Abstract
Diabetic retinopathy (DR) is a major cause of preventable blindness in the developed countries. Despite the advances in understanding and management of DR, it remains a challenging condition to manage. The standard of care for patients with DR include strict metabolic control of hyperglycemia, blood pressure control, normalization of serum lipids, prompt retinal laser photocoagulation and vitrectomy. For patients who respond poorly and who progressively lose vision in spite of the standard of care, intravitreal administration of steroids or/and anti-vascular endothelial growth factor (anti-VEGF) drugs appear to be a promising second-line of therapy. This review discusses the current concepts and the role of these novel therapeutic approaches in the management of DR.
Keywords: Diabetic retinopathy, Intravitreal steroids, Anti-VEGF drugs
1. Introduction
There is an epidemic of diabetes mellitus (DM) worldwide (Scanlon, 2009). Prevalence of diabetic retinopathy (DR) is also rising accordingly. DR is the major threat to sight in the working age population in the developed world (Zimmet et al., 2001). Furthermore, DR is increasing as a major cause of blindness in other parts of world including the eastern Mediterranean and middle eastern region representing an enormous public health problem (Scanlon, 2009; Zimmet et al., 2001).
The extent of visual impairment in diabetic patients with DR can undeniably be decreased with systemic and ocular therapeutic intervention as shown by many clinical trials. For last few decades, retinal laser photocoagulation has led a revolution in the management of diabetic retinopathy. Just as dramatic as laser photocoagulation, advances in instrumentation and vitreo-retinal surgical techniques have also been able to salvage vision in many patients with advanced stages of DR.
Since the DR is a complex entity with multi-factorial etiology it needs multipronged approach to treatment. Though the laser photocoagulation has remained as the mainstay of treatment for patients with DR, there is a distinct sub-group of eyes with DR which do not respond adequately to laser photocoagulation. This limitation has promoted interest to search for alternative treatment modalities. Several therapeutic modalities are under investigation presently. This article will address the current concepts in the management of DR with intravitreal administration of drugs.
2. Causes of visual loss in DR
Though the diabetic retinopathy progresses through various stages, as shown in Fig. 1, the treatment of DR in a patient depends on the cause/s of visual loss. The two main causes of visual loss/impairment in patients with diabetic retinopathy are: proliferative diabetic retinopathy (PDR) and diabetic macular edema (DME).
Figure 1.
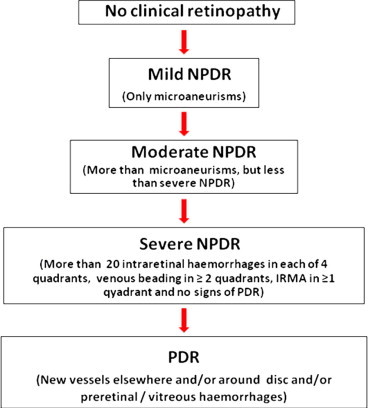
Classification of diabetic retinopathy.
Retinal neovascularization, a hallmark of proliferative diabetic retinopathy (PDR), is considered a major risk factor for severe vision loss in patients with DM (Abdulla and Fazwi, 2009). PDR can be further categorized as early, high-risk, or advanced, depending on the degree and severity of retinal new vessels, presence of vitreous or pre-retinal hemorrhage and retinal detachment.
The diabetic macular edema (DME) in the most common cause of moderate visual loss in patients with DM (Klein et al., 1984; Moss et al., 1988). DME may be associated with any of the stages of retinopathy. DME is defined as retinal thickening or presence of hard exudates within one disc diameter of the centre of the macula (The Early Treatment of Diabetic Retinopathy Study Research Group, 1985; Klein et al., 1991, 1995; Neelakshi et al., 2009). The Early Treatment of Diabetic Retinopathy Study (ETDRS) further classified DME as either clinically significant macular edema (CSME) or non-clinically significant, depending on its location and the presence of any associated exudates (Neelakshi et al., 2009; Wilkinson et al., 2003). DME becomes CSME if one or more of the following three conditions are present: (a) retinal thickening at or within 500 μm of the centre of the macula, (b) hard exudates at or within 500 μm of the centre of the macula if associated with thickening of the adjacent retina, (c) a zone or zones of retinal thickening of at least one disc diameter in size part of which is within one disc diameter of the centre of macula (The Early Treatment of Diabetic Retinopathy Study Research Group, 1985).
The CSME is further classified into focal or diffuse type depending on the pattern of the dye leakage on fluorescein angiography (FA) (Neelakshi et al., 2009). In focal CSME, focal leakage tends to occur from microaneurisms often with extravascular lipoproteins in circinate pattern around them; and well defined areas of fluorescein leakage from the microaneurisms are seen on the FA. These microaneurisms are thought to cause the retinal thickening. In contrast, the diffuse type of CSME results from a generalized breakdown of the blood–retinal barrier resulting into profuse leakage from the entire capillary bed in the posterior pole. The diffuse CSME is characterized by generalized intraretinal leakage from the retinal capillary bed and/or from intraretinal microvascular abnormalities (IRMAs) and/or from arterioles and venules (in severe cases), without any discrete areas of leakage from the microaneurisms. Hence diffuse CSME is more challenging to manage as compared to the focal type (Neelakshi et al., 2009).
3. Standard of care in DR
Several large, randomized, controlled clinical trials have provided the scientific basis for taking care of vision in the diabetic patients with DR (The Early Treatment of Diabetic Retinopathy Study Research Group, 1985; The Diabetes Control and Complications Trial Research Group, 1993; UK Prospective Diabetes Study (UKPDS) Group, 1998; The Diabetic Retinopathy Study Research Group, 1976, 1981, 1987; Early Treatment Diabetic Retinopathy Study Research Group, 1991). The guidelines set forth by these landmark studies have reduced the incidence of visual impairment/loss by helping the clinician in determining when and how to treat the DR (The Early Treatment of Diabetic Retinopathy Study Research Group, 1985; The Diabetes Control and Complications Trial Research Group, 1993; UK Prospective Diabetes Study (UKPDS) Group, 1998; The Diabetic Retinopathy Study Research Group, 1976, 1981, 1987; Early Treatment Diabetic Retinopathy Study Research Group, 1991).
The first step in managing DR is to control the underlying DM because prolonged hyperglycemia is a major risk factor for the development and progression of DR. Intensive metabolic control, as reflected by the HbA1c level, not only reduces the mean risk of developing retinopathy but also lowers the risk of progression (The Diabetes Control and Complications Trial Research Group, 1993; UK Prospective Diabetes Study (UKPDS) Group, 1998). The available data also suggests that proper management of hypertension can reduce diabetes-induced retinal complications (Funatsu and Yamashita, 2003; Matthews et al., 2004; Sheth et al., 2006). Hyperlipidemia has been linked to the presence of retinal hard exudates in patients with retinopathy and evidence suggests that lipid-lowering therapy may reduce hard exudates and microaneurisms (Sheth et al., 2006; Lyons et al., 2004; Miljanovic et al., 2004; Chew et al., 1996; Klein et al., 1991). It is important to appreciate that these treatments not only delay the onset of DR but also slow the progression of retinal lesions to more severe forms.
Over last 2–3 decades, laser photocoagulation has remained as the mainstay and the standard of care for managing patients with sight threatening DR: both PDR and DME (The Early Treatment of Diabetic Retinopathy Study Research Group, 1985; Neelakshi et al., 2009; The Diabetic Retinopathy Study Research Group, 1976, 1981, 1987). Panretinal photocoagulation (PRP) with lasers is the standard practice of managing PDR (The Diabetic Retinopathy Study Research Group, 1976, 1981, 1987). Laser photocoagulation reduces the oxygen demand of the outer layers of the retina and helps divert adequate oxygen and nutrients to the inner retinal layers, thus favorably altering the haemodynamics and introducing more choroidal oxygen to the ischemic inner retina, with a resultant reduction in hypoxia-mediated secretion of vascular endothelial growth factor (VEGF) and regression of neovascularization. In patients with DME too, the retinal laser photocoagulation in the form of focal laser for focal CSME or grid laser for diffuse CSME, as defined by the ETDRS, remains the standard of care (The Early Treatment of Diabetic Retinopathy Study Research Group, 1985; Neelakshi et al., 2009).
4. Intravitreal drugs for managing DR
Some patients with PDR and DME continue to lose vision despite the prompt laser treatment. Progression of visual loss continues to occur in 5% of patients in patients with PDR in spite of PRP (Aiello, 2005). In some patients of DME especially of diffuse CSME, the standard treatment with grid laser is somewhat less effective and more variable in outcome (Neelakshi et al., 2009). Thus, in day-to-day practice one commonly encounters some cases that are not/less responsive to the conventional laser therapy.
Many theories have been proposed to explain the clinico-pathological findings in PDR and DME, including biochemical, hemodynamic, endocrine, growth factors and inflammatory theories. Hence, it may be inadequate to treat PDR and DME with laser alone. These newer insights into the pathogenesis of DR have improved our understanding of the disease and helped devise new treatment options with alternative or adjunctive pharmacologic therapies for those cases that are not responsive to thermal laser therapy.
Different drugs and drug delivery systems are being tried in patients with DR. Some of them include: peribulbar steroid injections, intravitreal steroid injections, injection of sustained-release steroid intravitreal implants and intravitreal administration of anti-VEGF drugs. Most of them are being used as “off-label” therapy. But some of them appear to be having more convincing roles in the management of DR especially in the patients with DME who are refractory to laser photocoagulation. All of these drugs (as shown in Table 1) are in different levels of clinical trials. Currently none of these medications have received approval from the Federal Drug Agency (FDA, USA) to treat DR.
Table 1.
Intravitreal drugs for DR.
| Steroids |
| Triamcinolone acetonide |
| Triamcinolone acetonide implant (I-vation) |
| Flucinolone acetonide implant (Retisert) |
| Dexamethasone implant (Posidurex) |
| Anti-VEGFs |
| Bevacizumab (Avastin) |
| Ranibizumab (Lucentis) |
| Pegaptanib (Macugen) |
| VEGF Trap-eye |
| Enzymes |
| Hyaluronidase |
| Plasmin |
| Microplasmin |
Given the roles of up-regulated inflammatory mediators and vascular endothelial growth factors (VEGF) in the pathogenesis of DR, intravitreal steroids and intravitreal anti-VEGF therapy are commonly being used as second-line therapy for patients with DR which are not responsive to laser therapy. Hence, we will discuss the roles of intravitreal steroids and intravitreal anti-VEGF therapy in greater detail.
5. Intravitreal steroid injections (Silva et al., 2009)
The concept that DR is a low-grade chronic inflammatory condition is gaining acceptance. Corticosteroids are potent anti-inflammatory agents. In addition, they have been shown to inhibit the expression of VEGF, effectively reduce vascular permeability, prevent blood–retinal barrier breakdown and inhibit certain matrix metalloproteinases. This broad biologic activity and multiple pharmacologic effects of corticosteroids support the rationale behind its use for treatment for DME and PDR.
Among the corticosteroids being used in managing the DR, triamcinolone acetonide (TA) is more popular. TA can be administered by several routes, including intravitreal depot injection, periocular injection, posterior subtenon injection and intravitreal implant.
5.1. Intravitreal steroids for DME
Intravitreal administration of depot preparation of TA is an emerging therapy for persistent DME. Though it has been used in the dosages of 1–8 mg; the commonly used dosage is 4 mg. The DME often improves after injection along with the visual acuity. Intravitreal TA has demonstrated short-term efficacy for DME in multiple clinical trials. After depot injection, corticosteroid action peaks at 1 week, with residual activity persisting for 3–6 months. The two most common complications of intravitreal TA are cataract formation and raised intraocular pressure. The other less common complications reported with intravitreal TA injections are: endophthalmitis and rhegmatogenous retinal detachment. Peribulbar, rather than intravitreal, triamcinolone may reduce the risk of these adverse events. However, peribulbar triamcinolone appears to be less effective for DME than its intravitreal injection in multiple clinical trials.
Diabetic Retinopathy Clinical Research network (DRCR.net) which conducted a randomized clinical multicentric trial comparing intravitreal TA with macular laser treatment reported that the visual acuity seemed to improve faster in the 4-mg TA group than in the laser group (Diabetic Retinopathy Clinical Research Network, 2008). But, the mean visual acuity and the reduction in the central retinal thickness, as measured by optical coherence tomography (OCT), at 2 years after starting the treatment were better in the laser group compared to the TA group (Diabetic Retinopathy Clinical Research Network, 2008). Cataract formation was more in 4-mg TA group as compared to 1-mg TA group and laser group. This study indicated that focal/grid laser is a better treatment than TA in eyes with DME involving fovea with visual acuity between 20/40 and 20/320 (Diabetic Retinopathy Clinical Research Network, 2008).
Intravitreal TA injection is a promising therapy for DME unresponsive to laser therapy. But, some patients require re-injections as the therapeutic effect of TA diminishes after 3–6 months. Repeated injections carry risk and are inconvenient to patients. To reduce the need for repeated intravitreal injections, a non-biodegradable intravitreal implant, Retisert, has been developed for the extended-release of flucinolone acetonide within the posterior segment; and it is in phase 3 clinical trials. The other sustained-release steroid implants being evaluated for DME are: dexamethasone implants (Posidurex, Allergan, CA, USA) and TA implant (I-vation, Surmodics) both of which are in various levels of clinical trials.
5.2. Intravitreal steroids for PDR
PRP remains the current standard of care in the treatment of PDR. But, when PDR occurs concurrently with clinically significant DME, management becomes more complex. As PRP has been reported to cause or worsen CSME, some prospective trials have been conducted to evaluate the role of combination of intravitreal triamcinolone with PRP in the management of PDR coexisting with CSME. Several small, clinical trials demonstrated that the combination of laser photocoagulation (PRP laser and macular laser) with intravitreal TA was associated with improved visual acuity and decreased central macular thickness when compared with laser photocoagulation alone for the treatment of PDR and macular edema (Kang et al., 2006; Lam et al., 2007; Maia et al., 2009). Further studies are required to elucidate the role, long-term efficacy and safety of intravitreal injection of steroids in patients with PDR.
6. Anti-VEGF therapy in DR (Neelakshi et al., 2009; Jardeleza and Miller, 2009)
In the patho-physiologic cascade which leads to the DR, chronic hyperglycemia leads to ischemia which results in over-expression of a number of growth factors, including vascular endothelial growth factors (VEGF). Though blockade of all involved growth factors will likely be necessary to completely suppress the detrimental effects of ischemia, even isolated blockade of VEGF may have beneficial effects in DR.
VEGF is an endothelial-cell-specific angiogenic factor and it appears to play a major role in pathologic as opposed to physiologic, ocular neovascularization leading to PDR. VEGF is also a vasopermeable factor which increases vascular permeability by relaxing endothelial cell junctions and this mechanism is known to contribute to the development of DME. Inhibition of VEGF blocks these effects to some extent in DR, as demonstrated in several recent clinical trials and case series involving the anti-VEGF molecules. Currently, the anti-VEGF molecules which are commonly being studied in the management of DR are: pegaptanib (Macugen), ranibizumab (Lucentis), bevacizumab (Avastin) and VEGF Trap-eye. Of the available VEGF antagonists, bevacizumab is the most frequently used outside of a formal clinical trial because it is less expensive.
6.1. Bevacizumab
Bevacizumab is a full-length, recombinant, humanized antibody active against all isoforms of VEGF-A. Several studies reported the use of the off-label intravitreal injection of bevacizumab to treat DME and PDR. The commonly used typical dose is 1.25 mg, although doses as low as 6.2 μg and as high as 2.5 mg have been used.
Many studies have demonstrated beneficial effects following intravitreal bevacizumab in patients with DME. Increased visual acuity with decrease in central retinal thickness with a single injection of bevacizumab lasts for 4–6 weeks. Hence repeated injections may be required for a prolonged effect. However, bevacizumab’s safety for intravitreal use for DR has not been tested in large, randomized studies.
Intravitreal bevacizumab injection is an effective adjunct to conventional PRP in the treatment of PDR. Administering bevacizumab in conjunction with PRP for PDR results in greater and rapid regression of new vessels compared with PRP alone (Tonello et al., 2008; Mirshahi et al., 2008; Jorge et al., 2006). Bevacizumab also plays a role in the treatment of actively leaking new vessels refractory to adequately done laser in PDR. Some authors have studied the use of intravitreal bevacizumab in cases with dense vitreous hemorrhage that precludes the completion of PRP (Spaide and Fisher, 2006; Moradian et al., 2008). This approach was suggested as an option for patients who refuse surgery or are unable to undergo surgery due to their general condition (Abdulla and Fazwi, 2009). Bevacizumab has also shown to prevent or lessen PRP associated macular edema. Moreover, bevacizumab can be very helpful in PDR complicated by neovascular glaucoma (Abdulla and Fazwi, 2009).
Intravitreal bevacizumab injection a few days before the planned surgery facilitates surgical removal of fibrovascular membranes, reduces intra-operative bleeding, reduces intra-operative time, prevents re-bleeding, and helps in accelerating post-operative vitreous clear-up (Ishikawa et al., 2009; Yeoh et al., 2008; Chen and Park, 2006; Rizzo et al., 2008). However, since, tractional retinal detachment may occur or progress shortly following the intravitreal bevacizumab, the surgery should be done within few days after its pre-operative injection in these patients.
Persistent and recurrent vitreous hemorrhage after vitrectomy is a common complication associated with vitrectomy for diabetic retinopathy with an incidence ranging from 12% to 63% (Abdulla and Fazwi, 2009; Novak et al., 1984; Yang et al., 2008). Recurrent vitreous hemorrhage could delay visual rehabilitation and occasionally requires additional surgical procedures. It has been seen that the use of intravitreal bevacizumab at the end of surgery with or without supplementary endophotocoagulation reduces the incidence of re-bleeding.
6.2. Ranibizumab
Ranibizumab is a recombinant humanized antibody fragment that is active against all isoforms of VEGF-A. The commonly used intravitreal dosage of ranibizumab is 0.5 mg. Its usage is also off-label in DR in patients with DR. Like bevacizumab, ranibizumab is also being used for both DME and PDR. Some studies on intravitreal ranibizumab have demonstrated reduced foveal thickness and satisfactory visual outcome in patients with DME. Currently, READ-2 (Ranibizumab for Edema of the mAcula in Diabetes), a phase II study is ongoing in USA, to test the long-term safety and effectiveness of intraocular injections of ranibizumab in patients with DME. DRCR.net is also conducting randomized clinical trials to elucidate the role of ranibizumab in patients with PDR.
6.3. Pegaptanib
Pegaptanib is an aptamer that binds the VEGF-A 165 isoform. It differs from the above two anti-VEGF drugs in that instead of targeting all active VEGF-A isoforms, it prevents only VEGF-165 and larger isoforms from attaching to the VEGF receptors. Its intravitreal usage has shown good visual acuity outcomes, reduced central retinal thickness and reduced need for additional photocoagulation therapy in patients with DME. The retrospective analysis of the data of one study on patients who had concomitant DME and PDR at baseline, also demonstrated regression of new vessels after pegaptanib administration (Adamis et al., 2006).
Given the potential systemic side effects of VEGF blockade, some authors advocate pegaptanib over bevacizumab and ranibizumab in DR, since pegaptanib selectively blocks VEGF-165, which plays essential role in pathological, but not physiological neovascularization. This is especially significant in patients with DM since they may have co-morbidities such as increased cardiovascular events, proteinuria and hypertension.
6.4. VEGF Trap-eye
VEGF has two main receptors, VEGF receptor (VEGFR)-1 and VEGR-2, which bind VEGF-A, VEGF-B, VEGF-C, and placental growth factor (PGF) (Holash et al., 2002). VEGF Trap-eye is a recombinant fusion protein consisting of the VEGF binding domains of VEGFR-1 and VEGFR-2 fused to the Fc domain of human immunoglobulin-G. VEGF Trap-eye has a higher binding affinity for all VEGF-A isoforms, about 140 times greater than ranibizumab (Nguyen et al., 2006). In addition, VEGF Trap-eye maintains significant intravitreal VEGF-binding activity for 10–12 weeks after a single injection (Stewart and Rosenfeld, 2008). The theoretical advantages of VEGF Trap-eye over ranibizumab include higher binding affinity, longer half-life, and ability to inhibit other molecules such as PGF-1 and PGF-2 which may translate into clinical benefits of fewer intraocular injections and longer intervals between injections. Its single intravitreal injection has been found to be effective in patients with DME (Do et al., 2009).
7. Combination therapy with intravitreal steroids and anti-VEGF
To enhance the therapeutic effects of intravitreally administered steroids and anti-VEGF drugs, it is logical to administer both of them together in the vitreous cavity in one sitting. Hence their intravitreal combination is also being tried in patients of DR who are refractory to conventional therapy. Intravitreal combination of TA and bevacizumab seems to be effective in improving visual acuity and reducing the macular thickness in patients with DME who are unresponsive to laser therapy (Tsilimbaris et al., 2009).
8. Combination therapy with laser and intravitreal drugs
Many clinical trials are underway presently to see whether combination of laser with intravitreal drugs helps in any additional benefits in terms of efficacy and interval of treatments. Theoretically, this combination provides hope of combining the short term benefit of intravitreal drug (e.g. decreased retinal thickness and decreased fluid leakage) and the long term benefit of laser photocoagulation (e.g. reduction in fluid leakage). The DRCR.net is conducting a phase III multicenter clinical trial to compare the efficacy of sham intravitreal injection with laser versus laser combined with 4 mg intravitreal triamcinolone versus laser combined with 0.5 mg intravitreal ranibizumab versus 0.5 mg intravitreal ranibizumab with deferred laser.
9. Enzymatic vitreolysis
The vitreous plays a role in the development of PDR and DME. The vitreous in diabetic patients undergoes structural modifications secondary to enzymatic and non-enzymatic collagen glycation promoting collagen cross-linking and vitreomacular traction; and this can worsen the DME. Furthermore, the retinal new vessels use the posterior hyaloid face as a scaffold to grow. The retracting vitreous pulls on these vessels and is responsible for both vitreous hemorrhage and retinal detachment in PDR. If this vitreous could be detached early and liquefied, the extent of the complications in PDR can be reduced. Hence, enzymatic vitreolysis and induction of posterior vitreous detachment is being investigated as a minimally invasive non-surgical treatment for DR.
Vitreolysis, as a non-surgical treatment in DR, has been suggested by using many potential enzymes like hyaluronidase (Kuppermann et al., 2005), plasmin and microplasmin intravitreally. Hyaluronidase has been found to be non-toxic; and appears to be effective in the clearance of vitreous hemorrhage and treatment of DR in Phase III clinical trials (Kuppermann et al., 2005).
10. Conclusions
Diabetic retinopathy, a devastating retinal manifestation of diabetes mellitus, is a serious global public health problem that diminishes the quality of life. The number of people worldwide who are at risk for developing vision loss from diabetes, is predicted to double over the next 25 years. Since DR can progress in the absence of symptoms, producing irreversible damage to the retina, regular screening examinations play a major role in reducing the magnitude of DR related visual impairment in the community.
Once DR gets established, the evidence-based therapies which form the standard of care for DR include strict metabolic control of hyperglycemia, good blood pressure control, normalization of serum lipids, prompt retinal laser photocoagulation and vitrectomy.
Current techniques of improved laser photocoagulation and vitrectomy techniques will try in preserving the visual loss from DME and PDR. But, some patients may respond poorly and progressively lose vision in spite of this standard therapy. Newer insights into the biochemical changes and molecular events that occur with DM as well as with DR have led to novel treatments which may be effective in patients when the standard care fails. The therapies which are currently being used more frequently when the response to the standard care is un-satisfactory include intravitreal anti-VEGF and corticosteroid-based treatment strategies both of which form the second-line of therapy. Other new pharmacotherapies on the horizon also appear exciting at the moment. However, prospective randomized clinical trials are needed to study the role of all these novel therapies.
Disclosure
None of the authors have any financial interests to disclose. Each author has equally contributed in the preparation of the manuscript.
References
- Abdulla Walid, Fazwi Amani. Anti-VEGF therapy in proliferative diabetic retinopathy. Int. Ophthalmol. Clin. 2009;49:95–107. doi: 10.1097/IIO.0b013e31819fd84a. [DOI] [PubMed] [Google Scholar]
- Adamis A.P., Altaweel M., Bressler N.M. Changes in retinal neovascularization after pegaptanib (Macugen) therapy in diabetic individuals. Ophthalmology. 2006;113(1):23–28. doi: 10.1016/j.ophtha.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Aiello L.P. Angiogenic pathways in diabetic retinopathy. N. Engl. J. Med. 2005;353:839–841. doi: 10.1056/NEJMe058142. [DOI] [PubMed] [Google Scholar]
- Chen E., Park C.H. Use of intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina. 2006;26:699–700. doi: 10.1097/01.iae.0000225351.87205.69. [DOI] [PubMed] [Google Scholar]
- Chew E.Y., Klein M.L., Ferris F.L., III Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy: Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch. Ophthalmol. 1996;114:1079–1084. doi: 10.1001/archopht.1996.01100140281004. [DOI] [PubMed] [Google Scholar]
- Diabetic Retinopathy Clinical Research Network A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115(9):1447–1449. doi: 10.1016/j.ophtha.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do D.V., Nguyen Q.D., Shah S.M. An exploratory study of the safety, tolerability and bioactivity of a single intravitreal injection of vascular endothelial growth factor Trap-Eye in patients with diabetic macular oedema. Br. J. Ophthalmol. 2009;3:144–149. doi: 10.1136/bjo.2008.138271. [DOI] [PubMed] [Google Scholar]
- Early Treatment Diabetic Retinopathy Study Research Group Early photocoagulation for diabetic retinopathy. ETRDS Report Number 9. Ophthalmology. 1991;98:766–785. [PubMed] [Google Scholar]
- Funatsu H., Yamashita H. Pathogenesis of diabetic retinopathy and the renin–angiotensin system. Ophthal. Physiol. Opt. 2003;23(6):495–501. doi: 10.1046/j.1475-1313.2003.00134.x. [DOI] [PubMed] [Google Scholar]
- Holash J., Davis S., Papadopoulos N. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc. Natl. Acad. Sci. USA. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K., Honda S., Tsukahara Y. Preferable use of intravitreal bevacizumab as a pretreatment of vitrectomy for severe proliferative diabetic retinopathy. Eye. 2009;23:108–111. doi: 10.1038/sj.eye.6702983. [DOI] [PubMed] [Google Scholar]
- Jardeleza M.S.R., Miller J.W. Review of anti-VEGF therapy in proliferative diabetic retinopathy. Semin. Ophthalmol. 2009;24:87–92. doi: 10.1080/08820530902800330. [DOI] [PubMed] [Google Scholar]
- Jorge R., Costa R.A., Calucci D. Intravitreal bevacizumab (Avastin) for persistent new vessels in diabetic retinopathy (IBEPE study) Retina. 2006;26:1006–1013. doi: 10.1097/01.iae.0000246884.76018.63. [DOI] [PubMed] [Google Scholar]
- Kang S.W., Sa H.S., Cho H.Y., Kim J.I. Macular grid photocoagulation after intravitreal triamcinolone acetonide for diffuse diabetic macular edema. Arch. Ophthalmol. 2006;124(5):653–658. doi: 10.1001/archopht.124.5.653. [DOI] [PubMed] [Google Scholar]
- Klein R., Klein B.E., Moss S.E. Visual impairment in diabetes. Ophthalmology. 1984;91:1–9. [PubMed] [Google Scholar]
- Klein B.E., Moss S.E., Klein R., Surawicz T.S. The Wisconsin Epidemiologic Study of Diabetic Retinopathy, XIII: relationship of serum cholesterol to retinopathy and hard exudate. Ophthalmology. 1991;98:1261–1265. doi: 10.1016/s0161-6420(91)32145-6. [DOI] [PubMed] [Google Scholar]
- Klein R., Klein B.E., Moss S.E. The epidemiology of ocular problems in diabetes mellitus. In: Ferman S.S., editor. Ocular Problems in Diabetes Mellitus. Blackwell Publications; Boston: 1991. pp. 1–51. [Google Scholar]
- Klein R., Klein B.E.K., Moss S.E. The Wisconsin epidemiologic study of diabetic retinopathy. XV. The long term incidence of macular edema. Ophthalmology. 1995;102:7–16. doi: 10.1016/s0161-6420(95)31052-4. [DOI] [PubMed] [Google Scholar]
- Kuppermann B.D., Thomas E.L., de Smet M.D. Vitrase for Vitreous Hemorrhage Study Groups. Pooled efficacy results from two multinational randomized controlled clinical trials of a single intravitreous injection of highly purified ovine hyaluronidase (Vitrase) for the management of vitreous hemorrhage. Am. J. Ophthalmol. 2005;140(4):573–584. doi: 10.1016/j.ajo.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Lam D.S., Chan C.K., Mohamed S. Intravitreal triamcinoone plus sequential grid laser versus triamcinolone or laser alone for treating diabetic macular edema: six-month outcomes. Ophthalmology. 2007;114(12):2162–2167. doi: 10.1016/j.ophtha.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Lyons T.J., Jenkins A.J., Zheng D. Diabetic retinopathy and serum lipoprotein subclasses in the DCCT/EDIC cohort. Invest. Ophthalmol. Vis. Sci. 2004;45:910–918. doi: 10.1167/iovs.02-0648. [DOI] [PubMed] [Google Scholar]
- Maia O.O., Jr., Takahashi B.S., Costa R.A. Combined laser and intravitreal triamcinolone for proliferative diabetic retinopathy and macular edema: one-year results of a randomized clinical trial. Am. J. Ophthalmol. 2009;147(2):291–297. doi: 10.1016/j.ajo.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Matthews D.R., Stratton I.M., Aldington S.J., Holman R.R., Kohner E.M. Risk of progression of retinopathy and visual loss related to tight control of blood pressure in Type 2 diabetes mellitus. UKPDS 69. Arch. Ophthalmol. 2004;122(11):1631–1640. doi: 10.1001/archopht.122.11.1631. [DOI] [PubMed] [Google Scholar]
- Miljanovic B., Glynn R.J., Nathan D.M., Manson J.E., Schaumberg D.A. A prospective study of serum lipids and risk of diabetic macular edema in type 1 diabetes. Diabetes. 2004;53:2883–2892. doi: 10.2337/diabetes.53.11.2883. [DOI] [PubMed] [Google Scholar]
- Mirshahi A., Roohipoor R., Lashay A. Bevacizumab-augmented retinal laser photocoagulation in proliferative diabetic retinopathy: a randomized double-masked clinical trial. Eur. J. Ophthalmol. 2008;18:263–269. doi: 10.1177/112067210801800215. [DOI] [PubMed] [Google Scholar]
- Moradian S., Ahmadieh H., Malihi M. Intravitreal bevacizumab in active progressive proliferative diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2008;246:1699–1705. doi: 10.1007/s00417-008-0914-4. [DOI] [PubMed] [Google Scholar]
- Moss S.E., Klein R., Klein B.E. The incidence of vision loss in a diabetic population. Ophthalmology. 1988;95:1340–1348. doi: 10.1016/s0161-6420(88)32991-x. [DOI] [PubMed] [Google Scholar]
- Neelakshi Bhagat, Grigorian R.A., Tutela A. Diabetic macular edema: pathogenesis and treatment. Surv. Ophthalmol. 2009;54:1–32. doi: 10.1016/j.survophthal.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Nguyen Q.D., Shah S.M., Hafiz G. A phase I trial of an IV-administered vascular endothelial growth factor trap for treatment in patients with choroidal neovascularization due to age-related macular degeneration. Ophthalmology. 2006;113:1522. doi: 10.1016/j.ophtha.2006.05.055. [DOI] [PubMed] [Google Scholar]
- Novak M.A., Rice T.A., Michels R.G. Vitreous hemorrhage after vitrectomy for diabetic retinopathy. Ophthalmology. 1984;91:1485–1489. doi: 10.1016/s0161-6420(84)34099-4. [DOI] [PubMed] [Google Scholar]
- Rizzo S., Genovesi-Ebert F., Di Bartolo E. Injection of intravitreal bevacizumab (Avastin) as a preoperative adjunct before vitrectomy surgery in the treatment of severe proliferative diabetic retinopathy (PDR) Graefes Arch. Clin. Exp. Ophthalmol. 2008;246:837–842. doi: 10.1007/s00417-008-0774-y. [DOI] [PubMed] [Google Scholar]
- Scanlon P.H. Prologue. In: Scanlon P.H., Wilkinson C.P., Aldington S.J., Mathews D.R., editors. Diabetic Retinopathy Management. Wiley-Blackwell; West Sessex: 2009. pp. ix–xviii. [Google Scholar]
- Sheth H.G., Aslam S., Davies N. Lipid lowering drugs in diabetes: lipid lowering has ophthalmic benefits. Br. Med. J. 2006;332(7552):1272–1273. doi: 10.1136/bmj.332.7552.1272-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva P.S., Sun J.K., Aiello L.P. Role of steroids in the management of diabetic macular edema and proliferative diabetic retinopathy. Semin. Ophthalmol. 2009;24:93–99. doi: 10.1080/08820530902800355. [DOI] [PubMed] [Google Scholar]
- Spaide R.F., Fisher Y.L. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006;26:275–278. doi: 10.1097/00006982-200603000-00004. [DOI] [PubMed] [Google Scholar]
- Stewart M.W., Rosenfeld P.J. Predicted biological activity of intravitreal VEGF Trap. Br. J. Ophthalmol. 2008;92:667–668. doi: 10.1136/bjo.2007.134874. [DOI] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- The Diabetic Retinopathy Study Research Group Preliminary report on effects of photocoagulation therapy. Am. J. Ophthalmol. 1976;81:383–396. doi: 10.1016/0002-9394(76)90292-0. [DOI] [PubMed] [Google Scholar]
- The Diabetic Retinopathy Study Research Group Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study findings. DRS Report Number 8. Ophthalmology. 1981;88:583–600. [PubMed] [Google Scholar]
- The Diabetic Retinopathy Study Research Group Indications for photocoagulation treatment of diabetic retinopathy. Diabetic Retinopathy Study Report Number 14. Int. Ophthalmol. Clin. 1987;27:239–253. doi: 10.1097/00004397-198702740-00004. [DOI] [PubMed] [Google Scholar]
- The Early Treatment of Diabetic Retinopathy Study Research Group Photocoagulation for diabetic macular edema. The Early Treatment of Diabetic Retinopathy Study Report Number 1. Arch. Ophthalmol. 1985;103:1796–1806. [PubMed] [Google Scholar]
- Tonello M., Costa R.A., Almeida F.P. Panretinal photocoagulation versus PRP plus intravitreal bevacizumab for high-risk proliferative diabetic retinopathy (IBeHistudy) Acta Ophthalmol. 2008;86:385–389. doi: 10.1111/j.1600-0420.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- Tsilimbaris M.K., Pandeleondidis V. Intravitreal combination of triamcinolone acetonide and bevacizumab (kenacort-avastin) in diffuse diabetic macular edeme. Semin. Ophthalmol. 2009;24(6):225–230. doi: 10.3109/08820530903389775. [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with Type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- Wilkinson C.P., Ferris F.L., III, Klein R.E. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- Yang C.M., Yeh P.T., Yang C.H. Bevacizumab pretreatment and long-acting gas infusion on vitreous clear-up after diabetic vitrectomy. Am. J. Ophthalmol. 2008;146:211–217. doi: 10.1016/j.ajo.2008.04.028. [DOI] [PubMed] [Google Scholar]
- Yeoh J., Williams C., Allen P. Avastin as an adjunct to vitrectomy in the management of severe proliferative diabetic retinopathy: a prospective case series. Clin. Exp. Ophthalmol. 2008;36:449–454. [PubMed] [Google Scholar]
- Zimmet P., Alberti K.G., Shaw J. Global and societal implications of the diabetic epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]


