Abstract
The eosinophil is a multifunctional granulocyte best known for providing host defense against parasites. Paradoxically, eosinophils are also implicated in the pathogenesis of allergic inflammation, asthma, and hypereosinophilic syndromes. Emerging evidence also supports the potential for harnessing the cytotoxic power of eosinophils and redirecting it to kill solid tumors. Central to eosinophil physiology is interleukin-5 (IL-5) and its receptor (IL-5R) which is composed of a ligand-specific alpha chain (IL-5Rα) and the common beta chain (βc). Eosinophil activation can lead to their degranulation, resulting in rapid release of an arsenal of tissue-destructive proinflammatory mediators and cytotoxic proteins that can be both beneficial and detrimental to the host. This review discusses eosinophil immunobiology and therapeutic strategies for targeting of IL-5 and IL-5R, as well as the potential for harnessing eosinophil cytotoxicity as a tumoricide.
Keywords: Eosinophil, Interleukin-5 (IL-5), IL-5 receptor (IL-5R), Anti-IL-5 antibodies, Anti-IL-5R antibodies, Allergic inflammation, Asthma, Eosinophilic syndromes, Cancer, Therapeutic, Treatment, Hypereosinophilic disorders
INTRODUCTION
Eosinophils are innate immune effector cells best known for providing host defense against parasites, as well as playing a role in the pathogenesis of allergic diseases such as asthma, and in hypereosinophilic syndromes (1-4). In the past decade, additional physiologic roles for eosinophils have emerged, which include coordination of tissue remodeling events, orchestration of homeostatic functions, and regulation of innate and adaptive immunity [1-4]. Typically, eosinophils are found in low numbers in the blood (1-4% of total peripheral blood leukocytes; less than 500/cu mm), and under homeostatic conditions are also found within mucosal tissues, as well as primary and secondary lymphoid organs [5]. Eosinophils can be rapidly generated from bone marrow progenitors and recruited to sites of inflammation. The cytokine, interleukin-5 (IL-5), is essential for the differentiation and survival of eosinophils from hematopoietic progenitors [6]. Eosinophils and their progenitors express the IL-5R which is composed of a ligand specific alpha chain (IL-5Rα) and the common beta receptor (βc) which is shared by IL-3Rα and GM-CSFRα [7]. Chemotactic molecules are necessary for eosinophil recruitment and migration. Eotaxin-1 is an eosinophil-specific chemokine and is the most potent chemokine for eosinophils [8,9]. Other less selective chemokines include RANTES, eotaxin-2, eotaxin-3, MCP-2, and MCP-3, which also utilize the eotaxin-1 receptor (CCR3) [8,9].
Central to eosinophil effector functions is the capacity of these cells to immediately release their tissue-destructive cytoplasmic granules upon activation by various stimuli. Eosinophil granule secretion leads to the release of preformed pro-inflammatory mediators such as cytokines, chemokines, lipid and neuro-mediators, growth factors, and cationic proteins [1-5]. Eosinophils are characterized by the presence of specific granules that contain four classic cationic proteins: major basic protein (MBP), eosinophil peroxidase (EPO), eosinophilic cationic protein (ECP), and eosinophil-derived neurotoxin (EDN) [1-5]. The collective destructive power of these cytotoxic proteins provides efficacy against infectious organisms, accounts for the bystander damage to host tissue during eosinophilic inflammation, and makes them potentially attractive candidates for use as tumoricidals. Eosinophils mediate remodeling via profibrotic cytokines, such as TGFβ, and eosinophils are the largest producers of TGFβ in the airway [10,11]. TGFβ contributes to airway remodeling by detaching airway epithelial cells and increasing deposition of extracellular matrix proteins which causes fibrosis via matrix metalloproteinases (MMP) and IL-6 [10,11].
Eosinophils in Parasite Immunity
Eosinophils are central to host immunity against parasites [12-15]. In addition, eosinophils can effectively participate in immunity to - bacterial and viral infections via ligation of pattern recognition receptors by damage-associated molecular pattern molecules (DAMPs) and pathogen-associated molecular pattern molecules (PAMPs) to pattern recognition receptors [13,15]. Eosinophils are most effective against helminth parasites. When parasites infiltrate host tissues, a Th2 response is elicited which increases the generation of eosinophils under the influence of IL-5 [12,14]. Eosinophils are recruited to the site of infection by eotaxin-1. Once in contact with the parasite, the eosinophil degranulates to release reactive oxygen species and cytotoxic molecules such as EDN, EPO and MBP [14]. Eosinophils will also secrete lipid bodies which contain a variety of eicosanoids that are necessary (along with mast cells) for the smooth muscle modulation that occurs in peristalsis designed to expel parasites [14].
Eosinophils in Allergic Inflammation and Eosinophilic Syndromes
Eosinophils have a firmly established role in allergic inflammatory responses. In humans and mice, one of the hallmarks of asthma is eosinophilic infiltration of the bronchial mucosa and submucosa, and the number of airway eosinophils is directly associated with disease severity in asthmatic patients [16,17]. A role for eosinophils in the pathogenesis of asthma was experimentally supported using eosinophil-deficient mice, which had markedly diminished allergen-induced airway inflammation and markedly diminished bronchial hyper-reactivity [18,19]. Similar results were seen with IL-5-deficient mice, implicating the IL-5/eosinophil axis in allergic inflammation [20]. This axis was likewise implicated in humans by the presence of increased IL-5 in bronchoalveolar lavage fluid and bronchial biopsies of patients with allergic asthma [21-24].
Perhaps where eosinophilic pathology is most blatant is in hypereosinophilic syndromes (HES), which encompass a variety of disorders whose commonality is chronic elevation of blood eosinophil counts [25-27]. HES can cause complications such as cardiomyopathy, hepatosplenomegaly, neuropathy, skin lesions, and pulmonary disease. These symptoms can be fatal if untreated, and are secondary to the tissue damage caused by the eosinophils’ toxic mediators. Subtypes of HES are being delineated, and include those with the PGDF-FIPL1 fusion gene, and those secondary to increased IL-5 production, as well as idiopathic causes [25-27].
Other diseases characterized by eosinophilia include eosinophilic esophagitis (EE) and eosinophilic gastroenteritis (EG), referring to excess eosinophil infiltration of the esophagus and the stomach or intestines, respectively [28-30]. Experimentally, eotaxin-deficient mice had attenuated EE and IL-5-deficient mice had complete ablation of EE [31]. EG can occur in all parts of the gastrointestinal tract, and like EE, is usually caused by allergic responses. Churg Strauss syndrome (CSS) is also characterized by hypereosinophilia, and nasal polyposis is characterized by increased IL-5 production and infiltration of eosinophils in the polypoid tissue [32-34].
Duality of Eosinophil Physiology
The descriptions above demonstrate that eosinophils can be harmful or beneficial. There are two prevailing paradigms to explain this duality: either (1) the participation of eosinophils in allergic inflammation are part of a common physiologic Th2 immune response to environmental insults at the host-environment interface, or (2) eosinophils in allergic inflammation are a physiologically unintended or misdirected pathologic response that stems from the host’s use of the Th2 anti-parasite immune response pathway [35]. In support of the first option, the LIAR hypothesis specifically emphasized that the role of eosinophils is to provide “Local Immunity and Remodeling Repair,” explaining that the basal levels of eosinophils in the tissues are responsible for homeostatic remodeling [35,36]. Hence, the allergic response would be an over-exuberant, intended response to potentially harmful environmental antigens. By extension, Th2 immune responses may have also specifically evolved against non-infectious noxious agents or toxins as a mechanism to promote behavioral change (like the avoidance venomous stinging or biting animals). Whether Th2 immune responses evolved independently against parasites and non-infectious noxious agents, or are based on a shared mechanism, continues to be debated [35,36].
IL-5 and the IL-5 Receptor Complex
Eosinophils are critically dependent on IL-5 for their differentiation, activation, prolonged survival, increased adhesion to vascular endothelial cells, and augmentation of cytotoxic activity [1-4]. IL-5 is a glycoprotein homodimer that is produced by Th2 cells, as well as by NK cells, mast cells, basophils, and eosinophils [6]. Activated eosinophils produce IL-5 in an autocrine fashion to prolong their survival, and some evidence suggests that IL-5 is necessary for eosinophil migration along with chemokines like eotaxin-1 and RANTES [37].
IL-5 binds to a heteromeric receptor composed of a 65-kD, high affinity, ligand-specific IL-5Rα and a homodimeric 130kD βc, which is common to the GM-CSFR and the IL-3R [7]. Structurally, the IL-5 homodimer is composed of two four alpha helix motifs A-D and A’-D’ that are arranged in an up-up-down-down antiparallel configuration connected by loops [7,38]. The homodimer conformation interdigitates the A, B, and C helices from one molecule and the D’ helix from the other molecule, thereby yielding a molecule with a pair of four alpha helical bundle motifs with a C2-axis of symmetry [39]. Although this structure provides the homodimer with two potential binding domains for IL-5Rα and two for βc, only one IL-5Rα has been shown to directly bind to IL-5 [39]. The structural interactions of IL-5Rα-bound IL-5 to βc have yet to be solved, but are predicted to be limited to one βc. The IL-5 binding domain for IL-5Rα lies within the 1st and 3rd antiparallel loops [40], while the βc binding domain is anchored by the glutamate-13 residue (Glu-13) of IL-5 [39]. A recent crystallography study showed that steric hindrance is responsible for only one IL-5Rα being bound by the IL-5 homodimer [41]. It is predicted that IL-5 and the IL-5R complex forms in the same way that the GM-CSF and the GM-CSFR forms a dodecamer complex, with two IL-5/IL-5Rα complexes binding to one βc, followed by further aggregation of these ligand/receptor complexes, which enables engagement of adjacent βc, thereby facilitating transphosphorylation of JAK2 and signal transduction via STAT5 [42].
The IL-5Rα extracellular region consists of 3 fibronectin type III domains (D1, D2, D3). D2 binds IL-5’s M2 region using the D2 β1β2 loop while also binding at the hinge site between D2 and D3. It is D1 which is thought to be imperative for IL-5 binding, and dependent on Ile-161 [43]. After IL-5 binds to IL-5Rα via disulfide bonds, a conformational change occurs to allow IL-5 interaction with the βc. Both βc and IL-5R are constitutively associated with JAK kinases, and are responsible for signal transduction [44]. The main signaling pathways involved are the JAK/STAT, Ras/MAPK, p38/NF B, and the phosphoinositide 3-kinase (PI3K) pathways. These signaling pathways direct the transcription of various genes involved in eosinophil differentiation, activation, and survival [44].
STRATEGIES FOR ANTAGONIZING EOSINOPHILS
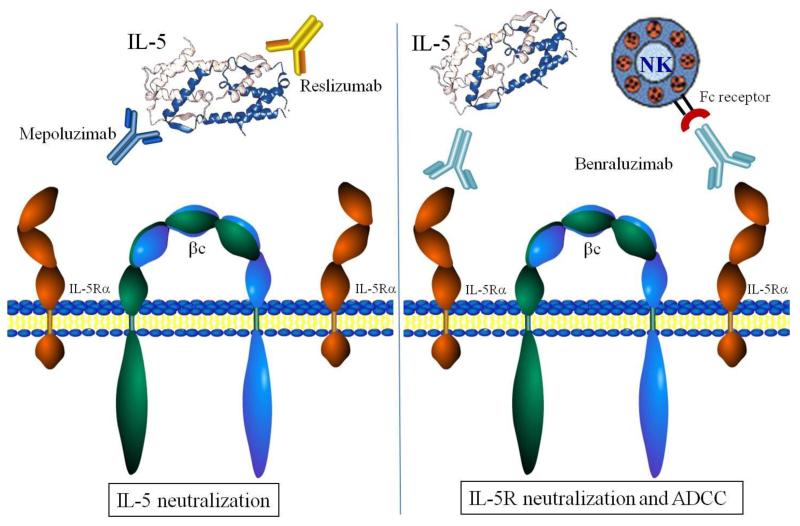
As eosinophils play a contributing role to allergic inflammation, asthma, and hypereosinophilic syndromes, eosinophil depletion has been a tantalizing target for treatment of these conditions. Since IL-5 is a specific mediator of eosinophil differentiation and survival, IL-5 and its receptor have evolved as drug targets (Fig. 1). Alternative strategies for antagonizing eosinophilic inflammation include targeting eotaxin, eosinophil adhesion molecules, or eosinophil signaling pathways. However, the greatest success has resided in targeting IL-5 or IL-5Rα.
Figure 1.
Current therapeutic strategies for antagonizing the IL-5/IL-5 receptor axis. In an inactivated state, the IL-5 receptor consists of two single IL-R chains (dark orange chains) and a c dimer (blue and green chains). Two different clinical approaches have been used to inhibit IL-5-induced signaling in eosinophils: (1) neutralization of IL-5 by humanized mAbs, mepolizumab and reslizumab (left panel); and (2) neutralization of the IL-5 receptor alpha chain (IL-5R) to block IL-5 binding and mediate ADCC lysis, benralizumab (right panel). IL-5 neutralization by mepolizumab and reslizumab involves the binding of these mAb to IL-5 domains which bind to IL-5R thereby blocking the formation of a signaling competent IL-5R complex (left panel). In contrast, Benralizumab actually binds the cell surface IL-5R to prevent IL-5 binding altogether. However, this approach also leads to antibody-dependent cellular cytoxicity (ADCC) caused by Fc receptor binding on NK cells to the anti-IL-5R mAb on eosinophils (right panel).
Targeting IL-5
Early on, it was determined in murine models that IL-5 neutralizing antibodies were effective down-regulators of eosinophilic inflammation, with similar favorable outcomes as those with IL-5-deficient or IL-5Rα-deficient mice [45]. In developing anti-IL-5 neutralizing monoclonal antibodies (mAb), the crucial epitopes are within the βc binding domain and the IL-5Rα binding domain. Targeting the βc binding domain on IL-5 would still allow IL-5 to bind to IL-5Rα on the surface of eosinophils, but signaling could not occur since engagement of the βc would be blocked. Conversely, targeting the IL-5Rα binding domain on IL-5 would block IL-5 binding to the eosinophil. Targeting either of these IL-5 domains would be predicted to be equally efficacious. Currently, there are only two IL-5 neutralizing mAb in human use, mepolizumab and reslizumab (Fig. 1). Both of these mAb have been humanized, bind to epitopes within the IL-5Rα binding domain (Table 1), and bind to IL-5 with similar affinity, 4.2pM and 20pM, respectively [46-49]. Mepolizumab is an IgG1κ antibody, while Reslizumab is an IgG4κ antibody, and hence they exhibit differences in their Fc biologic activity [47-49]. Whether their isotype differences will be clinically important is not clearly defined. Of note, there are no commercially developed mAb that bind the IL-5 βc binding domain, although this remains a viable target.
Table 1.
Targeting IL-5 and the IL-5R
| Target sites | Drug | Other names | Isotype | Mechanism | Human uses |
|---|---|---|---|---|---|
| IL-5 binding domain for IL- 5Rα |
Mepolizumab (GSK) | SB-240563 | IgG1k | Neutralizing antibody | (Phase 2) Asthma |
| (Phase 2) Adult eosinophilic esophagitis | |||||
| Bosatria | (Phase 2) Pediatric eosinophilic esophagitis |
||||
| (Phase 2) Churg Strauss syndrome | |||||
| (Phase 2) Nasal polyposis | |||||
| (Phase 3) Hypereosinophilic syndromes | |||||
| (Phase 3) Pediatric eosinophilic esophagitis | |||||
| (Phase 3) Rhinovirus induced asthma | |||||
| (Phase 3) COPD | |||||
| Reslizumab (Cephalon) | SCH55700 | IgG4k | Neutralizing antibody | (Phase 3) Asthma | |
| Cinquil | (Phase 2) Hypereosinophilic syndromes |
||||
| (Phase 3) Pediatric eosinophilic esophagitis | |||||
| (Phase 2) Loiasis | |||||
| IL-5 binding domain for βc |
------- | ------- | ------- | ---------------------------- | |
| IL-5R α | Benralizumab (MedImmune) |
MEDI-563 | IgG1k | ADCC, competitive inhibition |
(Phase 2) Asthma |
| (Phase 2) COPD | |||||
| IL-5R βc | TPI ASM8 (Pharmaxis) | ------- | Antisense gene target | (Phase 2) Asthma | |
| Eotaxin |
Mepolizumab
Clinical trials utilizing mepolizumab are summarized in Table 2. The initial mepolizumab trial targeted asthma patients. In this study, adults males with asthma received a single infusion of mepolizumab, and while peripheral blood eosinophil levels were reduced, there was no effect on clinical signs and symptoms [49,50]. Subsequent studies selected for patients with eosinophilic asthma and subgroups that were insufficiently controlled with corticosteroids. In prednisone-dependent asthmatics, an infusion of 750 mg mepolizumab was administered once a month for 5 months. Patients receiving the intervention had decreased blood and sputum eosinophils and improved asthma control as judged by decreased asthma exacerbations and lower requirements for prednisone [51]. In another study in adults with corticosteroid-refractory asthma, 12 monthly doses of mepolizumab resulted in fewer exacerbations, and patients improved their AQLQ score [52]. These trials also saw a subgroup improvement in patients with nasal polyposis. In an independent study on adults with severe nasal polyposis, patients who received two monthly infusions of 750 mg mepolizumab had a significant reduction in blood ECP and soluble IL-5Rα, and nasal IL-5Rα, IL-6, and IL-1β, which correlated with polyp improvement based on total polyp score (TPS) [53].
Table 2.
Human eosinophilic disease interventions
| Disease | Target | Intervention | Outcomes | Author |
|---|---|---|---|---|
| Asthma | Mild allergic asthma | Single dose IV 2.5 or 10 mg/kg mepolizumab |
Reduced blood and sputum eosinophilia, no effect on airway hyperreactivity. |
Leckie 2000 |
| Severe persistent asthma despite corticosteroids |
Single dose IV 1 mg/kg reslizumab |
After 2 days 50% eosinophil reduction, after 30 days only reduced by 18%. Unsustained improvement in FEV at 24 h. |
Kips 2003 |
|
| Asthma managed with corticosteroids |
3 monthly IV 250 or 750 mg mepolizumab |
Significant reduction in blood and sputum eosinophils. No significant clinical changes. |
Flood-Page 2007 |
|
| Mild atopic asthma | 4 once daily inhalation of 1,500 |ig TPI AMS8 |
46% reduction in sputum eosinophilila, reduction in EAR and LAR. Inhibition of sputum Pc mRNA and CCR3 mRNA. |
Gauvreau 2008 |
|
| Prednisone dependent asthma, sputum eos |
5 monthly IV 750 mg mepolizumab |
Reduced blood and sputum eosinophilia, reduced need for prednisone, fewer exacerbations. |
Nair 2009 |
|
| Refractory asthma | 12 monthly IV 750 mg mepolizumab |
Significantly fewer exacerbations, lower number blood and sputum eos, improved AQLQ score. |
Haldar 2009 |
|
| Mild atopic asthma | Single dose IV benralizumab (0.0003– 3 mg/kg) |
Dose dependent decrease in blood eosinophils, reduced levels of ECP, elevated CRP, CPK and IL-6. Transient reduction in WBC. |
Busse 2010 |
|
| Poorly controlled asthma and sputum eos |
4 monthly IV 3 mg/kg reslizumab |
Significant reduction of sputum eos. Nasal polyposis subgroup had improved ACQ scores. |
Castro 2011 |
|
| Mild allergic asthma | 4 days treatment with inhaled TPI ASM8 1– 4 mg bid vs 8 mg/day |
8 mg dose caused 60% reduction in sputum eos after 7 h, 68% reduction after 24 h |
Gauvreau 2011 |
|
| HES | HES refractory to other treatments |
Single dose IV 1 mg/kg reslizumab |
Reduction of eosinophilia, improved symptoms. Eosinophilia rebound and exacerbations post- treatment. |
Klion 2004 |
| Prednisone dependent HES, negative for FIP1L1–PDGFRA |
9 monthly IV 750 mg mepolizumab |
84% lowered their prednisone dosage to below 10 mg/day; 95% had fewer than 600 eos/ μL blood. Longer time to treatment failure. |
Rothenberg 2008 |
|
| Lymphocytic HES, negative for FIP1L1– PDGFRA |
750 mgIV mepolizumab every 4 weeks |
Reduce prednisone use ≤10 mg for 24 weeks (similar to non-lymphocytic HES); more likely to have <600 eos/μL blood than non- lymphocytic HES patients |
Roufousse 2010 |
|
| EE/EG | EE with dysphagia, and esophageal strictures. |
3 monthly IV 10mg/kg mepolizumab (up to 750 mg) |
An average 6 fold decrease in eosinophilia, improved clinical outcomes, decreased esophageal epithelial hyperplasia. |
Stein 2006 |
| EE with dysphagia | IV 750 mg mepolizumab at weeks 1 and 2. Then IV 1,500 mg at weeks 8 and 12. |
After two doses, reduction in esophageal eosinophilia. After 13 weeks, reduction in tenascin C and TGFβi in esophagus. Marginal clinical improvement. |
Straumann 2009 |
|
| Pediatric EE |
Children (2–17) with EE |
3 monthly IV 0.55, 2.5, or 10 mg/kg mepolizumab |
Endoscopy indicated improvement in patchy erythema, friability, furrows or vertical lines when there were <20 eosinophils/hpf. Eosinophilia recurred upon cessation. |
Assad 2011 |
| Children with moderate or severe EE |
4 monthly IV 1, 2, or 3 mg/kg reslizumab |
Significant reduction in esophageal eos; no significant clinical improvement. |
Spergel 2011 |
|
| Churg– Strauss Syndrome |
CSS with asthma, on corticosteroids |
Monthly IV 750 mg mepolizumab |
Reduction of eos to normal level, resolution of asthma, improvement of chest parenchyma. |
Kahn 2010 |
| CSS with eosinophilia | 4 monthly IV 750 mg mepolizumab, corticosteroid reduction |
64% reduction of corticosteroid use at 12 weeks, 61% decrease at 24 weeks. Reduced eosinophilia. Exacerbations recurred upon cessation. |
Kim 2010 |
|
| Nasal polyposis |
Severe nasal polyposis on corticosteroids. |
2 monthly IV 750 mg mepolizumab |
Significant reduction in blood ECP and IL-5Rα, nasal IL-5Rα, IL-6, IL-1β. Polyp improvement. |
Gevaert 2011 |
| Atopic dermatitis |
Moderate to severe AD | 2 weekly doses 750 mg mepolizumab |
Significant reduction of blood eos; no significant clinical improvement. |
Oldhoff 2005 |
| Moderate to severe AD | 2 weekly doses 750 mg mepolizumab |
No reduction in eczema lesion or tissue eos. | Oldhoff 2006 |
Mepolizumab has been used against a variety of eosinophil-mediated diseases, and studies have shown remarkable clearance of blood, lung, and bone marrow eosinophils. Among the HES, mepolizumab trials have focused on FIP1L1-PDGFRA-negative patients, since the FIP1L1-PDGFRA fusion gene promotes eosinophilia independent of IL-5 and is treated with the kinase inhibitor imitamib [54]. In patients requiring corticosteroid treatment for HES, 750 mg of mepolizumab was administered intravenously every 4 weeks for 36 weeks [54]. Of the patients who received mepolizumab, 84% lowered their prednisone dosage to below 10 mg/day as compared to 43% of the placebo group which achieved this end point. The intervention group also had lower blood eosinophil numbers (95% less than 600/μL), and the placebo group had a shorter time to treatment failure. Overall, hypereosinophilia was better controlled in the intervention group [54]. To determine if mepolizumab was equally effective for the lymphocytic and non-lymphocytic subsets of HES patients, 750 mg mepolizumab was administered every 4 weeks (55). This study showed that corticosteroid use could be reduced to a similar extent, but blood eosinophil numbers were not as attenuated in lymphocytic HES as they were in patients with non-lymphocytic HES.
When used to treat eosinophilic esophagitis, patients who were dysphagic (among other symptoms) received 10 mg/kg mepolizumab (up to 750 mg) every 4 weeks for 3 total treatments. All patients had improved clinical outcomes related to decreased dysphagia, blood eosinophil levels were decreased 6-fold, and three of the four patients had decreased esophageal epithelial hyperplasia [56]. In a study that looked more closely at the molecular modulations, Straumann demonstrated that the improvement in dysphagia was likely due to reduction in tenascin C and TGFβ1 in the esophagus, although this study showed only mild clinical improvements [57]. To determine if mepolizumab could be safely and effectively used in children, three monthly infusions of 0.55, 2.5, or 10 mg/kg mepolizumab were administered [58]. In children that had fewer than 20 eosinophils per high power field, there was an improvement in esophageal erythema, friability, and furrows or vertical lines.
Mepolizumab has also been used successfully for patients with Churg-Strauss syndrome (CSS) [59]. In a case report of a 28-year-old female, monthly infusions of 750 mg mepolizumab reduced eosinophils to normal levels, resolved the patient’s asthma, and improved lung parenchyma by chest radiographs [60]. In a clinical trial of patients with CSS and marked eosinophilia, four monthly infusions of 750 mg mepolizumab resulted in a 64% reduction of corticosteroid use at 12 weeks, and a 61% decrease at 24 weeks. Eosinophilia was also reduced, but upon cessation of the study exacerbations recurred [61].
Mepolizumab was unsuccessful in the treatment of atopic dermatitis [61,62]. In two studies by Oldhoff, mepolizumab did not improve patient prognosis as judged by physician global assessment (PGA), scoring atopic dermatitis SCORAD, and thymus- and activation-regulated chemokine (TARC) scores and by atopy patch test. In these studies, blood eosinophilia was reduced, but tissue eosinophilia was not [61,62].
Reslizumab
Clinical trials utilizing reslizumab are summarized in Table 2. In a reslizumab pilot study, 1 mg/kg reslizumab was administered intravenously once to patients with severe persistent asthma that was not controlled by corticosteroids [63]. Eosinophils were significantly reduced by about 50% after 2 days and slowly reestablished to about 18% 30 days after reslizumab intervention [63]. However, the only noticeable improvement was increased forced expiratory volume (FEV) at the 24-h post-treatment time point which was not sustained. In a later study of patients with poorly controlled asthma and sputum eosinophilia, the intervention group received monthly intravenous infusions of reslizumab. Results indicated that, while all patients had attenuated eosinophil numbers, only the nasal polyposis subgroup showed increased lung performance based on an Asthma Control Questionnaire (ACQ), which indicates that reslizumab may be an important therapeutic for certain disease subgroups [64].
In a limited study for HES, a single infusion of reslizumab (1 mg/kg) was administered to four adults with HES inadequately controlled by corticosteroids [65]. Three patients had significant reduction of eosinophilia, and two also had improved clinical symptoms. After cessation of treatment, eosinophil levels rebounded and exacerbations occurred. The fourth patient had no reduction in eosinophilia, with self-limited exacerbations [65].
Reslizumab treatment has also been used for pediatric eosinophilic esophagitis [66]. Patients received 1, 2 or 3 mg/kg reslizumab infusions monthly for 4 months. While all groups had a reduction of eosinophils, complete clearing of the esophagus did not occur and esophagitis improvement did not correlate with eosinophil reduction [66]. This study reported minimal adverse outcomes, the most common being cough, headache, congestion, and respiratory tract infection. Reslizumab is presently in further clinical trials for the aforementioned diseases to better elucidate the specificity of treatment, along with a clinical trial to evaluate its use in patients with loiasis, in an effort to limit host tissue damage associated with the loiasis-induced hypereosinophilia [67].
Targeting the IL-5 Receptor
There are two commercial therapeutics that target the IL-5R: benralizumab and TPI ASM8 [68-70]. Clinical trials with these agents are summarized in Table 2. Benralizumab is an IgG1κ mAb specific for IL-5Rα [68,69]. This drug has been developed to bind to the first fibronectin domain on IL-5Rα which attenuates eosinophil number by competitively inhibiting binding of IL-5 to the IL-5R, as well as by antibody-dependent cell-mediated cytotoxicity (ADCC) via Fcɣ RIIIa expressed by NK cells, macrophages, and neutrophils (see Fig. 1) [43,68,69]. The eosinophil-lowering capability is effective up to 56 days after administration. Benralizumab has been shown to be effective in clinical trials with asthma patients in whom it reduced eosinophil numbers in a dose-dependent manner, as well as reducing ECP levels. Benralizumab is also currently in phase II trials for the treatment of chronic obstructive pulmonary disease (COPD) [71].
TPI ASM8 is an antisense oligonucleotide that targets both the βc and eotaxin-1 [70]. While βc is an attractive target in that it would potentially inhibit the three Th2 cytokines, IL-5, IL-3, and GM-CSF, chronic treatment might result in pulmonary-alveolar proteinosis due to inadequate GM-CSF signaling [72,73]. Likewise, targeting βc would also target much broader subpopulations of leukocytes.
TPI ASM8 has been tested with 4-day and 14-day treatments [70]. These short-term treatment regimens and the short half-life of TPI ASM8 may mitigate potential chronic effects that could arise. In one study, it was determined that the half-life for the cocktail was less than 7 h and that the drug did not accumulate overtime [70]. In another study, mild asthmatics were antigen challenged and then inhaled TPI ASM8 with increasing doses for 4 days (twice daily for the first 3 days and then once on the fourth day after challenge). After 7 h there was a 60% reduction in sputum eosinophils and a 68% reduction after 24 h. Likewise, ECP levels were reduced after 3 days and early asthma response was attenuated, consequently so was late asthma response [70].
Hansen et al. have described an anti-βc mAb that antagonizes signaling in vitro [42]. The study showed that their anti-βc mAb inhibited GM-CSF-dependent colony formation by bone marrow cells from patients with chronic myelomonocytic leukemia, and hence might be a future therapeutic for such patients [42].
STRATEGIES FOR HARNESSING EOSINOPHIL-MEDIATED INFLAMMATION
Eosinophils and Cancer
Although most literature regarding the immune system and tumor cells concentrates on the role that CD8+ cytotoxic T cells and Th1 cytokines play in the tumor immune response, tumor-associated tissue eosinophilia (TATE) is widely recognized. [74-83]. The induction of a Th1 immune response (by M1 macrophages) is most commonly associated with increased tumor control and better prognosis of disease, while the Th2 immune response and its related molecules may exacerbate tumor growth and decrease tumor control [84,85]. Nonetheless, TATE in some cancers has been associated with an improved prognosis [74-80]. Hence, there is no agreement as to whether the presence of eosinophils is beneficial or detrimental to patient outcomes.
As reported by van Driel, TATE has a poor prognosis in cervical cancer, while Ishibashi reported that TATE in esophageal squamous cell carcinoma has no correlation with prognosis [81,82]. To investigate the potential effect of eosinophils on carcinomas, Wong compared the effect of IL-5 neutralizing antibody treatment to placebo on chemically induced squamous cell tumors in hamsters [83]. Eosinophil levels were decreased and the tumor burden was lower in anti-IL-5-treated hamsters. Thus, in this model, eosinophils appeared to be contributing to tumor pathogenesis. Eosinophils are involved in tissue remodeling and they produce VEGF as well as induce endothelial cell production of VEGF [86,87]. Furthermore, eosinophils produce pro-angiogenic cytokines: GM-CSF, FGF, and TGFα, as well as matrix degrading enzymes in the form of MMP, all of which are associated with remodeling [88-91]. Together, these data suggest that eosinophils could be used by tumor cells to promote their survival and expansion.
Conversely, patient survival or time to recurrence was improved in patients with TATE in some colon, breast, colorectal, nasopharyngeal, oral, gastric, and head and neck cancers [74-80], and metastasis was less frequent in colon cancer and head and neck cancer [75,76]. These studies have suggested that eosinophils could be used as a prognostic indicator such that patients with TATE could receive less aggressive interventions.
Eosinophils as a Tumoricide
Few studies have been performed to elucidate the connection between eosinophils and tumor prognosis. In mice, B16 OVA melanoma tumor clearance was dependent on eotaxin and degranulating eosinophils [92]. Additionally, IL-5 transgenic (Tg) mice, which overexpress IL-5 and have hypereosinophilia, had decreased tumor burden after fibrosarcoma induction, and eotaxin-deficient mice had greater fibrosarcoma burden. Eosinophil encapsulation of the tumor was prolonged in IL-5 Tg mice which may account for arrest of tumor growth [93]. Cormier furthers this observation in mice by demonstrating that eosinophil accumulation occurs early in subcutaneously injected melanoma tumors and localizes specifically to necrotic tumor areas and encapsulated areas [94]. A study performed in vitro on human-derived colon cancer cells demonstrated that eosinophils can kill tumor cells in a cell contact-dependent mechanism requiring the adhesion molecules CD11a and CD18, and that key molecules involved in the cytotoxic effects were TNF, ECP, and Granzyme A [95].
Given that TATE is well described in cancer patients, and can be associated with improved prognosis, the promotion of eosinophil effector function is a potentially viable strategy against tumors. Since the degranulation of eosinophils is key for eosinophil effector function, any strategy that would induce degranulation may be useful. The chemokine eotaxin can stimulate eosinophil degranulation, so eotaxin promotion might be beneficial. Indeed, an eosinophilotactic molecule was reportedly produced from a large-cell anaplastic carcinoma of the lung, so it may be possible to illicit a chemokine dependent response via modulation of the tumor [96]. Likewise, many cytokines are associated with eosinophil degranulation, including IL-5, IL-33, and GM-CSF [97, 98]. Another strategy would be to cross-link the surface receptors like FcαR using an antibody specific to the receptor [99].
Conclusions
IL-5 and IL-5Rα are clearly established effective targets for decreasing eosinophilia. Most strategies have successfully utilized mAb, but more recent strategies include using antisense oligonucleotides. Although initially developed for potential use in the treatment of allergic asthma, broader therapeutic potential is being demonstrated for subsets of HES. In addition, a new avenue of therapy is the use of anti-IL-5 therapeutics to dampen rather than ablate exuberant eosinophil responses to parasitic infections in an effort to prevent bystander host tissue damage. Furthermore, anti-IL-5Rα mAb capable of ADCC might be useful as treatment for eosinophilic leukemia. An important consideration in the use of eosinophil-modulating therapies is their potential adverse effects. Thus far, the agents currently in human trials have had minimal adverse effects reported. Other potential targets for modulating eosinophilic inflammation include chemokines like eotaxin and eosinophil adhesion molecules. The potential to harness eosinophils against cancers is an exciting frontier that could potentially lead to technological breakthroughs in clinical medicine.
Acknowledgment
This work is supported in part by National Institutes of Health grants R01 AI036936 and U19 AI071130.
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 2.Hogan SP, Rosenberg HF, Moqbel R, et al. Eosinophils: Biological properties and role in health and disease. Clin Exp Allergy. 2008;38(5):709–50. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard C, Rothenberg ME. Biology of the eosinophil. Adv Immunol. 2009;101:81–121. doi: 10.1016/S0065-2776(08)01003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shamri R, Xenakis JJ, Spencer LA. Eosinophils in innate immunity: an evolving story. Cell Tissue Res. 2011;343(1):57–83. doi: 10.1007/s00441-010-1049-6. An expert group of immunology, allergy, hematology, and pathology experts propose updated and refined criteria for the classification of eosinophilic disorders and related syndromes in this manuscript.
- 5.Gleich GJ, Adolphson CR, Leiferman KM. The biology of the eosinophilic leukocyte. Annu Rev Med. 1993;44:85–101. doi: 10.1146/annurev.me.44.020193.000505. [DOI] [PubMed] [Google Scholar]
- 6.Arai KI, Lee F, Miyajima A, et al. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- 7.Miyajima AT, Kitamura N, Harada T, Yokota T, Arai K. Cytokine receptors and signal transduction. Annu Rev Immunol. 1992;10:295–331. doi: 10.1146/annurev.iy.10.040192.001455. [DOI] [PubMed] [Google Scholar]
- 8.Komiya A, Nagase H, Yamada H, et al. Concerted expression of eotaxin-1, eotaxin-2, and eotaxin-3 in human bronchial epithelial cells. Cell Immunol. 2003;225(2):91–100. doi: 10.1016/j.cellimm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Amerio P, Frezzolini A, Feliciani C, et al. Eotaxins and CCR3 receptor in inflammatory and allergic skin diseases: therapeutical implications. Curr Drug Targets Inflamm Allergy. 2003;2(1):81–94. doi: 10.2174/1568010033344480. [DOI] [PubMed] [Google Scholar]
- 10.Halwani R, Al-Muhsen S, Al-Jahdali H, Hamid Q. Role of transforming growth factor-β in airway remodeling in asthma. American Journal of Respiratory Cell and Molecular Biology. 2011;44:127–33. doi: 10.1165/rcmb.2010-0027TR. [DOI] [PubMed] [Google Scholar]
- 11.Moore B, Murphy RF, Agrawal DK. Interaction of tgf-beta with immune cells in airway disease. Curr Mol Med. 2008;8(5):427–36. doi: 10.2174/156652408785160943. [DOI] [PubMed] [Google Scholar]
- 12.Klion AD, Nutman TB. The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol. 2004;113(1):30–7. doi: 10.1016/j.jaci.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 13.Lotfi R, Lee JJ, Lotze MT. Eosinophilic granulocytes and damage-associated molecular pattern molecules (DAMPs): role in the inflammatory response within tumors. Journal of Immunother. 2007;30:16–28. doi: 10.1097/01.cji.0000211324.53396.f6. [DOI] [PubMed] [Google Scholar]
- 14.Shin MH, Lee YA, Min DY. Eosinophil-mediated tissue inflammatory responses in helminth infection. Korean J Parasitol. 2009;47(Suppl):S125–31. doi: 10.3347/kjp.2009.47.S.S125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kvarnhammar AM, Cardell LO. Pattern Recognition Receptors in Human Eosinophils. Immunol. 2012;136(1):11–20. doi: 10.1111/j.1365-2567.2012.03556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohn L, Elias JA, Chupp GL. Asthma: Mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 17.Foster PS, Mould AW, Yang M, et al. Elemental Signals regulating eosinophil accumulation in the lung. Immunol. Reviews. 2001;179:173–181. doi: 10.1034/j.1600-065x.2001.790117.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee JJ, Dimina D, Macias MP, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305(5691):1773–76. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 19.Humbles AA, Lloyd CM, McMillan SJ, et al. A critical role for eosinophils in allergic airway remodeling. Science. 2004;305(5691):1776–79. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 20.Cho JY, Miller M, Baek KJ, et al. Inhibition of airway remodeling in IL-5-deficient mice. J Clin Invest. 2004;113:551–60. doi: 10.1172/JCI19133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson DS, Hamid Q, Ying S, et al. Predominant TH2-like brochoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 22.Hamelmann E, Oshiba A, Loader J, et al. Antiinterleukin-5 antibody prevents airway hyperresponsiveness in a murine model of airway sensitization. Am J Respir Crit Care Med. 1997;155:819–825. doi: 10.1164/ajrccm.155.3.9117011. [DOI] [PubMed] [Google Scholar]
- 23.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JJ, McGarry MP, Farmer SC, et al. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med. 1997;185:2143–2156. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klion AD. Hypereosinophilic syndrome: current approach to diagnosis and treatment. Annu Rev Med. 2009;60:293–306. doi: 10.1146/annurev.med.60.062107.090340. [DOI] [PubMed] [Google Scholar]
- 26.Simon HU, Rothenberg ME, Bochner BS, et al. Refining the definition of hypereosinophilic syndrome. J Allergy Clin Immunol. 2010;126(1):45–49. doi: 10.1016/j.jaci.2010.03.042. This article discusses the shortcomings of the old HES definition and proposes new considerations for HES classification.
- 27.Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012 Mar; doi: 10.1016/j.jaci.2012.02.019. Epub ahead of print. An expert group of immunology, allergy, hematology, and pathology experts propose updated and refined criteria for the classification of eosinophilic disorders and related syndromes in this manuscript.
- 28.Hogan SP, Rothenberg ME. Eosinophil Function in Eosinophil-associated Gastrointestinal Disorders. Curr Allergy Asthma Rep. 2006;6(1):65–71. doi: 10.1007/s11882-006-0013-8. [DOI] [PubMed] [Google Scholar]
- 29.DeBrosse CW, Rothenberg ME. Allergy and eosinophil-associated gastrointestinal disorders (EGID) Curr Opin Immunol. 2008;20(6):703–8. doi: 10.1016/j.coi.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dellon ES. Eosinophilic esophagitis: diagnostic tests and criteria. Curr Opin Gastroenterol. 2012;28(4):382–8. doi: 10.1097/MOG.0b013e328352b5ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest. 2001;107(1):83–90. doi: 10.1172/JCI10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pagnoux C, Guilpain P, Guillevin L. Churg-Strauss syndrome. Curr Opin Rheumatol. 2007;19(1) doi: 10.1097/BOR.0b013e3280119854. [DOI] [PubMed] [Google Scholar]
- 33.Baldini C, Talarico R, Della Rossa A, Bombardieri S. Clinical manifestations and treatment of Churg-Strauss syndrome. Rheum Dis Clin North Am. 2010;36:527–43. doi: 10.1016/j.rdc.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Blaiss MS. Expanding the evidence base for the medical treatment of nasal polyposis. J Allergy Clin Immunol. 2005;116:1272–4. doi: 10.1016/j.jaci.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Palm NW, Rosenstein RK, Medzhitov R. Allergic Host Defences. Nature. 2012;484(7395):465–72. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in health and disease: The LIAR hypothesis. Clinical and Experimental Allergy. 2010;40(4):563–575. doi: 10.1111/j.1365-2222.2010.03484.x. This provocative review challenges current dogma classifying eosinophils as simply end-stage effector cells and proposes a novel, counter concept in which accumulating tissue eosinophils are actually regulators of Local Immunity And/or Remodeling/Repair in both health and disease - the LIAR hypothesis.
- 37.Mattes J, Foster PS. Regulation of eosinophil migration and Th2 cell function by IL-5 and eotaxin. Current Drug Targets-Inflammation & Allergy. 2003;2(2):169–74. doi: 10.2174/1568010033484214. [DOI] [PubMed] [Google Scholar]
- 38.Bazan JF. Haemopoetic receptors and helical cytokines. Immunol Today. 1990;10:350–54. doi: 10.1016/0167-5699(90)90139-z. [DOI] [PubMed] [Google Scholar]
- 39.Dickason RR, Huston MM, Huston DP. Delineation of IL-5 domains, predicted to engage the IL-5 receptor complex. J Immunol. 1996;156:1030–7. [PubMed] [Google Scholar]
- 40.Carr PD, Conlan F, Ford S, Ollis DL, Young IG. An improved resolution structure of the human β common receptor involved in IL-3, IL-5 and GM-CSF signaling which gives better definition of the high-affinity binding epitope. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2006;62(6) doi: 10.1107/S1744309106016812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansen G, Hercus TR, McClure BJ, et al. The structure of the GM-CSF receptor complex reveals a distinct mode of cytokine receptor activation. Cell. 2008;134(3):496–507. doi: 10.1016/j.cell.2008.05.053. [DOI] [PubMed] [Google Scholar]
- 42.Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor α mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125(6):1344–53. doi: 10.1016/j.jaci.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Martinez-Moczygemba M, Huston DP. Biology of Common β receptor-signalling cytokine: IL-3, IL-5 and GM-CSF. J Allergy Clin Immunol. 2003;112:653–65. doi: 10.1016/S0091. [DOI] [PubMed] [Google Scholar]
- 44.Shardonofsky FR, Venzor J, 3rd, Barrios R, et al. Therapeutic efficacy of an anti-IL-5 monoclonal antibody delivered into the respiratory tract in a murine model of asthma. J Allergy Clin Immunol. 1999;104:215–21. doi: 10.1016/s0091-6749(99)70138-7. [DOI] [PubMed] [Google Scholar]
- 45.Zhang JI, Kuvelkar R, Murgolo J, et al. Mapping and characterization of the epitopes of Sch 55700, a humanized mAb, that inhibits human IL-5. International Immunology. 1999;11(12):1935–1943. doi: 10.1093/intimm/11.12.1935. [DOI] [PubMed] [Google Scholar]
- 46.Hart TK, Cook RM, Zia-Amirhosseini P, et al. Preclinical efficacy and safety of mepolizumab (SB-240563), a humanized monoclonal antibody to IL-5, in cynomolgus monkeys. J Allergy Clinical Immunol. 2001;108:2–250. doi: 10.1067/mai.2001.116576. [DOI] [PubMed] [Google Scholar]
- 47.Egan RW, Athwahl D, Chou CC, et al. Pulmonary biology of anti-interleukin 5 antibodies. Mem Inst Oswaldo Cruz. 1997;92:69–73. doi: 10.1590/s0074-02761997000800011. [DOI] [PubMed] [Google Scholar]
- 48.Flood-Page P, Swenson C, Faiferman I, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med. 2007;176(11):1062–71. doi: 10.1164/rccm.200701-085OC. [DOI] [PubMed] [Google Scholar]
- 49.Leckie MJ, ten Brinke A, Khan J, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper- responsiveness, and the late asthmatic response. Lancet. 2000;356(9248):2144–2148. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 50.Nair P, Pizzichini MMM, Kjarsgaard M, et al. Mepolizumab for Prednisone-Dependent Asthma with Sputum Eosinophilia. NEJM. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 51.Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. NEJM. 2009;360:973–84. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gevaert P, Van Bruaene N, Cattaert T, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011;128(5):989–95. doi: 10.1016/j.jaci.2011.07.056. [DOI] [PubMed] [Google Scholar]
- 53.Rothenberg ME, Klion AD, Roufosse FE, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. NEJM. 2008;358(12):1215–28. doi: 10.1056/NEJMoa070812. [DOI] [PubMed] [Google Scholar]
- 54.Roufosse F, de Lavareille A, Schandené L, et al. Mepolizumab as a corticosteroid-sparing agent in lymphocytic variant hypereosinophilic syndrome. J Allergy Clin Immunol. 2010;126(4):826–35. doi: 10.1016/j.jaci.2010.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stein ML, Collins MH, Villanueva JM, et al. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy CLin Immunol. 2006;118(6):1312–1319. doi: 10.1016/j.jaci.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 56.Straumann A, Conus S, Grzonka P, et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomized, placebo-controlled, double-blind trial. Gut. 2010;59:21–30. doi: 10.1136/gut.2009.178558. [DOI] [PubMed] [Google Scholar]
- 57.Assa’ad AH, Gupta SK, Collins MH, et al. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141(5):1593–1604. doi: 10.1053/j.gastro.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 58.Kahn JE, Grandpeix-Guyodo C, Marroun I, et al. Sustained response to mepolizumab in refractory Churg-Strauss syndrome. J Allergy Clin Immunol. 2010;125:267–70. doi: 10.1016/j.jaci.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 59.Kim S, Marigowda G, Oren E, Israel E, Wechsler M. Mepolizumab as a steroid-sparing treatment option in patients with Churg-Strauss syndrome. J Allergy Clin Immunol. 2010;125:1336–43. doi: 10.1016/j.jaci.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 60.Oldhoff JM, Darsow U, Werfel T, et al. Anti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitis. Allergy. 2005;60(5):693–696. doi: 10.1111/j.1398-9995.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- 61.Oldhoff JM, Darsow U, Werfel T, et al. No effect of anti-interleukin-5 therapy (mepolizumab) on the atopy patch test in atopic dermatitis patients. Int Arch Allergy Immunol. 2006;141:290–294. doi: 10.1159/000095299. [DOI] [PubMed] [Google Scholar]
- 62.Kips JC, O’Conner BJ, Langley SJ, Woodcock A. Effect of SCH55700, a humanized anti-human interleukin-5 antibody, in severe persistent asthma: A pilot study. Am J Resp Crit Med. 2003;167(12):1655–59. doi: 10.1164/rccm.200206-525OC. [DOI] [PubMed] [Google Scholar]
- 63.Castro M, Mathur S, Hargreave F, et al. Reslizumab for poorly controlled, eosinophilic asthma: A randomized, placebo controlled study. Am J Resp Crit Med. 2011;184:1125–32. doi: 10.1164/rccm.201103-0396OC. [DOI] [PubMed] [Google Scholar]
- 64.Klion AD, Law MA, Noel P, et al. Safety and efficacy of the monoclonal anti-interleukin-% antibody SCH55700 in the treatment of patients with hypereosinophilic syndrome. Blood. 2004;103:2939–41. doi: 10.1182/blood-2003-10-3620. [DOI] [PubMed] [Google Scholar]
- 65.Spergel JM, Rothenberg ME, Collins MH, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: Results of a double blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129(2):456–63. doi: 10.1016/j.jaci.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 66.NIAID . Internet. National Library of Medicine (US); Bethesda (MD): 2000. A randomized, placebo-controlled, double-blind pilot study of single-dose humanized anti-IL5 antibody (reslizumab) for the reduction of eosinophilia following diethylcarbamazine treatment of Loa Loa infection. ClinicalTrials.gov. [cited 2012 May 17]. Available from: http://clinicaltrials.gov/ct2/show/NCT01111305?term=reslizumab&rank=9 NLM Identifier: NCT01111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Devos R, Guisez Y, Plaetinck G, et al. Covalent modification of the interleukin-5 receptor by isothiazolones leads to inhibition of the binding of interleukin-5. Eur J Biochem. 1994;225(2):635–40. doi: 10.1111/j.1432-1033.1994.00635.x. [DOI] [PubMed] [Google Scholar]
- 68.Busse WW, Katial R, Gossage D, et al. Safety profile, pharmacokinetics, and biologic activity of MEDI-563, and anti-IL-5 receptor alpha antibody, in a phase I study of subjects with mild asthma. J Allergy Clin Immunol. 2010;125:1237–44. doi: 10.1016/j.jaci.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 69.Gauvreau GM, Pageau R, Seguin R, et al. Dose-response effects of TPI ASM8 in asthmatics after allergen. Allergy. 2011;66:1242–1248. doi: 10.1111/j.1398-9995.2011.02638.x. [DOI] [PubMed] [Google Scholar]
- 70.MedImmune LLC . Internet. National Library of Medicine (US); Bethesda (MD): 2000. A Study to Evaluate the Effectiveness of a Drug (MEDI-563) in Subjects With Chronic Obstructive Pulmonary Disease (COPD) ClinicalTrials.gov. [cited 2012 May 17]. Available from: http://clinicaltrials.gov/ct2/show/NCT01227278?term=MEDI-563&rank=5 NLM Identifier: NCT01227278. [Google Scholar]
- 71.Asquith KL, Ramshaw HS, Hansbro PM, et al. The IL-3/IL-5/GM-CSF common receptor plays a pivotal role in the regulation of Th2 immunity and allergic airway inflammation. J Immunol. 2008;180:1199–1206. doi: 10.4049/jimmunol.180.2.1199. [DOI] [PubMed] [Google Scholar]
- 72.Martinez-Moczygemba M, Huston DP. Immune Dysregulation in the Pathogenesis of Pulmonary Alveolar Proteinosis. Cur Allergy Asthma Rep. 2010;10:320–325. doi: 10.1007/s11882-010-0134-y. [DOI] [PubMed] [Google Scholar]
- 73.Dorta RG, Landman G, Kowalski LP, et al. Tumour-associated tissue eosinophilia as a prognostic factor in oral squamous cell carcinomas. Histopathology. 2002;41(2):152–7. doi: 10.1046/j.1365-2559.2002.01437.x. [DOI] [PubMed] [Google Scholar]
- 74.Pretlow TP, Keith EF, Cryar AK, et al. Eosinophil infiltration of human colonic carcinomas as a prognostic indicator. Cancer Res. 1983;43:2997–3000. [PubMed] [Google Scholar]
- 75.Goldsmith MM, Belchis DA, Cresson DH, MerrittWD III, Askin FB. The importance of the eosinophil in head and neck cancer. Otolaryngology Head and Neck Surgery. 1992;106:27–33. doi: 10.1177/019459989210600124. [DOI] [PubMed] [Google Scholar]
- 76.Ownby HE, Roi LD, Isenberg RR, Brennan MJ. Peripheral Lymphocyte and Eosinophil Counts as Indicators of Prognosis in Primary Breast Cancer. Cancer. 1983;52:126–130. doi: 10.1002/1097-0142(19830701)52:1<126::aid-cncr2820520123>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 77.Leighton SE, Teo JG, Leung SF, et al. Prevalence and prognostic significance of tumor-associated tissue eosinophilia in nasopharyngeal carcinoma. Cancer. 1996;77(3):436–40. doi: 10.1002/(SICI)1097-0142(19960201)77:3<436::AID-CNCR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 78.Fernandez-Acenero MJ, Galindo-Gallego M, Sanz J, Aljama A. Prognostic influence of tumor-associated eosinophilic infiltrate in colorectal carcinoma. Cancer. 2000;88:1544–8. [PubMed] [Google Scholar]
- 79.Iwasaki K, Torisu M, Fujimura T. Malignant tumor and eosinophils. I. Prognostic significance in gastric cancer. Cancer. 1986;58:1321–7. doi: 10.1002/1097-0142(19860915)58:6<1321::aid-cncr2820580623>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 80.van Driel WJ, Kievit-Tyson P, van den Broek, et al. Presence of an eosinophilic infiltrate in cervical squamous carcinoma results from a type 2 immune response. Gynecologic Oncology. 1999;74:188–95. doi: 10.1006/gyno.1999.5431. [DOI] [PubMed] [Google Scholar]
- 81.Ishibashi S, Ohashi Y, Suzuki T, et al. Tumor-associated tissue eosinophilia in human esophageal squamous cell carcinoma. Anticancer Res. 2006;26:1419–24. [PubMed] [Google Scholar]
- 82.Wong DTW, Bowen SM, Elovic A, Gallagher GT, Weller PF. Eosinophil ablation and tumor development. Oral Oncology. 1999;35:496–501. doi: 10.1016/s1368-8375(99)00023-8. [DOI] [PubMed] [Google Scholar]
- 83.Gordon S, Martinez FO. Alternative activation of macrophages: mechanisms and functions. Nat Rev Immunol. 2003;3(1) doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 84.Sica A, Mantovani A. Macrophage diversity and polarization: in vivo veritas. J Am Soc Hemat. 2006;108(2):408–409. [Google Scholar]
- 85.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The Role of Myeloid Cells in Tumour Angiogenesis. Nature Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 86.Puxeddu I, Alian A, Piliponsky AM, et al. Human peripheral blood eosinophils induce angiogenesis. Int J Biochem Cell Biol. 2005;37:628–36. doi: 10.1016/j.biocel.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 87.Wong DT, Weller PF, Galli SJ, et al. Human eosinophils express transforming growth factor alpha. J Exp Med. 1990;172:673–681. doi: 10.1084/jem.172.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hoshino M, Takahashi M, Aoike N. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. J Allergy Clin Immunol. 2001;107:295–301. doi: 10.1067/mai.2001.111928. [DOI] [PubMed] [Google Scholar]
- 89.Kita H, Ohnishi T, Okubo Y, et al. Granulocyte/macrophage colony-stimulating factor and interleukin 3 release from human peripheral blood eosinophils and neutrophils. J Exp Med. 1991;174:745–748. doi: 10.1084/jem.174.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ohno I, Ohtani H, Nitta Y, et al. Eosinophils as a source of matrix metalloproteinase-9 in asthmatic airway inflammation. Am J Resp Cell Molecular Biol. 1997;16(3):212–219. doi: 10.1165/ajrcmb.16.3.9070604. [DOI] [PubMed] [Google Scholar]
- 91.Mattes J, Hulett M, Xie W, et al. Immunotherapy of cytotoxic T cell resistant tumors by T helper 2 cells: an eotaxin and STAT 6-dependent process. J Exp Med. 2003;197:387–393. doi: 10.1084/jem.20021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Simson L, Ellyard JI, Dent LA, et al. Regulation of carcinogenesis by IL-5 and CCL11: A potential role for eosinophils in tumor surveillance. J Immunol. 2007;178(7):4222–4229. doi: 10.4049/jimmunol.178.7.4222. [DOI] [PubMed] [Google Scholar]
- 93.Cormier SA, Taranova AG, Bedient C, et al. Pivotal advance: eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J Leukoc Biol. 2006;9:1131–9. doi: 10.1189/jlb.0106027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Legrand F, Driss V, Delbeke M, et al. Human Eosinophils exert TNFa and Granzyme A-mediated tumorcidal activity toward colon carcinoma cells. J Immunol. 2010;185(12):7443–51. doi: 10.4049/jimmunol.1000446. This manuscript provides the first mechanistic evidence for innate responses of eosinophils against colon carcinoma cells.
- 95.Wasserman SI, Goetzl EJ, Ellman L, Austen KF. Tumor Associated Eosinophilotactic Factor. NEJM. 1974;290:420–424. doi: 10.1056/NEJM197402212900802. [DOI] [PubMed] [Google Scholar]
- 96.Cherry BW, Yoon J, Bartemes KR, Iijima K, Kita H. A Novel IL-1 Family Cytokine, IL-33, Potently Activates Human Eosinophils. J Allergy Clin Immunol. 2008;121(6):1484. doi: 10.1016/j.jaci.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carlson M, Peterson C, Venge P. The influence of IL-3, IL-5, and GM-CSF on normal human eosinophil and neutrophil C3b-induced degranulation. Allergy. 1993;48(6):437–42. [PubMed] [Google Scholar]
- 98.Pleass RJ, Lang ML, Kerr MA, Woof JM. IgA is a more potent inducer of NADPH oxidase activation and degranulation in blood eosinophils than IgE. Molecular Immunol. 2007;44:1401–1408. doi: 10.1016/j.molimm.2006.05.002. [DOI] [PubMed] [Google Scholar]