Abstract
Comitant strabismus is a common condition affecting infants, children and adults. Its impact on the affected patient may be severe resulting in visual loss, lack of binocularity, diplopia, social stigma and multiple corrective surgeries within the affected individual’s lifespan. It is therefore important that this prevalent disorder should be better understood.
We review the current understanding of the demographics and what is known of the etiology, risk factors and genetics of strabismus. We stress the importance of careful clinical assessment in classifying strabismus, and the common pitfalls in the measurement and pre-operative sensory work-up of the strabismic patient. The fact strabismus is comitant does not indicate it is benign: acute onset of comitant esotropia may be a presenting sign of pontine or cerebellar tumor.
Lastly, we review the impact of genetics on our understanding of strabismus. While the causes of many types of congenital incomitant strabismus have been elucidated through careful observation and genetic screening, the genetics of comitant strabismus are more complex and multifactorial. Only through careful study and recruitment of large groups of affected individuals and families can we start to answer the question: why is this group of patients pre-disposed to develop strabismus. Doing so will help identify patients at risk, to spare them from the significant morbidity associated with this common disorder.
Keywords: Strabismus, Comitant strabismus, Genetics, Management, Demographics
Defining comitant strabismus
Strabismus is best defined as a condition in which only one of the 2 eyes is directed at the object of interest. If the angle of ocular misalignment is equal in all fields of gaze, remains the same regardless of which eye is used for fixation, and if the eye movements are all full, the strabismus is described as comitant.1 Conversely, incomitant strabismus generally results from limitation of eye movement associated with paralytic or mechanical etiologies. Comitant strabismus develops most commonly in early childhood but can do so at any age particularly in the presence of monocular visual loss.
Demographics and prevalence
Population studies, mostly from Western European and North American populations suggest the mean prevalence of strabismus is between 2% and 5% with esodeviations outnumbering exodeviations in these populations.2 However, data from some Asian studies report slightly lower prevalence3–5 and exodeviations being more common than esodeviations.6,7
Recently some interesting trends have come to light regarding the number of new cases presenting for management as well as their diagnosis. Several studies from different countries have reported a decrease in the number of new cases being diagnosed as well in the number of surgical corrections performed.8–11 However, there is not yet conclusive evidence that the incidence of comitant strabismus is decreasing. A recent study from a single institution in America looked at a potential decrease to the number of annual surgical procedures for sub-types of comitant strabismus over a 20 year period ending in 2009.12 The number of annual procedures for infantile esotropia was stable. Surgery for esotropia decreased while it increased for exotropia.
Etiology
Comitant strabismus can be congenital or acquired. In the absence of obvious structural anomalies of the eye and brain, the etiology remains unclear. It is generally considered to be the result of a complex combination of several heterogeneous factors which can be part heredity and part environmental.
At present there are no identified anatomical anomalies associated with comitant strabismus in neurologically normal individuals. Most psychophysical and functional neuro-imaging studies to date are unfortunately affected in part by the co-existence of amblyopia, which has its own striate and extra-striate implications.
Recently, Lennerstrand13 reported that proprioceptive information from rectus muscles were processed differently in exotropic patients to normal subjects. However, the authors concluded this raised more questions than answered. The role of proprioceptive receptors in the extraocular muscles is unknown. Their findings suggest a putative role in strabismus, but whether this is causative or secondarily adaptive remains unclear.
Further understanding of some forms of strabismus has come from the identification of fibroelastic orbital muscle pulleys and subsequent identification of heterotopic pulley positions (by MRI) has challenged conventional understanding of strabismus.14,15 However, as yet this has not changed the practical management of strabismus for the majority of surgeons.
Risk factors
Comitant strabismus has significant public health significance since long-term consequences may include vision loss, reduced or absent depth perception, multiple life-long surgeries, stigma and social integration problems of affected individuals.16,17 Thus, it is important to identify potential risk factors, and to attempt to modify or prevent these.
Two large studies from America have examined associated risk factors.18,19 Both included a large population of children; one study examined children over serial visits between birth and the age of 7 years while the other study examined an ethnically diverse group of children at a single visit that ranged in age from 6 months to 5 years. Commonly associated risk factors include refractive errors; Hyperopia (3 diopters or greater) and anisometropia (1 diopter or greater) were found to be strong predictors of esotropia, while bilateral astigmatism of 1.5 diopter or more was a significant risk factor for exotropia. Older pre-school children (48–72 months of age) were most at risk of developing strabismus as compared to other age groups because of the frequent onset of accommodative esotropia at this age. Family history was only found to be a significant factor for exotropia.
An etiological mechanism cannot be identified for all cases of strabismus. However, there is strong evidence relating to environmental factors that impact the developing infant’s neurological status. Most reports put the prevalence of strabismus at less than 5% of the general population. However, when examining a cohort with altered neurological substrate this can increase to as much as 20–100%.20–22 Other factors such as low birth weight (especially <1500 g), prematurity (<33 weeks) and advanced maternal age also play a negative role. Even maternal smoking during pregnancy has been suggested to affect the incidence of strabismus, the likelihood of strabismus increasing with the average daily intake of cigarettes smoked by the pregnant mother, suggesting a dose-response effect.18 Further studies will probably reveal other potential risk factors for the development of childhood strabismus.
Strategies for improving management outcomes
The main goals in strabismus management are to optimize visual acuity and achieve binocularity. This generally includes performing accurate and repeated cycloplegic refractions, administering appropriate occlusion regimens, and restoring fusion and stereopsis by realigning the visual axes in order to normalize binocular input to the visual cortex. This either eliminates suppression or diplopia depending on the age of onset and duration of strabismus. Recently the advantages of surgical correction of strabismus in adults with childhood onset strabismus have been reviewed. Such treatment can no longer be considered purely ‘cosmetic’ since it also normalizes the binocular visual field and addresses the well-established psychosocial aspects of strabismus.17,23,24
In order to improve outcomes and minimize complications, it is essential to ensure the initial diagnosis is correct, the measurements are accurate and complete (near, distance, far distance, all cardinal positions of gaze) and the timing of surgery is appropriate. It is also important that individuals with an increased likelihood of developing binocularity problems following ophthalmological procedures are identified. For example, in the setting of comitant esotropia, it is now evident that the presence of dissociated vertical deviation (Fig. 1) and monocular smooth pursuit anomalies are reliable clinical indicators to distinguish between early versus late onset esotropia.25 This is particularly helpful in older patients in whom early history is either unknown or lost. One issue that still weighs heavily on the minds of most clinicians is when to fully investigate acute acquired esotropia for potential neurologic etiologies. Thus reminding us even in the face of comitant strabismus one needs to remain vigilant for ominous causes.26
Figure 1.
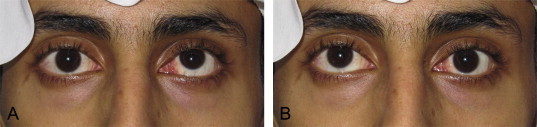
Dissociated vertical deviation (DVD). Patient with manifest DVD evident by elevation of either non-fixing eye. (A) Fixing with right eye with left eye elevated. (B) Fixing with left eye with right eye elevated.
Much attention has been paid to the timing of management, particularly surgical. Using infantile esotropia as an example, it is generally accepted that in order to optimize the likelihood of a binocular result, the eyes should be aligned before 10 months of age.27 Yet, randomized control studies are still needed to lay to rest the controversies regarding type of surgery and age of intervention.28 Nevertheless previous studies have yielded useful information and we now pay careful attention to additional variables that may affect outcome. Staying with the example of infantile esotropia, delaying surgery in an 8 month old because of variable strabismus is reasonable (Fig. 2). Also, surgery may be delayed for other patients with smaller angle esotropia to ensure that spontaneous resolution does not occur. Additionally, timing and amount of surgery will be influenced by the presence of neurological impairment or even by the presence of refractive errors such as simple myopia which is felt by some to be an indication for more aggressive surgery.29
Figure 2.
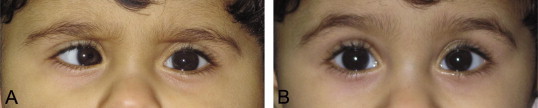
Infantile esotropia with variable angle. An 8 month old patient with onset of esotropia at 1 month of age with a cycloplegic refraction of +1.50 OU. Images A and B were taken at the same visit showing an extremely variable angle. (A) Right esotropia of 45 prism diopters. (B) Straight eyes moments later unrelated to visual attention or apparent accommodative effort.
Some variables such as high refractive errors are easier to control for than others and lead to improved data accuracy. For example patients with strabismus and high spectacle refractive errors must be re-evaluated with either contact lenses in place or the measured deviation obtained through the spectacles must be adjusted based on either the Prentice’s rule formula or using conversation tables taking into account direction of deviation and power of the spectacle correction. Similarly, adjustments are required for large angle strabismus when forced to split prisms before each eye.30,31 By taking such approaches we are documenting a much truer deviation which can then be used to generate a more accurate targeted surgical angle. Attention to such variables and the precautions taken to address them should now be a standard approach for all types of comitant strabismus.
We have also adopted several clinical tests to improve quantification of the strabismus and potential underlying mechanisms. These include the patch test, past 20 feet measurement or using +3.00 diopter lenses at near for exodeviations to distinguish between ‘true’ and ‘simulated’ distance near disparities. Similar strategies are also used for some forms of esotropia by differentiating between convergence excess type versus high AC/A ratio to determine if bifocals are warranted or in the selection of an appropriate surgical procedure. The Prism adaptation test is another method to minimize under-corrections and improve surgical outcomes.32 Other issues include the importance of pre-operative amblyopia treatment or the use of alternate occlusion for intermittent exotropia. These are but a few examples of the clinical strategies aimed at improving outcomes, however more work is still required as the full impact of these strategies remains unanswered without more evidence based scrutiny.
It is also important to recognize at the time of pre-operative assessment whether a patient is at risk for developing post-operative diplopia. A situation seen commonly in strabismus clinics in Saudi Arabia is an adult presenting with long-standing, often untreated childhood strabismus now seeking alignment surgery. The postoperative diplopia test can be helpful to predict if the patient is at risk of persisting postoperative diplopia if surgical realignment is performed (Fig. 3). Another situation involves adults with long-standing monocular visual loss. The common presentation is a patient with a history of traumatic cataract in older childhood who has had primary globe repair but left aphakic. Often there has been poor or no compliance to aphakic correction. If such a patient now seeks improvement of their visual acuity typically with secondary IOL implantation, he must be assessed preoperatively with a contact lens in place. This allows the clinician to determine if a sensory strabismus has developed and if there is evidence of fusion disruption in which a patient may achieve excellent monocular acuities, however fusion of the two images is impossible. Such patients require a full orthoptic assessment that incorporates a synoptophore evaluation and generally a trial of Fresnel prism correction of the strabismus prior to IOL implantation33 (Fig. 4).
Figure 3.
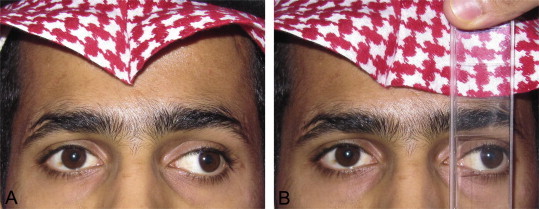
Postoperative diplopia test. Adult patient presenting with large angle exotropia and suppression of the deviating eye. (A) Left exotropia of 45 prism diopters as measured with the alternate prism cover test. (B) Postoperative diplopia test being performed preoperatively to determine the risk of potential diplopia if strabismus surgery is performed. With both eyes uncovered, a base-in prism bar is held in front of the deviating eye as increasing prism strength is introduced until either the objective angle of deviation is reached or patient begins to appreciate diplopia.
Figure 4.
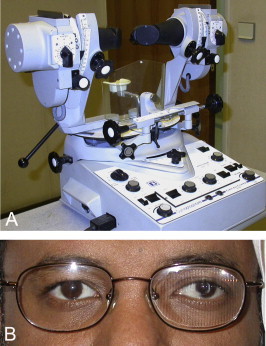
Tools for investigation of fusion potential. (A) Synoptophore: Binocular viewing device that can be used to determine fusion ability of individuals with diplopia who have had long-standing severe monocular visual loss in which the vision has been restored e.g. correction of aphakia. (B) Fresnel prism applied to left spectacle lens to permit overlapping of diplopic images to determine if fusion will occur.
More attention is now paid to the potential complications of refractive surgery in patients with a history of childhood strabismus having a well-established sensory adaptation. Such situations include creation of new monovision, even in patients with heterophorias, inadvertent switching of eye dominance, or inadvertently under correcting hyperopes with accommodative esotropia. Similar problems can occur with cataract surgery. Cataract surgeons now take appropriate precautions to minimize inducing anisometropia when performing IOL implants in patients with bilateral high refractive errors. However, disruption of an unrecognized significant phoria or sensory adaption may still occur. This was well highlighted in a series that looked at the etiology of diplopia following cataract surgery.34 This study was done at a time when cataract surgeons in this area were switching from local to topical anesthetic techniques. In the final 12 months of this 70 month study almost all procedures were done under topical anesthesia. However even after removing a potential etiological mechanism like intramuscular injection of anesthetic during the anesthetic block, diplopia still occurred in some patients. The main mechanism accounting for the diplopia now changed from primarily mechanical resulting in incomitant strabismus, to primarily sensory resulting in more comitant forms.
Future directions
We are in the midst of a genetic revolution; costs and available techniques are starting to make genetic studies aimed at unraveling at least some of the etiologies of comitant strabismus accessible for patient care.
Even since the time of Hippocrates (c. 460 BC–370 BC) there have been suspicions about heritability of strabismus.35 This is now supported by population, twin and family studies as summarized by Engle 2007.36 The results show differences in the frequency as well as the type of strabismus in different ethnic populations. However, population studies have limitations because they often include individuals with non-ocular disorders such as low birth weight or global CNS defects, and likely have incomplete identification of more subtle forms of strabismus such as phorias or microtropias. Twin studies have shown a high concordance rate in which both twins are affected. Monozygotic twins (73–82%) have a higher rate than dizygotic (35–47%) whose rate in turn is higher than siblings from different births (10–15%). Family studies have shown between 4% and 26% of strabismic patients have an affected first degree relative, the incidence depending of the type of strabismus.37 Families tend to be concordant for either esotropia or exotropia. This has been suggested to be the effect of two relatively common genes, one for eso and one for exo, however both forms have been reported within the same family, suggesting one strabismus gene but with variable expressivity.38,39 At this time, it seems family studies will yield our most useful data. This approach has already been applied to congenitally incomitant forms, now referred to as congenital cranial dysinnervation disorders (CCDDs), with great success as many of these disorders have turned out to be monogenetic with full penetrance and clear Mendelian inheritance patterns.40 Unfortunately this has not turned out to be the case for comitant strabismus forms. One study looked at the inheritance patterns in 173 pedigrees with infantile esotropia and reported this could be due to either two autosomal dominant genes with incomplete penetrance or from multifactorial inheritance.39 The first major gene mapping study came in 2003 in which the authors studied 7 families with multiple affected members using whole genome linkage analysis.41 The phenotypes were described only as nonsyndromic forms. Genetic results showed one family had linkage to chromosome 7p while this was not the case in the other 6 strabismic families. Their conclusions were that this could be a strabismus susceptibility locus and that non-linkage in the other families suggests genetic heterogeneity among families. In 2009, 12 families with nonsyndromic comitant esotropia were studied for linkage to this same locus.42 Linkage was found in one family, thus supporting 7p as an important strabismus locus but one that only accounts for a proportion of cases and that other genetic loci remain to be identified. More recently, Khan et al.43 studied a large inbred family and found linkage to chromosome 16p. Each affected family member had a different form of childhood strabismus and in some cases incomitant. Thus, current evidence indicates that the inheritance patterns of comitant strabismus are complex, arising from variation within multiple genes and their interaction with environmental factors. Part of the task in working through this complex puzzle falls to the clinician who must accurately decide the status of each family member. This is not a trivial task, as we have seen large families in whom labeling as affected or not affected has been difficult. For example, some families can include individuals with accommodative esotropia, infantile esotropia, esophoria without hyperopia and hyperopia without esotropia. It will only be through the on-going enrollment of more families, careful phenotypic descriptions of all members, not just probands, and with close communication between clinicians and geneticist that the most prominent phenotypic and genetic details will become evident. Therefore a key to the genetic elucidation of comitant strabismus will begin in the clinic. The adage of ‘good data in means good data out’ could not be truer here.
As with all medical conditions, understanding the underlying causes may help identify patients most at risk for losing fusion and developing amblyopia, or lead to new preventative or therapeutic approaches. We remain optimistic on-going and future genetic studies will provide some of these answers.
Summary
Our understanding of comitant strabismus continues to evolve. We now have more incidence and prevalence data. However the majority has been obtained from western populations and can be greatly influenced by environmental or genetic factors. More attention has been paid to identifying risk factors, which will hopefully lead to better directed management strategies. We have adopted many clinical strategies for improving management success and minimizing unexpected outcomes. Major advances are expected to come via the basic sciences and genetics, which will hopefully provide keys to better identifying those most at risk or even lead to new preventative or therapeutic approaches. The past decade has also seen advances in many other areas not addressed here such as improved technologies in automated vision screening devices, neuro-imaging in strabismus, changes in surgical techniques and improvements in anesthesia. This is an area of ophthalmology which for too long has been consigned to the backwaters of scientific discovery yet comitant strabismus is ready for a revolution in understanding which will likely come from the unraveling of its genetic causes. Answers to the important questions of causation and effective cure can only come after meticulous clinical observation and data collection on large populations of strabismic and non-strabismic patients.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Ansons A.M., Davis H. 3rd ed. Blackwell Science; Oxford: 2001. Diagnosis and management of ocular motility disorders. [Google Scholar]
- 2.Schiavi C. Comitant strabismus. Curr Opin Ophthalmol. 1997;8:17–21. doi: 10.1097/00055735-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Matsuo T., Matsuo C. The prevalence of strabismus and amblyopia in Japanese elementary school children. Ophthalmic Epidemiol. 2005;12:31–36. doi: 10.1080/09286580490907805. [DOI] [PubMed] [Google Scholar]
- 4.Chia A., Dirani M., Chan Y.H. Prevalence of amblyopia and strabismus in young Singaporean Chinese children. Invest Ophthalmol Vis Sci. 2010;51:3411–3417. doi: 10.1167/iovs.09-4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon K.C., Mun G.H., Kim S.D. Prevalence of eye diseases in South Korea: data from the Korea National Health and Nutrition Examination Survey 2008–2009. Korean J Ophthalmol. 2011;25:421–433. doi: 10.3341/kjo.2011.25.6.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chia A., Roy L., Seenyen L. Comitant horizontal strabismus: an Asian perspective. Br J Ophthalmol. 2007;91:1337–1340. doi: 10.1136/bjo.2007.116905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu C.B., Fan D.S., Wong V.W. Changing patterns of strabismus: a decade of experience in Hong Kong. Br J Ophthalmol. 2002;86:854–856. doi: 10.1136/bjo.86.8.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arora A., Williams B., Arora A.K. Decreasing strabismus surgery. Br J Ophthalmol. 2005;89:409–412. doi: 10.1136/bjo.2004.053678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacEwen C.J., Chakrabarti H.S. Why is squint surgery in children in decline? Br J Ophthalmol. 2004;88:509–511. doi: 10.1136/bjo.2002.016618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricci B., Coppola G., Ricci V., Ziccardi L. Nationwide study of hospitalization and surgical treatment for childhood strabismus in Italy between 1999 and 2004. Int Ophthalmol. 2009;29:153–156. doi: 10.1007/s10792-008-9209-3. [DOI] [PubMed] [Google Scholar]
- 11.Carney C.V., Lysons D.A., Tapley J.V. Is the incidence of constant esotropia in childhood reducing? Eye (Lond) 1995;9:40–41. [PubMed] [Google Scholar]
- 12.Weakley D.R., Dabes E.A., Birch E. Trends in surgical correction of strabismus: A 20-year experience, 1990–2009. J AAPOS. 2011;15:219–223. doi: 10.1016/j.jaapos.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Lennerstrand G., Tian S., Han Y. Effects of eye muscle proprioceptive activation on eye position in normal and exotropic subjects. Graefes Arch Clin Exp Ophthalmol. 1997;235:63–69. doi: 10.1007/BF00941731. [DOI] [PubMed] [Google Scholar]
- 14.Demer J.L., Miller J.M., Poukens V. Evidence for fibromuscular pulleys of the recti extraocular muscles. Invest Ophthalmol Vis Sci. 1995;36:1125–1136. [PubMed] [Google Scholar]
- 15.Miller J.M. Understanding and misunderstanding extraocular muscle pulleys. J Vis. 2007;7:10.1–10.5. doi: 10.1167/7.11.10. [DOI] [PubMed] [Google Scholar]
- 16.Kushner B.J. The efficacy of strabismus surgery in adults: a review for primary care physicians. Postgrad Med J. 2011;87:269–273. doi: 10.1136/pgmj.2010.108670. [DOI] [PubMed] [Google Scholar]
- 17.Olitsky S.E., Sudesh S., Graziano A. The negative psychosocial impact of strabismus in adults. J AAPOS. 1999;3:209–211. doi: 10.1016/s1091-8531(99)70004-2. [DOI] [PubMed] [Google Scholar]
- 18.Chew E., Remaley N.A., Tamboli A. Risk factors for esotropia and exotropia. Arch Ophthalmol. 1994;112:1349–1355. doi: 10.1001/archopht.1994.01090220099030. [DOI] [PubMed] [Google Scholar]
- 19.Cotter S.A., Varma R., Tarczy-Hornoch K. Risk factors associated with childhood strabismus: the multi-ethnic pediatric eye disease and Baltimore pediatric eye disease studies. Ophthalmology. 2011;118:2251–2261. doi: 10.1016/j.ophtha.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapiro M.B., France T.D. The ocular features of Down’s syndrome. Am J Ophthalmol. 1985;99:659–663. doi: 10.1016/s0002-9394(14)76031-3. [DOI] [PubMed] [Google Scholar]
- 21.Tamura E.E., Hoyt C.S. Oculomotor consequences of intraventricular hemorrhages in premature infants. Arch Ophthalmol. 1987;105:533–535. doi: 10.1001/archopht.1987.01060040103043. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor A.R., Stephenson T.J., Johnson A. Strabismus in children of birth weight less than 1701 g. Arch Ophthalmol. 2002;120:767–773. doi: 10.1001/archopht.120.6.767. [DOI] [PubMed] [Google Scholar]
- 23.Coats D.K., Paysse E.A., Towler A.J., Dipboye R.L. Impact of large angle horizontal strabismus on ability to obtain employment. Ophthalmology. 2000;107:402–405. doi: 10.1016/s0161-6420(99)00035-4. [DOI] [PubMed] [Google Scholar]
- 24.Nelson B.A., Gunton K.B., Lasker J.N. The psychosocial aspects of strabismus in teenagers and adults and the impact of surgical correction. J AAPOS. 2008;12(72–6):e1. doi: 10.1016/j.jaapos.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Schor C.M., Fusaro R.E., Wilson N., McKee S.P. Prediction of early-onset esotropia from components of the infantile squint syndrome. Invest Ophthalmol Vis Sci. 1997;38:719–740. [PubMed] [Google Scholar]
- 26.Lyons C.J., Tiffin P.A., Oystreck D. Acute acquired comitant esotropia: a prospective study. Eye (Lond) 1999;13(Pt 5):617–620. doi: 10.1038/eye.1999.169. [DOI] [PubMed] [Google Scholar]
- 27.Wong A.M. Timing of surgery for infantile esotropia: sensory and motor outcomes. Can J Ophthalmol. 2008;43:643–651. doi: 10.3129/i08-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elliott S., Shafiq A. Interventions for infantile esotropia. Cochrane Database Syst Rev. 2005:CD004917. doi: 10.1002/14651858.CD004917.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Shauly Y., Miller B., Meyer E. Clinical characteristics and long-term postoperative results of infantile esotropia and myopia. J Pediatr Ophthalmol Strabismus. 1997;34:357–364. doi: 10.3928/0191-3913-19971101-07. [DOI] [PubMed] [Google Scholar]
- 30.Thompson J.T., Guyton D.L. Ophthalmic prisms. Measurement errors and how to minimize them. Ophthalmology. 1983;90:204–210. doi: 10.1016/s0161-6420(83)34572-3. [DOI] [PubMed] [Google Scholar]
- 31.Scattergood K.D., Brown M.H., Guyton D.L. Artifacts introduced by spectacle lenses in the measurement of strabismic deviations. Am J Ophthalmol. 1983;96:439–448. doi: 10.1016/s0002-9394(14)77906-1. [DOI] [PubMed] [Google Scholar]
- 32.Prism Adaptation Study Research Group Efficacy of prism adaptation in the surgical management of acquired esotropia. Arch Ophthalmol. 1990;108:1248–1256. doi: 10.1001/archopht.1990.01070110064026. [DOI] [PubMed] [Google Scholar]
- 33.Digout L.G., Awad A.H. Restoration of binocular single vision after long-term fusion disruption. J AAPOS. 2003;7:185–189. doi: 10.1016/s1091-8531(03)00006-5. [DOI] [PubMed] [Google Scholar]
- 34.Nayak H., Kersey J.P., Oystreck D.T. Diplopia following cataract surgery: a review of 150 patients. Eye (Lond) 2008;22:1057–1064. doi: 10.1038/sj.eye.6702847. [DOI] [PubMed] [Google Scholar]
- 35.von Noorden G.K. Etiology of heterophoria and heterotropia. In: Campos E.C., editor. Binocular vision and ocular motility – theory and management of strabismus. 6th ed. Mosby; St. Louis: 2002. [Google Scholar]
- 36.Engle E.C. Genetic basis of congenital strabismus. Arch Ophthalmol. 2007;125:189–195. doi: 10.1001/archopht.125.2.189. [DOI] [PubMed] [Google Scholar]
- 37.Ziakas N.G., Woodruff G., Smith L.K., Thompson J.R. A study of heredity as a risk factor in strabismus. Eye (Lond) 2002;16:519–521. doi: 10.1038/sj.eye.6700138. [DOI] [PubMed] [Google Scholar]
- 38.Schlossman A., Priestley B.S. Role of heredity in etiology and treatment of strabismus. AMA Arch Ophthalmol. 1952;47:1–20. doi: 10.1001/archopht.1952.01700030004001. [DOI] [PubMed] [Google Scholar]
- 39.Maumenee I.H., Alston A., Mets M.B. Inheritance of congenital esotropia. Trans Am Ophthalmol Soc. 1986;84:85–93. [PMC free article] [PubMed] [Google Scholar]
- 40.Oystreck D.T., Engle E.C., Bosley T.M. Recent progress in understanding congenital cranial dysinnervation disorders. J Neuroophthalmol. 2011;31:69–77. doi: 10.1097/WNO.0b013e31820d0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parikh V., Shugart Y.Y., Doheny K.F. A strabismus susceptibility locus on chromosome 7p. Proc Natl Acad Sci USA. 2003;100:12283–12288. doi: 10.1073/pnas.2035118100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rice A., Nsengimana J., Simmons I.G. Replication of the recessive STBMS1 locus but with dominant inheritance. Invest Ophthalmol Vis Sci. 2009;50:3210–3217. doi: 10.1167/iovs.07-1631. [DOI] [PubMed] [Google Scholar]
- 43.Khan A.O., Shinwari J., Abu Dhaim N. Potential linkage of different phenotypic forms of childhood strabismus to a recessive susceptibility locus (16p13.12–p12.3) Mol Vis. 2011;17:971–976. [PMC free article] [PubMed] [Google Scholar]



