SUMMARY
Interleukin (IL) 10 and interferon-gamma (IFN-γ) levels in induced sputum supernatants of 21 tuberculosis (TB) patients at diagnosis and during chemotherapy were correlated to recurrence rates. IL-10 decreased until day 60 of treatment (T60), and between T60 and T180 it increased again in 7 cases (Pattern 1) and further decreased in 14 cases (Pattern 2). Follow-up of 69 months was performed in 20/21 cases; 6 had recurrence of TB, of which 5/7 (71%) had Pattern 1 and 1/13 (7.7%) Pattern 2 (OR 30.0, 95%CI 2.19–411.3, P = 0.0072). This was not observed for IFN-γ. High IL-10 levels at the end of treatment may function as a risk factor for TB recurrence.
Keywords: tuberculosis, IL-10, IFN-γ, induced sputum, recurrence
INTERLEUKIN (IL) 10 plays an important role in the negative regulation of IL-12 production and co-stimulatory molecule expression, thereby reducing the secretion of interferon-gamma (IFN-γ) by T-cells.1 The absence of IL-10 favors the increase of host resistance to intracellular organisms in the initial phase of tuberculosis (TB) infection.2 The World Health Organization (WHO) defines recurrent TB as a bacteriologically confirmed disease episode needing retreatment after a patient has been successfully treated or has defaulted during a previous disease episode.3 The present study extends the observations of Almeida et al. to establish a relationship between IL-10 patterns and recurrence during and at the end of anti-tuberculosis treatment in a cohort of patients with a long period of follow-up.4
PATIENTS AND METHODS
The study was approved by the institutional review boards of Weill Cornell Medical College, New York, NY, USA and the Federal University of Rio de Janeiro, Rio de Janeiro, RJ, Brazil.
A total of 21 TB cases, who received rifampicin, isoniazid, and pyrazinamide (RHZ) for 2 months followed by RH for 4 months, were evaluated. During treatment, patients were evaluated weekly for the first month and monthly thereafter. Induced sputum was obtained by inhalation of 2% nebulized saline solution at diagnosis and at 15, 30, 60, and 180 days of chemotherapy.5 Treatment of induced sputum samples and supernatant storage were performed as described, and cytokines were quantified from sputum supernatants using ELISA kits (Immunotech-Coulter, Marseille, France).4 At the end of treatment, all TB patients were deemed cured. Of the 21 patients, 20 were available for re-evaluation 68.9 (± 1.3) months after the end of treatment.
Statistical analyses were performed using Graph-Pad Prism 4.00 (GraphPad Software Inc., La Jolla, CA, USA).
RESULTS
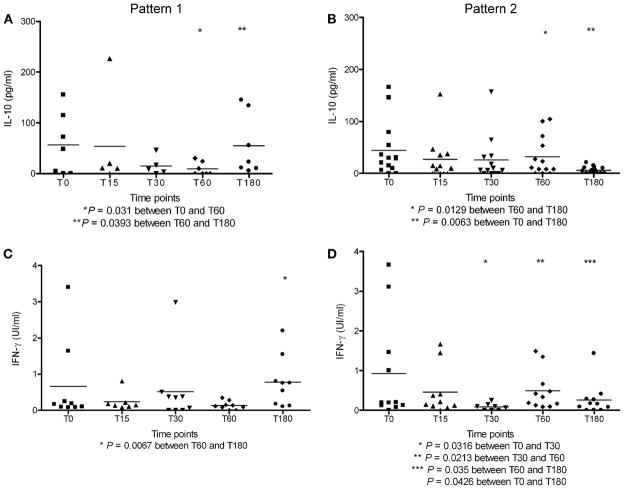
The Table shows demographic and clinical characteristic at diagnosis (T0) and compares them between patients who experienced at least one second TB episode during the follow-up period and those who remained free of TB. No clinical differences were seen between the two subgroups, apart from the higher frequency of previous TB episodes in those with recurrent TB (P = 0.0175). This article is a continuation of the study by Almeida et al., who presented IL-10/ IFN-γ data for healthy volunteers (Figure 7 A/B4). The analysis of IL-10 levels between day 60 of treatment (T60) and the end of treatment (T180) demonstrated that, after a decrease in IL-10 levels, 7 TB patients had experienced an increase of IL-10 at T180, called Pattern 1. A comparison between T0 (56.5 ± 23.2 pg/ ml; n = 7) and T60 (9.2 ±4.9 pg/ml; n = 7) showed a decrease in IL-10 (P = 0.031). Between T60 and T180, IL-10 increased again (T60 = 9.2 ± 4.9 pg/ml; n = 7; T180 = 54.9 ± 22.8 pg/ml; n = 7; P = 0.0393). IL-10 levels at diagnosis (T0) were similar to those at the end of treatment (T180; T0 = 65.87 ± 25.11 pg/ml, n = 7; T180 ± 54.9±22.8 pg/ml; n = 7, P > 0.05; Figure, A). The remaining 14 cases showed a consistent decrease in IL-10 levels at all time points, including T180 (T0 = 44.3 ± 13.9 pg/ml; n = 14; T180 = 6.1 ± 1.8 pg/ml; n = 14, P = 0.0063; Student’s t-test), called Pattern 2 (Figure, B).
Table.
Demographic, clinical, radiologic and bacteriologic findings in TB patients at the moment of diagnosis (T0) and comparison of initial findings in cases with or without a new TB episode after 5–6 years’ follow-up*
| Characteristic | TB (n = 21) n (%) |
Second episode† (n = 6) n (%) |
Cure‡ (n = 14) n (%) |
P value |
|---|---|---|---|---|
| Age, years, mean ± SEM | 32.81 ± 2.57 | 28.2 ± 6.9 | 33.8 ± 2.4 | NS§ |
| Male | 13 (61.9) | 4 (66.7) | 8 (57.1) | NS¶ |
| Smoker | 9 (42.9) | 2 (33.3) | 6 (42.9) | NS¶ |
| Alcoholism | 4 (19.0) | 1 (16.7) | 3 (21.4) | NS¶ |
| Asthma/rhinitis | 1 (4.8) | 0 | 1 (7.1) | NS¶ |
| Previous TB | 3 (14.3) | 3 (50.0) | 0 | 0.0175¶ |
| Duration of symptoms, weeks ± SEM | 8.91 ± 1.14 | 10.5 ± 3.3 | 8.0 ± 0.9 | NS§ |
| Extension > 1 lobe# | 11 (52.4) | 4 (66.7) | 7 (50.0) | NS¶ |
| Cavitary TB** | 8 (38.1) | 4 (66.7) | 4 (28.6) | NS¶ |
| Negative AFB in sputum | 5 (23.8) | 1 (16.7) | 3 (21.4) | NS¶ |
| Negative sputum culture | 3 (14.3) | 1 (16.7) | 1 (7.1) | NS¶ |
One TB case was lost to follow-up.
At least one new episode in 69 months of follow-up.
No new TB episode in 69 months of follow-up.
Student’s t-test.
Fisher’s exact test.
Number of pulmonary lobes affected by the disease.
Lung cavitation on chest X-ray.
TB = tuberculosis; SEM = standard error of mean; NS = non-significant; AFB = acid-fast bacilli.
Figure.
Patterns 1 and 2 of IL-10 and IFN-γ levels in the sputum of TB patients at the moment of diagnosis (T0) and during treatment. (Student’s t-test for paired samples, significance 95%. ELISA sensitivity for IL-10 = 5 pg/ml and for IFN-γ = 0.08 UI/ml.) In Pattern 1, the IL-10 levels had decreased at T60 and had increased again by the end of treatment (T180; A), whereas in Pattern 2, the IL-10 levels had decreased at T60 and had decreased further at the end of treatment (T180; B). Similar patterns were observed in the IFN-γ levels (C and D). C shows Pattern 1 with increased IFN-γ levels between T60 and T180 (T60 = 0.1348 ± 0.039 UI/ml; n = 9; T180 = 0.776 ± 0.233 UI/ml; n = 9; P = 0.0067). IFN-γ levels at diagnosis (T0) were similar to those at the end of treatment (T0 = 0.666 ± 0.38 UI/ml, n = 9; T180 = 0.776 ± 0.233 UI/ml; n = 9; P = NS). The remaining 11 were Pattern 2 cases showing a consistent drop in IFN-γ at all time points, including T180 (T0 = 0.92 ± 0.394 UI/ml; n = 11; T180 = 0.261 ± 0.124 UI/ml; n = 11, P = 0.0426; D). In the case of IL-10, Pattern 1 was associated with increased TB recurrence rates. For IFN-γ, the 6 recurrent cases were distributed equally between Patterns 1 and 2. IL = interleukin; IFN-γ = interferon-gamma; TB = tuberculosis.
The same analysis was performed for IFN-γ: nine cases followed Pattern 1, and 11 followed Pattern 2 (Figure, C and D).
Five years after enrollment, 20/21 patients returned for consultation. Of these, 6 had experienced at least one second TB episode during the period (five had had two episodes), and 14 had remained free of TB. All episodes were treated with standard RHZ for 6 months. At the last visit, all were well and the chest X-ray had limited fibrosis, compatible with TB sequelae. TB recurrence was linked to the IL-10 and IFN-γ patterns described above. Regarding IL-10, of the six TB patients with at least a second episode of disease, five presented Pattern 1 (5/7, 71%), whereas just one (1/13, 7.7%; one Pattern 2 case lost to follow-up) showed Pattern 2 (OR 30.0; 95%CI 2.19–411.3; P = 0.0072, Fisher’s exact test). No such relationship was seen for IFN-γ.
DISCUSSION
A key element of the protective response against TB involves secretion of pro-inflammatory cytokines, such as tumor necrosis factor-α, IL-12, IL-1β and IFN-γ.1 To protect against excess damage caused by these pro-inflammatory cytokines, secretion of anti-inflammatory cytokines, such as transforming growth factor-β and IL-10, is essential in this process.6 If these anti-inflammatory cytokines are disproportionally represented at the beginning of the anti-tuberculosis immune process, they will contribute to suppression of a protective response, even in the presence of pro-inflammatory cytokines.6 This study demonstrated that IL-10 presents different patterns of temporal evolution which are associated with an important epidemiological outcome: TB recurrence. In some patients, IL-10 concentrations fell between T0 and T60, followed by a new increase at the end of treatment to levels similar to those at diagnosis. We wondered if these different behaviors could influence clinical outcomes. Pattern 1 showed a strong association between increased IL-10 concentrations at the end of treatment and a second episode of TB up to 6 years after discharge. No similar observation was made for IFN-γ.
Through its capacity to promote the deactivation of previously activated macrophages, IL-10 plays a strong regulatory role in immune response, thereby contributing to the control of inflammatory reaction, and limiting the damage induced by inflammation.7,8 IL-10 is therefore linked to the ability of mycobacteria to evade immune responses, and mediates long-term infections in the lung.9
Recurrent TB is a public health problem in high-burden countries. A study in South Africa found that 14% of patients who completed treatment had a second episode of TB, and the majority of patients presented re-infection with a mycobacterial strain that was different from the first episode.10 Although the recurrence rate seen in the present study was higher than expected, the study was not designed to ascertain this point, nor were clinical isolates available for genotyping to verify if they were cases of relapse or re-infection. The possibility that genetic changes in the host facilitate recurrence, probably by single nucleotide polymorphisms in the relevant genes, including IL-10, needs further clarification.
Acknowledgments
Funding was provided by National Institutes of Health grants R01 HL61960 and R21 AI063147 (to J L Ho), ICOHRTA AIDS/TB # 5U2RTW006883 (PI JRLS), Coordenação de Aperfeicoamento de Pessoal de Nivel Superior (CAPES, Ministry of Education, Brazil), the Brazilian Research Council/CNPq, the Brazilian Research Council/World Bank Millennium Institutes of Science, Programa de Apoio a Núcleos de Excelência (PRONEX/FAPERJ).
References
- 1.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 2.Murray PJ, Young RA. Increased antimycobacterial immunity in interleukin-10-deficient mice. Infect Immun. 1999;67:3087–3095. doi: 10.1128/iai.67.6.3087-3095.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization/International Union Against Tuberculosis and Lung Disease. A WHO/The Union monograph on TB and tobacco control: joining efforts to control two related global epidemics. Geneva, Switzerland: WHO; 2007. [Accessed August 2011]. p. 6. http://whqlibdoc.who.int/publications/2007/9789241596220_eng.pdf. [Google Scholar]
- 4.Almeida AS, Lago PM, Boéchat N, et al. Tuberculosis is associated with a down-modulatory lung immune response that impairs Th1-type immunity. J Immunol. 2009;183:718–731. doi: 10.4049/jimmunol.0801212. [DOI] [PubMed] [Google Scholar]
- 5.Bigby TD, Margolskee D, Curtis JL, et al. The usefulness of induced sputum in the diagnosis of Pneumocystis carinii pneumonia in patients with the acquired immunodeficiency syndrome. Am Rev Respir Dis. 1986;133:515–518. doi: 10.1164/arrd.1986.133.4.515. [DOI] [PubMed] [Google Scholar]
- 6.Bonecini-Almeida MG, Ho JL, Boechat N, et al. Down-modulation of lung immune responses by interleukin-10 and transforming growth factor beta (TGF-beta) and analysis of TGF-beta receptors I and II in active tuberculosis. Infect Immun. 2004;72:2628–2634. doi: 10.1128/IAI.72.5.2628-2634.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huard RC, Chitale S, Leung M, et al. The Mycobacterium tuberculosis complex-restricted gene cfp32 encodes an expressed protein that is detectable in tuberculosis patients and is positively correlated with pulmonary interleukin-10. Infect Immun. 2003;71:6871–6883. doi: 10.1128/IAI.71.12.6871-6883.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho JL, Lapa e Silva JR. Promotion of a down-modulated lung immune state may be a strategy by M. tuberculosis to foster active disease and persistence. Discov Med. 2010;9:34–41. [PubMed] [Google Scholar]
- 9.Redford PS, Murray PJ, O’Garra A. The role of IL-10 in immune regulation during M. tuberculosis infection. Mucosal Immunol. 2011;4:261–270. doi: 10.1038/mi.2011.7. [DOI] [PubMed] [Google Scholar]
- 10.Verver S, Warren RM, Beyers N, et al. Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis. Am J Respir Crit Care Med. 2005;171:1430–1435. doi: 10.1164/rccm.200409-1200OC. [DOI] [PubMed] [Google Scholar]