GA) fixation. (E) Vibratome section. (F) Celloidin section. Note that none of the sections in B–F indicate any major loss of particles from the section surfaces (surface bins).
Paraffin Sections
The trochlear nucleus was previously reconstructed from serial sections in a P1 hatchling chick and this “gold standard” yielded, on one side in one individual animal, 922 trochlear motor neurons (Hatton and von Bartheld, 1999). To replicate these findings and to determine interanimal variability in the number of trochlear motoneurons, we first counted motor neurons in six trochlear nuclei that were fixed with Methacarn and embedded in paraffin, as in the previous study (Hatton and von Bartheld, 1999). Using the unbiased counting rule of the optical disector (Gundersen et al., 1988; Howard and Reed, 1998), we then counted each trochlear motoneuron in serial sections (range of the number of sections for each trochlear nucleus is 13– 14). For this study, we did not sample and did not apply the average sample density to the volume of the trochlear nucleus, to avoid any variability introduced by defining and measuring the precise reference volume. This approach makes the resulting numbers entirely a reflection of the ability to identify trochlear motoneurons and of true interanimal variability, but renders the study “immune” to any variability in identification of the precise borders of the reference volume or errors in measuring section thickness (Guillery, 2002; Guillery and August, 2002). This approach is possible in a small nucleus such as the chick trochlear nucleus, because the number of neurons is relatively small (<1,000 total).
Methacarn fixation produces high-quality tissue sections with excellent histological detail, as shown in Figure 1A. The average final paraffin section thickness was 31.7 μm. z-Axis analyses showed complete stain penetration (Fig. 2B). Motoneurons were readily identified based on the location of the cells within the compact trochlear nucleus, the large cell size, characteristic large Nissl bodies, and distinct and relatively large nucleoli. Total neuron counts from serial sections of paraffin-embedded trochlear nuclei fixed with Methacarn (n = 6) were 904 ± 15 (SEM). Counts ranged from 856 to 942 with the extreme values within 5.4% of the average (904). For details of numerical values see Table 1. There was no statistically significant difference between numbers from the right and the left side of the brain. This is important because the right side of the embryo is typically situated upright in the egg and is more exposed to light, which leads to some right–left asymmetries in the avian visual system (Gunturkun, 1997).
To determine how Methacarn fixation compares with PFA fixation, we counted three additional trochlear nuclei in serial paraffin sections from PFA-perfused animals. The morphology of PFA-fixed paraffin sections was of an acceptable quality (Fig. 1B). The average final section thickness was 24.4 μm (n = 65). This is consistent with our experience that PFA-fixed tissue shrinks postsectioning, while Methacarn-fixed tissue shrinks primarily presectioning. The z-axis analysis showed complete stain penetration (data not shown). The counts of trochlear neurons produced an average of 909 ± 26 neurons, with extremes of 876–960 neurons. The numbers from PFA-fixed brains were statistically not different (F1,6 < 1, not significant = NS) from the Methacarn-fixed brains, and therefore were combined for subsequent statistical analyses (906 ± 12, SEM, n = 9). We conclude from these counts that interanimal and right–left variability was low (less than 5%, see Table 1), thus making the trochlear nucleus an excellent model system for the subsequent comparisons of different tissue processing protocols. The validity of the number close to 900 trochlear neurons is further supported by previous studies on the counts of all nerve fibers in the trochlear nerve of the posthatch chicken, which was 861 ± 26 (Sohal et al., 1985) and 905 ± 15, SEM (Croes et al., 2007). We conclude that embedding in paraffin after fixation with either Methacarn or PFA yields numbers that are accurate within about 5%. This percentage does not take into account potential sampling errors or errors due to inaccurate volume measurements (Guillery, 2002; Guillery and August, 2002, see also Discussion).
Cryosections
Among different types of tissue sections, cryosections are currently most often used for optical disector counting (Baryshnikova et al., 2006), despite their inferior morphology. To determine if inferior tissue quality of cryosections affects numerical estimates, eight complete series of cryosections through trochlear motoneurons fixed with PFA were assessed. A representative cryosection shows the tissue quality obtained (Fig. 1C). The average final section thickness was 21.7 μm. Z-axis analyses showed complete stain penetration (Fig. 2C). The number of sections per trochlear nucleus ranged from 9 to 10. Cryosectioned trochlear nuclei fixed with PFA alone (n = 8) revealed an average motor neuron count of 812 ± 12 (SEM). The counts ranged from 762 to 861. The average number of 812 was 10.4% lower than for those embedded in paraffin (906 for all paraffin cases combined, n = 9, see earlier), and the lowest value was within 16% of 906. The difference between the paraffin-derived numbers and the cryosection-derived numbers was statistically significant (F1,15 = 28.0, P < 0.001).
Addition of as little as 0.01–0.1% GA to PFA fixative is known to significantly improve the tissue quality (Hockfield et al., 1993; Stuart and Oorschot, 1995). To determine if the tissue quality (and the neuron counts) obtained from cryosections could be significantly improved by addition of 0.1% GA in the fixative, animals were fixed with 4% PFA and 0.1% GA (n = 5), and cryosectioned trochlear neurons (Fig. 1D) were counted. The average number obtained with the optical disector method from the cryosectioned trochlear nuclei fixed with 4% PFA and 0.1% GA (n = 5) was 867 ± 17 (SEM) motor neurons. Counts ranged from 818 to 917 and the average number of 867 was within 4.3% of the average paraffin value of 906 and the lowest value within 10%. The difference between total motor neuron counts from the two groups (fixed with 4% PFA only or 4% PFA and 0.1% GA) was statistically significant (F1,11 = 7.5, P < 0.05), so they were analyzed separately. Z-axis analysis of both fixation protocols showed complete stain penetration and no loss of particles at section surfaces (Figs. 2C and 2D). The difference between the two estimates for the different fixation protocols indicates that the addition of 0.1% GA resulted in improved tissue quality with improved recognition of neurons in the tissue (cf. Baryshnikova et al., 2006). The numerical data are summarized in graph form in Figure 3. We conclude that cryosections of PFA-fixed tissue produce an undercount of about 10%, but with additional fixation of 0.1% GA, numerical estimates can be brought within less than 5% of the true value. The measured value in this instance approaches a value that is different from the accurate value, but there is not a statistically significant difference (F1,12 = 3.5, P < 0.10).
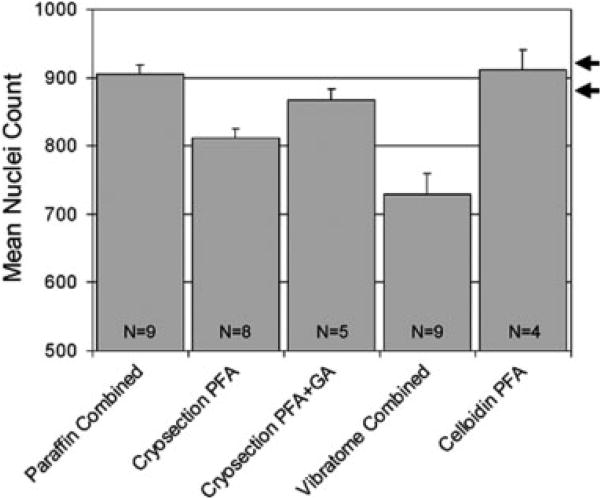
Fig. 3.
Synopsis of neuron numbers obtained by counting every neuronal nucleus with an unbiased counting rule in complete series of tissue sections through the trochlear nucleus of hatchling chickens in five different tissue processing conditions: paraffin, cryosection with paraformaldehyde (PFA) fixation, cryosection with additional glutaraldehyde (GA) fixation, vibratome section, and celloidin section. Error bars = SEM. The number (N) of trochlear nuclei counted for each condition is indicated on the bars. The range of “accurate” numbers of neurons is indicated by the two arrows on the right side, based on one serial section reconstruction as well as reports on the number of nerve fibers in the trochlear nerve of posthatch chickens.
Vibratome Sections
Vibratome sections are known for their relatively poor morphology and chatter marks during sectioning, but are popular with immunohistochemical applications due to enhanced antibody penetration. The typical appearance of a trochlear motoneuron in a vibratome section is shown in Figure 1E. The average final section thickness of PFA-fixed vibratome sections was 17.3 μm. Z-axis analyses confirmed complete stain pen etration (Fig. 2E). The number of sections through the trochlear nucleus ranged from seven to nine. PFA-fixed trochlear motor nuclei sectioned on a vibratome (n = 6) resulted in an average motor neuron number of 747 ± 39 (SEM). The numbers ranged from 614 to 836 and the average number of 747 was within 18% of the aver age number for paraffin sections (906) and the lowest value was within 33%. The difference between the av erage vibratome number and the average paraffin number was statistically significant (F1,13 ± 20.9, P < 0.001). We also tested, similar to the cryosections, whether the addition of 0.1% GA to 4% PFA as fixative (n = 3) would improve the numbers of trochlear motoneurons, but this was not the case (Table 1). Taken all vibratome sections together (n = 9), the neuron counts were 729 ± 31 (SEM), a deficit of about 20%. We conclude that vibratome sections produce a 20% deficit in numerical estimates, and this deficit is not improved by fixation with GA. Again, these deficits do not take into account any additional potential errors due to sampling or volume measurements.
Celloidin Sections
Celloidin resin (plastic) sections have superb morphology, but they are not very popular among investigators (Baryshnikova et al., 2006) because of the elaborate and slow embedding procedure and limited antibody penetration in these plastic sections (Williams et al., 2003). To determine if celloidin sections produce similar optical disector-derived numbers as the paraffin sections, four series of sections through the trochlear nucleus were analyzed. A typical trochlear neuron from a celloidin section is shown in Figure 1F. Average final section thickness was 26.2 μm. Z-axis analyses showed complete stain penetration (Fig. 2F). The number of sections through each trochlear nucleus ranged from 11 to 13. Celloidin sections produced an average motor neuron number of 912 ± 28 (SEM). The numbers ranged from 853 to 981 and the average number of 912 was within 1% of the average number for paraffin sections (906) and the lowest value was within 6%. The difference between the average celloidin-derived num ber and the average paraffin-derived number was statistically not significant (F1,11 < 1, NS).
The numerical data are summarized in graph form in Figure 3. We conclude that paraffin and celloidin sections are similarly well suited to recognize neurons and to obtain accurate numerical data for the first step in optical disector counting; this does not exclude the possibility of potential sampling errors or errors in volume measurements when numerical estimates are calculated. Vibratome sections, however, and to some extent cryosections, violate the general requirement for unbiased particle number estimation, namely that it is possible to observe and recognize all particles of interest in the containing space (Dorph-Petersen et al., 2001).
Discussion
Methods that deliver efficient, accurate, and reliable numerical estimates of particles in sectioned tissues are in great demand in quantitative morphology. This need is demonstrated by the large number of citations (Institute for Scientific Information) for key publications of modern stereological methods with citation numbers that combined now exceed 10,000 for the past two decades (e.g., Coggeshall and Lekan, 1996; Gundersen et al., 1988; Howard and Reed, 1998). When the disector technology was introduced, it raised great expectations, as it promised unbiased numerical esti mates (Howard and Reed, 1998; Mouton, 2002). However, calibration studies and discrepancies between results from different teams of investigators have revealed a number of potential sources of bias in the past decade. Optical disector counting is by no means “fool-proof.” This becomes most obvious by the wide range of numerical estimates from different reports that vary by two to threefold between the lower and the higher numerical estimates, and thus differ little, if at all, in this respect from traditional corrected profile counts (Coggeshall and Lekan, 1996). Our work reveals and quantifies one important source of bias: the degree of preservation of tissue quality that is produced by different embedding and sectioning techniques.
Technical Considerations
Several technical issues need to be addressed in this context. In our study, we compared the obtained number with the calibrated “accurate” number. We are confident that our paraffin sections produce reasonably “accurate” numbers of trochlear motoneurons for the following reasons. First, the number was verified by serial section reconstruction, which is considered the “gold standard” in particle counting (Coggeshall and Lekan, 1996; Coggeshall et al., 1990; Farel, 2002; Geuna, 2000, 2005; Guillery, 2002; Hatton and von Bartheld, 1999; Heller et al., 2001; von Bartheld, 2001, 2002; West, 1999). Second, the number of nerve fibers in the trochlear nerve (Croes et al., 2007; Sohal et al., 1985) is virtually identical with the paraffin- and celloidin section-derived number, further confirming the validity of the numbers obtained.
Another important question is whether the tissue sections generated for the present analysis produced “typical” section quality, or whether they could have been compromised by some systematic, lab-specific protocol deviation. We maintain that our cryosections and vibratome sections were of “typical” section quality. As shown in Figure 1, tissue quality was what we consider standard (based on 25+ years of experience of the senior investigator, von Bartheld, with tissue sections generated and prepared in several different labs on three continents). Several cryosection assessment and improvement papers (Ishii et al., 1993; Revilla and Jones, 2002; Wahle, 2002) were carefully studied and sections generated in our lab were compared with sections generated in leading histology labs worldwide (e.g., Karolinska, Heidelberg, San Diego, Seattle, Göttingen, Brisbane). Thus, we are confident that our tissue sections are of standard quality that matches those produced throughout the world. This suggests that the results of our study are relevant for a large majority of cryosection and vibratome sectioning protocols.
We did not attempt to quantify serial sections embedded in methacrylate. Many of the initial optical disector studies used methacrylate sections, but the fraction of optical disector studies employing methacrylate sections is steadily declining (Baryshnikova et al., 2006). Yet the main reason for omission of methacrylate sections in our study is a technical one. It is virtually impossible to generate serial sections of appropriate thickness from methacrylate-embedded tissue (Hatton and von Bartheld, 1999; personal experience and Nyengaard, personal communication). Therefore, it would have been impossible to generate numerical estimates in the same way as done in our study for the other four section types.
Possible Mechanisms of Numerical Differences
Our study shows significant differences in the numerical yield between sections obtained by different tissue processing protocols. Which factor(s) are responsible for these differences? Two general categories need to be considered: theoretically, the section quality may impact particle retention or particle recognition. As in previous studies, including a multi-center international study (Baryshnikova et al., 2006), our z-axis analyses did not provide any evidence for significant loss of particles or particle fragments (“lost caps,” Hedreen, 1998) from the surfaces of our tissue sections in the case of vibratome or cryosections. Therefore, we do not believe that particle retention is a major factor responsible for the observed differences. Rather, the recognition of particles appears to be compromised in the vibratome sections and in the cryosections that were fixed with PFA only.
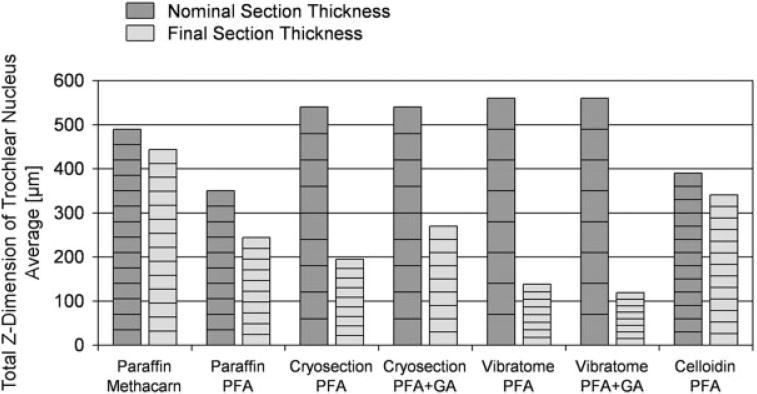
A major factor that may be relevant is the collapse in the z-axis of vibratome and cryosections during tissue processing. As shown in Table 1 and Figure 4, vibratome sections compacted from their nominal section thickness to a final section thickness of 20–25%, and cryosections to a final thickness of 35–50%, while par affin sections and celloidin sections retained 70–90% of their original thickness. This obviously makes it more challenging to identify particles in vibratome and cryosections, because particles are much more tightly stacked within the same final space. One countermeasure could be to cut the original vibratome or cryosection even thicker—in the range of 80–100 μm. However, this would further diminish the total number of tissue sections through the reference volume, which is already somewhat lower for vibratome and cryosections (8–9) versus 10–14 for paraffin and celloidin sections (Table 1 and Fig. 4). We were striving for a reasonable balance between the number of tissue sections and their final section thickness, to make the parameters analyzed most comparable between the conditions.
Fig. 4.
Graphs show the original (nominal) combined thickness of sections (dark gray) through the trochlear nucleus, compared with the final (combined) section thicknesses (light gray), for each of the tissue processing conditions. Averages from three to eight cases (actual variations from individual cases are not shown). Note that the underestimates are particularly pronounced in those cases where the section thickness shrinks to one half or less. PFA, paraformaldehyde; GA, glutaraldehyde.
During counting, we subjectively noticed the differences in preservation of tissue morphology, and this was correlated with the numbers of neurons counted. Our subjective impressions are most consistent with the notion that, even with only slightly reduced tissue preservation, some particles may present “borderline cases” that cannot be identified with certainty due to resemblance of artifacts or smaller cellular debris. Compaction within a tighter space due to shrinkage may be primarily responsible for the reduced number of neuronal nuclei. Alternatively, we cannot formally exclude the possibility that some sectioning devices (such as the vibratome) may chatter and disrupt major fragments of neuronal nuclei that then may not be recognizable in one or both of the two adjacent tissue sections.
Sources of Bias in Optical Disector Counting
Optical disector counting was and still is advertised to generate “unbiased” numbers (Howard and Reed, 1998; Mouton, 2002)—thus, potentially misleading investigators and evoking a false sense of security and expectations (Guillery and August, 2002; Saper, 1999; von Bartheld, 1999). A number of factors have been identified that may be responsible for numerical discrepancies between studies. These include interanimal variability, z-axis distortion of tissue sections and use of inappropriate guard spaces, loss of particles from surface regions, errors in measuring section thickness, errors in assessing representative samples, errors in measuring the precise reference volume (border of populations of cells), and investigator errors such as fatigue (Geuna, 2005; Guillery, 2002; Guillery and August, 2002; Mouton, 2002). Previous calibration analyses have shown considerable systematic biases because of z-axis distortion of “softer” tissue sections and/or loss of particles from section surfaces (Baryshnikova et al., 2006; Gardella et al., 2003). Our study adds to this list of potential biases, the bias that results from different tissue processing protocols and inherent differences in preservation of tissue qualities. Such differences have received remarkably little attention, and no attempts appear to have been made to quantify such differences. Therefore, the goal of our study was to objectively measure the effect of histological tissue section quality because of different embedding/sectioning protocols on total neuron counts and, by implication, on the numerical estimates that would be obtained by optical disector counting.
Our study shows that the four major types of tissue sections currently used in optical disector counting are not equivalent in yielding true numerical estimates. Those section types that are generally known to produce lower quality sections (cryosections and vibratome sections) indeed produced significantly lower particle numbers. In particular, vibratome sections produced substantially lower counts, nearly 20% underestimate on average, without considering any potential additional sources of errors in optical disector counting (Guillery, 2002; Guillery and August, 2002). Interestingly, addition of 0.1% GA as a fixative in addition to 4% PFA for fixation of brains processed for cryosections significantly improved the tissue quality, reduced the collapse of the section thickness, and apparently enhanced the recognition of particles.
Importantly, a large majority of current optical disectorcounting is performed on cryo- and vibratome sections (Baryshnikova et al., 2006). Thus, nearly two-thirds of current optical disector counting studies likely underestimate true numbers by 10–20%. Indeed, some studies using vibratome sections report about 10–15% fewer tyrosine-hydroxylase immunoreactive neurons in the murine substantia nigra when compared with studies employing cryosections or paraffin sections (Schober et al., 2007, and references cited therein). One reason that the undercount with vibratome and cryosections has not been recognized earlier may be due to the fact that optical disector counting in paraffin sections can likewise lead to an undercount of 10–25% when inappropriate guard spaces are used (Gardella et al., 2003; Hatton and von Bartheld, 1999; von Bartheld, 1999). Inappropriate guard spaces may have been widely used (and may still be used), because problems and consequences of the use of inappropriate guard zones were not realized until 1999 (Gardella et al., 2003; Hatton and von Bartheld, 1999; von Bartheld, 1999, 2002), when the importance of z-axis anal yses emerged. Thus, data from paraffin and cryo- or vibratome studies seemed to match at relatively too low counts and seemed to, falsely, confirm each other. This reasoning may also apply to explain apparent matching of numerical estimates from cryosections and methacrylate sections (Schmitz and Hof, 2005).
Conclusions
Whenever possible, either paraffin or celloidin sections should be used to obtain valid numerical estimates with optical disector counting. If cryosections have to be used, the tissue should be fixed in addition with 0.1% GA to improve the numerical yield. We recognize that when particles require staining by immunolabeling, the addition of GA or use of paraffin sections may not be advised, because some antibodies do not work with paraffin sections or with particular fixatives such as GA. Whenever this is an option, paraffin or celloidin sections should be preferred. When GA fixation does not interfere with antigen binding by the antibody, this approach should be tested. Furthermore, the underestimate due to inferior tissue section quality should be considered when comparisons are made between studies, and investigators should be aware of the possibility of significant underestimates when cryo-or vibratome sections are used in optical disector counting.
Acknowledgments
The authors thank Christopher Pung and Stephanie Chin for technical assistance, and William Hatton for comments.
Contract grant sponsor: NIH grants; Contract grant numbers: EY 12841, DA 021131; Contract grant sponsor: Sanford Center for Aging, Reno.
References
- Abercrombie M. Estimation of nuclear populations from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Aldana Marcos HJ, Ferrari CC, Benitez I, Affanni JM. Standardization of fixation, processing and staining methods for the central nervous system of vertebrates. Biocell. 1996;20:265–272. [PubMed] [Google Scholar]
- Baryshnikova LM, von Bohlen und Halbach O, Kaplan S, von Bartheld CS. Two distinct events, section compression and loss of particles (“lost caps”), contribute to z-axis distortion and bias in optical disector counting. Microsc Res Tech. 2006;69:738–756. doi: 10.1002/jemt.20345. [DOI] [PubMed] [Google Scholar]
- Clarke PGH, Oppenheim RW. Neuron death in vertebrate development: In vitro methods. Methods Cell Biol. 1995;46:277–321. [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: A case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, La Forte R, Klein CM. Calibration of methods for determining numbers of dorsal root ganglion cells. J Neurosci Methods. 1990;35:187–194. doi: 10.1016/0165-0270(90)90123-w. [DOI] [PubMed] [Google Scholar]
- Croes SA, Baryshnikova LM, Kaluskar SS, von Bartheld CS. Acute and long-term effects of botulinum neurotoxin on the function and structure of developing chick extraocular muscles. Neurobiol Dis. 2007;25:649–664. doi: 10.1016/j.nbd.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Nyengaard JR, Gundersen HJ. Tissue shrinkage and unbiased stereological estimation of particle number and size. J Microsc. 2001;204:232–246. doi: 10.1046/j.1365-2818.2001.00958.x. [DOI] [PubMed] [Google Scholar]
- Durham D, Woolsey TA, Kruger L. Cellular localization of 2-[3H]deoxy-d-glucose from paraffin-embedded brains. J Neurosci. 1981;1:519–526. doi: 10.1523/JNEUROSCI.01-05-00519.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farel PB. Trust, but verify: The necessity of empirical verification in quantitative neurobiology. Anat Rec. 2002;269:157–161. doi: 10.1002/ar.10111. [DOI] [PubMed] [Google Scholar]
- Gardella D, Hatton WJ, Rind HB, Rosen GD, von Bartheld CS. Differential distortion of the z-axis in tissue sections: Implications for optical disector counting. J Neurosci Methods. 2003;124:45–59. doi: 10.1016/s0165-0270(02)00363-1. [DOI] [PubMed] [Google Scholar]
- Geuna S. Appreciating the difference between design-based and model-based sampling strategies in quantitative morphology of the nervous system. J Comp Neurol. 2000;427:333–339. [PubMed] [Google Scholar]
- Geuna S. The revolution of counting “tops”: Two decades of the disector principle in morphological research. Microsc Res Tech. 2005;66:270–274. doi: 10.1002/jemt.20167. [DOI] [PubMed] [Google Scholar]
- Guillery RW. On counting and counting errors. J Comp Neurol. 2002;447:1–7. doi: 10.1002/cne.10221. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Herrup K. Quantification without pontification: Choosing a method for counting objects in sectioned tissues. J Comp Neurol. 1997;386:2–7. doi: 10.1002/(sici)1096-9861(19970915)386:1<2::aid-cne2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Guillery RW, August BK. Doubt and certainty in counting. Prog Brain Res. 2002;135:25–42. doi: 10.1016/S0079-6123(02)35005-2. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sørensen FB, Vesterby A, West MJ. The new stereological tools: Disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. Acta Pathol Microbiol Immunol Scand. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Gunturkun O. Visual lateralization in birds: From neurotrophins to cognition? Eur J Morphol. 1997;35:290–302. [PubMed] [Google Scholar]
- Hatton WJ, von Bartheld CS. Analysis of cell death in the trochlear nucleus of chick embryos: Calibration of the optical disector counting technique reveals systematic bias. J Comp Neurol. 1999;409:169–186. [PubMed] [Google Scholar]
- Hedreen JC. Lost caps in histological counting methods. Anat Rec. 1998;25:366–372. doi: 10.1002/(SICI)1097-0185(199803)250:3<366::AID-AR11>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Heller B, Schweingruber F, Guvenc D, Heller A. Computer experiments to determine whether over- or under-counting necessarily affects the determination of difference in cell number between experimental groups. J Neurosci Methods. 2001;106:91–99. doi: 10.1016/s0165-0270(01)00333-8. [DOI] [PubMed] [Google Scholar]
- Hockfield S, Carlson S, Evans C, Levitt P, Pintar J, Silberstein L. Selected methods for antibody and nucleic acid probes. New York: Cold Spring Harbor Laboratory Press; 1993. Immunocytochemistry; pp. 111–226. [Google Scholar]
- Howard CV, Reed MG. Three-dimensional measurement in microscopy. New York: Springer; 1998. Unbiased stereology; p. 246. [Google Scholar]
- Ishii T, Kasama K, Kondo M, Takahashi T. Cryostat sectioning of formalin-fixed brain: Further attempt to improve section quality by previous infiltration with O.C.T. compound. Tohoku J Exp Med. 1993;171:101–105. doi: 10.1620/tjem.171.101. [DOI] [PubMed] [Google Scholar]
- Konigsmark BW. Methods for the counting of neurons. In: Nauta WJH, Ebbesson SOE, editors. Contemporary research methods in neuroanatomy. New York: Springer; 1970. pp. 315–340. [Google Scholar]
- Medeiros LR, Rosa DD, Edelweiss MI, Stein AT, Bozzetti MC, Zelmanowicz A, Pohlmann PR, Meurer L, Carballo MT. Accuracy of frozen-section analysis in the diagnosis of ovarian tumors: A systematic quantitative review. Int J Gynecol Cancer. 2005;15:192–202. doi: 10.1111/j.1525-1438.2005.15203.x. [DOI] [PubMed] [Google Scholar]
- Mouton PR. Principles and practices of unbiased stereology An introduction for bioscientists. Baltimore: Johns Hopkins University Press; 2002. p. 214. [Google Scholar]
- Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2004;127:569–580. doi: 10.1530/rep.1.00095. [DOI] [PubMed] [Google Scholar]
- Pinto PB, Andrade LA, Derchain SF. Accuracy of intraoperative frozen section diagnosis of ovarian tumors. Gynecol Oncol. 2001;81:230–232. doi: 10.1006/gyno.2001.6133. [DOI] [PubMed] [Google Scholar]
- Prieto VG, Argenyi ZB, Barnhill RL, Duray PH, Elenitsas R, From L, Guitart J, Horenstein MG, Ming ME, Piepkorn MW, Rabkin MS, Reed JA, Selim MA, Trotter MJ, Johnson MM, Shea CR. Are en face frozen sections accurate for diagnosing margin status in melanocytic lesions? Am J Clin Pathol. 2003;120:203–208. doi: 10.1309/J1Q0-V35E-UTMV-R193. [DOI] [PubMed] [Google Scholar]
- Revilla V, Jones A. Cryostat sectioning of brains. Int Rev Neurobiol. 2002;47:61–70. doi: 10.1016/s0074-7742(02)47052-3. [DOI] [PubMed] [Google Scholar]
- Saper CB. Unbiased stereology: Three-dimensional measurement in microscopy. Trends Neurosci. 1999;22:94–95. Book review. [Google Scholar]
- Schmitz C, Hof PR. Design-based stereology in neuroscience. Neuroscience. 2005;130:813–831. doi: 10.1016/j.neuroscience.2004.08.050. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Korr H, Heinsen H. Design-based counting techniques: The real problems. Trends Neurosci. 1999;22:345–346. doi: 10.1016/s0166-2236(99)01418-6. [DOI] [PubMed] [Google Scholar]
- Schober A, Peterziel H, Simon H, von Bartheld CS, Krieglstein K, Unsicker K. GDNF applied to the MPTP-lesioned nigrostria-tal system requires TGF-β for its neuroprotective action. Neurobiol Dis. 2007;25:378–391. doi: 10.1016/j.nbd.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Sohal GS, Knox TS, Allen JC, Jr, Arumugam T, Campbell LR, Yamashita T. Development of the trochlear nucleus in quail and comparative study of the trochlear nucleus, nerve, and innervation of the superior oblique muscle in quail, chick, and duck. J Comp Neurol. 1985;239:227–236. doi: 10.1002/cne.902390209. [DOI] [PubMed] [Google Scholar]
- Stuart DA, Oorschot DE. Embedding, sectioning, immunocytochemical and stereological methods that optimise research on the lesioned adult rat spinal cord. J Neurosci Methods. 1995;61:5–14. doi: 10.1016/0165-0270(95)00017-o. [DOI] [PubMed] [Google Scholar]
- Tandrup T. Unbiased estimates of number and size of rat dorsal root ganglion cells in studies of structure and cell survival. J Neuro-cytol. 2004;33:173–192. doi: 10.1023/b:neur.0000030693.91881.53. [DOI] [PubMed] [Google Scholar]
- Taylor CR. Immunohistochemistry for the age of molecular morphology. Appl Immunohistochem Mol Morphol. 2001;9:1–2. [PubMed] [Google Scholar]
- von Bartheld CS. Systematic bias in an ‘unbiased’ neuronal counting technique. Anat Rec (New Anat) 1999;257:119–120. doi: 10.1002/(SICI)1097-0185(19990815)257:4<119::AID-AR2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- von Bartheld CS. Comparison of 2D- and 3D-counting: The need for calibration and common sense. Trends Neurosci. 2001;24:504–506. doi: 10.1016/s0166-2236(00)01960-3. [DOI] [PubMed] [Google Scholar]
- von Bartheld CS. Counting particles in tissue sections: Choices of methods and importance of calibration to minimize biases. Histol Histopathol. 2002;17:639–648. doi: 10.14670/HH-17.639. [DOI] [PubMed] [Google Scholar]
- Wahle P. Combining non-radioactive in situ hybridization with immunohistological and anatomical techniques. Int Rev Neurobiol. 2002;47:203–238. doi: 10.1016/s0074-7742(02)47061-4. [DOI] [PubMed] [Google Scholar]
- Ward TS, Rosen GD, von Bartheld CS. Optical disector counting in cryosections and vibratome sections underestimates neuron numbers: Effects of tissue quality. Soc Neurosci Abstr. 2007;845:10. doi: 10.1002/jemt.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ. Stereological methods for estimating the total number of neurons and synapses: Issues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- Williams RW, Rakic P. Three-dimensional counting: Accurate and direct method to estimate numbers of cells in sectioned material. J Comp Neurol. 1988;278:344–352. doi: 10.1002/cne.902780305. [DOI] [PubMed] [Google Scholar]
- Williams RW, von Bartheld CS, Rosen GD. Counting cells in sectioned material: A suite of techniques, tools and tips. Curr Protocols Neurosci. 2003;1:11, 1–29. doi: 10.1002/0471142301.ns0111s24. [DOI] [PubMed] [Google Scholar]
- Wootipoom V, Dechsukhum C, Hanprasertpong J, Lim A. Accuracy of intraoperative frozen section in diagnosis of ovarian tumors. J Med Assoc Thai. 2006;89:577–582. [PubMed] [Google Scholar]