Abstract
A critical step in phacoemulsification (as well as extracapsular cataract extraction) is making a window in anterior capsule wall (i.e. anterior capsulotomy). Continuous Curvilinear Capsulorhexis (CCC) has become recognized as the standard method of anterior capsulectomy. Techniques employed for CCC have undergone sustained evolution. The present review evaluates elementary principles of CCC. Management of CCC in the presence of small pupil and pseudoexfoliation syndrome is discussed. Main differences of pediatric CCC from its adult-style counterpart and finally several techniques of rescue of an extending capsulorhexis are also reviewed.
Abbreviations: ICCE, intracapsular cataract extraction; ECCE, extracapsular cataract extraction; IOL, intraocular lens; CCC, Continuous Curvilinear Capsulorhexis; OVDs, Ophthalmic Viscosurgical Devices; PCCC, Posterior Continuous Curvilinear Capsulorhexis; PXF, pseudoexfoliation syndrome; TIPP, 2-incision push–pull
Keywords: Capsulorhexis, Phacoemulsification, Cataract extraction, Pseudoexfoliation syndrome, Pediatric cataract surgery, Complications
Introduction
Cataract surgery is a rapidly evolving area in ophthalmology. Several decades ago, the most commonly performed surgical intervention for cataract was lens removal (in its entirety) through a large limbal incision. This technique, later named as intracapsular cataract extraction (ICCE) involved severing of zonular attachments (either mechanically or enzymatically) in an attempt to remove the entire lens through a large corneal scleral incision. Following several days of hospitalization patients who were lucky enough to escape numerous probable surgical complications, would typically be left with suboptimal sight dependent on aphakic spectacles. Naturally this technique was accompanied by quite a high risk of vitreous loss, hemorrhage, retinal detachment, chronic cystoid macular edema, and high astigmatism.43 In fact removing the entire lens through a large corneal incision along with keeping the anterior vitreous face undamaged was a major challenge in ICCE. Improvements in surgical accessories paved the way for extracapsular cataract extraction (ECCE) to be recognized by ophthalmologists worldwide. ECCE was based on creating an opening “capsulotomy” in the anterior capsular bag through which the lens nucleus could be “prolapsed”. Obviously this technique required a relatively smaller corneal incision and even more importantly the intact posterior capsule was remained as a safeguard against probable vitreous loss. Ultimately introduction of the intraocular lens (IOL) elucidated another superiority of ECCE, i.e. the remaining capsular bag could often be employed as a support for an IOL. While yet in its infancy, a major concern threatened ECCE’s popularity. Sometimes it could get quite difficult to adequately remove the lens material. Retained cortical material would trigger a severe inflammation and subsequently could lead to dense posterior membranous opacification. However, Improvements in automated irrigation–aspiration systems, innovations in capsulotomy techniques, and the advent of phacoemulsification represented significant leaps forward in the evolution of ECCE. Thanks to the great contribution of ophthalmologists worldwide ECCE by phacoemulsification flourished into one of the safest, most successful, and most commonly performed outpatient surgeries at the turn of the twentieth century.18,42 Having overviewed briefly the history of modern cataract surgery this article proceeds to focus on “anterior capsulotomy”, an innovation that changed cataract surgery forever.
Anterior capsulotomy
A critical step in ECCE (either ECCE by Phacoemulsification or the conventional ECCE) is making a window in anterior capsule wall (i.e. anterior capsulotomy). Techniques employed for this task have undergone sustained evolution. The primitive technique for capsulotomy was Vogt’s technique. He utilized toothed forceps for grasping and ripping out a part of anterior capsule. This could often lead to unpredictable and even catastrophic outcomes.75 In 1968 Kelman introduced “Christmas tree” approach in which a dull cystitome was used to peel anterior capsule cortex and tore that in triangular or Christmas tree morphology instead of cutting the cortex.41 This more acceptable capsulotomy technique was replaced by novel and popular “can-opener” technique. The can-opener technique uses a cystitome for interconnecting perforations of anterior capsule to create a circular window. The sequential and stepwise can-opener technique provides exact control of size and shape of capsule window.75 The Galand “letterbox” technique was later developed which used two steps and anterior capsule window was not completed until after implantation of the IOL and was more attractive for planned ECCE.26
Although these techniques fulfilled the goal of making a window on central part of anterior capsule, they were based on incisions which left multiple ragged edges any of which could potentially promote catastrophic tears proceeding outward. In fact surgical manipulations during phacoemulsification or conventional extraction of nucleus material almost inevitably would lead to unintentional tearing of peripheral anterior capsular rim. These tears could often extend to the capsular equator or even into the posterior capsule cortex. Posterior capsule tears were associated with vitreous loss and shifting of nucleus toward vitreous. Moreover, unfavorable tear extensions could produce some separated flaps on the anterior capsule cortex which disturbed aspiration of peripheral cortical residues. In addition, anterior capsule tears could result in decentration. Endeavors to overcome these drawbacks did not bring about a significant progress until middle 1980s when Howard Gimbel and Thomas Neuhann introduced capsulorhexis, which soon became recognized as the standard method of anterior capsulectomy. Capsulorhexis was later termed ‘Continuous Curvilinear Capsulorhexis’ (CCC), the term describing the exact surgical technique. Prior to this innovation it was well known that tear extensions most often occurred in the V-shape notches of the anterior capsulotomy rim. The core superiority of capsulorhexis over older methods simply lays in the fact that when done correctly, CCC does not leave any edges.28 Therefore any force applied to the anterior rim distributes in different directions and fail to extend a tear. Gimbel and Neuhann independently applied the same circular concept in different techniques. Prior to the development of viscoelatics, Gimbel demonstrated that anterior capsule could more favorably be incised in arc-like sections leaving small bridges to stabilize the flap in the face of turbulence flow of irrigation until the circle was mostly formed.27 Gimbel presented his technique at the American Intraocular Implant Society film festival in April 1985. Concurrently Neuhann developed and reported a technique which started capsulotomy by creating a single needle puncture at the imaginary circumference of the target circular capsulotomy and subsequently two arcs of incisions were initiated from this puncture point in opposite directions until circular capsulotomy is accomplished. Neuhann’s technique was presented at the German Society of Ophthalmology meeting in 198528 and subsequently published in German Medical Journal.54 Neuhann primarily named this technique as Capsulorhexis. The rhexis is a Greek suffix which means “to tear”. Next year Calvin Fercho introduced another technique for circular capsulotomy in Welsh Cataract Congress and named the technique as “Continuous Circular Tear Anterior Capsulotomy”.28 Ultimately Gimbel and Neuhann coined the name continuous circular capsulorhexis (CCC), to fully cover the concept.29,30
It is worth mentioning that although CCC generally refers to anterior capsulotomy, in particular conditions (such as developmental cataract) a portion of posterior capsule can also be removed by a similar technique.
Capsulorhexis physics and techniques
Lens capsule acts just like a cellophane. As an unfavorable consequence it tears easily at sharp angles, making straight and round cuts difficult to achieve. On the other hand, however, it can adequately cover and support artificial lens when stretch over it, even when large posterior capsule defects exist.4,9,40,48,62,76 Apprehension of elastic physical behavior is helpful for capsulorhexis expertise. Stretching an elastic strip like cellophane by two hands at both ends will lengthen the strip until a critical moment when it tears at an unpredictable spot associated with the lowest resistance. In addition, the cumulative force required to overcome elastic resistance would be accumulated and stored in elastic fibers and thus may extend the tear in an undesired direction even after the extrinsic stretching force halts.3 This explains how an initial puncture point will provide more precise control of tear direction. In fact a puncture made by a cystitome on the anterior capsule decreases the resistance of elastic fibers at that point. Therefore, in the absence of a significant amount of potential energy built up in the elastic fibers, sum of the extrinsic vector forces will be the determinant of the direction of tearing.69 It is worthy to bear in mind that surgeon’s hand is not the only source of extrinsic force during capsulorhexis. Another major vector of force pertains to zonular fibers applying centrifugal force which tends to divert the tear direction toward the periphery.10 The zonular fiber force is augmented if the lens moves anteriorly as a result of increased posterior pressure by vitreal thrust or decreased anterior pressure by leakage of Ophthalmic Viscosurgical Devices (OVDs) from anterior chamber.
Extrinsic force applied by surgeon can be classified as “stretching” and “shearing”. Stretching force is applied by a cystitome i.e. typically a double bended 27 gauge insulin needle. Double curve facilitates manual maneuver and puts the tip of the needle on the anterior capsule. “Stretching” represents applying the force in the same plane as radial zonular force. If the needle of cystitome is stuck on the external surface of anterior capsule and subsequently pulled toward the center of capsule, the opposed radial zonular force at the contact point would extend the tear toward periphery. Accordingly it is advisable to apply cystitome stretch in a degree of less than 180° in regard to the centrifugal vector of zonular force. As a rule of thumb, the cystitome stretch vector should be rotated at least once for each 45–50° of anterior capsule tearing to accomplish a well-shaped capsulotomy. In summary the needle (cystitome) technique is started by making a horizontal puncture on the anterior capsule. A radial incision is then started from this puncture point centrifugally extending as near as 1 mm (but not closer) to the desired imaginary border of capsulorhexis. Subsequently a small triangular shaped capsular flap is made by putting the needle beneath the anterior capsule and precise slight elevation of the needle tip. When the flap is formed it should be extended by pull/push forces until complete circular capsulotomy is accomplished and the flap’s base reaches the start point.10
“Shearing” denotes ripping force applied perpendicular to the plane of tearing, usually via forceps. In this technique the surgeon elevates the edge of the preformed puncture or tear perpendicular to the plane of anterior capsule. Hence a 90° angle will form between the shear force and the zonular vector force. Due to specific organization of elastic fibers in capsule lens, anterior capsule shows maximum resistance in the zonular plane. Contrarily in the perpendicular vector, fibers are most vulnerable to tear. Hence, the direction of tear extension closely obeys the direction of forceps movement. Thus forceps can yield much easier and continuous tearing.10 The disadvantage of forceps technique may be its dependence on using OVDs to preserve anterior chamber size which might impair surgeon’s precise sight. Leakage of viscoelastic substance through insertion site of the forceps may also potentially constitute a disadvantage.74 The entry site to the anterior chamber may be at the scleral spur, near trabecular meshwork, Schwalbe’s line or through Descemet’s membrane with their specific advantages and disadvantages.43 Different types of forceps are utilized for capsulorhexis with either sharp or blunt tips. Popular forceps for capsulorhexis include Utrata (with a straight blunt tip), Corydon (with an acute-angled tip), Buratto (with an acute-angled tip) and Buratto (with a round valve). To insert the forceps into the eye’s anterior chamber a surgeon has to make a larger scleral wound compared with the needle technique. Therefore forceps technique is almost inevitably associated with aqueous humor leakage and surgeons usually have to fill the anterior chamber with OVDs.10 Before a blunt forceps is utilized, a cystitome is applied to puncture the anterior capsule and subsequently to cut the capsule toward the 3 o’clock (by definition, the entrance spot of needle/forceps into the anterior chamber is referred as 12 o’clock). Having formed a small triangular flap (the same as needle technique), blunt forceps are utilized to grasp the flap and extend it continuously until a CCC is accomplished. Concurrent by flap’s extension, forceps should be repeatedly released and again regrasped at flap’s base to maintain a precise control on tear direction. Grasping the flap more proximally is especially helpful when the flap is approaching the insertion point of the forceps into the anterior chamber. In this phase forceps handling usually leads to significant astigmatism and ensuing poor visibility of the tear edge. Capsulorhexis terminates at 360° or may be further extended if capsulotomy enlargement is intended.10
When sharp forceps are employed the surgeon needs no needle for the initial puncture. Having punctured central anterior capsule the surgeon grasps and drags the forceps toward 12 o’clock. By starting and terminating the capsulorhexis at 12 o’clock, forceps handling will not impair the visibility of the untorn capsule. However some surgeons, even with sharp forceps in their hands, prefer to initiate the flap at 3 o’clock.10
Capsule staining
Basically red reflex visualizes the round rim of rhexis. Staining serves as a treasured adjunct to enhance visualization of the anterior capsule especially in cases with impaired red reflex. The mature or white cataracts, opalescent cortical material, dense posterior subcapsular opacification, vitreous hemorrhage, or corneal clouding are the common reasons for red reflex impairment.31 Of the numerous dyes encountered in the capsulorhexis literature (including Indocyanine green (ICG), trypan blue, fluorescein, crystal violet, gentian violet and Brilliant Blue G (BBG), only trypan blue is US Food and Drug Administration (FDA)-approved. The most common technique of staining involves injection of trypan blue under an air bubble and subsequently washing the excess dye out.47 The dye can also be applied beneath an OVD,17 with OVD in a mixture form,39 through a three steps method (in which Trypan blue stains anterior capsule via a BSS shell that forms on the anterior capsule after applying OVD),46 or by dispersing a dye droplet on the anterior capsule by a delicate spatula which hinders dye diffusion into the anterior chamber.11 American Academy of Ophthalmology reported in 2006 that there was level II evidence for staining capsule before capsulorhexis in pediatrics less than 5 years and white cataracts.35 It is notable that trypan blue may stiffen the anterior capsule and thus increase unwanted tears of capsulorhexis21
CCC through small pupil
Small pupil for capsulorhexis (pupil which has failed to dilate more than 4 mm), is associated with increased risk of complications.23 This is mainly due to restricted surgeon’s sight over the marginal edge of torn anterior capsule. Cataract surgeons face this condition most commonly in patients suffering from pseudoexfoliation syndrome.7 Other instances include iris scarring or posterior synechiae secondary to trauma, anterior uveitis with posterior synechiae, age related iris sphincter sclerosis, diabetes, chronic syphilis and long term mitotic consumption (e.g. for glaucoma treatment) leading to papillary fibrosis.38 Miotic agents generally should be halted at least 2 weeks before surgery. Tamsulosin, a selective alpha 1A-adrenorecptor antagonist, can cause pupil miosis as long as 28 days accompanied with floppy iris syndrome; therefore, it should be avoided way prior to cataract surgery.13,58
At the surgery day, management of pupils resistant to dilation begins with mydriatic application (e.g. 1% tropicamide, 1% cyclopentolate hydrochloride, 2.5% phenylephrine or 0.5% ketorolac tromethamine) with adequate frequency, usually given in 5–15 min intervals up to four times prior to surgery. For refractory pupils one droplet of phenylepherine may prove quite effective, provided that no cardiac concerns exist.24 Furthermore some methods can aid surgeons during the surgery. Expert surgeons can guess the hidden margin of rhexis by scrutinizing the folded anterior capsule flap. By applying centripetal forces during capsulorhexis, it is possible to move the whole lens toward the center of pupil to provide better vision while a tight rein is yet kept on the size of rhexis. Some other techniques are based on manipulating the iris by flexible hook72 or irrigating iris retractor.8 Some surgeons prefer to complete CCC in two stages, by first creating a small size capsulotomy and then proceeding to extend it.24 Excess OVDs usage is also reported to successfully dilate the pupil.20,37,77 Some devices have also been innovated to overcome small pupil. The Perfect Pupil system, a polyurethane tension ring supported by a silicon ring, can attach to papillary margins and dilate it with no significant iris. This devise sustains circular shape of the iris.5,38 Malyugin Pupil Expansion is a newer device for pupil dilation. Although easier to work than Perfect Pupil, it is associated with more deformation during surgery.12 Another silicone pupil expander known as Graether is also available.32 Finally In appropriate cases, surgeon may resort to especial surgical interventions such as lysis of synechiae, and multiple sphincterotomies.22
Thickened calcified capsules
Based on our experience thickened and calcified capsules are usually seen in the setting of traumatic cataracts or neglected congenital membranous cataracts. In some cases the lens material is absorbed and the anterior and posterior capsules are fused together. This may result in a very thick and/or calcified membrane which makes it seemingly impossible for surgeons to rip. In these cases one may find it helpful to start with a minute opening made with a sharp 23 gauge needle. A sharp stab (usually 15° stab) may be needed. To enlarge the aperture retinal (intraocular) scissors, usually used for removing thick intraocular bands and membranes, may be helpful.
Pseudoexfoliation syndrome
Pseudoexfoliation syndrome (PXF) was first described by Lindberg in 1917. The preliminary pathology in PXF is unbridled production of extracellular material in the anterior segment as well as other tissues of the body. Related deposits have been demonstrated on lens epithelium, IOL, corneal endothelium, papillary border, iridocorneal angle, ciliary processes and zonules. Ophthalmic manifestations of PXF including glaucoma, small pupil, zonular friability and cataract become more evident as age increases.71
Cataract surgery can be quite challenging in the background of PXF’s specifications. Small pupil, shallow anterior chamber, hyper-deep anterior chamber, vitreous prolapse, zonules dialysis and capsule fragility collectively increase cataract surgery complications.71 Extracellular matrix sedimentation may even form some fragile false superficial layers on the anterior capsule which cannot readily be distinguished from the true anterior capsule without Trypan blue staining. However, among all, small pupil and zonules weakness have the greatest impact on cataract surgery prognosis in PXF syndrome.53 Small pupil in PXF may reflect mechanical resistance of iris to mydriasis secondary to extracellular infiltrations as well as degeneration of the dilator and sphincter muscles.63 Zonular fragility on the other hand has been attributed to accumulation of matrix molecules at zonular origins and insertions.66,67 Scuderi et al.68 have reported that 2% ibopamine – a dopamine agonist – may significantly facilitate pupil dilation in PXF patients with a concurrent increase of 4 mmHg in intraocular pressure. A cataract surgeon may face the impact of zonular weakness on capsulorhexis as soon as he/she intends to create the initial anterior capsule puncture. In the absence of effective zonular constraint, surgeons may have to fix the lens with a micrograsper so that the cystitome can puncture rather than tilt away the lens. Furthermore diminished zonular force makes progress of rhexis unusually difficult. Capsule retractor along and capsular hooks have been employed to address this issue.71
Pediatric capsulorhexis
Cataract surgery in pediatrics age group offers several differences from its counterpart in adults. These differences begin with the initial puncture of CCC which is sensibly more difficult in children because of higher elasticity of the anterior capsule. Surgeons will face even more trouble in cases of deficient zonular counteraction such as Marfanoid children. An appropriate technique in this situation would be using two needles in a crossed-sword configuration which limits excessive movements of the lens.73 Capsulorhexis in children is associated with a greater risk of peripheral extension which is mainly attributed to more elasticity of anterior capsule as well as higher posterior vitreous pressure.2,80,81,82 Yet, surgeons have to overcome difficulties of anterior and posterior Continuous Curvilinear Capsulorhexis (CCC) in children on their way to successful cataract extraction and IOL implantation. Successful primary IOL implantation in the capsular bag necessitates a well centered, regular CCC of appropriate size with acceptable resistance to probable tear extension.1,34,56
Several methods have been examined in pediatric capsulorhexis including but not limited to vitrectorhexis (capsulotomy via vitrectomy probe), use of a cystitome and a capsule forceps, CCC with a 27-gauge needle, and the 2-incision push–pull (TIPP) technique.33,55 Surveys of the American Society of Cataract and Refractive Surgery (1993, 2001 and 2003) demonstrated that the preferred method for surgeons all over the world was manual CCC, vitrectorhexis and the combination of manual with vitrector.6
Utilizing the vitrectomy probe for capsulotomy although quite straightforward even for naive surgeons, is associated with a greater risk of radial tear in comparison with manual CCC.34,78 Most tears occur during implantation of IOL rather than vitrectorhexis itself.83 Other techniques such as electrocatheterization and Fugo plasma blade capsulotomy are also less resistant than manual CCC.34 Performing a curvilinear capsulorhexis with a 27-gauge needle or a capsule forceps in children usually leads to capsulotomies of unpredictable size, essentially because of commonly occurring radial extensions. In general it is recommended to use vitrectorhexis method for children younger than 6 years of age and manual CCC for 6-year or older.
The TIPP approach, introduced by Nischal,55 Hamada et al.33 bears interesting features. However it may lead to CCCs of unpredictable size and shape. In our experience, the technique usually resulted in oval capsulorhexis. Finally a 4-incision CCC technique introduced by the first author of the current article is reviewed (Figs. 1–3). Initially four arcuate incisions of 1–2 mm length are made on the anterior capsule with a bent 27-gauge needle. Afterward each incision is grasped by a capsular forceps and pulled toward the center of rhexis. The resulted flaps are consequently joined together. Four-incision capsulorhexis appeared to be a safe technique for well-centered, optimum size anterior and posterior capsulorhexis in children especially in cases of hypermature cataracts.50
Figure 1.
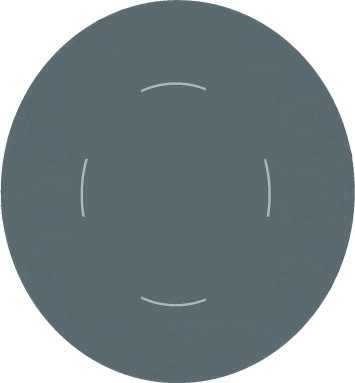
Four arcuate 1.0–2.0 mm incisions are made on the anterior capsule.
Figure 2.
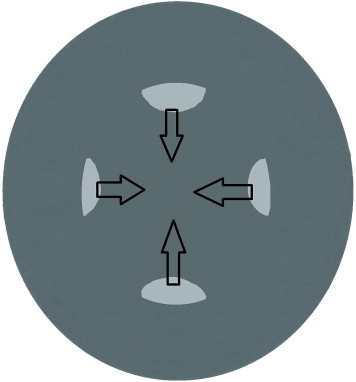
The apex of each incision is grasped by a capsule forceps and pulled toward the central anterior capsule.
Figure 3.
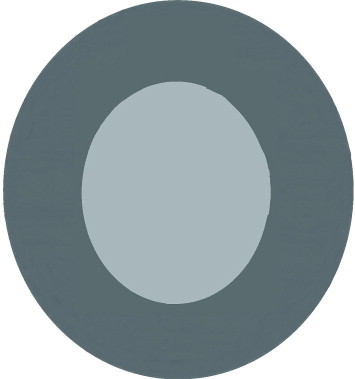
A complete 4-incision CCC.
As mentioned earlier another culprit for higher vulnerability of CCC in children to radial tears is known to be their higher vitreous pressure. Since sclera in young eyes shows less rigidity appropriate application of OVDs to deal with scleral collapse would be an important step in controlling vitreous pressure.80 The superviscous Healon5 can be considered a good option to maintain anterior chamber depth without leakage.36
Another major difference between pediatric and adult-style cataract surgery lays in management of the posterior capsule. Prior to advent of Posterior Continuous Curvilinear Capsulorhexis (PCCC) in 198357 it was well established that posterior capsule opacification is a rapid and virtually inevitable source of poor visual outcome in young children when posterior capsule is left intact (resembling adult style surgery). To perform PCCC following phacoemolisification surgeons should fill the remained capsular bag by OVDs to flatten the posterior capsule. PCCC is usually found to be a difficult procedure to perform, and disruption of the vitreous face is the main complication.14,55 There are various methods for creating posterior capsulectomy among which manual capsulorhexis provides better outcomes with less uncontrolled tearing and more regular and stable rim.2,19,81 Essentially similar techniques to anterior capsulorhexis are employed for PCCC. With a cystitome the anterior face of the posterior capsule should be ripped from center toward periphery being most careful not to depress the posterior capsule toward vitreous. Vitreous face disruption would be recognized by visible strands floating or adherent to the flap or pupil or by deformation of capsulorhexis margin.61 The rhexis is then completed by a forceps. The diameter of posterior rhexis should generally be less than the anterior rhexis (approximately 3–4 mm).19 Modern methods of posterior capsule management include pars plicata posterior capsulorhexis, sutureless vitrectomy, sealed-capsule irrigation, and bag-in-the-lens IOL. Presently primary posterior capsulotomy and vitrectomy are considered routine surgical steps, especially in children younger than 3 years old. For children at ages 3–7, simple PCCC is usually sufficient. For older children there is no need for preventive PCCC.79.
Rescue of rhexis
There are many factors that may play a role in occurrence of radial tears during CCC; including a shallow anterior chamber due to inadequate viscoelastic injection (low quantity or poor quality of viscoelastic material), weak zonules (as in PXF syndrome), high positive vitreous pressure (e.g. either due to excessive injection of anesthesia or inadequate anesthesia that may lead to patients discomfort and subsequent squeezing), large CCC that may disrupt the anterior zonules, intumescent and hypermature cataracts, pediatric cataracts with elastic anterior capsules and inexperienced surgeons for CCC.4,15,16,59,60,65,70 Radial tears in turn may lead to a series of complications such as zonular rupture, posterior capsular tear, vitreous presentation, insufficient capsular support for IOL implantation, and even nucleus drop during phacoemulsification.
Numerous measures have been introduced for managing a radial tear. Changing the procedure to a conventional can-opener capsulotomy and subsequent ECCE is an option. Another one is to restart from the opposite direction. Shifting the procedure to an ECCE, especially when temporal approach is applied, may cause substantial postoperative against-the-rule astigmatism. Restarting the capsulorhexis in an opposite direction is a difficult procedure and finally the rhexis may be decentered and notched out. Irregularity of CCC’s rim may cause further extension of the rhexis during hydrodissection or phacoemulsification. In order to prevent these complications, we suggest performing a bimanual automated aspiration of available lens materials (instead of hydrodissection) prior to commencing phacoemulsification.49 An alternative for rescuing radial tears is based on making a midway tangential anterior capsular flap and connecting it to the initial flap.51 Little et al.44 described their own method of rescuing radial extension in main steps of unfolding the capsulorhexis and grasping and pulling it toward the center for directing the tear toward the center; after this maneuver rhexis can be continued regularly.
Other important issues regarding rescue of rhexis includes the preferred IOL type, site of implantation (capsular bag vs. ciliary sulcus) and long term effects of various anterior capsulotomies and radial tears on IOL centration. Oner et al.56 compared the outcomes of different anterior capsulotomies for guaranteeing IOL centration and found that the highest values of tilt and decentration occur in envelope capsulotomy. They reported that CCC with one radial tear although not ideal, is satisfactory regarding IOL centration. Some authors suggest a 6.5 mm optic PMMA IOL in the case of radial tears in CCC to prevent later decentration.1 We suggest not performing any additional opposing radial tears; one should simply continue with decophaco and finally implant a 3-piece foldable IOL with a 6-mm optic and overall diameter of 13 mm. This has the advantage of using a foldable hydrophobic acrylic IOL with an acceptable optic size and resistant haptics that minimizes the risk of IOL decentration and may be implanted safely in the ciliary sulcus even in children.6 In conditions that rhexis is completely decentered and/or the zonular integrity is poor (as in PXF syndrome), we prefer to put the IOL in the ciliary sulcus to prevent further IOL decentration due to asymmetric zonular support and capsular contraction following surgery.
Femtosecond assisted capsulotomy
Various lasers have been evaluated in ophthalmology. The femtosecond laser originally applied in laser in situ keratomileusis and penetrating keratoplasty, is best known for its ability to create precise incisions with minimal collateral damage. Gradually this technology attracted the attention of cataract surgeons as well. The capsulotomies created by the laser have been reported to be more precise in size and shape than the manual CCC.25,45 Currently two commercial systems are available for femtosecond rhexis using optical coherence tomography and off-axis cameras for viewing the capsule anatomy during procedure. The later provides better view for the posterior capsule at the same time.52 More uniform capsulotomies made by Femtosecond technology although appealing turned out to be associated with capsular block syndrome (CBS). It is suggested that sudden, uninvited movement of the lens nucleus during hydrodissection may block the hydrodissection fluid leaving no exit pathway for the flow, hence an explosive tear in the posterior capsule. On the other hand the femtosecond laser produces intracapsular gas which might augments this phenomenon.64 Apart from safety protocols, economic concerns (e.g. cost-effectiveness, health system finance and payment methods) are also among important issues that have to be addressed before accepting this technology as the standard method of capsulotomy.
Financial disclosure
No author has any financial or proprietary interest in any materials or methods mentioned.
References
- 1.Al-Attar L., Smiddy W.E., Schiffman J.C. Foldable versus rigid intraocular lenses in conjunction with pars plana vitrectomy and other vitreoretinal procedures. J Cataract Refract Surg. 2004;30(5):1092–1097. doi: 10.1016/j.jcrs.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Andreo L.K., Wilson M.E., Apple D.J. Elastic properties and scanning electron microscopic appearance of manual continuous curvilinear capsulorhexis and vitrectorhexis in an animal model of pediatric cataract. J Cataract Refract Surg. 1999;25(4):534–539. doi: 10.1016/s0886-3350(99)80051-0. [DOI] [PubMed] [Google Scholar]
- 3.Arshinoff S. Mechanics of capsulorhexis. J Cataract Refract Surg. 1992;18(6):623–628. doi: 10.1016/s0886-3350(13)80456-7. [DOI] [PubMed] [Google Scholar]
- 4.Assia E.I., Apple D.J., Tsai J.C. The elastic properties of the lens capsule in capsulorhexis. Am J Ophthalmol. 1991;111(5):628–632. doi: 10.1016/s0002-9394(14)73710-9. [DOI] [PubMed] [Google Scholar]
- 5.Auffarth G.U., Reuland A.J., Heger T. Cataract surgery in eyes with iridoschisis using the Perfect Pupil iris extension system. J Cataract Refract Surg. 2005;31(10):1877–1880. doi: 10.1016/j.jcrs.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 6.Bartholomew L.R., Wilson M.E., Jr., Trivedi R.H. Pediatric anterior capsulotomy preferences of cataract surgeons worldwide: comparison of 1993, 2001, and 2003 surveys. J Cataract Refract Surg. 2007;33(5):893–900. doi: 10.1016/j.jcrs.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Belovay G.W., Varma D.K., Ahmed I.I. Cataract surgery in pseudoexfoliation syndrome. Curr Opin Ophthalmol. 2010;21(1):25–34. doi: 10.1097/ICU.0b013e328332f814. [DOI] [PubMed] [Google Scholar]
- 8.Bohm P., Horvath J., Zahorcova M. Irrigating iris retractor for complicated cataract surgery. J Cataract Refract Surg. 2009;35(3):419–421. doi: 10.1016/j.jcrs.2008.10.056. [DOI] [PubMed] [Google Scholar]
- 9.Budning A., Rosen J. The elastic properties of the lens capsule in capsulorhexis. Am J Ophthalmol. 1991;112(4):474–475. doi: 10.1016/s0002-9394(14)76270-1. [DOI] [PubMed] [Google Scholar]
- 10.Buratto L. 2nd ed. SLACK Inc.; Thorofare, NJ: 2003. Phacoemulsification: principles and techniques. [Google Scholar]
- 11.Caporossi A., Balestrazzi A., Alegente M. Trypan blue staining of the anterior capsule: the one-drop technique. Ophthal Surg Lasers Imaging. 2005;36(5):432–434. [PubMed] [Google Scholar]
- 12.Chang D.F. Use of Malyugin pupil expansion device for intraoperative floppy-iris syndrome: results in 30 consecutive cases. J Cataract Refract Surg. 2008;34(5):835–841. doi: 10.1016/j.jcrs.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 13.Chang D.F., Braga-Mele R., Mamalis N. ASCRS White Paper: clinical review of intraoperative floppy-iris syndrome. J Cataract Refract Surg. 2008;34(12):2153–2162. doi: 10.1016/j.jcrs.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 14.Comer R.M., Abdulla N., O’Keefe M. Radiofrequency diathermy capsulorhexis of the anterior and posterior capsules in pediatric cataract surgery: preliminary results. J Cataract Refract Surg. 1997;23(Suppl. 1):641–644. doi: 10.1016/s0886-3350(97)80047-8. [DOI] [PubMed] [Google Scholar]
- 15.Corey R.P., Olson R.J. Surgical outcomes of cataract extractions performed by residents using phacoemulsification. J Cataract Refract Surg. 1998;24(1):66–72. doi: 10.1016/s0886-3350(98)80076-x. [DOI] [PubMed] [Google Scholar]
- 16.Cruz O.A., Wallace G.W., Gay C.A. Visual results and complications of phacoemulsification with intraocular lens implantation performed by ophthalmology residents. Ophthalmology. 1992;99(3):448–452. doi: 10.1016/s0161-6420(92)31954-2. [DOI] [PubMed] [Google Scholar]
- 17.Dada V.K., Sudan R., Sharma N. Trypan blue with a viscoelastic agent. J Cataract Refract Surg. 2002;28(2):205–206. doi: 10.1016/s0886-3350(01)01337-2. [DOI] [PubMed] [Google Scholar]
- 18.Desai P., Minassian D.C., Reidy A. National cataract surgery survey 1997–8: a report of the results of the clinical outcomes. Br J Ophthalmol. 1999;83(12):1336–1340. doi: 10.1136/bjo.83.12.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dholakia S.A., Praveen M.R., Vasavada A.R. Completion rate of primary posterior continuous curvilinear capsulorhexis and vitreous disturbance during congenital cataract surgery. J AAPOS. 2006;10(4):351–356. doi: 10.1016/j.jaapos.2006.01.212. [DOI] [PubMed] [Google Scholar]
- 20.Dick H.B., Krummenauer F., Augustin A.J. Healon5 viscoadaptive formulation: comparison to Healon and Healon GV. J Cataract Refract Surg. 2001;27(2):320–326. doi: 10.1016/s0886-3350(00)00482-x. [DOI] [PubMed] [Google Scholar]
- 21.Dick H.B., Aliyeva S.E., Hengerer F. Effect of trypan blue on the elasticity of the human anterior lens capsule. J Cataract Refract Surg. 2008;34(8):1367–1373. doi: 10.1016/j.jcrs.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 22.Fine I.H. Pupilloplasty for small pupil phacoemulsification. J Cataract Refract Surg. 1994;20(2):192–196. doi: 10.1016/s0886-3350(13)80165-4. [DOI] [PubMed] [Google Scholar]
- 23.Fine I.H., Hoffman R.S. Phacoemulsification in the presence of pseudoexfoliation: challenges and options. J Cataract Refract Surg. 1997;23(2):160–165. doi: 10.1016/s0886-3350(97)80336-7. [DOI] [PubMed] [Google Scholar]
- 24.Fishkind WJ, editor. Complications in phacoemulsification: avoidance, recognition, and management. New York: Thieme; 2002.
- 25.Friedman N.J., Palanker D.V., Schuele G. Femtosecond laser capsulotomy. J Cataract Refract Surg. 2011;37(7):1189–1198. doi: 10.1016/j.jcrs.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 26.Galand A. A simple method of implantation within the capsular bag. J. Am. Intraocul. Implant. Soc. 1983;9(3):330–332. doi: 10.1016/s0146-2776(83)80071-8. [DOI] [PubMed] [Google Scholar]
- 27.Gimbel H.V. Capsulotomy method eases in-the-bag PCL. Ocular Surg. News. 1985;3(13):2–3. [Google Scholar]
- 28.Gimbel H.V. The history of the capsulorhexis technique. Cataract Refract Surg Today. 2007;7:39–41. [Google Scholar]
- 29.Gimbel H.V., Neuhann T. Development, advantages, and methods of the continuous circular capsulorhexis technique. J Cataract Refract Surg. 1990;16(1):31–37. doi: 10.1016/s0886-3350(13)80870-x. [DOI] [PubMed] [Google Scholar]
- 30.Gimbel H.V., Neuhann T. Continuous curvilinear capsulorhexis. J Cataract Refract Surg. 1991;17(1):110–111. doi: 10.1016/s0886-3350(13)81001-2. [DOI] [PubMed] [Google Scholar]
- 31.Goldman J.M., Karp C.L. Adjunct devices for managing challenging cases in cataract surgery: capsular staining and ophthalmic viscosurgical devices. Curr Opin Ophthalmol. 2007;18(1):52–57. doi: 10.1097/ICU.0b013e3280121b24. [DOI] [PubMed] [Google Scholar]
- 32.Graether J.M. Graether pupil expander for managing the small pupil during surgery. J Cataract Refract Surg. 1996;22(5):530–535. doi: 10.1016/s0886-3350(96)80004-6. [DOI] [PubMed] [Google Scholar]
- 33.Hamada S., Low S., Walters B.C. Five-year experience of the 2-incision push–pull technique for anterior and posterior capsulorrhexis in pediatric cataract surgery. Ophthalmology. 2006;113(8):1309–1314. doi: 10.1016/j.ophtha.2006.03.057. [DOI] [PubMed] [Google Scholar]
- 34.Izak A.M., Werner L., Pandey S.K. Analysis of the capsule edge after Fugo plasma blade capsulotomy, continuous curvilinear capsulorhexis, and can-opener capsulotomy. J Cataract Refract Surg. 2004;30(12):2606–2611. doi: 10.1016/j.jcrs.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 35.Jacobs D.S., Cox T.A., Wagoner M.D. Capsule staining as an adjunct to cataract surgery: a report from the American Academy of Ophthalmology. Ophthalmology. 2006;113(4):707–713. doi: 10.1016/j.ophtha.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 36.Jeng B.H., Hoyt C.S., McLeod S.D. Completion rate of continuous curvilinear capsulorhexis in pediatric cataract surgery using different viscoelastic materials. J Cataract Refract Surg. 2004;30(1):85–88. doi: 10.1016/S0886-3350(03)00669-2. [DOI] [PubMed] [Google Scholar]
- 37.Jhanji V., Sharma N., Vajpayee R.B. Management of intraoperative miosis during pediatric cataract surgery using Healon 5. Middle East Afr J Ophthalmol. 2011;18(1):55–57. doi: 10.4103/0974-9233.75888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kershner R.M. Management of the small pupil for clear corneal cataract surgery. J Cataract Refract Surg. 2002;28(10):1826–1831. doi: 10.1016/s0886-3350(02)01206-3. [DOI] [PubMed] [Google Scholar]
- 39.Khokhar S., Pangtey M.S., Panda A. Painting technique for staining the anterior lens capsule. J Cataract Refract Surg. 2003;29(3):435–436. doi: 10.1016/s0886-3350(02)01607-3. [DOI] [PubMed] [Google Scholar]
- 40.Krag S., Thim K., Corydon L. Strength of the lens capsule during hydroexpression of the nucleus. J Cataract Refract Surg. 1993;19(2):205–208. doi: 10.1016/s0886-3350(13)80943-1. [DOI] [PubMed] [Google Scholar]
- 41.Kwitko ML, Kelman CD, editors. In: Kugler, editor. The history of modern cataract surgery. The Netherlands: The Hague; 1998.
- 42.Leaming D.V. Practice styles and preferences of ASCRS members – 1999 survey. J Cataract Refract Surg. 2000;26(6):913–921. doi: 10.1016/s0886-3350(00)00469-7. [DOI] [PubMed] [Google Scholar]
- 43.Linebarger E.J., Hardten D.R., Shah G.K. Phacoemulsification and modern cataract surgery. Surv Ophthalmol. 1999;44(2):123–147. doi: 10.1016/s0039-6257(99)00085-5. [DOI] [PubMed] [Google Scholar]
- 44.Little B.C., Smith J.H., Packer M. Little capsulorhexis tear-out rescue. J Cataract Refract Surg. 2006;32(9):1420–1422. doi: 10.1016/j.jcrs.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 45.Mamalis N. Femtosecond laser: the future of cataract surgery? J Cataract Refract Surg. 2011;37(7):1177–1178. doi: 10.1016/j.jcrs.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 46.Marques D.M., Marques F.F., Osher R.H. Three-step technique for staining the anterior lens capsule with indocyanine green or trypan blue. J Cataract Refract Surg. 2004;30(1):13–16. doi: 10.1016/S0886-3350(03)00499-1. [DOI] [PubMed] [Google Scholar]
- 47.Melles G.R., de Waard P.W., Pameyer J.H. Trypan blue capsule staining to visualize the capsulorhexis in cataract surgery. J Cataract Refract Surg. 1999;25(1):7–9. doi: 10.1016/s0886-3350(99)80004-2. [DOI] [PubMed] [Google Scholar]
- 48.Meng Y., Magd S.A., Waring G.O. A study on stretching capacity of continuous circular capsulorhexis and nucleus delivery. Zhonghua Yan Ke Za Zhi. 1995;31(4):277–279. [PubMed] [Google Scholar]
- 49.Mohammadpour M. Management of radial tears during capsulorhexis. Tech Ophthalmol. 2006;4(2):56–60. [Google Scholar]
- 50.Mohammadpour M. Four-incision capsulorhexis in pediatric cataract surgery. J Cataract Refract Surg. 2007;33(7):1155–1157. doi: 10.1016/j.jcrs.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 51.Mohammadpour M. Rescue of an extending capsulorrhexis by creating a midway tangential anterior capsular flap: a novel technique in 22 eyes. Can J Ophthalmol. 2010;45(3):256–258. doi: 10.3129/i09-260. [DOI] [PubMed] [Google Scholar]
- 52.Naranjo-Tackman R. How a femtosecond laser increases safety and precision in cataract surgery? Curr Opin Ophthalmol. 2011;22(1):53–57. doi: 10.1097/ICU.0b013e3283415026. [DOI] [PubMed] [Google Scholar]
- 53.Naumann G.O. Exfoliation syndrome as a risk factor for vitreous loss in extracapsular cataract surgery (preliminary report). Erlanger–Augenblatter-Group. Acta Ophthalmol. 1988;184(Suppl.):129–131. doi: 10.1111/j.1755-3768.1988.tb02645.x. [DOI] [PubMed] [Google Scholar]
- 54.Neuhann T. Theory and surgical technic of capsulorhexis. Klin Monbl Augenheilkd. 1987;190(6):542–545. doi: 10.1055/s-2008-1050454. [DOI] [PubMed] [Google Scholar]
- 55.Nischal K.K. Two-incision push–pull capsulorhexis for pediatric cataract surgery. J Cataract Refract Surg. 2002;28(4):593–595. doi: 10.1016/s0886-3350(01)01125-7. [DOI] [PubMed] [Google Scholar]
- 56.Oner F.H., Durak I., Soylev M. Long-term results of various anterior capsulotomies and radial tears on intraocular lens centration. Ophthal Surg Lasers. 2001;32(2):118–123. [PubMed] [Google Scholar]
- 57.Parks M.M. Posterior lens capsulectomy during primary cataract surgery in children. Ophthalmology. 1983;90(4):344–345. doi: 10.1016/s0161-6420(83)34549-8. [DOI] [PubMed] [Google Scholar]
- 58.Parssinen O., Leppanen E., Keski-Rahkonen P. Influence of tamsulosin on the iris and its implications for cataract surgery. Invest Ophthalmol Vis Sci. 2006;47(9):3766–3771. doi: 10.1167/iovs.06-0153. [DOI] [PubMed] [Google Scholar]
- 59.Pingree M.F., Crandall A.S., Olson R.J. Cataract surgery complications in 1 year at an academic institution. J Cataract Refract Surg. 1999;25(5):705–708. doi: 10.1016/s0886-3350(99)00017-6. [DOI] [PubMed] [Google Scholar]
- 60.Prasad S. Phacoemulsification learning curve: experience of two junior trainee ophthalmologists. J Cataract Refract Surg. 1998;24(1):73–77. doi: 10.1016/s0886-3350(98)80077-1. [DOI] [PubMed] [Google Scholar]
- 61.Praveen M.R., Vasavada A.R., Koul A. Subtle signs of anterior vitreous face disturbance during posterior capsulorhexis in pediatric cataract surgery. J Cataract Refract Surg. 2008;34(1):163–167. doi: 10.1016/j.jcrs.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 62.Rao H., Wang L., Zhou S. [An experimental study of stretching capacity of lens anterior capsule] Zhonghua Yan Ke Za Zhi. 1997;33(1):56–58. [PubMed] [Google Scholar]
- 63.Repo L.P., Naukkarinen A., Paljarvi L. Pseudoexfoliation syndrome with poorly dilating pupil: a light and electron microscopic study of the sphincter area. Graefes Arch Clin Exp Ophthalmol. 1996;234(3):171–176. doi: 10.1007/BF00462029. [DOI] [PubMed] [Google Scholar]
- 64.Roberts T.V., Sutton G., Lawless M.A. Capsular block syndrome associated with femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2011;37(11):2068–2070. doi: 10.1016/j.jcrs.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 65.Saber H.R., Butler T.J., Cottrell D.G. Resistance of the human posterior lens capsule and zonules to disruption. J Cataract Refract Surg. 1998;24(4):536–542. doi: 10.1016/s0886-3350(98)80298-8. [DOI] [PubMed] [Google Scholar]
- 66.Schlotzer-Schrehardt U., Naumann G.O. A histopathologic study of zonular instability in pseudoexfoliation syndrome. Am J Ophthalmol. 1994;118(6):730–743. doi: 10.1016/s0002-9394(14)72552-8. [DOI] [PubMed] [Google Scholar]
- 67.Schlotzer-Schrehardt U., Naumann G.O. Ocular and systemic pseudoexfoliation syndrome. Am J Ophthalmol. 2006;141(5):921–937. doi: 10.1016/j.ajo.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 68.Scuderi G.L., Regine F., Perdicchi A. Efficacy of 2% ibopamine on the dilation of patients with pseudoexfoliation syndrome. Eur J Ophthalmol. 2010;20(1):120–123. doi: 10.1177/112067211002000116. [DOI] [PubMed] [Google Scholar]
- 69.Seibel B.S. 2nd ed. SLACK; Thorofare, NJ: 1995. Phacodynamics: mastering the tools and techniques of phacoemulsification surgery. [Google Scholar]
- 70.Seward H.C., Dalton R., Davis A. Phacoemulsification during the learning curve: risk/benefit analysis. Eye (Lond.) 1993;7(Pt. 1):164–168. doi: 10.1038/eye.1993.35. [DOI] [PubMed] [Google Scholar]
- 71.Shingleton B.J., Crandall A.S., Ahmed I.I. Pseudoexfoliation and the cataract surgeon: preoperative, intraoperative, and postoperative issues related to intraocular pressure, cataract, and intraocular lenses. J Cataract Refract Surg. 2009;35(6):1101–1120. doi: 10.1016/j.jcrs.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 72.Smith G.T., Liu C.S. Flexible iris hooks for phacoemulsification in patients with iridoschisis. J Cataract Refract Surg. 2000;26(9):1277–1280. doi: 10.1016/s0886-3350(00)00523-x. [DOI] [PubMed] [Google Scholar]
- 73.Snyder M.E., Lindsell L.B. Crossed-swords, capsule-pinch technique for capsulotomy in pediatric and/or loose lens cataract extraction. J Cataract Refract Surg. 2010;36(2):197–199. doi: 10.1016/j.jcrs.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 74.Spaeth G.L. 3rd ed. Saunders; Philadelphia: 2003. Ophthalmic surgery: principles and practice. [Google Scholar]
- 75.Steinert R.F., Fine I.H. 1st ed. Saunders; Philadelphia: 1995. Cataract surgery: technique, complications & management. [Google Scholar]
- 76.Thim K., Krag S., Corydon L. Stretching capacity of capsulorhexis and nucleus delivery. J Cataract Refract Surg. 1991;17(1):27–31. doi: 10.1016/s0886-3350(13)80980-7. [DOI] [PubMed] [Google Scholar]
- 77.Tognetto D., Cecchini P., Ravalico G. Survey of ophthalmic viscosurgical devices. Curr Opin Ophthalmol. 2004;15(1):29–32. doi: 10.1097/00055735-200402000-00006. [DOI] [PubMed] [Google Scholar]
- 78.Trivedi R.H., Wilson M.E., Jr., Bartholomew L.R. Extensibility and scanning electron microscopy evaluation of 5 pediatric anterior capsulotomy techniques in a porcine model. J Cataract Refract Surg. 2006;32(7):1206–1213. doi: 10.1016/j.jcrs.2005.12.144. [DOI] [PubMed] [Google Scholar]
- 79.Vasavada A.R., Praveen M.R., Tassignon M.J. Posterior capsule management in congenital cataract surgery. J Cataract Refract Surg. 2011;37(1):173–193. doi: 10.1016/j.jcrs.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 80.Wilson M.E. Anterior capsule management for pediatric intraocular lens implantation. J Pediatr Ophthalmol Strabismus. 1999;36(6):314–319. doi: 10.3928/0191-3913-19991101-05. (quiz 342–313) [DOI] [PubMed] [Google Scholar]
- 81.Wilson M.E., Jr. Anterior lens capsule management in pediatric cataract surgery. Trans Am Ophthalmol Soc. 2004;102:391–422. [PMC free article] [PubMed] [Google Scholar]
- 82.Wilson M.E., Bluestein E.C., Wang X.H. Comparison of mechanized anterior capsulectomy and manual continuous capsulorhexis in pediatric eyes. J Cataract Refract Surg. 1994;20(6):602–606. doi: 10.1016/s0886-3350(13)80646-3. [DOI] [PubMed] [Google Scholar]
- 83.Wilson M.E., Jr., Trivedi R.H., Bartholomew L.R. Comparison of anterior vitrectorhexis and continuous curvilinear capsulorhexis in pediatric cataract and intraocular lens implantation surgery: a 10-year analysis. J AAPOS. 2007;11(5):443–446. doi: 10.1016/j.jaapos.2007.03.012. [DOI] [PubMed] [Google Scholar]


