Abstract
Pancreatic ductal adenocarcinoma is a devastating disease, and patient outcomes have not improved in decades. Treatments that target tumor cells have largely failed. This could be because research has focused on cancer cells and the influence of the stroma on tumor progression has been largely ignored. The focus of pancreatic cancer research began to change with the identification of pancreatic stellate cells, which produce the pancreatic tumor stroma. There is compelling in vitro and in vivo evidence for the influence of pancreatic stellate cells on pancreatic cancer development; several recent preclinical studies have reported encouraging results with approaches designed to target pancreatic stellate cells and the stroma. We review the background and recent advances in these areas, along with important areas of future research that could improve therapy.
Keywords: Pancreatic Stellate Cells, Pancreatic Cancer, Inflammation, Immune Surveillance
The cellular and molecular features of pancreatic cancer cells have been studied for more than 3 decades. Unfortunately, despite development of therapies to target specific pathways in cancer cells (in addition to surgical and radiotherapy approaches), there has been little improvement in the outcomes of patients with pancreatic ductal adenocarcinoma (PDAC), possibly because an important element of the tumor, the abundant desmoplasia, had been largely ignored, and most studies have focused on cancer cells themselves. Most experimental models used to develop therapeutics for pancreatic cancer do not accurately replicate the dense stroma that is part of human tumors. In recent years, with the identification of the cells that produce the stroma, researchers have been able to turn their attention to the role of desmoplasia in pancreatic cancer pathobiology.1
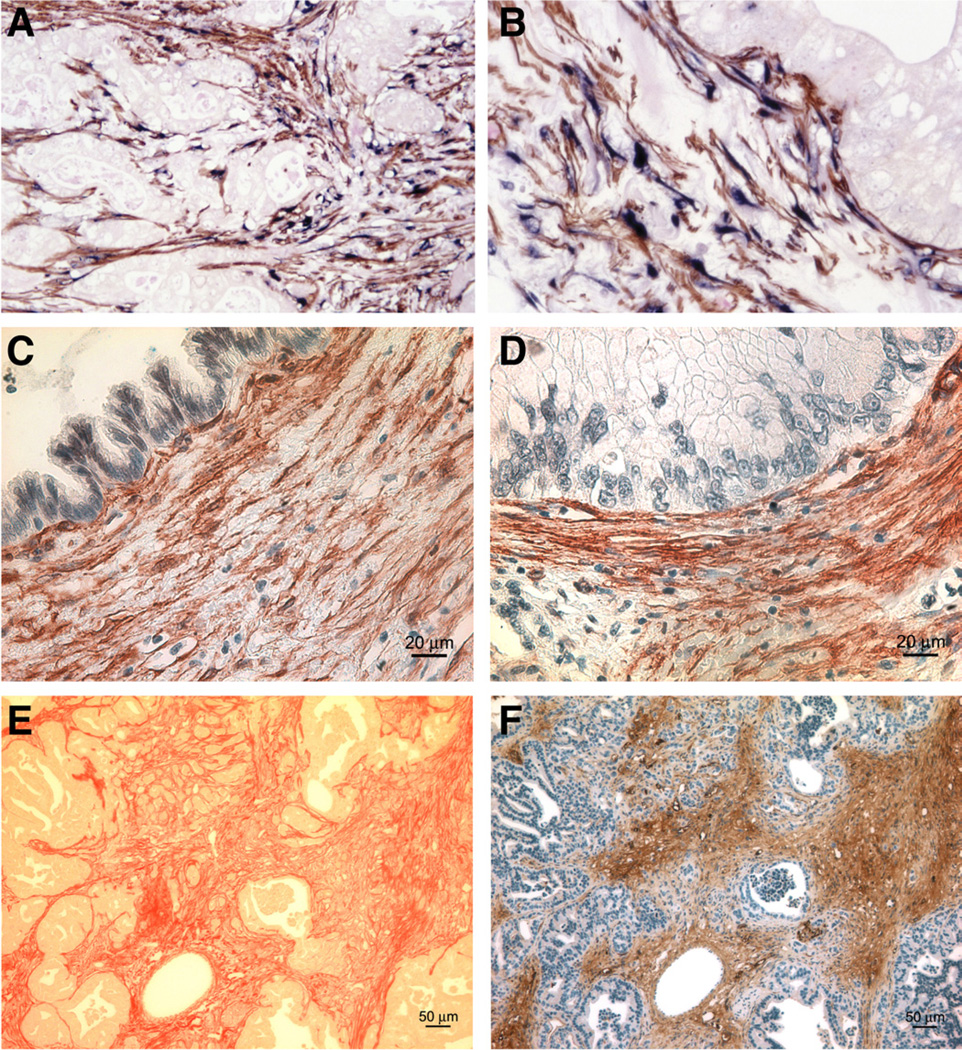
In 2004, pancreatic stellate cells (PaSCs), which contribute to fibrosis during development of chronic pancreatitis, were found to produce the extracellular matrix (ECM) proteins that comprise the pancreatic tumor stroma.2 Using immunohistochemical analyses of human PDAC sections for stellate cell-selective markers (ie, desmin, glial fibrillary acidic protein, nestin, and neural cell adhesion molecule), as well as α–smooth muscle actin as a marker of activation, Apte et al2 demonstrated the presence of activated PaSCs in the tumor stroma. They found that activated PaSCs were the specific cellular source of ECM proteins, and that α–smooth muscle actin messenger RNA and collagen messenger RNA were restricted to the tumor stroma. They also showed that activated PaSCs were the predominant source of collagen in the tumor stroma (Figure 1).
Figure 1.
Identification of activated PaSCs in the stroma of PDAC and PanIN. (A) Immuno-histochemistry for α–smooth muscle actin (αSMA) (brown) and procollagen α1(I) (blue) on human PDAC tissues indicates that active PaSCs are the main source of collagens in the tumor stroma. Low- (A) and high- (B) power views of a representative section of human pancreatic cancer tissue. (C,D) Immunohis-tochemical staining for αSMA (brown) reveals abundant activated PaSCs surrounding Pan-INs in human pancreas. (E, F) Sirius red (red; E) and αSMA (brown; F) staining on pancreatic tissue from a GEM model (KrasLSL-G12D/+; Pdxcre/+) illus- trates the abundance of collagens and activated PaSCs surrounding PanINs.
More recently, activated PaSCs were found to surround human pancreatic intraepithelial neoplastic lesions (Pan-INs) (Figure 1; unpublished observations, AL, SJP, and David Dawson, UCLA), indicating that they might function during early stages of cancer development. Activated stellate cells also surround PanINs that develop in genetically engineered mice (KrasLSL-G12D/+; Pdxcre/+),3 and are likely to be of pancreatic origin. However, bone marrow–derived cells have also been reported to localize to the injured pancreas in response to chemotactic signals (albeit in relatively small numbers) in rodent models of pancreatitis,4,5 as well as in mice after induction of pan-creatic cancer with a combination of the carcinogen dim-ethylbenzanthracene and pancreatitis-inducer cerulein.6
Because of the close proximity of PaSCs and cancer cells within the tumor, there have been many studies to investigate how they might interact.
Interactions Between PaSC and Cancer Cells in Culture
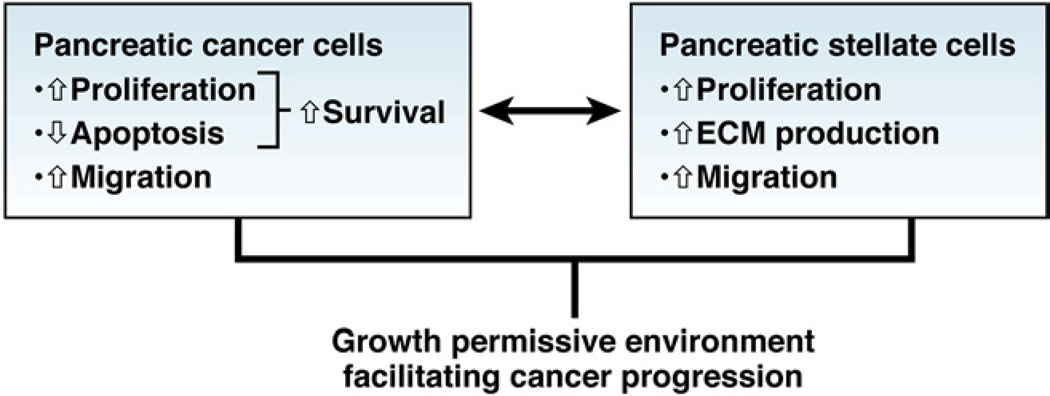
Coculture experiments with PaSCs and pancreatic cancer cell lines, or in which one cell type is exposed to conditioned medium from the other, support the concept that pancreatic cancer cells recruit PaSCs, which promote tumor growth and local invasion (Figure 2). Although it has been proposed that under conditions of chronic inflammation, fibroblasts can induce transformation of epithelial cells, this concept has not been tested with PaSCs and pancreatic epithelial cells.
Figure 2.
Close relationship between pancreatic cancer cells and PaSCs pancreatic cancer cells recruit PaSCs to their immediate vicinity and promote fibrogenic responses in PaSCs. PaSCs reciprocate by facilitating cancer cell growth as well as local invasion.
Pancreatic cancer cells stimulate proliferation and migration of PaSCs in culture, as well as their production of ECM components.1 The cancer cell—induced increase in ECM synthesis by PaSCs is likely to be mediated by transforming growth factor (TGF)–β1 and fibroblast growth factor, whereas proliferation of PaSC is promoted by platelet-derived growth factor.7 Other studies have reported that cyclo-oxygenase−28 and trefoil factor 19 (a secretory protein that is up-regulated by pancreatic cancer cells, but not expressed by normal pancreas cells) promote proliferation of PaSC in response to factors secreted by cancer cells. Cyclo-oxygenase–2 is up-regulated in PaSCs exposed to the pancreatic cancer cell line PANC-1, and inhibition of cyclo-oxygenase−2 prevents PANC-1–induced proliferation of PaSC. Extracellular signal-regulated kinases 1 and 2 regulate cancer cell-induced proliferation of PaSC.10–12 Pandol et al3 reported that PanIN cells isolated from genetically engineered KrasLSL−G12D/+; Pdxcre/+ mice that develop pancreatic cancer induced proliferation and fibrogenic responses in mouse PaSC, indicating that preneoplastic lesions are able to activate PaSCs early during tumor development.
PaSCs, in turn, stimulate cancer cell proliferation and inhibit cancer cell apoptosis, to increase the population of cancer cells.13 PaSCs also promote migration and the epithelial–mesenchymal transition in cancer cells, indicated by their reduced expression of epithelial markers, such as E-cadherin and increased expression of mesenchymal markers, such as vimentin and snail.14 The ability of PaSC to induce the epithelial–mesenchymal transition in cancer cells might account for the increase in cancer cell migration observed after their exposure to PaSCs. The factors that mediate the effects of PaSCs on cancer cells remain to be characterized. However, PaSC-induced proliferation of cancer cells is thought to be mediated, at least in part, by platelet-derived growth factor.15 Other factors secreted by PaSC that require further study include insulin-like growth factor, epidermal growth factor, hepato-cyte growth factor, TGFβ1, and other inflammatory cytokines.
Ikenaga et al16 observed functional heterogeneity among human PaSCs that can determine their effects on cancer cells. They identified a subset of PaSCs, isolated from resected human pancreatic tissue, that express increased levels of CD10 (a cell membrane–associated matrix metalloproteinase). CD10-expressing PaSCs had a greater inductive effect on cancer cell invasion and proliferation than CD10-negative PaSCs.
Hypoxia might also affect PaSC function in pancreatic tumors. Pancreatic tumors are hypovascular, and cancer cells are thought to thrive under hypoxic conditions. In an in vitro study, Eguchi et al17 showed that the effect of PaSCs on cancer cell invasion is significantly greater under hypoxic (1% oxygen) than normoxic (21% oxygen) conditions. Hypoxia was shown to increase secretion of connective tissue growth factor (CTGF) by PaSCs, and knockdown of CTGF in PaSCs reduced cancer cell invasion under hypoxic conditions. These findings indicate that PaSC-induced cancer cell invasion in the hypoxic environment of the tumor might be mediated by CTGF produced by PaSCs.
PaSCs might also be involved in the resistance of pancreatic cancer to chemo- and radiotherapy and recurrence after treatment. Using a coculture system, Mantoni et al18 demonstrated that PaSCs protect cancer cells from radiation and increase their clonogenic survival via a pathway that requires β1-integrin. Reducing β1-integrin and focal adhesion kinase activity in cancer cells using small interfering RNAs blocked the radioprotective effects of PaSCs on cancer cells.
Recurrence of pancreatic cancer has been attributed to a cancer stem cell niche that remains unaffected by therapy. Hamada et al19 found that PaSCs increase the stem cell phenotype of cancer cells based on increased expression of markers, such as nestin, ABCG2, and LIN28. It is possible that PaSC-induced stemness of a pool of cancer cells facilitates recurrence of the disease.
Most of the described studies were performed in 2-di-mensional cultures of PaSCs and cancer cells. In 3-dimensional culture systems,20,21 PaSCs and cancer cells are embedded in collagen and Matrigel to better simulate the tumor microenvironment. PaSCs induce changes in cell adhesion molecules in cancer cells, such as decreasing expression of E-cadherin, increasing levels of β-catenin, and translocating Ezrin to basal surface of the cells, which was associated with increased invasive activity.21
In Vivo Studies
Although there is strong evidence from in vitro studies that cancer cell interact with PaSCs, it is important that these observations are validated in animal models, such as mice with tumor xenografts or genetic-engineered mouse (GEM) models. Bachem et al7 subcutaneously injected human pancreatic cancer cells, alone or in combination with human PaSCs, into the flanks of immunocompromised mice. They found that cancer cells coinjected with PaSCs proliferated and formed tumors more rapidly than cancer cells injected alone. In histologic analysis, the tumors formed from the cancer cells were found to have a prominent stromal reaction, supporting the in vitro observations of PaSC-induced cancer cell proliferation. However, subcutaneous models do not have physiologically relevant tumor microenvironment, and are not useful for studies of metastasis—one of the prominent features of pancreatic cancer. To overcome these drawbacks, researchers developed orthotopic and GEM models of pancreatic cancer in which tumors develop in an anatomically relevant location (within an appropriate microenvironment) and metastasize to locoregional and distant sites.
In one of the earliest studies to identify a stromal reaction in orthotopic pancreatic tumors, Lohr et al22 injected PANC-1 cells transfected with TGFβ1 complementary DNA into the pancreas of nude mice. They found that that increased TGFβ1 expression by the cells induced formation of extensive desmoplasia around the tumor. Although the role of PaSCs was not assessed in this study, it is likely that TGFβ1, a fibrogenic cytokine, increased the activation of PaSCs, which increased deposition of ECM proteins around the tumor.
Some years later, Apte et al13 developed an orthotopic model by injecting a suspension of human pancreatic cancer cells (MiaPaCa-2, AsPC-1), alone or in combination with primary human PaSCs, directly into mouse pancreas. Hwang et al23 developed a similar model using the pancreatic cancer cell line BxPC-3 and immortalized PaSCs.
Both studies showed that human PaSCs within the tumor stimulated fibrosis, local tumor growth, and importantly, increased regional and distant metastasis. This model was a significant improvement over previous orthotopic models because the tumors formed stroma and became metastatic.
In vitro studies have shown that PaSCs can migrate through an endothelial cell monolayer and that this trans-endothelial migration is increased significantly in the presence of cancer cells—an effect likely mediated by plate-let-derived growth factor secreted by cancer cells.15 These results indicated that PaSCs had the capacity to intrava-sate and extravasate through blood vessels and metastasize. Xu et al15 injected a mixture of female pancreatic cancer cells and male PaSCs into the pancreas of female nude mice. Using fluorescent in situ hybridization, the authors detected Y-chromosome—positive PaSCs in metastatic nodules at distant sites, including the liver, diaphragm, and mediastinum. PaSCs from the primary tumor are therefore able to travel to metastases (possibly with cancer cells), where they likely facilitate the seeding, survival, and proliferation of cancer cells. Notably, similar observations have been reported in a model of lung cancer.24 These results challenge the concept that metastasis is mediated by only cancer cells, and indicate a need for new therapeutic approaches to target not only cancer cells, but also stromal cells.
GEM models of pancreatic cancer have been created in which tumors have a prominent stromal reaction, similar to human disease. These include:
KrasLSL-G12D/+; Trp53LSL-R172H/+; Pdxcre/+ (KPC) mice
KrasLSL-G12D/+; Trp53LSL-R172H/+ ; R26lsl-gfp/+; Pdxcre/+ (KPGC) mice
Pancreas-specific type II receptor for TGFβ (Tgfbr2) knockout mice that express activating Kras mutation (KrasLSL-G12D/+; Tgfbr2floxflox ;Ptf1acre/+). The tumors in these models, which develop spontaneously, progress from preinvasive ductal lesions to overt invasive carcinomas and metastases.11,25,26 They also have a progressive increase in desmoplasia that contains activated PaSCs.11
PaSCs are believed to be involved in resistance of tumors to chemo- and radiotherapy. In KPC mice, gemcit-abine (a first-line chemotherapeutic for pancreatic cancer) was found to be sequestered in the stroma of the pancreatic tumors, and was not able to reach all cancer cells.27 In addition, subcutaneous tumors that form from a mixture of cancer cells and PaSCs are more resistant to single doses or fractionated radiation than tumors produced by cancer cells alone.18
Roles for PaSCs and Leukocytes
Clinical and experimental evidence indicates that inflammation is a significant risk factor for PDAC. Patients with chronic pancreatitis have an increased risk of developing PDAC,28–30 and 40% of patients with hereditary pancreatitis develop pancreatic cancer.31–33 Consistent with these clinical observations, induction of chronic pancreatitis with cerulein in different Kras overexpressing GEM models of PDAC speed the development of the primary cancer and formation of metastases.34–38 Given the role of activated PaSCs in development of chronic pancreatitis, it is likely that these cells promote PDAC not only via direct interactions with cancer cells, but also by influencing the inflammatory process.
Activated PaSCs produce multiple ligands that regulate the inflammatory response, including TGFβ, tumor necrosis factor (TNF) — α, CTGF, monocyte chemoattractant factor-1, interleukin (IL)-1β, IL-6, IL-8, IL-15, and regulated and normal T-cell regulated and secreted (RANTES).39–41 In addition, several of these mediators further activate PaSCs through autocrine mechanisms, to amplify the inflammatory effect.39,42
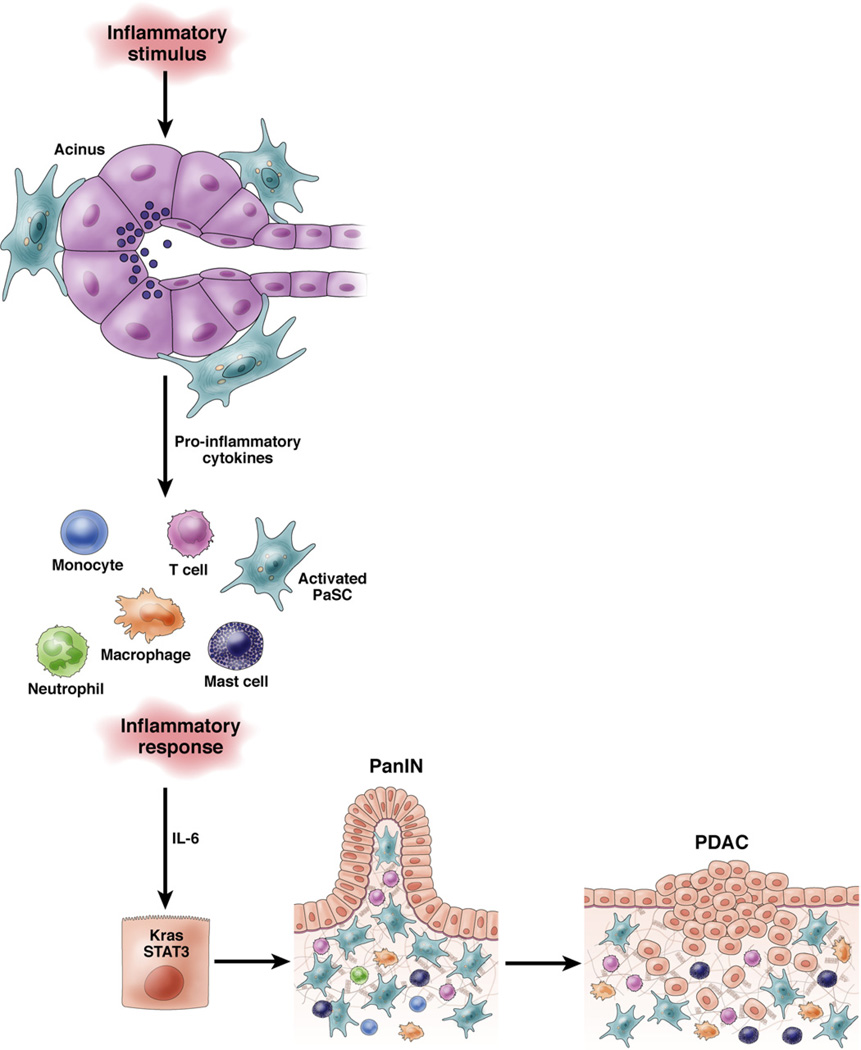
Serum levels of IL-6 are increased in patients with PDAC and tumor-associated macrophages are the main source of IL-6 in human PDAC tissues and GEM models.43,44 Secreted IL-6 promotes formation of PanIN lesions and PDAC in a KrasG12D mice by IL-6 —dependent activation of signal transducer and activator of transcription 3 in the pancreas44,45 (Figure 3).
Figure 3.
Role of inflammation in progression of PDAC. An inflammatory stimulus activates PaSCs in the periacinar area, leading to recruitment of inflammatory cells (monocytes, T cells, neutrophils, macrophage, and mast cells). These cells produce IL-6, which activate signal transducer and activator of transcription 3 (STAT3) to promote development of PanIN and PDAC in susceptible tissue (such as with activating mutations in Kras). The activated PaSCs also produce galectin-1, which causes apoptosis of T cells to prevent anti-tumor immunity.
Most patients with PDAC have no history of pancreatitis, even though there is evidence for inflammation in tumor samples. It will be important to determine how other risk factors for PDAC, such as smoking, diabetes, and obesity, might activate inflammatory signaling systems to promote carcinogenesis. Activation of Kras in pancreatic cells promotes inflammatory signaling and development of precancerous lesions in the pancreas of mice.46 Subclinical and local inflammatory processes might therefore promote carcinogenesis in tissues that have activated oncogenes or inactivated tumor suppressors.
Development of PDAC also requires evasion from immune surveillance.47 In brief, mast cells and tumor-associated macrophages localize to the edge of the tumor, where they accelerate invasion and metastasis. Myeloid-derived suppressor cells are also located at the tumor’s edge, where they inhibit the anti-tumor CD8+ cytotoxic T cells. Immunosuppressive regulatory T cells were also found in high abundance in PDAC.
How might PaSCs contribute to immune evasion by PDAC? Production of galectin-1 (a β-galactoside—binding protein) has been specifically localized to activated PaSCs in PanINs and PDAC.48,49 Galectin-1 appears to regulate the immune-suppressed environment of the tumor,50 inducing apoptosis in effector T cells by binding to N-acetyllactosamine on membrane glycoproteins.51 Knockdown or overexpression of galectin-1 in PaSCs induced apoptosis of T cells involved in tumor surveillance.
Fibroblast activation protein–α has also been reported to disrupt anti-tumor immunity.52 Studies in a GEM model showed that expression of tumor-derived granulocyte-macrophage colony-stimulating factor by PDAC cells recruited myeloid-derived suppressor cells, which suppressed tumor immune surveillance.53 Interactions between PaSCs and cancer cells were shown to reduce the anti-tumor immune response in patients with PDAC.
Interactions Between PaSCs and Endothelial Cells
Clinical and experimental studies have shown that that pancreatic cancer cells produce the angiogenic factors vascular endothelial growth factor (VEGF) and fibroblast growth factor.54 Overexpression of VEGF in tumor tissues has been linked to poor prognosis of patients with PDAC.55 However, despite evidence of increased angiogenic signaling, PDACs also have vascular abnormalities that contribute to poor perfusion and hypoxia.27,56 Pancreatic cancer cells appear to thrive under hypoxic conditions, which activate genetic and metabolic changes that support their survival in low-oxygen microenvironments. Histologic analysis of human PDAC samples showed low vascularity in areas with a dense desmoplastic reaction, and higher levels of vascularity at the periphery of tumors and areas adjacent to neoplastic tissues. Within the tumor stroma, direct contact between cancer cells and blood vessels was rare or absent.10,27 Similar hypovascularity has been observed in GEM models that develop pancreatic tumors with extensive desmoplasia.10,27
Although the mechanisms that underlie these histo-pathologic features are not well understood, the spatial differences in vascularity might be related to dynamic changes during tumor progression. Solid pressure, generated by proliferating cancer cells and other cell types in the stroma, as well as progressive deposition of ECM proteins, could reduce the diameter of blood vessels and vascular flow to ultimately reduce delivery of nutrients and oxygen and cause hypoxia.57 In support of this concept, in mice, pancreatic xenograft or autograft tumors that have little stroma (ie, low content of PaSC and ECM deposition) have a high density of blood vessels, with neoplastic cells in direct contact with endothelial cells, and vascular hyperpermeability in response to VEGF.27,58 This hypervascular phenotype could account for the efficacy of anti-tumor agents against xenograft tumors in mice, but not against human PDAC. Human PDACs are also refractory to many chemotherapies and reagents that block VEGF signaling.59
PaSCs affect the vascular features of pancreatic tumors via different mechanisms. PaSCs express angiogenic factors, including VEGF, angiopoietin-1, and periostin.60,61 In addition, hypoxia stimulates the production of hypoxia-inducible factor-1 and profibrotic and proangiogenic responses in cultured PaSC.60,61 Injection of PaSCs into pancreas of nude mice promoted the ability of coinjected AsPC-1 cells to form metastatic tumors.15 This study also found that PaSCs stimulated angiogenesis in primary tumors and in coculture with endothelial cells. Other studies have shown that PaSCs produce angiostatic factors, such as endostatin, especially when cultured along with cancer cells.60,62 PaSCs might therefore alter the balance between pro- and anti-angiogenic signals in the tumor microenvironment.
Hedgehog (Hh) signaling is another important regulator of stellate cells and desmoplasia.23,27,63 In the KPC model of pancreatic cancer, which has a stromal reaction similar to that of human PDAC, systemic administration of the Hh inhibitor IPI926 significantly reduced tumor levels of α—smooth muscle actin—positive PaSCs and collagen deposition, which increased intratumoral vascularity and delivery of gemcitabine.64 These findings support a model in which the stroma impedes drug delivery and mediates chemoresistance of pancreatic cancer.
ECM proteins secreted by PaSCs and other cells can directly affect angiogenesis. Activation of VEGF ligands, sequestered in the matrix, requires matrix-degrading enzymes, such as matrix metalloproteinase-9.65 Matrix proteins can also affect tumor vascularity and the hemodynamic properties of blood vessels within the tumor. It has been proposed that the extensive deposition of collagens and glycosaminoglycans, such as hyaluronan, which is found in the stroma of PDAC,66,67 leads to high interstitial fluid pressure and changes the diameter and structure of vessels and microcapillaries. These changes increases endothelial barrier integrity and alter vascular permeability to nutrients and therapeutic drugs.10,27,57
Two recent studies examined the effect of enzymatic degradation of intrapancreatic hyaluronan (alone and in combination with gemcitabine) in a GEM model of pancreatic cancer by systemic administration of a PEGylated human recombinant PH20 hyaluronidase.10,11 The results were remarkably similar in that PH20 reduced tumor volume by increasing delivery of gemcitabine. Provenzano et al11 reported that PH20 increased the diameter of tumor vessels (without associated endothelial proliferation), while Jacobetz et al10 demonstrated that PH20 increased tumor vascularity, led to changes in interendothelial junctional gaps, and increased endothelial permeability to macromolecules. These studies indicate the potential for combined chemotherapeutic strategies designed to target cancer cells, PaSCs, and ECM proteins in the tumor microenvironment.
Roles for Neural Components
The pancreas is a highly innervated organ that receives neural inputs from preganglionic fibers of the sympathetic and parasympathetic systems and from postganglionic fibers that emanate from intrapancreatic ganglia. Terminal axons surround almost all acini, in close proximity with periacinar PaSC, pancreatic ducts, blood vessels, and islets.68 Histopathologic analysis of pancreatic tissue samples from patients with PDAC frequently reveals extensive neural remodeling within the tumor stroma, such as increased neural density, intrapancreatic neural hypertrophy, and neuritis.69,70 Similar neuroplasticity has been found in neurons cocultured with pancreatic tumor tissue extracts and in specimens from patients with chronic pancreatitis.71 One feature of PDAC is the invasion of pancreatic nerves by cancer cells. The degree of perineural invasion varies among patients, but it is generally associated with poor prognosis and disease recurrence after surgical resection.72,73
The few studies of the interactions between neural components and neoplastic cells or PaSCs in PDAC support the concept of a close relationship between neural components and cancer cells that promotes tumor growth and invasion. Nerve-derived trophic factors, including nerve growth factor, and glial cell line—derived neurotrophic factor are overexpressed in human PDAC—in cancer cells and nerves within the stroma, and in nerve fibers in the periphery of the tumor.70,74 Nerve growth factor or glial cell line—derived neurotrophic factor stimulate mitogenic signaling by cultured pancreatic cancer cells and promote cell invasion.75,76 Cholinergic and sympathetic inputs also affect growth signaling by pancreatic cancer cells, via nicotinic and muscarinic acetylcholine and β-adrenergic receptors.77,78 Finally, the neurokinin-1 receptor and its ligand, substance P, promote inflammation and growth in several tumor types, including PDAC.79
PaSCs express the neural markers glial fibrillary acidic protein and nestin, and the neurotrophic factors nerve growth factor, brain-derived neutrophic factor, and neu-rotrophin 45.66,80,81 PaSC might therefore act as neural elements in the tumor stroma, affecting the growth of nerves and survival of cancer cells that express receptors for these factors. In support of this concept, Ceyhan et al reported a positive correlation between the extent of desmoplasia and the degree of neural invasion in human PDAC, although there was no clear relationship between desmoplasia and nerve remodeling.69 Other studies showed that neuron growth and elongation was influenced by ECM components, such as collagen, fibronectin, and hyaluronic acid, which are all present in large amounts in pancreatic desmoplasia.82,83 PaSC might therefore affect remodeling of nerves in PDAC by regulating changes in the composition of the ECM.
Interestingly, cultured human and rat PaSC produce acetylcholine (ACh) in response to cholecystokinin and, in turn, ACh stimulates enzyme secretion in pancreatic acinar cells cocultured with PaSCs.84 Although the functional significance of ACh production by PaSCs is unclear, these findings support the interaction among cancer cells, neurons, and PaSC, via ACh receptors.
Potential for PaSCs to Mediate the Effects of Risk Factors
Risk factors for PDAC include age, chronic pancreatitis, smoking, obesity, diabetes, and, to a lesser extent, heavy alcohol intake without pancreatitis.3,85 Alcohol abuse and smoking are associated with development of chronic inflammatory and fibrosing pancreatitis, which involves activation of PaSCs. Activation of PaSCs by alcohol abuse or smoking might therefore be involved in pancreatic carcinogenesis.1,39 However, most patients with PDAC with one or more risk factors do not have clinical evidence of pancreatitis. It will be interesting to investigate whether PaSCs still promote carcinogenesis in the presence of environmental risks factors not associated with pancreatitis. In mice with activated Kras, we observed that either a high-fat and high-calorie diet or administration of smoking compounds promoted formation of advanced PanIN lesions with activated PaSCs and development of PDAC.3,86 Importantly, activated PaSCs were observed in human low-grade PanIN lesions (see Figure 1), indicating the cells function during early stages of tumor formation.
There are reasons to propose roles for PaSCs in mediating the effects of risk factors for PDAC. For example, alcohol abuse induces the metabolite acetaldehyde and increases circulating levels of lipopolysaccharide (LPS), a metabolite of the intestinal microflora—both activate PaSCs in vitro and in vivo.87,88 This activation could promote development of PDAC directly, through chronic pancreatitis, or through a clinically silent inflammatory process. Alcohol abuse is also associated with increased circulating levels of oxidized low-density lipoprotein (oxLDL)89 and TNFa,90 which each have potential effects on stellate cell activation. Most importantly, obesity, diabetes, metabolic syndrome, and high-fat diet are associated with increased circulating levels of LPS, oxLDL, TNFα, leptin, IL-6, and insulin-like growth factor—1. 91–95 There have been no studies to investigate the effect of smoking compounds, alone or in combination with alcohol metabolites, on PaSC activation.
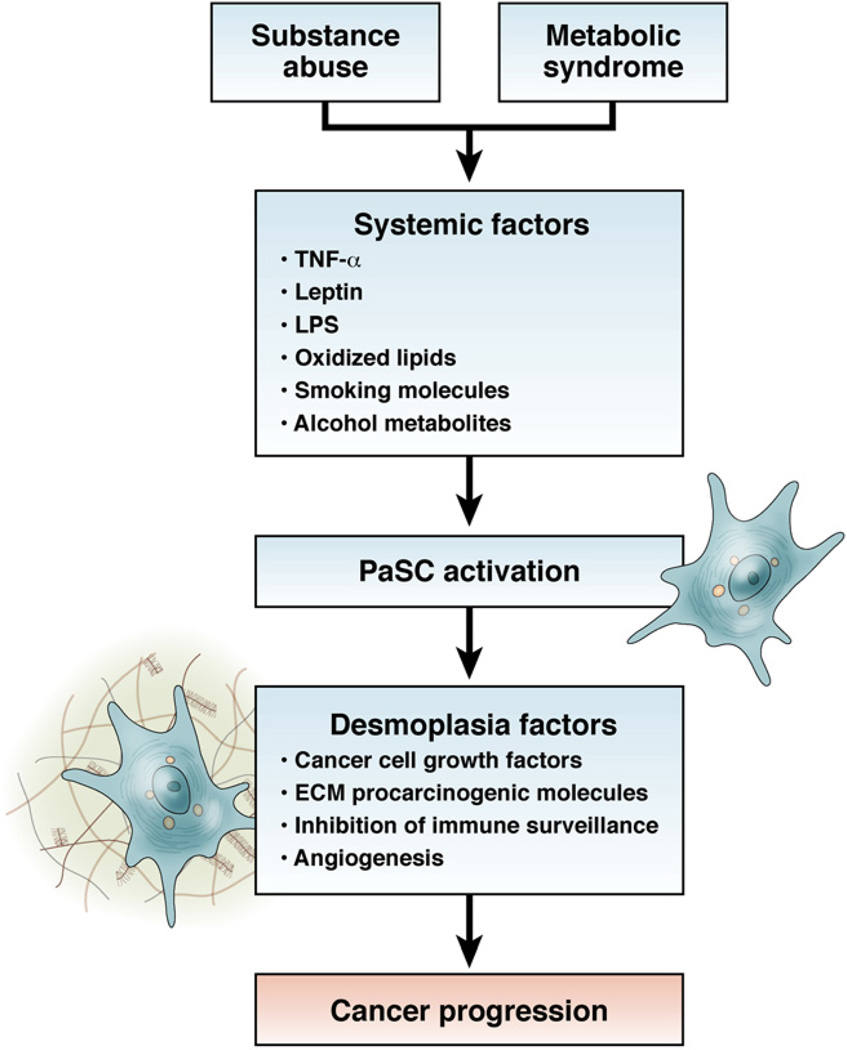
Although some studies have addressed the effects of LPS, TNFα, and IL-6 on PaSC responses, most of the information related with the risk factors for PDAC come from studies in hepatic stellate cells (HSCs), which share many characteristics with PaSCs.66 Both HSC and PaSC have Toll-like receptors, such as Toll-like receptor—4, that interact with LPS, and receptors that mediate TNFα signaling, leading to activation responses.96 Leptins have profibrogenic effects in the liver. 92 Insulin-like growth factor—1 has been shown to promote proliferation of HSC.95 HSCs express oxLDL receptors, and respond to oxLDL by increasing production of ECM proteins. 94,97,98 Figure 4 depicts a model of how PaSCs might affect risk for pancreatic cancer.
Figure 4.
Role of PaSCs in mediating the effects of environmental risk factors for pancreatic cancer. Potential relationships between environmental risk factors and the activation state of PaSCs that could promote tumor progression.
Targeting PaSCs in the Microenvironment
It is clear that the pancreatic tumor stroma promotes pancreatic cancer development and is an important target for new therapeutics. Recent preclinical studies of reagents that target PaSCs have reported some encouraging results. Von Hoff et al99 reported significant depletion of the stromal reaction after nanoparticle albumin-bound paclitaxel (nab-paclitaxel) was administered to subcutaneous, patient-derived xenograft tumors in mice. The stromal depletion was accompanied by the presence of dilated blood vessels within the tumor, increased expression of the endothelial cell marker mNestin, and a 2.8-fold increase in intratumoral concentrations of gemcitabine, compared with mice given only gemcitabine. These findings support the concept that the stromal compartment hinders drug delivery to tumor cells. However, these studies must be repeated in appropriate orthotopic models of pancreatic cancer, which would also allow assessment of the effects of nab-paclitaxel on regional and distant metastasis.
All-trans retinoic acid, an inducer of quiescence in PaSCs, significantly decreased tumor growth in the KPC transgenic model of pancreatic cancer.100 The reduction was associated with decreased cancer cell proliferation, increased cancer cell apoptosis, and reduced Wnt signaling to β-catenin, resulting in decreased cancer cell invasion (although the effect of all-trans retinoic acid on metastasis was not assessed in this study).
The Hh pathway has been also targeted to disrupt the effects of PaSCs on cancer cells. In the KPC model,27 oral administration of IP-926, an inhibitor of Smoothened (one of the 2 transmembrane receptors in the Hh pathway), reduced PaSC activation, leading to stromal depletion, increased tumor vasculature, and improved delivery of gemcitabine to cancer cells. However, this was a transient effect; tumor size was reduced for 1 to 2 weeks, but tumors began growing again in subsequent weeks. In an orthotopic model of pancreatic cancer produced by injection of mice with BxPC3 cells alone or a mixture of BxPC3 and PaSCs, AZD8542 (another Smoothened inhibitor) significantly reduced tumor size, increased tumor vascularity, and reduced metastasis to liver, but only in tumors formed by a 3:1 ratio of PaSCs to cancer cells. The specific mechanisms of the observed effect are unclear because the compound did not have a direct inhibitory effect on PaSC activation in vivo.
Enzymatic targeting of the stromal component hyaluronan (in combination with gemcitabine) in KPC mice was recently reported to decrease tumor volumes and increase overall survival time. This was associated with a significant reduction in the amount of fibrillar collagen and number of activated PaSCs in tumor stroma.10,11
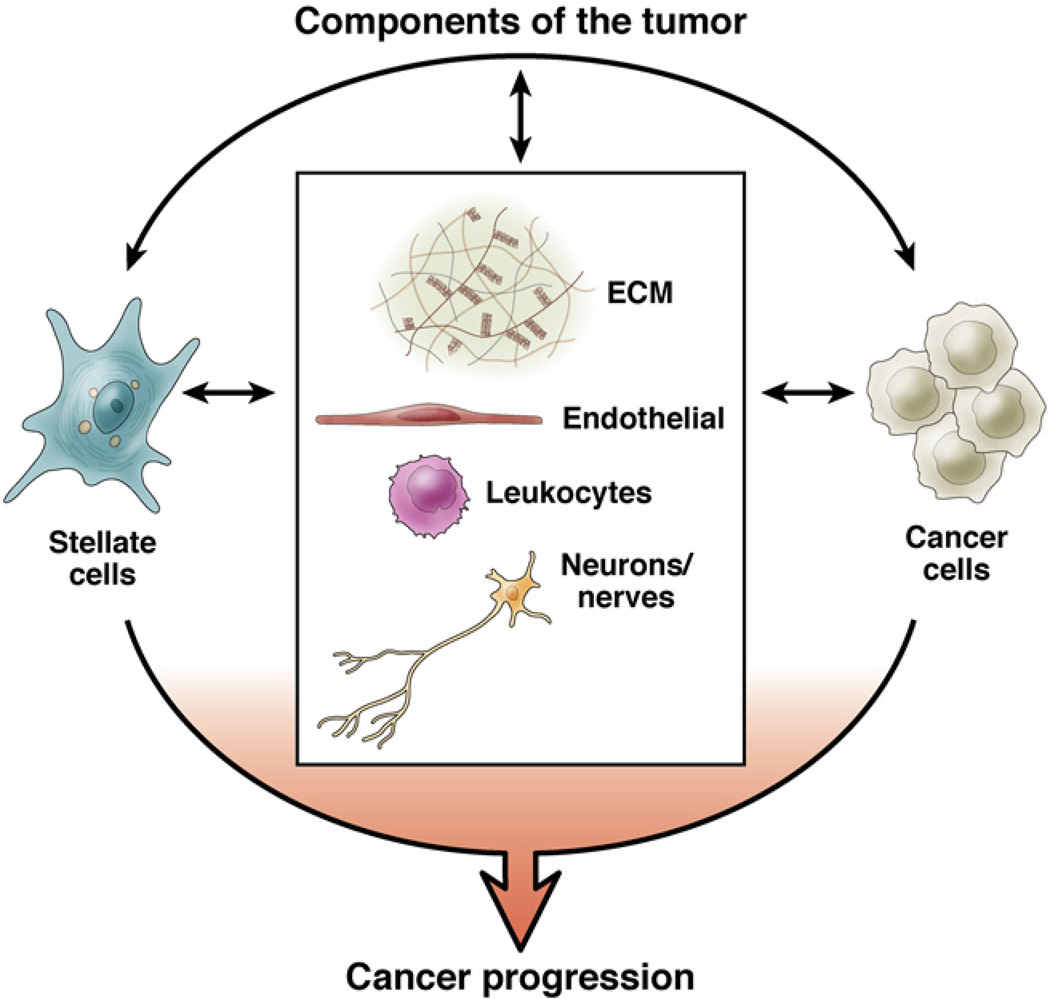
Based on all studies of the pancreatic tumor stroma (notwithstanding the design limitations in each), it is clear that combined targeting of the stroma and cancer cells should be a goal for therapies. As more-specific mediators of the interactions between PaSC and cancer cells are identified and characterized, new approaches will be designed to inhibit the effects of PaSCs on cancer cells, along with disease progression. Figure 5 illustrates the components of PDAC and potential interactions between these that might be targeted in therapeutic approaches.
Figure 5.
Components of the tumor. The components of PDAC and potential interactions between the stroma and cancer cells should be considered in developing therapeutic approaches for pancreatic cancer.
Abbreviations used in this paper
- ACh
acetylcholine
- CTGF
connective tissue growth factor
- ECM
extracellular matrix
- GEM
genetic-engineered mouse
- Hh
Hedgehog
- HSC
hepatic stellate cell
- IL
interleukin
- LPS
lipopolysaccharide
- oxLDL
oxidized low-density lipoprotein
- PanIN
pancreatic intraepithelial neoplastic lesions
- PaSC
pancreatic stellate cells
- PDAC
pancreatic ductal adenocarcinoma
- TGF
transforming growth factor
- Tgfbr2
transforming growth factor–βtype II receptor
- TNF
tumor necrosis factor
- VEGF
vascular endothelial growth factor
Biographies

Minoti V. Apte

Jeremy S. Wilson

Aurelia Lugea

Stephen J. Pandol
Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Apte MV, Wilson JS. Dangerous liaisons: pancreatic stellate cells and pancreatic cancer cells. J Gastroenterol Hepatol. 2012;27(Suppl 2):69–74. doi: 10.1111/j.1440-1746.2011.07000.x. [DOI] [PubMed] [Google Scholar]
- 2.Apte MV, Park S, Phillips PA, et al. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29:179–187. doi: 10.1097/00006676-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Pandol S, Gukovskaya A, Edderkaoui M, et al. Epidemiology, risk factors, and the promotion of pancreatic cancer: role of the stellate cell. J Gastroenterol Hepatol. 2012;27(Suppl 2):127–134. doi: 10.1111/j.1440-1746.2011.07013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marrache F, Pendyala S, Bhagat G, et al. Role of bone marrow-derived cells in experimental chronic pancreatitis. Gut. 2008;57:1113–1120. doi: 10.1136/gut.2007.143271. [DOI] [PubMed] [Google Scholar]
- 5.Sparmann G, Kruse ML, Hofmeister-Mielke N, et al. Bone marrow-derived pancreatic stellate cells in rats. Cell Res. 2010;20:288–298. doi: 10.1038/cr.2010.10. [DOI] [PubMed] [Google Scholar]
- 6.Scarlett CJ, Colvin EK, Pinese M, et al. Recruitment and activation of pancreatic stellate cells from the bone marrow in pancreatic cancer: a model of tumor-host interaction. PLoS One. 2011;6:e26088. doi: 10.1371/journal.pone.0026088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachem MG, Schunemann M, Ramadani M, et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907–921. doi: 10.1053/j.gastro.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida S, Ujiki M, Ding XZ, et al. Pancreatic stellate cells (PSCs) express cyclooxygenase-2 (COX-2) and pancreatic cancer stimulates COX-2 in PSCs. Mol Cancer. 2005;4:27. doi: 10.1186/1476-4598-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arumugam T, Brandt W, Ramachandran V, et al. Trefoil factor 1 stimulates both pancreatic cancer and stellate cells and increases metastasis. Pancreas. 2011;40:815–822. doi: 10.1097/MPA.0b013e31821f6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobetz MA, Chan DS, Neesse A, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–120. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida S, Yokota T, Ujiki M, et al. Pancreatic cancer stimulates pancreatic stellate cell proliferation and TIMP-1 production through the MAP kinase pathway. Biochem Biophys Res Commun. 2004;323:1241–1245. doi: 10.1016/j.bbrc.2004.08.229. [DOI] [PubMed] [Google Scholar]
- 13.Vonlaufen A, Joshi S, Qu C, et al. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res. 2008;68:2085–2093. doi: 10.1158/0008-5472.CAN-07-2477. [DOI] [PubMed] [Google Scholar]
- 14.Kikuta K, Masamune A, Watanabe T, et al. Pancreatic stellate cells promote epithelial-mesenchymal transition in pancreatic cancer cells. Biochem Biophys Res Commun. 2010;403:380–384. doi: 10.1016/j.bbrc.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 15.Xu Z, Vonlaufen A, Phillips PA, et al. Role of pancreatic stellate cells in pancreatic cancer metastasis. Am J Pathol. 2010;177:2585–2596. doi: 10.2353/ajpath.2010.090899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikenaga N, Ohuchida K, Mizumoto K, et al. CD10+ pancreatic stellate cells enhance the progression of pancreatic cancer. Gastroenterology. 2010;139:1041–1051. doi: 10.1053/j.gastro.2010.05.084. 1051 e1-e8. [DOI] [PubMed] [Google Scholar]
- 17.Eguchi D, Ikenaga N, Ohuchida K, et al. Hypoxia enhances the interaction between pancreatic stellate cells and cancer cells via increased secretion of connective tissue growth factor. J Surg Res. 2012 Jul 6; doi: 10.1016/j.jss.2012.06.051. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Mantoni TS, Lunardi S, Al-Assar O, et al. Pancreatic stellate cells radioprotect pancreatic cancer cells through beta1-integrin signaling. Cancer Res. 2011;71:3453–3458. doi: 10.1158/0008-5472.CAN-10-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamada S, Masamune A, Takikawa T, et al. Pancreatic stellate cells enhance stem cell-like phenotypes in pancreatic cancer cells. Biochem Biophys Res Commun. 2012;421:349–354. doi: 10.1016/j.bbrc.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Froeling FE, Marshall JF, Kocher HM. Pancreatic cancer organotypic cultures. J Biotechnol. 2010;148:16–23. doi: 10.1016/j.jbiotec.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Froeling FE, Mirza TA, Feakins RM, et al. Organotypic culture model of pancreatic cancer demonstrates that stromal cells modulate E-cadherin, beta-catenin, and Ezrin expression in tumor cells. Am J Pathol. 2009;175:636–648. doi: 10.2353/ajpath.2009.090131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lohr M, Schmidt C, Ringel J, et al. Transforming growth factor-beta1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Res. 2001;61:550–555. [PubMed] [Google Scholar]
- 23.Hwang RF, Moore TT, Hattersley MM, et al. Inhibition of the hedgehog pathway targets the tumor-associated stroma in pancreatic cancer. Mol.Cancer Res. 2012;10:1147–1157. doi: 10.1158/1541-7786.MCR-12-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duda DG, Duyverman AM, Kohno M, et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci U S A. 2010;107:21677–21682. doi: 10.1073/pnas.1016234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 26.Ijichi H, Chytil A, Gorska AE, et al. Inhibiting Cxcr2 disrupts tumor-stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma. J Clin Invest. 2011;121:4106–4117. doi: 10.1172/JCI42754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433–1437. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 29.Lowenfels AB, Maisonneuve P. Epidemiologic and etiologic factors of pancreatic cancer. Hematol Oncol Clin North Am. 2002;16:1–16. doi: 10.1016/s0889-8588(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 30.McKay CJ, Glen P, McMillan DC. Chronic inflammation and pancreatic cancer. Best Pract Res Clin Gastroenterol. 2008;22:65–73. doi: 10.1016/j.bpg.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Greer JB, Whitcomb DC. Inflammation and pancreatic cancer: an evidence-based review. Curr Opin Pharmacol. 2009;9:411–418. doi: 10.1016/j.coph.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Whitcomb D, Greer J. Germ-line mutations, pancreatic inflammation, and pancreatic cancer. Clin Gastroenterol Hepatol. 2009;7(Suppl):S29–S34. doi: 10.1016/j.cgh.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 33.Vitone LJ, Greenhalf W, Howes NR, et al. Hereditary pancreatitis and secondary screening for early pancreatic cancer. Rocz Akad Med Bialymst. 2005;50:73–84. [PubMed] [Google Scholar]
- 34.Adhami VM, Syed DN, Khan N, et al. Dietary flavonoid fisetin: a novel dual inhibitor of PI3K/Akt and mTOR for prostate cancer management. Biochem Pharmacol. 2012;84:1277–1281. doi: 10.1016/j.bcp.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guerra C, Collado M, Navas C, et al. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell. 2011;19:728–739. doi: 10.1016/j.ccr.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guerra C, Schuhmacher AJ, Canamero M, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Morris JP, 4th, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer. 2010;10:683–695. doi: 10.1038/nrc2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rhim AD, Stanger BZ. Molecular biology of pancreatic ductal adenocarcinoma progression: aberrant activation of developmental pathways. Prog Mol Biol Transl Sci. 2010;97:41–78. doi: 10.1016/B978-0-12-385233-5.00002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Apte MV, Pirola RC, Wilson JS. Pancreatic stellate cells: a starring role in normal and diseased pancreas. Front Physiol. 2012;3:344. doi: 10.3389/fphys.2012.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andoh A, Takaya H, Saotome T, et al. Cytokine regulation of chemokine (IL-8, MCP-1, and RANTES) gene expression in human pancreatic periacinar myofibroblasts. Gastroenterology. 2000;119:211–219. doi: 10.1053/gast.2000.8538. [DOI] [PubMed] [Google Scholar]
- 41.Shek FW, Benyon RC, Walker FM, et al. Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am J Pathol. 2002;160:1787–1798. doi: 10.1016/s0002-9440(10)61125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masamune A, Watanabe T, Kikuta K, et al. Roles of pancreatic stellate cells in pancreatic inflammation and fibrosis. Clin Gastroenterol Hepatol. 2009;7(Suppl):S48–S54. doi: 10.1016/j.cgh.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 43.Lesina M, Kurkowski MU, Ludes K, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456–469. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Ebrahimi B, Tucker SL, Li D, et al. Cytokines in pancreatic carcinoma: correlation with phenotypic characteristics and prognosis. Cancer. 2004;101:2727–2736. doi: 10.1002/cncr.20672. [DOI] [PubMed] [Google Scholar]
- 45.Fukuda A, Wang SC, Morris JP, 4th, et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell. 2011;19:441–455. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daniluk J, Liu Y, Deng D, et al. An NF-κB pathway-mediated positive feedback loop amplifies Ras activity to pathological levels in mice. J Clin Invest. 2012;122:1519–1528. doi: 10.1172/JCI59743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans A, Costello E. The role of inflammatory cells in fostering pancreatic cancer cell growth and invasion. Front Physiol. 2012;3:270. doi: 10.3389/fphys.2012.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan S, Chen R, Reimel BA, et al. Quantitative proteomics investigation of pancreatic intraepithelial neoplasia. Electrophoresis. 2009;30:1132–1144. doi: 10.1002/elps.200800752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen R, Pan S, Ottenhof NA, et al. Stromal galectin-1 expression is associated with long-term survival in resectable pancreatic ductal adenocarcinoma. Cancer Biol Ther. 2012;13:899–907. doi: 10.4161/cbt.20842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang D, Yuan Z, Xue X, et al. High expression of Galectin-1 in pancreatic stellate cells plays a role in the development and maintenance of an immunosuppressive microenvironment in pancreatic cancer. Int J Cancer. 2012;130:2337–2348. doi: 10.1002/ijc.26290. [DOI] [PubMed] [Google Scholar]
- 51.Cedeno-Laurent F, Dimitroff CJ. Galectin-1 research in T cell immunity: past, present and future. Clin Immunol. 2012;142:107–116. doi: 10.1016/j.clim.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kraman M, Bambrough PJ, Arnold JN, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330:827–830. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 53.Bayne LJ, Beatty GL, Jhala N, et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21:822–835. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abdollahi A, Schwager C, Kleeff J, et al. Transcriptional network governing the angiogenic switch in human pancreatic cancer. Proc Natl Acad Sci U S A. 2007;104:12890–12895. doi: 10.1073/pnas.0705505104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seo Y, Baba H, Fukuda T, et al. High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer. 2000;88:2239–2245. doi: 10.1002/(sici)1097-0142(20000515)88:10<2239::aid-cncr6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 56.Sofuni A, Iijima H, Moriyasu F, et al. Differential diagnosis of pancreatic tumors using ultrasound contrast imaging. J Gastroenterol. 2005;40:518–525. doi: 10.1007/s00535-005-1578-z. [DOI] [PubMed] [Google Scholar]
- 57.Michl P, Gress TM. Improving drug delivery to pancreatic cancer: breaching the stromal fortress by targeting hyaluronic acid. Gut. 2012;61:1377–1379. doi: 10.1136/gutjnl-2012-302604. [DOI] [PubMed] [Google Scholar]
- 58.Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108(Pt 6):2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- 59.Bach DR, Kindler J, Strik WK. Elevated bilirubin in acute and transient psychotic disorder. Pharmacopsychiatry. 2010;43:12–16. doi: 10.1055/s-0029-1237376. [DOI] [PubMed] [Google Scholar]
- 60.Erkan M, Reiser-Erkan C, Michalski CW, et al. Cancer-stellate cell interactions perpetuate the hypoxia-fibrosis cycle in pancreatic ductal adenocarcinoma. Neoplasia. 2009;11:497–508. doi: 10.1593/neo.81618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Masamune A, Kikuta K, Watanabe T, et al. Hypoxia stimulates pancreatic stellate cells to induce fibrosis and angiogenesis in pancreatic cancer. Am J Physiol Gastrointest Liver Physiol. 2008;295:G709–G717. doi: 10.1152/ajpgi.90356.2008. [DOI] [PubMed] [Google Scholar]
- 62.Brammer RD, Bramhall SR, Eggo MC. Endostatin expression in pancreatic tissue is modulated by elastase. Br J Cancer. 2005;92:89–93. doi: 10.1038/sj.bjc.6602234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bailey JM, Swanson BJ, Hamada T, et al. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res. 2008;14:5995–6004. doi: 10.1158/1078-0432.CCR-08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Omary MB, Lugea A, Lowe AW, et al. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117:50–59. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Theocharis AD, Tsara ME, Papageorgacopoulou N, et al. Pancreatic carcinoma is characterized by elevated content of hyaluronan and chondroitin sulfate with altered disaccharide composition. Biochim Biophys Acta. 2000;1502:201–206. doi: 10.1016/s0925-4439(00)00051-x. [DOI] [PubMed] [Google Scholar]
- 68.Solomon TE. Control of exocrine pancreatic secretion. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Vol. 1. New York: Raven Press; 1994. pp. 1499–1529. [Google Scholar]
- 69.Ceyhan GO, Bergmann F, Kadihasanoglu M, et al. Pancreatic neuropathy and neuropathic pain—a comprehensive pathomorphological study of 546 cases. Gastroenterology. 2009;136:177–186. doi: 10.1053/j.gastro.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 70.Ceyhan GO, Schafer KH, Kerscher AG, et al. Nerve growth factor and artemin are paracrine mediators of pancreatic neuropathy in pancreatic adenocarcinoma. Ann Surg. 2010;251:923–931. doi: 10.1097/SLA.0b013e3181d974d4. [DOI] [PubMed] [Google Scholar]
- 71.Demir IE, Wang K, Tieftrunk E, et al. Neuronal plasticity in chronic pancreatitis is mediated via the neurturin/GFRalpha2 axis. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1017–G1028. doi: 10.1152/ajpgi.00517.2011. [DOI] [PubMed] [Google Scholar]
- 72.Chatterjee D, Katz MH, Rashid A, et al. Perineural and intraneural invasion in posttherapy pancreaticoduodenectomy specimens predicts poor prognosis in patients with pancreatic ductal ade-nocarcinoma. Am J Surg Pathol. 2012;36:409–417. doi: 10.1097/PAS.0b013e31824104c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hirai I, Kimura W, Ozawa K, et al. Perineural invasion in pancreatic cancer. Pancreas. 2002;24:15–25. doi: 10.1097/00006676-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 74.Zhu Z, Kleeff J, Kayed H, et al. Nerve growth factor and enhancement of proliferation, invasion, and tumorigenicity of pancreatic cancer cells. Mol Carcinog. 2002;35:138–147. doi: 10.1002/mc.10083. [DOI] [PubMed] [Google Scholar]
- 75.Okada Y, Eibl G, Guha S, et al. Nerve growth factor stimulates MMP-2 expression and activity and increases invasion by human pancreatic cancer cells. Clin Exp Metastasis. 2004;21:285–292. doi: 10.1023/b:clin.0000046131.24625.54. [DOI] [PubMed] [Google Scholar]
- 76.Veit C, Genze F, Menke A, et al. Activation of phosphatidylinositol 3-kinase and extracellular signal-regulated kinase is required for glial cell line-derived neurotrophic factor-induced migration and invasion of pancreatic carcinoma cells. Cancer Res. 2004;64:5291–5300. doi: 10.1158/0008-5472.CAN-04-1112. [DOI] [PubMed] [Google Scholar]
- 77.Schuller HM. Neurotransmission and cancer: implications for prevention and therapy. Anticancer Drugs. 2008;19:655–671. doi: 10.1097/CAD.0b013e3283025b58. [DOI] [PubMed] [Google Scholar]
- 78.Weddle DL, Tithoff P, Williams M, et al. Beta-adrenergic growth regulation of human cancer cell lines derived from pancreatic ductal carcinomas. Carcinogenesis. 2001;22:473–479. doi: 10.1093/carcin/22.3.473. [DOI] [PubMed] [Google Scholar]
- 79.Friess H, Zhu Z, Liard V, et al. Neurokinin-1 receptor expression and its potential effects on tumor growth in human pancreatic cancer. Lab Invest. 2003;83:731–742. doi: 10.1097/01.lab.0000067499.57309.f6. [DOI] [PubMed] [Google Scholar]
- 80.Demir IE, Friess H, Ceyhan GO. Nerve-cancer interactions in the stromal biology of pancreatic cancer. Front Physiol. 2012;3:97. doi: 10.3389/fphys.2012.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haas SL, Fitzner B, Jaster R, et al. Transforming growth factor-beta induces nerve growth factor expression in pancreatic stellate cells by activation of the ALK-5 pathway. Growth Factors. 2009;27:289–299. doi: 10.1080/08977190903132273. [DOI] [PubMed] [Google Scholar]
- 82.Gritsenko PG, Ilina O, Friedl P. Interstitial guidance of cancer invasion. J Pathol. 2012;226:185–199. doi: 10.1002/path.3031. [DOI] [PubMed] [Google Scholar]
- 83.Preston M, Sherman LS. Neural stem cell niches: roles for the hyaluronan-based extracellular matrix. Front Biosci (Schol Ed) 2011;3:1165–1179. doi: 10.2741/218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Phillips PA, Yang L, Shulkes A, et al. Pancreatic stellate cells produce acetylcholine and may play a role in pancreatic exocrine secretion. Proc Natl Acad Sci U S A. 2010;107:17397–17402. doi: 10.1073/pnas.1000359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an update. Dig Dis. 2010;28:645–656. doi: 10.1159/000320068. [DOI] [PubMed] [Google Scholar]
- 86.Pandol SJ, Apte MV, Wilson JS, et al. The burning question: why is smoking a risk factor for pancreatic cancer? Pancreatology. 2012;12:344–349. doi: 10.1016/j.pan.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vonlaufen A, Xu Z, Daniel B, et al. Bacterial endotoxin: a trigger factor for alcoholic pancreatitis? Evidence from a novel, physiologically relevant animal model. Gastroenterology. 2007;133:1293–1303. doi: 10.1053/j.gastro.2007.06.062. [DOI] [PubMed] [Google Scholar]
- 88.Apte MV, Phillips PA, Fahmy RG, et al. Does alcohol directly stimulate pancreatic fibrogenesis? Studies with rat pancreatic stellate cells. Gastroenterology. 2000;118:780–794. doi: 10.1016/s0016-5085(00)70148-x. [DOI] [PubMed] [Google Scholar]
- 89.Alho H, Sillanaukee P, Kalela A, et al. Alcohol misuse increases serum antibodies to oxidized LDL and C-reactive protein. Alcohol Alcohol. 2004;39:312–315. doi: 10.1093/alcalc/agh059. [DOI] [PubMed] [Google Scholar]
- 90.Gonzalez-Quintela A, Campos J, Loidi L, et al. Serum TNF-alpha levels in relation to alcohol consumption and common TNF gene polymorphisms. Alcohol. 2008;42:513–518. doi: 10.1016/j.alcohol.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 91.Ghoshal S, Witta J, Zhong J, et al. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res. 2009;50:90–97. doi: 10.1194/jlr.M800156-JLR200. [DOI] [PubMed] [Google Scholar]
- 92.Lanthier N, Horsmans Y, Leclercq IA. The metabolic syndrome: how it may influence hepatic stellate cell activation and hepatic fibrosis. Curr Opin Clin Nutr Metab Care. 2009;12:404–411. doi: 10.1097/MCO.0b013e32832c7819. [DOI] [PubMed] [Google Scholar]
- 93.Kern PA, Ranganathan S, Li C, et al. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 94.Wiesner P, Choi SH, Almazan F, et al. Low doses of lipopolysaccharide and minimally oxidized low-density lipoprotein cooperatively activate macrophages via nuclear factor kappa B and activator protein-1: possible mechanism for acceleration of atherosclerosis by subclinical endotoxemia. Circ Res. 2010;107:56–65. doi: 10.1161/CIRCRESAHA.110.218420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gentilini A, Lottini B, Brogi M, et al. Evaluation of intracellular signalling pathways in response to insulin-like growth factor I in apoptotic-resistant activated human hepatic stellate cells. Fibrogenesis Tissue Repair. 2009;2:1. doi: 10.1186/1755-1536-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Masamune A, Shimosegawa T. Signal transduction in pancreatic stellate cells. J Gastroenterol. 2009;44:249–260. doi: 10.1007/s00535-009-0013-2. [DOI] [PubMed] [Google Scholar]
- 97.Kang Q, Chen A. Curcumin eliminates oxidized LDL roles in activating hepatic stellate cells by suppressing gene expression of lectin-like oxidized LDL receptor-1. Lab Invest. 2009;89:1275–1290. doi: 10.1038/labinvest.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schneiderhan W, Schmid-Kotsas A, Zhao J, et al. Oxidized low-density lipoproteins bind to the scavenger receptor, CD36, of hepatic stellate cells and stimulate extracellular matrix synthesis. Hepatology. 2001;34:729–737. doi: 10.1053/jhep.2001.27828. [DOI] [PubMed] [Google Scholar]
- 99.Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548–4554. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Froeling FE, Feig C, Chelala C, et al. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-beta-catenin signaling to slow tumor progression. Gastroenterology. 2011;141:1486–1497. doi: 10.1053/j.gastro.2011.06.047. [DOI] [PubMed] [Google Scholar]