Abstract
Purpose
To review the clinical experience of fungal keratitis cases at King Khaled Eye Specialist Hospital (KKESH) in Riyadh, Saudi Arabia.
Methods
Retrospective observational review and analysis of 124 patient charts with confirmed diagnosis of fungal keratitis between 1984 and 2004.
Results
One hundred and twenty four eyes of 124 patients had proven fungal infection; 101 eyes had fungal keratitis and 23 eyes had fungal endophthalmitis complicating keratitis. Estimated proportion of fungal keratitis and endophthalmitis was 10.3%. Mean age was 55 years with male predominance (79.0%). Commonly associated factors were previous intraocular surgery (38.7%) and trauma (20.9%). Major risk factor for progressing to endophthalmitis was previous intraocular surgery (65.2%), p < 0.001. Initial laboratory results were fungal positive only in 30.6% (p < 0.001). Commonest organisms isolated were Aspergillus spp. (29.8%) followed by Trichophyton sp. (16.1%), then Candida and Fusarium sp. Comparison of both phases of the study showed improvement in the rate of successfully treated cases from 34.6% to 58.3%, and a decline in cases progressing to endophthalmitis from 25.0% to 13.9%. Therapeutic penetrating keratoplasty increased from 26.9% to 73.6% (p < 0.001). Thirteen eyes required enucleation or evisceration.
Conclusions
In contrast to other studies on fungal keratitis, Aspergillusspp. and Trichophyton sp. were the most commonly isolated fungal pathogens; the former carries the worst prognosis. Risk factors included previous intraocular surgery and trauma. Poor outcome was associated with Aspergillus spp., delayed presentation, previous intraocular surgery and late surgical intervention. This study recommends early surgical intervention to improve the outcome.
Keywords: Fungal keratitis, Fungal endophthalmitis, Risk factors, Aspergillus spp., Trichophyton sp., Therapeutic keratoplasty
1. Introduction
Keratomycosis is recognized worldwide as a vision-threatening corneal infection. Poor visual outcome is related to delay in clinical diagnosis, virulence of fungal organisms and limitations of effective anti-fungal agents.
The increase in encountered fungal keratitis over the past three decades has been dramatic (Liesegang and Forster, 1980; Rosa et al., 1994; Dunlop et al., 1994; Bharathi et al., 2003; Gopinathan et al., 2002; Leck et al., 2002; Wong et al., 1997; Coster et al., 1981; Asbell and Stenson, 1982; Carmichael et al., 1985; Khairallah et al., 1992; Mselle, 1999; Tanure et al., 2000). This is attributable to greater awareness by the ophthalmic community, better diagnostic recognition and improved laboratory techniques, as well as the indiscriminate use of topical broad-spectrum antibiotics, corticosteroids, immunosuppressive drugs and surgical trauma.
The incidence of fungal keratitis varies between tropical and temperate climatic conditions (Liesegang and Forster, 1980; Rosa et al., 1994; Dunlop et al., 1994; Bharathi et al., 2003; Gopinathan et al., 2002; Leck et al., 2002; Wong et al., 1997; Coster et al., 1981; Asbell and Stenson, 1982; Carmichael et al., 1985; Khairallah et al., 1992; Mselle, 1999; Tanure et al., 2000; Harris et al., 1998; Keyhani et al., 2005; Thomas, 2003; Ou and Acharyya, 2007; Chander and Sharma, 1994; Hagan et al., 1995; Ormerod et al., 1987). In tropical climates (Liesegang and Forster, 1980; Rosa et al., 1994; Dunlop et al., 1994; Bharathi et al., 2003; Gopinathan et al., 2002; Leck et al., 2002; Wong et al., 1997) such as South Florida, Bangladesh and South India, the incidence of fungal keratitis is reported to be from 17% to 40%. In temperate climates (Coster et al., 1981; Asbell and Stenson, 1982; Carmichael et al., 1985) such as London, Northern United States and the high altitude of Johannesburg, the proportion of filamentous fungi causing suppurative keratitis is very low. In Saudi Arabia (Khairallah et al., 1992), the previously estimated number of fungal keratitis cases was 14% (27/191) out of the total number of microbial corneal ulcers presented to KKESH.
Corneal trauma has remained the main predisposing factor. In recent years HIV-positive cases and chronic ocular surface disease have been recognized to a greater extent as risk factors for fungal keratitis in certain areas (Mselle, 1999; Tanure et al., 2000). Aspergillus, Fusarium and Candida species remain the commonest ‘organisms’ isolated worldwide (Liesegang and Forster, 1980; Rosa et al., 1994; Dunlop et al., 1994; Bharathi et al., 2003; Gopinathan et al., 2002; Leck et al., 2002; Wong et al., 1997; Coster et al., 1981; Asbell and Stenson, 1982; Carmichael et al., 1985; Khairallah et al., 1992; Tanure et al., 2000; Ou and Acharyya, 2007; Chander and Sharma, 1994; Hagan et al., 1995; Ormerod et al., 1987; Ritterbrand et al., 2006).
The incidence of fungal keratitis after penetrating keratoplasty is 1.8–6.0% and is mainly associated with positive corneoscleral rim cultures (Harris et al., 1998; Keyhani et al., 2005; Thomas, 2003; Xie et al., 2001).
Early diagnosis, proper medical management and timely surgical intervention of these cases remain a challenge to the ophthalmologist who aims to save vision and globe integrity.
The aim of this study is to evaluate all cases of fungal keratitis and endophthalmitis presented at the KKESH. A tertiary care eye hospital based in Riyadh, Saudi Arabia, accepts referrals of cases from across Saudi Arabia as well as its neighboring countries.
2. Methods
After approval of the Institutional Review Board, charts of patients diagnosed with fungal keratitis and endophthalmitis at KKESH from 1984 until the end of 2004 were retrospectively reviewed. Charts were identified based on medical records coding of infectious keratitis, corneal ulcer, and infectious endophthalmitis; out of these, charts with fungal etiology were identified and reviewed.
Cases with smear, culture-positive or histopathology confirmed fungal infection were included in the study. Eyes with clinical diagnosis and no supportive laboratory results of fungal infection were excluded from the study. Positive laboratory results included one or more of the findings below:
-
1.
Smear positive cases meaning the microscopic evidence of fungal element on one or more of Gram, Giemsa, calcofluor white (CFW), or Gomori methenamine silver (GMS) stains.
-
2.
Culture growth of fungal elements in one or more culture media.
-
3.
Histopathology evidence of fungal elements in the specimens provided.
Out of 1200 charts identified by the coding system, 124 cases that had proven fungal keratitis and/or endophthalmitis were reviewed and analyzed. Data were extracted with a uniform data retrieval form. Charts review was done separately in two intervals: the first in 1997, to cover a period from 1984 until the end of 1996; and the second in 2005, to cover the rest of the study period from 1997 until 2004. Data analysis combined both study periods, as well as dividing it into the two time intervals for comparative purposes whenever it was significant.
The rate of occurrence of fungal keratitis and endophthalmitis was identified as the proportion of cases of microbial keratitis presented to KKESH that are caused by fungi and given in percentage.
Demographic data collected for each patient included age at time of infection, gender, place of residence and occupation. A detailed recording of the history of presented complaints included history of trauma, predisposing or risk factors, time and onset of symptoms, contact lens wear, medications used, past medical and surgical history and referral information when available. Ophthalmic evaluation on admission, during hospitalization, and on discharge was recorded and analyzed.
Microbiology study site, results and type of fungal pathogen were recorded. Management data contained information on topical and systemic medications used, in addition to time and type of surgical intervention if any. Finally, the sequel of the infection, recurrence and the need to enucleate or eviscerate were documented.
Initially Gram and Giemsa stains were performed on corneal scrapings taken from all patients presented with corneal ulcer to the Emergency Room. If fungal etiology was suspected then additional slides for CFW and GMS stains were taken. Routine culturing of specimens using culture media was performed on all given specimens provided from corneal scraping, anterior chamber tap, vitreous tap, corneal tissue, or from the globe or its contents. KKESH microbiology laboratory was the only laboratory involved.
The mycological diagnosis was through using the same standard inoculation methods to Sabouraud’s dextrose agar without cyclohexamide but containing gentamicin, 5% sheep blood agar, chocolate agar, brain/heart infusion medium and on thyoglycate liquid medium. Culture plates were maintained in the laboratory for 21 days before considered negative.
Cases with positive smear and negative cultures that were not responding to medical management were indicated for repeat corneal scraping or biopsy.
Medical treatment protocol was to start either single or a combination of topical antifungal therapy based on a positive laboratory result. Systemic treatment is case selective depending on the severity and extension of the infection.
Therapeutic PKP was indicated in cases with an overwhelming fungal infection without significant response to medical therapy, infiltrated ulcer approaching the limbus, corneal perforation or impending perforation, or in cases of recurrence while on medical therapy.
Successfully treated cases were defined as cases with healed corneal ulcer which were managed by medical or surgical intervention and absence of infiltration or inflammation.
For significance testing: proportions or categories were compared using the Chi-square test, the fisher exact test was used when a value in the categories was equal to or less than 5.
3. Results
3.1. Epidemiology
Out of 1200 cases of infectious keratitis, 124 eyes of 124 patients with fungal etiology were identified. On presentation, 101 eyes had fungal keratitis, 15 eyes had fungal endophthalmitis complicating keratitis and 8 eyes had keratitis that progressed to endophthalmitis during hospitalization. None of the cases had fungal endophthalmitis without keratitis. Of the patients, 79% were men; the average age at presentation was 55 years, ranging from 4 to 100 years of age.
While seasonal variation was not statistically significant, the highest number of keratitis cases was presented during spring and more endophthalmitis cases were presented during summer.
3.2. Presenting clinical features
Average duration of symptoms related to keratitis before presented or referred to KKESH was 21.6 days (range, 1–126 days). The average duration of symptoms in cases presented with fungal endophthalmitis was 36.6 days (range, 20–60 days). Eleven cases had perforated corneal ulcer; one was diagnosed with endophthalmitis on presentation. Of those, five were infected with Aspergillus spp., 1 with Trichophyton and 1 with Paecilomyces.
Only 19.6% of the referral letters to KKESH specified keratitis or endophthalmitis related to fungal etiology with a significant p < 0.001 value. The rest were diagnosed as either non-specific keratitis or microbial keratitis.
3.3. Risk factors
Table 1 lists the associated and predisposing risk factors of fungal keratitis and endophthalmitis.
Table 1.
Risk factors of fungal keratitis and endophthalmitis in order of decreasing frequency of occurrences.
| Risk factors | No. of cases (%) |
|---|---|
| Previous ocular surgery | 48 (38.7) |
| Trauma | 26 (20.9) |
| Topical corticosteroids | 21 (16.9) |
| Diabetes Mellitus | 15 (12) |
| Trachoma sequels and entropion | 11 (8.87) |
| Systemic diseasea | 9 (7) |
| Topical and systemic steroids | 3 (2.41) |
| Therapeutic contact lens wear | 1 (0.8) |
Other than diabetes.
Previous ocular surgery, a major risk association factor in our study, was reported in 48 cases (38.7%). Table 2 lists details of surgeries performed prior to ulcer.
Table 2.
Subcategories of previous intraocular surgeries performed in order of decreasing frequency.
| Previous ocular surgery | No. of cases (%) |
|---|---|
| None | 76 (61.3) |
| Extra capsular cataract extraction | 20 (16.1) |
| Penetrating keratoplastya (PKP) | 18 (14.5) |
| Trabeculectomya | 4 (3.22) |
| Lamellar keratoplastya (LKP) | 2 (1.61) |
| Repair of corneal laceration | 2 (1.61) |
| Corneal debridement with BCL | 1 (0.8) |
| Pterygium excision | 1 (0.8) |
Bandage contact lens (BCL).
All LKP and trabeculectomy cases and 5 of the PKP cases were combined with Extra capsular cataract extraction.
Of the 48 cases with previous ocular surgery, 20 had extra capsular cataract extraction (ECCE); none of the cases were done as phacoemulsification. Five of the 20 patients developed endophthalmitis. The shortest time interval between surgery and infection was two weeks. Penetrating keratoplasty (PKP) was the second most common intraocular surgery reported in 18 cases, of which five were combined with cataract extraction and seven developed endophthalmitis.
Twenty-three eyes developed endophthalmitis; of these 15 had previous intraocular surgical intervention in the form of ECCE, PKP, trabeculectomy and repair of corneal laceration after trauma with a significant p < 0.001 value.
Trauma was reported in 20.9% of cases (26 eyes) of those; 12 eyes had corneal abrasions, 9 eyes had penetrating or perforating injury, and 5 eyes had blunt trauma. Trauma with organic material was reported in 6 eyes out of the 26 trauma cases, while trauma with non-organic material was noted in 15 eyes.
Twenty-four (19.3%) cases were on topical steroids in addition to topical broad-spectrum antibiotics on presentation. Of these, three had additional systemic steroids.
3.4. Laboratory diagnosis
Out of 124 corneas scraped, only 38 eyes were positive for fungal elements on initial smears or cultures. When indicated, a further examination was carried out on initially negative cases utilizing corneal biopsies in 12 eyes and corneal buttons in 67 eyes; all biopsy specimens were culture and/or histopathology positive for fungal elements.
Anterior chamber taps combined with other interventional procedures were done on 9 cases; similarly vitreous taps were done on 9 cases of suspected endophthalmitis.
Microbiology results were mainly growing Aspergillus and Candida species; additionally 3 of the anterior chamber taps grew Rhizopus, Fusarium, and Scedosporium apiospermum species. All patients received intracameral or intravitreal injections of amphotericin B 5–10 μg in 0.1 ml.
Specimens from eyes that underwent keratoplasty were sent to microbiology and histopathology laboratories; all given specimens were positive for fungal elements.
Table 3 lists all fungal isolates in addition to a few of its sequelae. The most common genus isolated was Aspergillus, accounting for 29.8% (37/124) of cases; Aspergillus flavus was the most commonly isolated species 64.9% (24/37). Twenty-seven eyes had keratitis while endophthalmitis was diagnosed in 10 eyes. Aspergillus spp. was the organism isolated in most cases requiring enucleation or evisceration (8/13).
Table 3.
Fungal organisms Isolated between January 1984 and December 2004, in order of decreasing frequency of occurrence.
| Final organisms isolated | Number of isolates (%) | E&E | Corneal perforation | Therapeutic PKPb | ||
|---|---|---|---|---|---|---|
| Organisms | Keratitis | Keratitis & Endophthalmitis | Total | Number of eyes | Number of eyes | Number of eyes |
| Aspergillus | 28 | 9 | 37 | 8 | 4 | 24 |
| A. flavus | 20 | 4 | 24 | 6 | ||
| A. niger | 4 | 3 | 7 | |||
| A. fumigatus | 2 | 2 | ||||
| A. terreus | 1 | 2 | 3 | 2 | ||
| A. sp. | 1 | 0 | 1 | |||
| Trichophyton | 20 | 0 | 20 | 3 | 12 | |
| T. schoenleinii | 14 | 0 | 14 | |||
| T. verrucosum | 1 | 1 | ||||
| T. sp. | 5 | 5 | ||||
| Candida | 11 | 3 | 14 | 3 | 1 | 8 |
| C. albicans | 5 | 2 | 7 | 1 | ||
| C. parapsilosis | 2 | 2 | ||||
| C. tropicalis | 1 | 1 | ||||
| C. sp. | 3 | 1 | 4 | |||
| Fusarium | 12 | 1 | 13 | 2 | 8 | |
| F. solani | 2 | 2 | ||||
| F. sp. | 10 | 1 | 11 | |||
| Alternaria sp. | 6 | 0 | 6 | 3 | ||
| Scedosporium sp. | 3 | 0 | 3 | 2 | ||
| Acremonium | 2 | 0 | 2 | 1 | ||
| Basidiobolus ranarum | 0 | 1 | 1 | 1 | ||
| Pseudallescheria boydii | 1 | 0 | 1 | 1 | ||
| Mucor sp. | 1 | 1 | ||||
| Paecilomyces | 2 | 2 | ||||
| Rhizopus | 1 | 1 | ||||
| Phoma sp. | 1 | 1 | ||||
| Scytalidium dimidatuma | 1 | 1 | ||||
| Others | 18 | 3 | 21 | 2 | 1 | 9 |
E/E: Enucleation or Evisceration.
Reported from KKESH in Br. J. Ophthalmol. 1993;77:388–390.
Five cases had more than one PKP.
The second most commonly isolated organism was Trichophyton sp., accounting for 20 cases of keratitis. Of these, 16 were medically cleared and one progressed to endophthalmitis. Two cases had corneal perforation that was managed surgically.
The third most common isolates were Candida (14 cases) and Fusarium sp. (13 cases).
Three cases developed endophthalmitis; 2 of them were caused by Candid sp.
Eleven Fusarium cases were cleared while 1 case developed corneal perforation requiring surgical intervention.
Mixed bacterial-fungal culture positive infections were found in 23.6% cases. Isolated Gram-positive bacterial organisms were mainly staphylococci (20.8%) and streptococci (2.8%). One case had concomitant culture growth of Pseudomonas sp. and another had growth of Morexella sp. None of the cases had documented herpetic-fungal infection. In addition, none of the cultures showed simultaneous growth of two different fungal elements.
3.5. Medical treatment
Throughout the study period 36 patients were medically treated without any surgical intervention.
The initial topical antifungal agents commonly used were; amphotericin B 1.5–2.5 mg/ml in 73 patients and natamycin 5% suspension (Natacyn®, Alcon, 50 mg/ml) in 69 patients. Natamycin was used alone as topical treatment in 44/69 patients, and combined with other topical antifungal agents in 25/69 patients. Topical miconazole 10 mg/ml was used in 40/124 patients.
Systemic antifungal therapy in the form of oral ketoconazole 200 mg daily or fluconazole 100–400 mg daily was used in 57 patients. 5-flucytosine was used in only 3 patients.
Sub-conjunctival antifungal agents were either amphotericin B 0.5 mg/ml or miconazole 10 mg/ml. Amphotericin B 10 μg/0.1 ml and miconazole 25 μg/0.1 ml were given through intracameral or intravitreuos routes in selected cases; mainly for patients going for intraocular interventional procedures or endophthalmitis cases.
Three patients had been started on topical steroids while on antifungal therapy; 2 cases eventually had therapeutic PKP.
The average hospitalization stay was 25.5 days ranging from 1 to 90 days. One hundred and sixteen patients (93.5%) were followed up for 21.8 months after hospital discharge; range of follow-up was from 1 to 99 months. Eight patients did not show up for follow-up, three of them had evisceration and one patient died of pulmonary embolism.
3.6. Surgical treatment
Sixty-seven patients (54%) required therapeutic PKP either alone or in combination with another procedure (vitrectomy, peripheral iridectomy, total or subtotal iridectomy). Of these patients, 14 (26.9%) were done in the first phase of the study, while 53 cases (73.6%) were done in the second phase with a significant p < 0.001 value. Five eyes required repeat therapeutic PKP. Pars plana vitrectomy alone or combined with PKP was done in 9 cases.
Of the 124 patients, 13 required enucleation or evisceration for an overwhelming fungal infection secondary to: Aspergillus flavus in 6 patients, Aspergillus terreus in 2, Candida in 1, Basidiobolus ranarum in 1, and Pseudallescheria boydii in 1 patient. Organisms were unspecified in 2 patients.
3.7. Outcome/sequel
Of the 124 cases, 60 (48.4%) were labeled as cleared clinically or successfully treated. Of these, 18 (34.6%) were from phase one of the study and 42 (58.3%) from the second phase with a significant p < 0.001 value.
Corneal perforation requiring surgical intervention occurred in 11/124 patients. Of these cases, 4 were due to Aspergillus spp., 3 due to Tricophyton schoeneleini, 2 due to Fusarium and 1 due to Candida. Two cases progressed to endophthalmitis.
Complications included secondary glaucoma in 13 eyes, and corneal graft failure, or scarring in 45 patients. Loss of globe integrity occurred in 16 cases; of these 13 had enucleation or evisceration and 3 developed phthisis bulbi.
Positive fungal growth of the donor rim following therapeutic PKP was cultured in 2 patients growing Candida sp. Both patients required the PKP urgently for an overwhelming filamentous fungal infection and were treated with systemic fluconazole 200 mg, intracameral amphotericin B in addition to topical antifungal agents. A third patient had positive bacterial rim growth. All cases had clear corneal grafts with no signs of infection after the transplantation.
Fig. 1 compares between visual acuity on presentation and final acuity, showing improvement in the number of eyes gaining useful vision after treatment.
Figure 1.
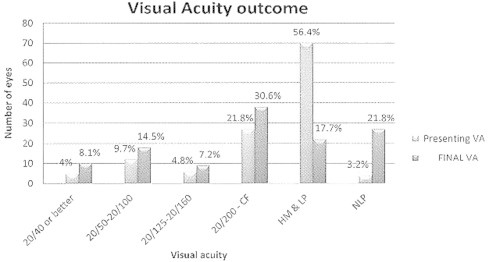
Comparitive graph between visual acuity on presentation and final acuity. Useful vision in the range of 20/160 or better has improved from 23 cases at presentation to 37 cases after treatment. HM: hand motion; CF: counting fingers; NLP: no light perception; LP: light perception.
4. Discussion
This study reports trends management and the outcomes of the largest series of fungal keratitis and related endophthalmitis with the longest follow-up period in the Middle East region. One hundred and twenty four cases were diagnosed and managed in a tertiary eye care hospital in Riyadh, Saudi Arabia over a period of 20 years. During that period the estimated percentage of fungal keratitis in proportion to the overall microbial keratitis cases was 10.3%. Of these patients, 18.5% progressed to endophthalmitis.
In 1987 the previously estimated proportion of fungal keratitis in Saudi Arabia (Khairallah et al., 1992) was 14% over a period of 3 years; both studies used similar admission criteria.
These findings are consistent with reports from temperate climates (Coster et al., 1981; Asbell and Stenson, 1982; Carmichael et al., 1985) and is within the range of incidences in the developed (6–35%) and developing world (4–60%). The variation in incidence is believed to be related to differences in the climate and geography of each region (Gopinathan et al., 2002; Tanure et al., 2000; Thomas, 2003; Ou and Acharyya, 2007).
Preliminary accurate diagnosis of fungal keratitis in local regional clinics remains deficient and challenging with only 19.6% of patients referred as fungal keratitis. While the majority of patients, 80% were inaccurately diagnosed and treated with topical antibiotics and/or steroids prior to the presentation to KKESHs Cornea Service.
Topical steroids usage remains a major risk factor in our study; this is consistent with other studies (Tanure et al., 2000; Iyer et al., 2006) that reported the frequent use of topical steroids before a definitive diagnosis is established. This practice should be discouraged until the pathogen is isolated and the eye responds to antimicrobial treatment. Emphasis on educational campaigns and the implementation of outreach programs to reach all remote areas of the country is one of the means taken to overcome this problem.
The value of the Gram and Giemsa-stained and other smears done from corneal scrapings on admission is of considerable importance. Initial smear studies raised suspicion of fungal infection by showing few hyphal elements that could not initially be confirmed by culture in 69.35% of cases (p ⩽ 0.001). However, they were later confirmed by a biopsy taken from the infected cornea or after keratoplasty was done. Therefore, clinical judgment in addition to a positive smear is important in the initial diagnosis and management of fungal keratitis. This is consistent with other reported sensitivity rates for the detection of fungi on initial stain (Khairallah et al., 1992; Tanure et al., 2000; Iyer et al., 2006).
The most frequent fungus isolated was Aspergillus spp. (37/124 cases). Aspergillus flavus (24/37 cases) carried the worst prognosis among keratitis and endophthalmitis cases. Aspergillus spp. remains the most common and most virulent fungal agent in all regions of Saudi Arabia in this current previous study (Khairallah et al., 1992). Aspergillus; survives very hot summer temperatures (Liesegang and Forster, 1980; Rosa et al., 1994; Dunlop et al., 1994; Bharathi et al., 2003; Gopinathan et al., 2002; Khairallah et al., 1992; Ou and Acharyya, 2007; Chander and Sharma, 1994) changing seasons and sand storms help spread its spores explaining why Aspergillus-related cases peaked in spring and summer.
This agrees with a report from India (Chander and Sharma, 1994) where Aspergillus predominates. However; it contrasts with reports from South Florida (Liesegang and Forster, 1980; Rosa et al., 1994), South India (Gopinathan et al., 2002) and Ghana (Hagan et al., 1995) where Fusarium predominates and with reports from Southern California (Ormerod et al., 1987) and Pennsylvania (Tanure et al., 2000), where Candida was the predominant organism.
Tricophyton sp. was the second most common organism isolated (20/124 cases). Of these, 14 were T. schoenleinii, 1 patient developed endophthalmitis. A series of 5 Trichophyton-infected cases associated with progressive keratolysis, scleral extension and endophthalmitis (Mohammed et al., 2006) were reported previously from KKESH. Few other cases of Trichophyton keratitis have been reported (Shenoy et al., 2003; Quaintenne, 1970) but this is the only study that shows the predominance of this organism in the region.
Major reviews (Liesegang and Forster, 1980; Rosa et al., 1994; Dunlop et al., 1994; Bharathi et al., 2003; Gopinathan et al., 2002; Leck et al., 2002; Wong et al., 1997; Coster et al., 1981; Asbell and Stenson, 1982; Carmichael et al., 1985; Khairallah et al., 1992; Tanure et al., 2000; Harris et al., 1998; Ou and Acharyya, 2007; Chander and Sharma, 1994; Hagan et al., 1995; Ormerod et al., 1987; Ritterbrand et al., 2006; Xie et al., 2001) from tropical and climatic areas indicate that this organism is not a major cause of keratomycosis. Most cases infected with this organism in our study recovered with favorable outcome. Trichophyton is a dermatophyte which inhabits the soil, humans or animals. These fungi are highly adoptive to nonliving keratinized tissues of nail, hair, and stratum corneum of the skin (Rayan, 1984).
Although this organism may be endemic in certain parts of the world, the definitive explanation to its predominance as fungal keratitis in our region is unclear. In general, hand hygiene plays a role in this dermatophytes transmission and infection.
Previous intraocular surgery is the major associated factor in fungal keratitis. The interesting finding in our study is not the development of keratitis in these eyes but the higher incidence of endophthalmitis (15/23 cases) in eyes with previous intraocular surgery regardless of the interval between the surgery and the infectious process.
The interval between the intraocular surgery and the development of infection with endophthalmitis ranged between two weeks to a few years; all short-interval cases (two weeks) were after ECCE. Prior intraocular surgery was noticed previously to be the most common predisposing factor in cases with bacterial keratitis studied at KKESH (Shehri et al., 2009). These eyes may have predisposing ocular surface pathology; in addition prior surgery may provide a route for organism to penetrate into the eye even if the surgery was done a long time ago. Based on this the authors recommend prompt therapeutic medical and surgical intervention to minimize the chances of progression to endophthalmitis in eyes with previous intraocular surgery.
Trauma is the second most common risk factor directly related to the development of fungal keratitis. This contrasts with most other studies where trauma is cited as the major risk factor (Liesegang and Forster, 1980; Rosa et al., 1994; Gopinathan et al., 2002; Leck et al., 2002; Wong et al., 1997; Ou and Acharyya, 2007). In developing countries trauma is reported in 90% of fungal ulcers. Of those, 60% are related to vegetative injuries (Gopinathan et al., 2002). In developed countries it is reported in 11–44% of cases, specifically related to agricultural trauma (Rosa et al., 1994; Xie et al., 2001).
In this study, trauma with organic matter was only reported in 6 of the 26 trauma eyes. Other causes of trauma were related to abrasive, penetrating or perforating injury. Whatever the cause of trauma, it should raise high suspicion of fungal pathogen involvement even if not with organic matter. Fungal pathogens require an epithelial abrasion that attracts fungi to invade a predisposed cornea.
Alternatively, in contrast to other reports (Rosa et al., 1994; Ou and Acharyya, 2007), none of the cases reported in our study had contact lens related fungal keratitis, except for one case of therapeutic contact lens that was applied after superficial keratectomy. The relation of contact lens wear to microbial keratitis has changed after the study period and more cases of contact lens related microbial keratitis, especially in younger patients, were observed. During the study period and afterward (2005–2006 world wide epidemic) there was no outbreak of contact lens associated Fusarium keratitis reported in Saudi Arabia.
Medical management of fungal keratitis in both phases of the study at KKESH was very similar. A combined approach with two or more antifungal agents was used. This may have been because of late presentation and severity of these cases. Amphotericin B and natamycin were the commonly used topical antifungal agents, used either alone or in combination with other systemic agents. Dual medications, although from the same group, are used to provide a broader coverage to both filamentous and yeast fungal infections (Tanure et al., 2000; Iyer et al., 2006).
Therapeutic PKP was performed in 67 eyes (54%) in our study compared to 25–27% in other studies (Rosa et al., 1994; Ou and Acharyya, 2007; Xie et al., 2001). It is encouraged in cases responding poorly to medical management with large extensive ulcers and with corneal perforation. Therapeutic PKP was indicated more frequently in the second phase of the study, this practice may have improved the infection control and final outcome in these cases.
Successfully treated cases increased from 34.6% to 58.3% and cases of endophthalmitis declined from 25.0% to 13.9%. While this improvement could be multifactoral, the most important factor is therapeutic surgical intervention, which increased from 26.9% to 73.6%. As fungal keratitis is considered a surgical disease, proper timing of the surgical intervention is crucial to complete the treatment successfully.
This study showed that fungal keratitis remains challenging in terms of diagnosis and management and still carries the worst outcome than other forms of microbial keratitis. Aspergillus and Trichophyton species were the most commonly isolated pathogens. The value of smears on initial corneal scrapings remains essential for early detection of fungal keratitis. Prior intraocular surgery was a major risk factor associated with fungal keratitis and a major factor in cases progressing to endophthalmitis. While the medical treatment remained similar in both study phases, surgical management has changed significantly and improved the successful outcome of cases.
References
- Asbell P., Stenson S. Ulcerative keratitis: Survey of 30 years laboratory experience. Arch. Ophthalmol. 1982;100:77–80. doi: 10.1001/archopht.1982.01030030079005. [DOI] [PubMed] [Google Scholar]
- Bharathi M.J., Ramakrishnan R., Vasu S., Meenakshi R., Palaniappan R. Epidemiological characteristics and laboratory diagnosis of fungal keratitis: a three-year study. Indian J. Ophthalmol. 2003;51:315–321. [PubMed] [Google Scholar]
- Carmichael T.R., Wolpert M., Koornhof H.J. Corneal ulceration at an urban African hospital. Br. J. Ophthalmol. 1985;69:920–926. doi: 10.1136/bjo.69.12.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chander J., Sharma A. Prevalence of fungal ulcers in northern India. Infection. 1994;22:207–209. doi: 10.1007/BF01716706. [DOI] [PubMed] [Google Scholar]
- Coster, D.J., Wilhelmus, K., Peacock, J., Jones, B.R., 1981. Suppurative keratitis in London. In: IVth Congress of the European Society of Ophthalmology. Royal Society of Medicine Internal Congress and Symposium Series No. 40, London, pp. 395–398.
- Dunlop A.A., Wright E.D., Howlader S.A., Nazrul I., Husain R., McCellan Suppurative corneal ulceration in Bangladesh: a study of 142 cases examining the microbiological diagnosis, clinical and epidemiological features of bacterial and fungal keratitis. Aust. N. Z. J. Ophthalmol. 1994;22:105–110. doi: 10.1111/j.1442-9071.1994.tb00775.x. [DOI] [PubMed] [Google Scholar]
- Gopinathan U., Garg P., Fernandes M., Sharma S., Athmanathan S., Rao G.N. The epidemiological features and laboratory results of fungal keratitis: a 10-year review at a referral eye care center in south India. Cornea. 2002;21:555–559. doi: 10.1097/00003226-200208000-00004. [DOI] [PubMed] [Google Scholar]
- Hagan M., Wright E., Newman M., Dolin P., Johnson G. Causes of suppurative keratitis in Ghana. Br. J. Ophthalmol. 1995;79:1024–1028. doi: 10.1136/bjo.79.11.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D.J., Jr., Stulting R.D., Waring G.O., Wilson L.A. Late bacterial and fungal keratitis after corneal transplantation: spectrum of pathogens, graft survival, and visual prognosis. Ophthalmology. 1998;95:1450–1457. doi: 10.1016/s0161-6420(88)33008-3. [DOI] [PubMed] [Google Scholar]
- Iyer S.A., Tuli S.S., Wagoner R.C. Fungal keratitis: emerging trends and treatment outcomes. Eye Contact Lens. 2006;32(6):267–271. doi: 10.1097/01.icl.0000249595.27520.2e. [DOI] [PubMed] [Google Scholar]
- Keyhani K., Seedor J.A., Shah M.K., Terraciano A.J., Ritterband D.C. The incidence of fungal keratitis and endophthalmitis following penetrating keratoplasty. Cornea. 2005;24(3):288–291. doi: 10.1097/01.ico..0000138832.3486.70. [DOI] [PubMed] [Google Scholar]
- Khairallah S.H., Byrne K.A., Tabbara K.F. Fungal keratitis in Saudi Arabia. Doc. Ophthalmol. 1992;79:269–276. doi: 10.1007/BF00158257. [DOI] [PubMed] [Google Scholar]
- Leck A.K., Thomas P.A., Hagan M., Kaliamurthy J., Ackuaku E., John M., Newman M.J., Codjoe F.S., Opintan J.A., Kalavathy C.M., Essuman V., Jesudasan C.A.N., Johnson G.J. Etiology of suppurative corneal ulcers in Ghana and south India, and epidemiology of fungal keratitis. Br. J. Ophthalmol. 2002;86:1211–1215. doi: 10.1136/bjo.86.11.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesegang T.J., Forster R.K. Spectrum of microbial keratitis in South Florida. Am. J. Ophthalmol. 1980;90:38–47. doi: 10.1016/s0002-9394(14)75075-5. [DOI] [PubMed] [Google Scholar]
- Mohammed A., Al-Rajhi A., Wagoner M.D. Trichophyton fungal keratitis. Cornea. 2006;25(1):118–122. doi: 10.1097/01.ico.0000164834.77291.51. [DOI] [PubMed] [Google Scholar]
- Mselle J. Fungal keratitis as an indicator of HIV infection in Africa. Trop. Doct. 1999;29(3):133–135. doi: 10.1177/004947559902900303. [DOI] [PubMed] [Google Scholar]
- Ormerod L.D., Hertzmark E., Gomez D.S., stabiner R.G., Schanzlin D.J., Smith R.E. Epidemiology of microbial keratitis in Southern California: a multivariate case analysis. Ophthalmology. 1987;94:1322–1333. doi: 10.1016/s0161-6420(87)80019-2. [DOI] [PubMed] [Google Scholar]
- Ou J.I., Acharyya N.R. Epidemiology and treatment of fungal corneal ulcers. Int. Ophthalmol. Clin. 2007;47(3):7–16. doi: 10.1097/IIO.0b013e318074e727. [DOI] [PubMed] [Google Scholar]
- Quaintenne E.J. Mycotic keratitis caused by Trichophyton sabouraudi. Arch. Ophthalmol. B. Aires. 1970;45(8):318–321. [PubMed] [Google Scholar]
- Rayan K.J. Elsevier Science Publishing Co., Inc.; New York: 1984. Medical Microbiology an Introduction to Infectious Diseases. [Google Scholar]
- Ritterbrand D.C., Seedor J.A., Shah M.K. Fungal keratitis at the New York eye and ear infirmary. Cornea. 2006;25:264–267. doi: 10.1097/01.ico.0000177423.77648.8d. [DOI] [PubMed] [Google Scholar]
- Rosa R.H., Jr., Miller D., Alfonso E.C. The changing spectrum of fungal keratitis in South Florida. Ophthalmology. 1994;101:1005–1013. doi: 10.1016/s0161-6420(94)31225-5. [DOI] [PubMed] [Google Scholar]
- Shehri A., Jastaneiah S.S., Wagoner M.D. Changing trends in the clinical course and outcome of bacterial keratitis at King Khaled Eye Specialist Hospital. Int. Ophthalmol. 2009;29(3):143–152. doi: 10.1007/s10792-008-9206-6. [DOI] [PubMed] [Google Scholar]
- Shenoy R., Shenoy U.A., Al Mahrooqi Z.H. Keratomycosis due to Trichophyton mentagrophytes. Mycoses. 2003;46(3-4):157–158. doi: 10.1046/j.1439-0507.2003.00863.x. [DOI] [PubMed] [Google Scholar]
- Tanure M.A., Cohen E.J., Sudesh S., Rapuano C.J., Laibson P.R. Spectrum of fungal keratitis at Wills Eye Hospital, Philadelphia, Pennsylvania. Cornea. 2000;19(3):307–312. doi: 10.1097/00003226-200005000-00010. [DOI] [PubMed] [Google Scholar]
- Thomas P.A. Fungal infections of the cornea. Eye. 2003;17(8):852–862. doi: 10.1038/sj.eye.6700557. [DOI] [PubMed] [Google Scholar]
- Wong T.Y., Ng T.P., Fong K.S., Tand T.H. Risk factors and clinical out comes between fungal and bacterial keratitis: a comparative study. CLAO. 1997;23:275–281. [PubMed] [Google Scholar]
- Xie L., Dong X., Shi W. Treatment of fungal keratitis by penetrating keratoplasty. Br. J. Ophthalmol. 2001;85:1070–1074. doi: 10.1136/bjo.85.9.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]


