Abstract
Dacryocystorhinostomy (DCR) is a procedure of choice for nasolacrimal duct obstruction and chronic dacryostenosis in the setting of patent canaliculi and a functional lacrimal pump. Two major approaches are utilized: external, via a transcutaneous incision and endonasal endoscopically guided. The surgery has a high success rate via both approaches. We review the history, evolution, current techniques, complications and future directions of DCR.
Keywords: Dacryocystorhinostomy, Endoscopic dacryocystorhinostomy, Balloon dacryoplasty, Dacryocystitis, Nasolacrimal duct obstruction
1. Introduction
The main indications for external DCR are clinically significant epiphora in the presence of nasolacrimal duct obstruction (NLDO), chronic conjunctivitis in the presence of nasolacrimal duct obstruction, dacryocystitis, and dacryoliths in lacrimal sac causing periodic episodes of nasolacrimal duct obstruction. Other causes of nasolacrimal obstruction include lacrimal sac tumors, nasal and facial fractures involving the nasolacrimal canal.
2. History
Surgical treatment of dacryocystitis stretches back nearly 2000 years (Chandler, 1936; Carter and Nerad, 1996). Celsus, in the first century, described a way of creating an artificial passageway into the nose by using hot cautery to puncture through the lacrimal bone. A similar procedure was performed by Galen in the second century. Better understanding of lacrimal physiology and nasal and lacrimal anatomy through the centuries led to development of more modern techniques starting in the eighteenth century. Some of the procedures described, such as canaliculotomy or dacryocystectomy would no longer be considered indicated for cases of NLDO or dacryocystitis under normal circumstances. However, in very sick debilitated patients, patients who cannot stop anticoagulation therapy and in patients with lacrimal sac tumors dacryocystectomy may be the procedure of choice.
Several avenues had been tried by the early part of the 20th century. An interesting approach involved attempts to drain the lacrimal sac into the maxillary sinus. Several small successful series were published in early 20th century. Intranasal approach operations had also been described (Girgis, 1968). Many variations were attempted with some advocating opening or resection of the lower aspect of the nasolacrimal canal as well as use of glass tubes or wire to keep the new passageway patent. West and Polyak who originated one type of such operation reported 90% and 85% success rates respectively. Others applying similar techniques had success rates ranging from as low as 63% to as high as 100% (Chandler, 1936; Henry, 1933).
The earliest operation that would resemble a modern external DCR was attempted by Woolhouse in England in the 18th century. He advocated extirpating the sac, perforating the lacrimal bone and placing a drain made of gold, lead or silver. By the early 20th century others attempted to open the sac without removing most of it. Various stenting materials were used to maintain the patency of the ostium. These included leaving a thread, placing a gold cannula, placing a ball of catgut suture and placing gauze wicks which were periodically exchanged. Recreating a duct by placing a skin graft wrapped around a piece of wax had also been tried. Some authors reported success rates of 70–85% (Chandler, 1936).
Toti in 1904 published what is considered the first modern description of external DCR (Chandler, 1936; Carter and Nerad, 1996; Girgis, 1968; Picó, 1971). An external incision was made, the periosteum and the sac were elevated. A bony ostium was created using a punch. The medial wall of the sac was excised using a canalicular probe as a guide. A corresponding piece of nasal mucosa was removed. Suturing instead of excising of lacrimal sac and nasal mucosal flaps was described as early as 1914. Depuy-Dutemps and Bourguet in France and Ohm in Germany independently published what became the basis of truly modern DCR in the 1920’s (Girgis, 1968; Picó, 1971). These surgeons advocated suturing both the posterior and the anterior flaps. Depuy-Dutemps and Bourguet reported success rates of 94% (Chandler, 1936; Carter and Nerad, 1996; Girgis, 1968; Picó, 1971).
The difficulty in suturing both posterior and anterior flaps as well as early fears of significant bleeding when the angular vessels were encountered led to various modifications being developed throughout the 20th century (Picó, 1971; Iliff, 1971). Issues such as incision placement, elevation of medial canthal tendon, use of chisels (Williams and Hill, 1944), rongeurs, bone trephines (Picó, 1971; Iliff, 1971) or burrs (Girgis, 1968), placement of stenting material, flap sutures, cautery of posterior flaps (Veirs, 1969) and whether to suture the posterior flaps were debated.
Our technique has evolved as well. In the 1970’s we analyzed all the DCRs performed at Wills Eye Hospital between January 1971 and January 1979 (McLachlan et al., 1980). Of the 291 procedures included in the study, 18 (6%) were anatomical failures. The technique was similar to that of (Iliff, 1971) with the exceptions being the placement of incision (medial to the angular vessels in our case vs. along the medial orbital rim) and only approximately one-half of the patients having French catheter stent placement vs. nasal gauze packing in the case of the other half. Only the anterior flaps were sutured. The posterior flaps were excised. Importantly, in the study protocol, even the asymptomatic patients were probed and irrigated in order to establish anatomic success. In that series we observed that 14 of the 18 failures (78%) had blockage proximal to or at the common canaliculus. The other four were at the ostium site. Despite the 94% success rate with the technique used at that time, we have since become more aware of and respectful of the canalicular system. It was felt that excessive probing, manipulation and iatrogenic trauma to the common canaliculus and internal punctum may have been responsible for the high rate of canalicular involvement in failed cases. It is now thought that the most common site of failure is closure of the ostium by scar tissue growth (Carter and Nerad, 1996) (and rarely bony regeneration) (Iliff, 1971; McLachlan et al., 1980). We have modified our technique to avoid damaging the common internal punctum. Below, we present our current technique.
3. Current technique
3.1. Diagnosis and work up
DCR is the procedure of choice in patients with chronic epiphora or intermittent or chronic dacryocystitis caused by nasolacrimal duct obstruction. A normally functioning lacrimal pump, properly positioned and patent puncta as well as present and patent canaliculi are required for the operation to be successful. The preoperative assessment is aimed at establishing the presence of the above factors as well as at ruling out other potential causes of chronic epiphora.
Chronic epiphora without signs of dacryocystitis may be a result of reflex tearing caused by dry eye syndrome, irregular corneal surface, corneal exposure, lid malposition such as ectropion or entropion, trichiasis, blepharitis or ocular allergies. Punctal stenosis, or presence of punctal or canalicular plugs (Lee and Flanagan, 2001) may also result in compromised tear drainage. Rarely, primary tear hypersecretion can occur.
Probing and irrigation of the lacrimal system is performed following topical anesthesia. A forceful stream through the opposite punctum indicates a complete or near-complete obstruction. Reflux through the same punctum indicates obstruction at the level of the involved canaliculus or common internal punctum. Partial obstruction may also be observed where reflux through the opposite punctum is noted, yet the patient is able to taste some of the fluid. In these cases the amount of obstruction is graded subjectively as 90%, 70%, 80%, 50% or less. In cases of complete or near complete (70–90%) obstruction DCR is the procedure of choice. In cases of mild obstruction some alternative procedures (described below) may be considered.
We no longer routinely utilize dacryocystography as probing and irrigation provides sufficient clinically relevant information. We obtain CT scans in cases of prior trauma, surgery, suspected neoplasm or significant sinus disease.
Acute dacryocystitis is treated medically first and with incision and curettage of abscess if necessary (Fig. 1). A submuscular pocket of abscess may be encountered (Boulos and Rubin, 2008). Once active infection is cleared, the patient may proceed to definitive surgery to resolve the underlying condition: nasolacrimal duct obstruction.
Figure 1.
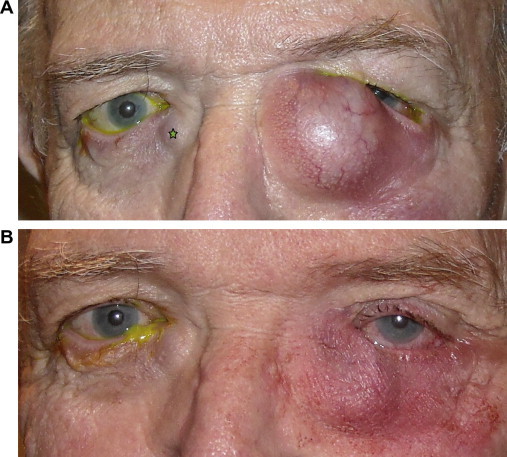
Acute and chronic dacryocystitis and lacrimal sac abscess. (A) A grossly distended, tender left lacrimal sac abscess is seen. Note chronically distended right lacrimal sac (∗). (B) Immediately after office based incision and drainage of the left lacrimal sac abscess. The mucopurulent material was cultured and revealed methcillin-resistant Staphylococcus aureus (MRSA). The patient was treated medically and underwent an uncomplicated external DCR on the left followed 1–2 months later by an external DCR on the right.
3.2. Indications
We utilize the external approach in the vast majority of our primary (first time) DCR cases. We generally reserve the endoscopic approach for secondary cases, for patients who are overly concerned about the possibility of a scar or in cases of tumor involving the lacrimal sac as well as nasal passages and sinuses (Table 1).
Table 1.
Indications and contraindications for dacryocystorhinostomy.
| Indications | Contraindications |
|---|---|
| Clinically significant epiphora in presence of nasolacrimal duct obstruction | Patient is on anti-coagulation medications and is unable to stop perioperatively |
| Chronic conjunctivitis in presence of nasolacrimal duct obstruction | Active dacryocystitis |
| Dacryocystitis | Tumor of lacrimal sac |
| Dacryoliths in lacrimal sac causing periodic episodes of nasolacrimal duct obstruction |
3.3. Anesthesia considerations
The surgery may be performed under general anesthesia or local anesthesia with sedation. Regional (supratrochlear and infraorbital) blocks may also be utilized when surgery is performed under sedation. The local and block anesthetic consists of 1% or 2% lidocaine with 1:200,000 epinephrine and hyaluronidase. Oxymetazoline nasal spray (Afrin)-soaked or 4% cocaine-soaked cottonoids are used to pack the naris in order to vasoncostrict the mucosa and augment anesthesia whether general or local anesthesia is used.
3.4. Description of procedure
Once the patient is sedated or intubated, he/she is then prepped and draped in the standard fashion. The incision is marked using a fine-tip surgical marking pencil. A vertically oriented curvilinear incision is made approximately 4 mm anterior to the medial canthal angle and extended down for approximately 10 mm. The upper extent of the incision should not be close to the upper eyelid in order to prevent web formation.
The skin incision is made with a #15 Bard-Parker blade. Dissection to the periosteum is carried out using either tenotomy scissors or unipolar electrocautery on cut mode. Angular vessels are avoided if possible, but may be cauterized if necessary. An assistant retracts the wound with fine rakes or a self retaining speculum is placed. A periosteal (Cottle or Freer) elevator is used to reflect the periosteum and the superficial (anterior) head of the medial canthal tendon. The lacrimal sac is encountered and is carefully reflected laterally exposing the fossa. The previously placed nasal packing is removed. A natural point of weakness is present at the juncture of the maxillary and lacrimal bone within the lacrimal sac fossa. This fact is exploited in surgery: firm pressure with the periosteal elevator is frequently sufficient to infracture the bone and start the bony ostium. Alternatively a fine osteotome and mallet may be utilized. The osteotomy is then enlarged using various sized rongeurs (we typically use a Citelli punch or Kerrison). The osteotomy is made approximately 15 × 15 mm in size.
The puncta are then dilated. A Bowman probe (usually 0–0 or #1) is then passed into the lacrimal sac and used to tent up the lacrimal sac wall (Fig. 2A). The assistant holds the probe. The surgeon incises the lacrimal sac wall using a #65 Beaver blade or a #11 Bard-Parker blade (Fig. 2B). Westcott scissors are then used to remove the posterior lacrimal sac wall (Fig. 2C, D). This is sent for permanent pathologic evaluation. If stones (dacryoliths) or any other unusual lacrimal sac contents are encountered they are sent for pathologic evaluation as well. If pus is present cultures and sensitivities may be obtained. In cases of chronic dacryocystitis a markedly thickened and multilayered lacrimal sac wall may be found. Sharp dissection is carried out until the Bowman probe tip is visualized. The opposite punctum may then be probed simultaneously and its entry into the lacrimal sac visualized. In approximately 90% of patients (Yacizi and Yacizi, 2000) a common canaliculus is present and the two probe tips will be seen entering the sac together (Fig. 2E). This structure is carefully preserved.
Figure 2.
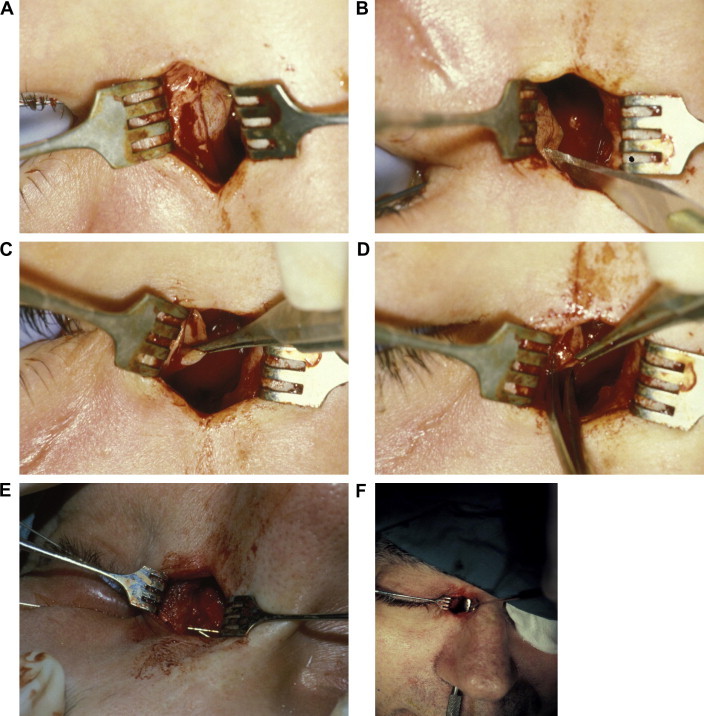
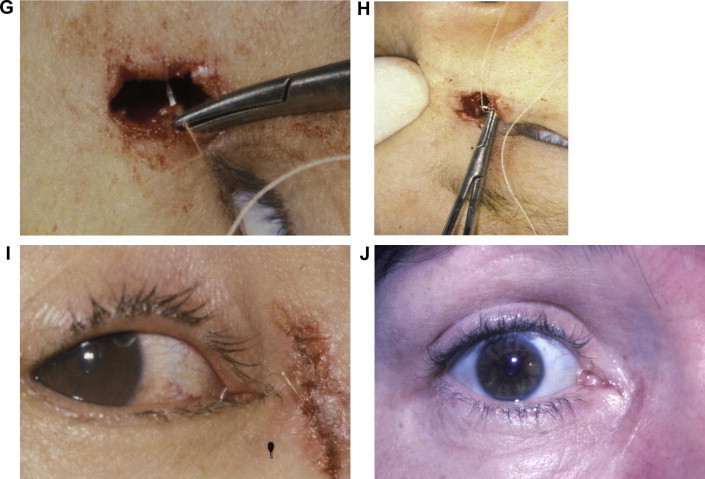
Key steps in external DCR. (A) Tenting of lacrimal sac wall with Bowman probe. (B) Incision into lacrimal sac with #11 Bard-Parker blade. (C & D). Posterior flap is grasped and excised. (E) Bowman probes passed through upper and lower canaliculi demonstrating a common internal punctum. (F) The bony ostium is demonstrated with Freer elevator passed up through the nose. (G) Suturing the anterior sac flap to anterior nasal mucosal flap with 5-0 polyglactin suture on P2 needle. (H) Surgeon’s view. (I) Immediate postoperative appearance. Skin is closed with interrupted 6-0 plain suture. Crawford tube is in place. (J) Six week postoperative appearance prior to tube removal. A faint incision line without scar hypertrophy or webbing is noted.
A periosteal elevator is passed into the naris and is used to tent up the nasal mucosa. A #65 Beaver or a #11 Bard-Parker blade is used to incise the nasal mucosa in a line parallel to the lacrimal sac incision. A flap is then fashioned by placing two cuts perpendicular to the first. The posterior flap is excised (Fig. 2F).
Silicone stents (Older, 1982) (Crawford Bicanaliculus Intubation, FCI Ophthalmic, Marshfield Hill, MA, USA) are passed through both puncta into the wound. A silicone bolster is passed over the introducers. The two ends are tied securely with multiple square knots. The knot is externalized into the nasal cavity with bayonet forceps. The bolster may be sutured to the nasal septum with 5-0 polyglactin suture. Gelatin matrix/Thrombin hemostatic agent (Floseal Hemostatic Matrix, Baxter, Deerfield, IL, USA) is placed into the wound for hemostasis. The anterior lacrimal sac and mucosal flaps are then sutured using 5-0 polyglactin suture (Fig. 2G, H). 5-0 polyglactin suture may also be used to reconstitute the anterior crus of the medial canthal tendon which is usually detached during the initial dissection. The skin is then closed using interrupted 6-0 plain gut suture (Fig. 2I). The entire procedure, including set up and anesthesia time usually takes 45 minutes.
3.5. Postoperative Care
The patients are placed on an oral antibiotic, an antibiotic-steroid combination ophthalmic drop (e.g. neomycin-polymixin-dexamethesone or tobramycin-dexamethasone) in the operated eye and an antibiotic-steroid combination ointment to be applied to the wound twice a day. The patients are instructed to use the drops and ointment until the bottle and tube run out (approximately 3–4 weeks). Recently, we have started placing patients on nasal steroid sprays (e.g. fluticasone propionate) in order to decrease nasal congestion and help prevent postoperative scarring.
The silicone stent is kept in place for at least one to three weeks. This is done to minimize the chance of soft tissue scarring occluding the newly formed common canaliculus. In cases of canalicular stenosis silicone tubing may be kept in place longer. We also generally keep silicone tubes in place for six months in patients undergoing secondary operations. In rare instances of patients being unable to tolerate the silicone stents due to stents becoming dislodged, we remove them prior to the planned time period.
The external scar usually heals very well and is generally barely discernible (Fig. 2J) This has been our as well as published experience (Sharma et al., 2005). Measures taken to minimize scarring include inquiring about the history of keloid formation preoperatively, placement of the wound, meticulous skin closure as well as use of steroid containing ointment postoperatively. If a hypertrophic scar is encountered measures such as digital massage, continued application of mild steroid ointment (such as flourometholone 0.1% ophthalmic ointment) and steroid injections may be utilized. Later, trichloroacetic acid chemical peels can also be applied. Scar revision is rarely necessary.
Success of the surgery is assessed both subjectively and objectively. Subjectively, resolution of epiphora is considered to be success. Objectively, restoration of unobstructed flow with irrigation confirms the patency of the system. We irrigate all patients who complain of persistent epiphora following removal of silicone stents. Paradoxically, sometimes patients may have subjective resolution of their symptoms with only partial removal of blockage on objective testing. Subjective success is likely more important as one cannot “cure an asymptomatic patient.” Nevertheless, these two definitions of surgical success must be kept in mind when reviewing literature and comparing various procedures and modifications.
4. Alternative approaches
4.1. Probing and irrigation
Probing of the entire length of the nasolacrimal system is typically not curative in adults. In newborns a common cause of NLDO is the presence of a membrane at the valve of Hasner: the distal tip of the nasolacrimal duct below the inferior turbinate. This obstruction often resolves spontaneously or with local massage and topical antibiotic ointment or drops within the first twelve months of life (Nelson et al., 1985). In cases of incomplete resolution, probing can puncture through the membrane and resolve the obstruction (Pediatric Eye Disease Investigator Group, 2008). In cases of recurrent NLDO following probing, balloon dacryoplasty may be performed. Silicone stents (e.g. Crawford or Ritleng, FCI Ophthalmics) may be placed and left for as long as one year. In older children, if all of the above approaches fail, DCR may need to be performed (Harrison and Mukherjee, 1967). Concerns had been raised regarding the possibility of inducing facial asymmetry or dysmorphia by removing bone as part of DCR in a child. In our clinical experience that is not the case (Nowinski et al., 1986; Barnes, 2001).
4.2. Balloon dacryoplasty
In patients with incomplete obstruction of ∼50% or less (see Section 3.1 Diagnosis and Work Up) we consider balloon dacryoplasty. The puncta are dilated and Bowman probes are passed in the same manner as in pediatric probing. A 3 mm LacriCATH (Quest Medical, Allen, TX, USA) balloon catheter is then passed into the nasolacrimal canal (Fig. 3). Once the 30 mm mark is encountered the balloon is inflated to a pressure of 7 atm for 90 seconds twice. We then retract the balloon to the 20 mm mark and inflate to 7 atm for 90 seconds, once. At the 20 mm mark the balloon straddles the lacrimal sac/nasolacrimal duct junction. In cases of mild canalicular stenosis we sometimes inflate the balloon while within the canaliculus as well. We typically place either a bicanalicular (Crawford or Ritleng) or unicanalicular (Ritleng) silicone stent at the end of the procedure.
Figure 3.
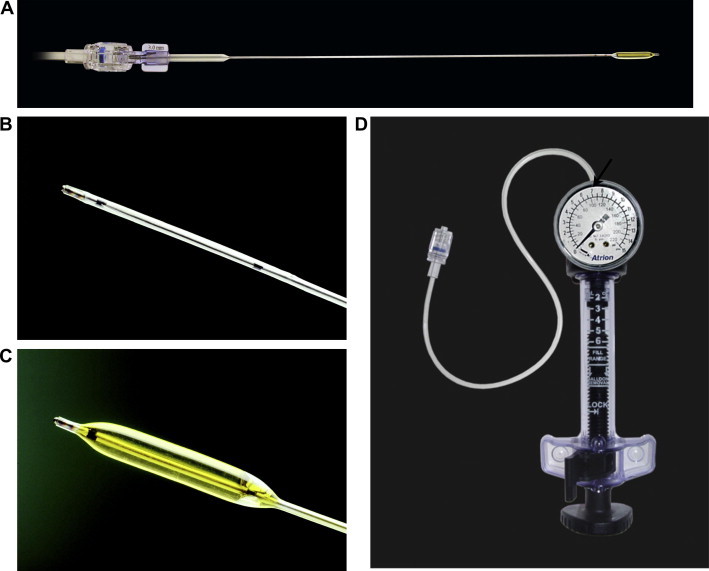
Balloon dacryoplasty apparatus. (A) 3 mm (diameter) balloon (expanded). (B) Balloon tip in a deflated state. (C) Balloon tip inflated. (D) The pump apparatus with gauge. Arrow indicates the 7 atm mark. Photos courtesy of Quest Medical, Inc., Allen, TX, USA.
This has been employed with some success in children as well. In children more than 24 months of age who had undergone a previous probing, one study showed a success rate greater than 95.1% (Tao et al., 2002). Adult success rate has been noted by one study to be as high as 73% objectively (Perry et al., 1998). However, there has been a wide range of degrees of success, varying from 20% to 90% (Robinson et al., 1993; Liermann et al., 1996). Patients who fail a balloon dacryoplasty are offered the option of standard DCR.
4.3. Endoscopic
Endoscopic transnasal dacryocystorhinostomy was first described in 1989 by McDonogh and Meiring (1989). The procedure has been gaining popularity recently compared to conventional external dacryocystorhinostomy. External and endoscopic dacryocystorhinostomy have the same goal, to create a bypass of the blocked nasolacrimal duct by creating a fistula that allows the internal common punctum to communicate directly into the nasal cavity through the lateral nasal wall. Most surgeons utilize a 30 degree fiber-optic endoscope to identity the lacrimal sac fossa. The sac is covered by the lacrimal bone and is removed during the surgical approach to lacrimal sac. Once the lacrimal bone is exposed, an ostium is made that allows passage of tears from the lacrimal sac into the nasal cavity.
While endonasal DCR has been performed by both ophthalmic surgeons and otorhinolaryngologists (ENT) in the past, the advancement of nasal endoscope and functional endoscopic sinus surgery (FESS) has led to more ENT surgeons performing endoscopic DCR. For ophthalmologists performing an endoscopic DCR, there is a learning curve both in instrumentation and anatomical variations amongst individuals via the endoscopic approach (Wesley and Bond, 1986). With external DCR, there is an unobstructed view and working space of the lacrimal anatomy, making it technically easier. With study and experience, the surgeon becomes better at maneuvering and individualizing the bony and soft tissue opening endoscopically (Wormald et al., 2000).
Another obstacle that can limit that usefulness of endoscopic dacryocystorhinostomy is the cost of instrumentation. Endoscopic DCR equipment include a rigid fiberoptic endoscope, a video display monitor, instrumentation for bone and soft tissue removal including various laser sources and fibers, a high speed drill, radiofrequency cautery and electrodes, and endonasal rongeurs and curettes. In certain circumstances, if a suspicious lacrimal system neoplasm or sinonasal disease cannot be excluded, radiologic studies such as dacryocystography and computed tomography may be required. Radiographic guidance systems can also be used for assistance with intraoperative orientation (Day et al., 2008) but are also expensive. Anari et al. (2008) refute this in their manuscript, stating that in spite of additional instrumentation, an additional surgeon, and lower success rate; endoscopic DCR is more cost-effective because it can decrease operative time allowing an experienced surgeon a higher number of cases per day. This has not been our experience as we discuss below.
Endoscopic dacryocystorhinostomy has certain advantages over external dacryocystorhinostomy. The most recognizable advantage is that the endoscopic approach is cosmetically more appealing due to the absence of a visible scar and bruising. Without a skin and orbicularis incision, there is faster return to normal daily activities and patient’s satisfaction. It has been hypothesized that endonasal approach may be more effective because it does not violate the lacrimal pump system therefore minimizing collateral damage to skin, muscle and surrounding structures (Hartikainen et al., 1998). Since it also provides a direct view of the nasal anatomy, it can be potentially more precise and atraumatic.
Patients with NLD obstruction and a previous history of sinus surgery, facial trauma, or failed external DCR are good candidates for endoscopic DCR. In cases of previously failed DCR, endoscopy can help visualize previous scarring (Orcutt et al., 1990). Adolescents with anatomical variations from atypical forms of congenital dacryostenosis may also benefit from endoscopic DCR (Wong et al., 1999). Acute dacryocystitis with abscess formation is a contraindication for external DCR, and in this case, an endoscopic approach is favored by some authors (Lee and Woog, 2001). Numerous studies have reported that endoscopic DCR has a decreased amount of intraoperative hemorrhaging (Hartikainen et al., 1998; Shun-Shin, 1998). With these advantages, ophthalmologists have been divided in their view of external vs. endoscopic DCR.
Initially, it was believed that external dacryocystorhinostomy had a higher success rate (85–100%) (Mathew et al., 2004). With the advancement of technology and the design of better nasal endoscopes, endoscopic dacryocystorhinostomy has become the procedure of choice for otolaryngologists as well as a myriad of ophthalmologists. Some recent manuscripts have shown that endoscopic DCR success rates were equal to or better than external DCR. Agarwal reported in a retrospective study of 300 patients with NLD obstruction, who underwent endoscopic DCR a success rate of 94% after the initial surgery (Agarwal, 2009). Feretis et al. (2009) gave their patients a questionnaire regarding their post-operative epiphora, and found no statistically significant differences between results for the external and endoscopic procedures.
To further enhance the success rate of endoscopic dacryocystorhinostomy, surgeons have been recently utilizing a semiconductor diode laser (Plaza et al., 2007). Laser-assisted procedures are faster and provide excellent hemostasis throughout the surgery. However, it has been theorized that a laser-assisted procedure induces fibroblastic activity causing excessive scarring and stenosis of the rhinostomy compared to non-laser dissection. Maini et al. did a prospective study of 60 patients that underwent endoscopic potassium titanyl phosphate (KTP) laser DCR and 66 underwent endoscopic surgical DCR (Maini et al., 2007). The study showed that initially within the first 3 months the laser DCR group had a higher success rate at 81.7% compared to the 75.8% surgical DCR. But at the 12 month interval surgical endoscopic DCR patients had a higher symptomatic success rate at 74.3% compared to the 68.3% laser group. Sadiq et al. (1996) performed endoscopic Holmium YAG laser DCR and had a success rate of 78.5% at 3 months, but that decreased to 59% at 12 months. Various new procedures and techniques have been described in literature regarding the success of endoscopic DCR. Surgical endoscopic DCR techniques use a range of instruments and drills to create rhinostomies. Likewise, different types of lasers have been described for endonasal laser DCR. There also seems to be conflicting data as to which type of laser and duration one can use. At this time it appears that laser endoscopic DCR has a lower success rate than non-laser endoscopic or external DCR.
We reserve endoscopic DCR for those patients excessively concerned about the possibility of scar formation, those with previously failed external DCR, and those with concomitant sinonasal pathology. We perform endoscopic DCR together with an ENT surgeon. The ENT surgeon approaches the nasal landmarks. We then place a fiberoptic light (20 gauge vitreoretinal light pipe or laser work as well) through one of the canaliculi into the lacrimal sac. This transilluminates the lacrimal sac fossa and guides the ENT surgeon in bone and/or soft tissue removal. Once the ostium is created, we place Bowman probes and guide the ENT surgeon in making a sufficiently large opening for unobstructed passage of probes. Crawford tubes are then passed into the nasal cavity and retrieved and tied as usual. Irrigation with endoscopic visualization can also be performed.
In our practice, the main advantages of the combined approach are direct visualization of scar tissue in previously failed cases and the ability to have the patient receive all the necessary surgical treatment in one sitting if sinus surgery is indicated. The main disadvantages are logistical. Because we perform this procedure in combination with another surgeon, more time is required. Endoscopic equipment, and if utilized, image guidance systems requires set up time. Additional sinonasal work, if performed, also adds to the total operative time. For these reasons and due to its high success rate we continue to perform external DCR as our preferred primary procedure (Table 2).
Table 2.
Comparison of advantages and disadvantages of endoscopic DCR.
| Advantages | Disadvantages |
|---|---|
| No external scar | Expensive equipment |
| Direct visualization of endonasal anatomy | Increased operative time |
| Direct visualization of scar tissue in cases of primary failure | Need for (further) imaging |
| Ability to perform concomitant sinus surgery if indicated | Requires extensive knowledge of endonasal anatomy. Therefore often done in conjunction with ENT |
4.4. Conjunctivodacryocystorhinostomy
Patent canaliculi and a functional lacrimal pump are required for success in DCR. When those conditions are not met a conjunctivodacryocystorhinostomy (CDCR) may be indicated. A CDCR may be utilized when a large segment of the canaliculus is obstructed or there is canalicular agenesis, such that it is unable to be used in the rebuilding of the tear drainage system apparatus. The cause of obstruction may also be related to infection (i.e., herpes), trauma, tumors, inflammation, chemo- and radiation therapy, and facial nerve palsy (Freitag and Woog, 2000; Lim et al., 2004; Jones, 1962).
This procedure bypasses the lacrimal drainage system. After an external or endonasal DCR is performed, a Jones tube (made of Pyrex glass, Weiss Scientific Glass Blowing Company, Portland, OR, U.S.A.) is placed through an opening made in the inferior half of the caruncle then through to the middle nasal meatus via an osteotomy site (Jones, 1962; Dailey and Tower, 2005). Once inserted, the Jones tube is left in place permanently (Fig. 4A). Newer versions of Jones tubes are available with modifications such as an eyelet for suture placement and frosted flange to promote adhesions between the tube and soft tissues along the tract (Dailey and Tower, 2005).
Figure 4.
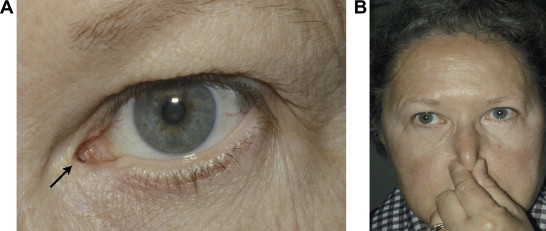
(A) A well positioned Jones tube is demonstrated (arrow). (B) Daily maintenance of Jones tube. After instilling 2–3 drops of artificial tears forced inspiration through the nose with the nose occluded is performed.
There are complications in this procedure, including: extrusion, obstruction, and hypermobility of the tube, migration of the tube as well as pyogenic granuloma formation. Less frequent complications include infection, discomfort, and diplopia (Freitag and Woog, 2000; Lim et al., 2004; Rosen et al., 1994).
This procedure is not popular because postoperative care is cumbersome – the patient must manually perform a forced inspiration with the mouth and nose closed to get air through the tube into the nasal airway to clear debris, once a day (Fig. 4B). In addition, patients are often dissatisfied because of the frequency of postoperative visits, tearing in the supine position and fogging of spectacles especially when sneezing or blowing their nose (Lim et al., 2004; Rosen et al., 1994). Nevertheless, this is a procedure that can be highly effective in relieving epiphora and obtaining a comfortable eye in carefully selected patients.
5. Complications
As with any surgical procedure a potential for complications exists (Table 3). Below we discuss the most common and significant complications of dacryocystorhinostomy.
Table 3.
Complications of dacryocystorhinostomy.
| Primary failure | Infection |
|---|---|
| Scarring | Meningitis |
| Bleeding | CSF leak |
| Orbital emphysema |
5.1. Primary failure
The cause of primary failure is usually due to soft tissue scarring over the rhinostomy (Carter and Nerad, 1996). In our early experience obstruction was frequently found proximal to the common internal punctum (McLachlan et al., 1980). A recent study found that in patients who had late failure after primary dacryocystorhinostomy lacrimal system obstruction occurs at the common canaliculus (McMurray et al., 2010). We again emphasize the importance of avoiding damage to the common internal punctum as well as preoperative assessment of canalicular patency. Overall, very high success rates ranging from 80% to nearly 100% are consistently reported.
A study by Fayers et al recently compared success rates in surgeries performed by an experienced surgeon vs. cases performed by trainees (fellows and residents) (Fayers et al., 2009). They found an overall lower rate of success for trainees in terms of both functional (64.4%) and anatomic (68.1%) improvement as compared to 80.6% functional and 87.1% anatomic success rate for the consultant surgeon.
5.2. Excessive scarring
A recent study showed that there was a relatively low percentage of patients who complained of a scar (Sharma et al., 2005). In response to this article, Weinberg offered an alternative: a medial lower lid incision, just above the junction between the cheek and eyelid skin which would help to conceal the scar (Weinberg, 2006). This appears similar to the incision described by (Iliff, 1971) in the 1950s. In our experience the placement of the incision anterior to the medial canthal tendon as well as avoidance of extension of the incision into the upper lid produces a minimally noticeable and cosmetically satisfying scar.
5.3. Bleeding
One study showed a rate of secondary hemorrhage as high as 3.8%, attributing these complications to use of NSAIDs, immunocompromised states, and non-routine operations (Tsirbas and McNab, 2000). Some studies have shown that patients undergoing DCR may have delayed epistaxis but this should not be related to their taking anticoagulant agents if their use is stopped within a defined period of time before and after the surgery (Ben Simon et al., 2010).
5.4. Infection
An uncommon complication of external dacryocystorhinostomy is wound infections. A United Kingdom study reported cellulitis rates of 8% to 18% after external DCR when systemic antibiotic prophylaxis was not administered (Walland and Rose, 1994). A five time reduction in this rate can be achieved with routine administration of antibiotics after surgery. The utilization of silicone tubing does not increase the risk of infection (Ma’luf et al., 2001). In 1992, a survey was performed amongst oculoplastic surgeons and approximately 50% favored the use of routine antibiotic prophylaxis for external DCR (Hurley et al., 1992).
5.5. Cerebrospinal fluid leakage
Cerebrospinal fluid (CSF) leakage is an unusual complication of orbital surgery. The majority of CSF leaks that have been reported occur with orbital exenterations and decompressions. Their incidence rates are 1.6–16.7% and 0–10%, respectively (Limawararut et al., 2008). However there have been several CSF leaks reported with both endonasal (Badilla and Dolman, 2007) and external DCR. A low cribriform plate or propagation of cracks during osteotomy creation may predispose to CSF leaks and meningitis. Therefore, when using biting rongeurs for bone removal, torquing or twisting motions must be avoided.
A retrospective review of 2456 DCRs, performed over a 14 year period, revealed only one case of CSF leak, which spontaneously resolved: an incidence of 0.04% (Badilla and Dolman, 2007). In another case a CSF fistula developed in a patient with a known meningoencephalocele (Yilmaz et al., 2008) following external DCR. CSF leaks may be missed and may spontaneously heal, but a potential complication that one can develop is meningitis typically caused by Streptococcus pneumonia. Chronic rhinorrhea especially if associated with headache following DCR should raise suspicion of a CSF leak. The fluid can be tested for β2-transferrin to determine if it is CSF. The majority of leaks spontaneously resolve within 24 hours. If meningitis is suspected, the patient should be placed on broad-spectrum antibiotics with good blood-brain barrier penetration and a neurosurgical consultation should be obtained (Badilla and Dolman, 2007).
5.6. Meningitis
This is a rare complication with 2 cases in the published literature. Usul et al. (2004) reported a case of a patient who on postoperative day one following an external DCR developed pneumocephalus in the anterior cranial fossa. This was caused by a fracture of the fovea ethmoidalis. A diagnostic lumbar puncture was performed, and the cerebrospinal fluid cultures showed S. pneumonia. Disruption of the posterior-medial aspect of the orbital roof results in a lack of a protective barrier between the meninges and orbit. The same mechanism was postulated by Beiran et al. (1994) in a case where a 9 year old girl developed meningitis 9-days post-operatively.
5.7. Orbital emphysema
Orbital emphysema has been reported in patients following lacrimal surgery. Laryngospasm following anesthesia, blowing of the nose, or Valsalva maneuver, such as sneezing, can cause a high positive pressure, which is forced from the nasal cavity via the DCR ostium into the orbit or subcutaneous tissue. Ajit et al. (2004) reported a case of orbital emphysema following balloon dacryoplasty. Ghosheh and Kathuria (2005) reported on a DCR patient that developed periorbital crepitus postoperatively. The superficial musculoaponeurotic system (SMAS) is an extension of the superficial cervical fascia in the neck, and invests the facial mimetic muscles. When air enters the subcutaneous tissue, the SMAS provides a one-way pathway for air to dissect into the neck and face. Careful observation and supportive care is the main treatment for orbital emphysema. However one must be cautious that the cause of crepitus is not from necrotizing fasciitis. CT scanning can demonstrate the presence of air pockets.
6. Future directions
Despite the long history of the procedure debate about various steps continues. Issues such as whether or not to suture the flaps, which flaps to suture (Baldeschi et al., 2004; Erdoğan et al., 2010), how to place certain sutures (Evereklioglu et al., 2007; Ciftci et al., 2010) where to place the incision (Picó, 1971; Iliff, 1971; Sharma et al., 2005; Weinberg, 2006), and whether the external or endoscopic approach is better (Hartikainen et al., 1998; Feretis et al., 2009; Goldberg, 2004; Woog et al., 2001) continue to be debated in the literature.
Various tools are advocated for the removal of bone. Burrs and trephines have been described for use in DCR for over 100 years (Chandler, 1936; Carter and Nerad, 1996; Picó, 1971; Iliff, 1971). An ultrasound device for bone removal in DCR was constructed in the Soviet Union in the 1960’s (Krasnov, 1971). More recently, a Japanese neurosurgical ultrasound device (Sonopet OMNI) has been applied to DCR (Sivak-Callcott et al., 2005). The latest version of the machine utilizes both a longitudinal and torsional motion of the tip (Sonopet, 2010) similar to the modern phacoemulsification machines. While these are exciting developments we continue to rely on rongeurs for bone removal. Rongeurs are reliable, low technology, widely available instruments that do not require any extra set up time unlike any powered tools described above.
An additional development is the use of anti-fibrotic agents such as mitomycin C (MMC). While we do not have personal experience using MMC several methods have been reported. Use of MMC is familiar to many ophthalmologists performing glaucoma, pterygium and refractive surgery but is less common in oculoplastic surgery.
Recently, some have advocated the use of MMC as it has shown to improve success rates in both external and endoscopic DCRs. In a retrospective study, surgical outcomes in a group of 193 endonasal DCRs with MMC applied, soaked on a 0.8mm cottonoid in a concentration for 0.5 mg/ml for 10 minutes, achieved a 95% success rate with a mean follow-up period of 18.3 months (Dolmetsch, 2010). No complications, including delayed wound healing infection or abnormal bleeding were noted post-operatively. Yildirim et al performed a prospective randomized controlled study for external DCR where 18 (90%) of the 20 eyes in the MMC group remained symptom-free; while 12 (60%) of 20 eyes in the control group were reported to be symptom-free. In the MMC group 0.2 mg/ml MMC was applied to the osteotomy site for 30 min, and the success rate 95% compared with 85% in the control group (Yildirim et al., 2007). The published literature on MMC use in DCR, presents multiple variations in the method of application, duration of application as well as the concentration of mitomycin C. Use of MMC is a novel treatment modality for both external and endoscopic DCR, however further studies will need to define the optimal dosing and application regimen.
7. Summary
As our technique has evolved, we have settled on the following elements
-
•
Making a curvilinear incision anterior to the medial canthal tendon.
-
•
Detaching the anterior crus of the medial canthal tendon during dissection.
-
•
Using rongeurs for bone removal.
-
•
Fashioning and suturing anterior lacrimal sac and nasal mucosa flaps when possible.
-
•
Placing silicone stents in virtually all cases.
The history and development, indications, complications and future directions of DCR have been discussed. External DCR were noted a very successful procedure. It remains our preferred primary procedure in the treatment of nasolacrimal duct obstruction and chronic dacryocystitis. An additional benefit to the classic external DCR is that it does not require expensive high technology equipment and can therefore be performed in places with developing medical infrastructure. Where access to endoscopic equipment is available, endonasal DCR can serve as an alternative primary or secondary procedure. However, external DCR as described in the article remains our primary operation of choice due to high success rate, reasonable operative time and patient comfort.
Conflict of interest
None declared.
Contributor Information
Vladimir S. Yakopson, Email: vovik24@yahoo.com.
Joseph C. Flanagan, Email: lwebb@willseye.org.
Daniel Ahn, Email: dannon@gmail.com.
Betsy P. Luo, Email: betsyluo@gmail.com.
References
- Agarwal S. Endoscopic dacryocystorhinostomy for acquired nasolacrimal duct obstruction. J. Laryngol. Otol. 2009;123:1226–1228. doi: 10.1017/S0022215109990776. [DOI] [PubMed] [Google Scholar]
- Ajit R., Inkster C., Tuck J., Mortzos P. Orbital emphysema: an unusual complication of balloon dacryocystoplasty. Br. J. Radiol. 2004;77(924):1057–1058. doi: 10.1259/bjr/86898521. [DOI] [PubMed] [Google Scholar]
- Anari S., Ainsworth G., Robson A.K. Cost-efficiency of endoscopic and external dacryocystorhinostomy. J. Laryngol. Otol. 2008;122:476–479. doi: 10.1017/S0022215107009954. [DOI] [PubMed] [Google Scholar]
- Badilla J., Dolman P.J. Cerebrospinal fluid leaks complicating orbital or oculoplastic surgery. Arch. Ophthalmol. 2007;125(12):1631–1634. doi: 10.1001/archopht.125.12.1631. [DOI] [PubMed] [Google Scholar]
- Baldeschi L., Macandie K., Hintschich C.R. The length of unsutured mucosal margins in external dacryocystorhinostomy. Am. J. Ophthalmol. 2004;138(5):840–844. doi: 10.1016/j.ajo.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Barnes E.A., Abou-Rayyah Y., Rose G.E. Pediatric dacryocystorhinostomy for nasolacrimal duct obstruction. Ophthalmology. 2001;108(9):1562–1564. doi: 10.1016/s0161-6420(01)00699-6. [DOI] [PubMed] [Google Scholar]
- Beiran I., Pikkel J., Gilboa M., Miller B. Meningitis as a complication of dacryocystorhinostomy. Br. J. Ophthalmol. 1994;78:417–418. doi: 10.1136/bjo.78.5.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Simon G.J., Cheung N., McNab A.A. Delayed epistaxis in external dacryocystorhinostomy: rate and risk factors. Arch. Otolaryngol. Head Neck Surg. 2010;136(2):183–186. doi: 10.1001/archoto.2009.200. [DOI] [PubMed] [Google Scholar]
- Boulos P.R., Rubin P.A. A lacrimal sac abscess incision and drainage technique. Arch. Ophthalmol. 2008;126(9):1297–1300. doi: 10.1001/archopht.126.9.1297. [DOI] [PubMed] [Google Scholar]
- Carter K.D., Nerad J.A. Primary Acquired Nasolacrimal Duct Obstruction. In: Bosniak S., editor. Principles and Practice of Ophthalmic Plastic and Reconstructive Surgery. Saunders; Philadelphia: 1996. pp. 784–796. [Google Scholar]
- Chandler P.A. Dacryocystorhinostomy. Trans. Am. Ophthalmol. Soc. 1936;34:240–263. [PMC free article] [PubMed] [Google Scholar]
- Ciftci F., Dinc U.A., Ozturk V. The importance of lacrimal diaphragm and periosteum suturation in external dacryocystorhinostomy. Ophthal. Plast. Reconstr. Surg. 2010;26(4):254–258. doi: 10.1097/IOP.0b013e3181bb5942. [DOI] [PubMed] [Google Scholar]
- Dailey R.A., Tower R.N. Frosted jones pyrex tubes. Ophthal. Plast. Reconstr. Surg. 2005;21(3):185–187. doi: 10.1097/01.iop.0000160595.34837.0c. [DOI] [PubMed] [Google Scholar]
- Day S., Hwang T.N., Pletcher S.D., Bhatki A., McCulley T.J. Interactive image-guided endoscopic dacryocystorhinostomy. Ophthal. Plast. Reconstr. Surg. 2008;24(4):338–340. doi: 10.1097/IOP.0b013e31817e6133. [DOI] [PubMed] [Google Scholar]
- Dolmetsch A.M. Nonlaser endoscopic endonasal dacryocystorhinostomy with adjunctive mitomycin C in nasolacrimal duct obstruction in adults. Ophthalmology. 2010;117:1037–1040. doi: 10.1016/j.ophtha.2009.09.028. [DOI] [PubMed] [Google Scholar]
- Erdoğan G., Unlü C., Vural E.T. Inferior flap anastomosis in external dacryocystorhinostomy. Ophthal. Plast. Reconstr. Surg. 2010;26(4):277–280. doi: 10.1097/IOP.0b013e3181c3252c. [DOI] [PubMed] [Google Scholar]
- Evereklioglu C., Oner A., Somdaş M.A. Figure-of-eight vertical mattress suture technique for anterior flap suspension to overlying tissues in external dacryocystorhinostomy. Am. J. Ophthalmol. 2007;143(2):328–333. doi: 10.1016/j.ajo.2006.09.057. [DOI] [PubMed] [Google Scholar]
- Fayers T., Laverde T., Tay E., Olver J.M. Lacrimal surgery success after external dacryocystorhinostomy: functional and anatomical results using strict outcome criteria. Ophthal. Plast. Reconstr. Surg. 2009;25(6):472–475. doi: 10.1097/IOP.0b013e3181b81e9f. [DOI] [PubMed] [Google Scholar]
- Feretis M., Newton J.R., Ram B., Green F. Comparison of external and endonasal dacryocystorhinostomy. J. Laryngol. Otol. 2009;123:315–319. doi: 10.1017/S0022215108002685. [DOI] [PubMed] [Google Scholar]
- Freitag S.K., Woog J.J. Congenital nasolacrimal duct obstruction. Ophthalmol. Clin. North Am. 2000;13(4):705–718. [Google Scholar]
- Ghosheh F., Kathuria S. Massive subcutaneous emphysema mimicking necrotizing fasciitis after dacryocystorhinostomy. Ophthal. Plast. Reconstr. Surg. 2005;21:389–391. doi: 10.1097/01.iop.0000176267.81599.d8. [DOI] [PubMed] [Google Scholar]
- Girgis I.H. Dacryocystorhinostomy. J. Laryngol. Otol. 1968;82(2):149–152. doi: 10.1017/s0022215100068602. [DOI] [PubMed] [Google Scholar]
- Goldberg R.A. Endonasal dacryocystorhinostomy: is it really less successful? Arch. Ophthalmol. 2004;122(1):108–110. doi: 10.1001/archopht.122.1.108. [DOI] [PubMed] [Google Scholar]
- Harrison M.S., Mukherjee A.K. Dacryocystorhinostomy in children and infants. J. Laryngol. Otol. 1967;81(1):45–50. doi: 10.1017/s0022215100066755. [DOI] [PubMed] [Google Scholar]
- Hartikainen J., Grenman R., Puukka P., Seppa H. Prospective randomized comparison of external dacryocystorhinostomy and endonasal laser dacryocystorhinostomy. Ophthalmology. 1998;105:1106–1113. doi: 10.1016/S0161-6420(98)96015-8. [DOI] [PubMed] [Google Scholar]
- Henry L.M. Results of intranasal dacryocystorhinostomy. Br. J. Ophthalmol. 1933;17(9):550–552. doi: 10.1136/bjo.17.9.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley L.D., Westfall C.T., Shore J.W. Prophylactic use of antibiotics in oculoplastic surgery. Int. Ophthalmol. Clin. 1992;32:165–178. [PubMed] [Google Scholar]
- Iliff C.E. A simplified dacryocystorhinostomy. 1954–1970. Arch. Ophthalmol. 1971;85(5):586–591. doi: 10.1001/archopht.1971.00990050588011. [DOI] [PubMed] [Google Scholar]
- Jones L.T. The cure of epiphora due to canalicular disorders, trauma and surgical failures on the lacrimal passages. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1962;66:506–524. [PubMed] [Google Scholar]
- Krasnov M.M. Ultrasonic dacryocystorhinostomy. Am. J. Ophthalmol. 1971;72(1):200–201. doi: 10.1016/0002-9394(71)91614-x. [DOI] [PubMed] [Google Scholar]
- Lee J., Flanagan J.C. Complications associated with silicone intracanalicular plugs. Ophthal. Plast. Reconstr. Surg. 2001;17(6):465–469. doi: 10.1097/00002341-200111000-00017. [DOI] [PubMed] [Google Scholar]
- Lee T.S., Woog J.J. Endonasal dacryocystorhinostomy in the primary treatment of acute dacryocystitis with abscess formation. Ophthal. Plast. Reconstr. Surg. 2001;17:103–106. doi: 10.1097/00002341-200105000-00006. [DOI] [PubMed] [Google Scholar]
- Liermann D., Berkefeld J., Fries U. Balloon dacryoplasty: an alternative treatment for obstructed tear ducts. Ophthalmologica. 1996;210:319–324. doi: 10.1159/000310732. [DOI] [PubMed] [Google Scholar]
- Lim C., Marin P., Benger F. Lacrimal canalicular bypass surgery with the lester jones tube. Am. J. Ophthalmol. 2004;137(1):101–108. doi: 10.1016/j.ajo.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Limawararut V., Valenzuela A.A., Sullivan T.J. Cerebrospinal fluid leaks in orbital and lacrimal surgery. Surv. Ophthalmol. 2008;53:274–284. doi: 10.1016/j.survophthal.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Maini S., Raghava N., Youngs R. Endoscopic endonasal laser versus endonasal surgical dacryocystorhinostomy for epiphora due to nasolacrimal duct obstruction: prospective, randomized, controlled trial. J. Laryngol. Otol. 2007;121:1170–1176. doi: 10.1017/S0022215107009024. [DOI] [PubMed] [Google Scholar]
- Ma’luf R.N., Dbaibo G., Araj G.F., Noureddin B. Bacterial flora of the ocular fornix in patients with lacrimal silicone tubes. Ann. Ophthalmol. 2001;33(1):31–34. [Google Scholar]
- Mathew M.R., McGiness R., Webb L.A. Patient satisfaction in our initial experience with endonasal endoscopic non-laser dacryocystorhinostomy. Orbit. 2004;23:77–85. doi: 10.1080/01676830490501415. [DOI] [PubMed] [Google Scholar]
- McDonogh M., Meiring J.H. Endoscopic transnasal dacryocystorhinostomy. J. Laryngol. Otol. 1989;100:585–587. doi: 10.1017/s0022215100109405. [DOI] [PubMed] [Google Scholar]
- McLachlan D.L., Shannon G.M., Flanagan J.C. Results of dacryocystorhinostomy: analysis of the reoperations. Ophthalmic Surg. 1980;11(7):427–430. [PubMed] [Google Scholar]
- McMurray, C.J., McNab, A.A., Selva, D., 2010. Late Failure of Dacryocystorhinostomy. Ophthal. Plast. Reconstr. Surg. [DOI] [PubMed]
- Nelson L.B., Calhoun J.H., Menduke H. Medical management of congenital nasolacrimal duct obstruction. Ophthalmology. 1985;92:1187–1190. doi: 10.1016/s0161-6420(85)33878-2. [DOI] [PubMed] [Google Scholar]
- Nowinski T.S., Flanagan J.C., Mauriello J. Pediatric dacryocystorhinostomy. Arch. Ophthalmol. 1986;103:1226–1229. doi: 10.1001/archopht.1985.01050080138035. [DOI] [PubMed] [Google Scholar]
- Older J.J. Routine use of a silicone stent in a dacryocystorhinostomy. Ophthalmic Surg. 1982;13(11):911–915. [PubMed] [Google Scholar]
- Orcutt J.C., Hillel A., Weymuller E.A., Jr Endoscopic repair of failed dacryocystorhinostomy. Ophthal. Plast. Reconstr. Surg. 1990;6:197–202. doi: 10.1097/00002341-199009000-00009. [DOI] [PubMed] [Google Scholar]
- Pediatric.Eye.Disease Investigator Group Primary treatment of nasolacrimal duct obstruction with probing in children younger than 4 Years. Ophthalmology. 2008;115:577–584. doi: 10.1016/j.ophtha.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J.D., Maus M., Nowinski T.S., Penne R.B. Balloon catheter dilation for treatment of adults with partial nasolacrimal duct obstruction: a preliminary report. Am. J. Ophthalmol. 1998;126:811–816. doi: 10.1016/s0002-9394(98)00278-5. [DOI] [PubMed] [Google Scholar]
- Picó G. A modified technique of external dacryocystorhinostomy. Am. J. Ophthalmol. 1971;72(4):679–690. doi: 10.1016/0002-9394(71)90001-8. [DOI] [PubMed] [Google Scholar]
- Plaza G., Betere F., Nogueira A. Transcanalicular dacryocystorhinostomy with diode laser: long-term results. Ophthal. Plast. Reconstr. Surg. 2007;23:179–182. doi: 10.1097/IOP.0b013e31804bdef9. [DOI] [PubMed] [Google Scholar]
- Robinson R., Turner, Brettle P. The treatment of epiphora with balloon dacryocystoplasty. Eye. 1993;7:687–690. doi: 10.1038/eye.1993.156. [DOI] [PubMed] [Google Scholar]
- Rosen N., Ashkenazi I., Rosner M. Patient dissatisfaction after functionally successful conjunctivodacryocystorhinostomy with Jones tube. Am. J. Ophthalmol. 1994;117:636–642. doi: 10.1016/s0002-9394(14)70070-4. [DOI] [PubMed] [Google Scholar]
- Sadiq S.A., Hugkulstone C.E., Jones N.S., Downes R.N. Endoscopic holmium YAG laser dacryocystorhinostomy. Eye. 1996;10:43–46. doi: 10.1038/eye.1996.6. [DOI] [PubMed] [Google Scholar]
- Sharma V., Martin P.A., Benger R. Evaluation of the cosmetic significance of external dacryocystorhinostomy scars. Am. J. Ophthalmol. 2005;140(3):359–362. doi: 10.1016/j.ajo.2005.04.039. [DOI] [PubMed] [Google Scholar]
- Shun-Shin G.A. Endoscopic dacryocystorhinostomy: a personal technique. Eye. 1998;12:467–470. doi: 10.1038/eye.1998.106. [DOI] [PubMed] [Google Scholar]
- Sivak-Callcott J.A., Linberg J.V., Patel S. Ultrasonic bone removal with the Sonopet Omni: a new instrument for orbital and lacrimal surgery. Arch. Ophthalmol. 2005;123(11):1595–1597. doi: 10.1001/archopht.123.11.1595. [DOI] [PubMed] [Google Scholar]
- Sonopet OMNI Catalog, 2010. Accessed at <http://www.synergeticsusa.com/index.php?option=com_flippingbook&view=book&id=12> Aug 22nd.
- Tao S., Meyer D.R., Simon J.W. Success of balloon catheter dilation as a primary or secondary procedure for congenital nasolacrimal duct obstruction. Ophthalmology. 2002;109:2108–2111. doi: 10.1016/s0161-6420(02)01216-2. [DOI] [PubMed] [Google Scholar]
- Tsirbas A., McNab A.A. Secondary haemorrhage after dacryocystorhinostomy. Clin. Exp. Ophthalmol. 2000 Feb;28(1):22–25. doi: 10.1046/j.1442-9071.2000.00249.x. [DOI] [PubMed] [Google Scholar]
- Usul H., Kuzeyli K., Cakir E. Meningitis and pneuomocephalus. A rare complication of external dacryocystorhinostomy. J. Clin. Neurosci. 2004;11:901–902. doi: 10.1016/j.jocn.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Veirs E.R. The use of cautery in external dacryocystorhinostomy. Arch. Ophthalmol. 1969;82(4):489–490. doi: 10.1001/archopht.1969.00990020491013. [DOI] [PubMed] [Google Scholar]
- Walland M.J., Rose G.E. Soft tissue infections after open lacrimal surgery. Ophthalmology. 1994;101:608–611. doi: 10.1016/s0161-6420(13)31269-x. [DOI] [PubMed] [Google Scholar]
- Weinberg D.A. Response to evaluation of the cosmetic significance of external dacryocystorhinostomy scars. Am. J. Ophthalmol. 2006;141(4):780. doi: 10.1016/j.ajo.2005.11.061. [DOI] [PubMed] [Google Scholar]
- Wesley R.E., Bond J.B. Intranasal procedures for successful lacrimal surgery. Ophthal. Plast. Reconstr. Surg. 1986;2:153–157. doi: 10.1097/00002341-198601060-00007. [DOI] [PubMed] [Google Scholar]
- Williams J.L., Hill B.G. A simplified external dacryocystorhinostomy. Br. J. Ophthalmol. 1944;28(8):407–410. doi: 10.1136/bjo.28.8.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J.F., Woog J.J., Cunningham M.J. A multidisciplinary approach to a typical lacrimal obstruction in childhood. Ophthal. Plast. Reconstr. Surg. 1999;15:293–298. doi: 10.1097/00002341-199907000-00011. [DOI] [PubMed] [Google Scholar]
- Woog J.J., Kennedy R.H., Custer P.L. Endonasal dacryocystorhinostomy: a report by the American Academy of Ophthalmology. Ophthalmology. 2001;108(12):2369–2377. doi: 10.1016/s0161-6420(01)00945-9. [DOI] [PubMed] [Google Scholar]
- Wormald P.J., Kew J., van Hasselt A. Intranasal anatomy of the nasolacrimal sac in endoscopic dacryocystorhinostomy. Otolaryngol. Head Neck Surg. 2000;123:307–310. doi: 10.1067/mhn.2000.105416. [DOI] [PubMed] [Google Scholar]
- Yacizi B., Yacizi Z. Frequency of the common canaliculus. A Radiological Study. Arch. Ophthalmol. 2000;(118):1381–1385. doi: 10.1001/archopht.118.10.1381. [DOI] [PubMed] [Google Scholar]
- Yildirim C., Yaylali V., Esme A., Ozden S. Long-term results of adjunctive use of mitomycin C in external dacryocystorhinostomy. Int. Ophthalmol. 2007;27(1):31–35. doi: 10.1007/s10792-007-9057-6. [DOI] [PubMed] [Google Scholar]
- Yilmaz Y.F., Ozcan M., Titiz A., Unal A. Meningoencephalocele as a rare cause of cerebrospinal fluid fistula during dacryocystorhinostomy. Ophthal. Plast. Reconstr. Surg. 2008;24:240–241. doi: 10.1097/IOP.0b013e3181710962. [DOI] [PubMed] [Google Scholar]


