Abstract
A wide variety of space occupying lesions may be encountered in the orbit. CT and MR imaging frequently help confirm the presence of a mass and define its extent. Characteristic imaging features may help distinguish among lesions that have overlapping clinical presentations. This review focuses on some of the common orbital masses. Common vascular lesions that are reviewed include: capillary (infantile) hemangioma, cavernous hemangioma (solitary encapsulated venous-lymphatic malformation), and lymphangioma (venous-lymphatic malformation). Benign tumors that are reviewed include: optic nerve sheath meningioma, schwannoma, and neurofibroma. Malignancies that are reviewed include: lymphoma, metastasis, rhabdomyosarcoma, and optic glioma. Key imaging features that guide radiological diagnosis are discussed and illustrated.
Keywords: Benign orbital masses, Orbital vascular lesions, Malignant orbital masses, Pediatric orbital masses
Introduction
A wide variety of processes can produce space-occupying lesions in and around the orbit. These include benign neoplasms, malignant neoplasms, vascular lesions, inflammatory disease, congenital lesions, and infection, among other causes. Imaging can be used to precisely localize a lesion, to help establish a diagnosis or generate a differential diagnosis that guides management, to follow a known lesion for progression, or some combination of these.
The first step in establishing a differential diagnosis is obtaining an accurate clinical history. CT is excellent for confirming the presence of a mass, is well tolerated, can be rapidly obtained at most medical centers, and gives clues to the specific diagnosis. Although MRI can be more difficult to perform, it can reveal additional key imaging features that allow one to more confidently distinguish between similar appearing lesions, and often arrive at a single most likely diagnosis.
Our goal is to review the most commonly encountered lesions to make the reader aware of their typical appearances and presentations, with emphasis on key imaging features that may distinguish among similar lesions.
This review focuses on the most common vascular masses, benign neoplasms, and malignant neoplasms. Due to space constraints, infection1–3 and inflammatory disease3–6 are not discussed here. In addition, a number of other vascular lesions and neoplasms are not discussed, including orbital venous varix,3,7,8 arteriovenous malformation,3,9 cavernous sinus thrombosis,3,10 carotid cavernous fistula,3,7,8 a variety of benign and malignant mesenchymal tumors,3,11,12 epithelial neoplasms of the lacrimal apparatus,3,7,13–15 and ocular tumors that secondarily involve the orbit.3,7 One may also encounter other masses that are not true neoplasms, such as dermolipomas,3,16,17 dermoid cysts,3,18–20 and epidermoid cysts.3,20 For discussion of these lesions, and others, we direct the reader to the referenced sources.
Vascular lesions
Capillary hemangioma (infantile hemangioma)
Clinicopathologic features
Although the term “hemangioma” is sometimes used to describe certain vascular malformations, it more appropriately describes a true neoplasm with vascular channels lined by proliferating endothelial cells.21 Capillary hemangiomas are the commonest tumors of infancy,7,22–24 typically appearing as a reddish macule in the first 6 months of life. A proliferative phase occurs up to 10 months, followed by a slow involution phase for up to 10 years.21,25,26 Though typically sporadic, they may occur as part of a genetic syndrome such as PHACES syndrome.25
Imaging findings
Contrast enhanced MRI is the preferred modality for imaging hemangioma, although CT can also be considered if it is not possible to sedate the patient for MRI. The diagnosis is typically well-established clinically, but imaging is indicated to assess the extent of the lesion and mass effect on adjacent structures. With deep lesions, the clinical diagnosis may be challenging. Capillary hemangioma typically shows: (1) lobular contour borders, (2) bright T2 signal with T2 dark septa between lobules, (3) fine internal flow voids, (4) intense, homogeneous enhancement, and (5) preservation of adjacent bone (Fig. 1). Capillary hemangioma can have an atypical appearance without all of these features. Nevertheless, if these features are not all present, then alternative diagnoses should be considered, including rhabdomyosarcoma. DWI may help distinguish these lesions in challenging cases, as rhabdomyosarcoma typically shows lower ADC and brighter DWI signal than hemangioma.27–29 CT may demonstrate bony orbital expansion or scalloping with rapidly growing lesions. True bone invasion and calcifications are rarely seen.
Figure 1.
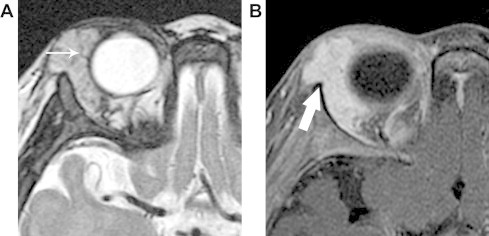
A 7 month-old girl with capillary (infantile) hemangioma. A. Axial T2 shows a well-defined, lobular, hyperintense mass with fine internal signal void (arrow) that likely represents an internal high-flow vessel. Septa in between lobules are slightly more linear. B. Axial fat-suppressed post contrast T1 shows intense, homogeneous enhancement typical of a proliferative phase lesion. The intact bony cortex at the zygoma is well demonstrated as a hypointense line (arrow).
CT and MR may both demonstrate fat deposition and heterogeneous appearing enhancement, if imaging is performed during the involution phase.9,23–25
Differential diagnosis
Rhabdomyosarcoma, vascular malformation, infantile fibromatosis, and infantile fibrosarcoma.
Key features
Bright T2 signal, lobular borders, fine internal flow voids, extraconal location, and intense homogeneous enhancement.
‘Cavernous hemangioma’ (solitary, encapsulated venous-lymphatic malformation)
Clinicopathologic features
‘Cavernous hemangioma’ is a term that is widely used to describe a solitary, encapsulated venous-lymphatic malformation, the most common vascular lesion of the orbit.30 The typical clinical presentation is of mostly painless proptosis (mean 5–6 mm), pain, lid swelling, diplopia, lump, and recurrent obstructed vision. Middle-aged women are the most commonly affected group, and the average duration from symptom onset to presentation is 4 years.22 The most frequent locations are the retrobulbar muscle cone, especially the lateral aspect of the intraconal space.31 However, a small minority (less than 10%) of these lesions are extraconal.32 Cavernous hemangiomas rarely bleed33 due to firmer texture from the surrounding support of rich fibrous tissue.34 Orbital cavernous hemangiomas are typically solitary and unilateral, though multifocal and bilateral lesions have been reported.35–38
Imaging appearance
Cavernous hemangioma typically appears as a well-circumscribed intraconal mass.39–42 Although most lesions are ovoid or round, larger lesions have lobulated margins. Larger lesions will distort surrounding structures, as opposed to lymphoma which molds around structures. CT shows homogeneous soft tissue density, and may show small calcifications or phleboliths.39 MR shows isointense T1 signal, bright T2 signal, dark internal septations, and a dark circumferential rim that represents a fibrous pseudocapsule (Fig. 2).22,34,43,44
Figure 2.
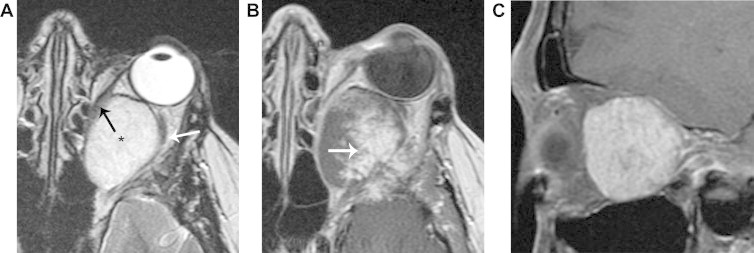
Cavernous hemangioma (solitary encapsulated venous-lymphatic malformation). A. Axial T2 shows an ovoid, homogeneous hyperintense mass (*) that distorts the medial rectus muscle (black arrow) and the optic nerve (white arrow). B. Axial T1 with contrast, obtained immediately after contrast injection, shows nodular enhancement. C. Para-sagittal oblique fat suppressed T1 with contrast, obtained 12 min after contrast injection, shows more uniform contrast enhancement. This slowly progressive nodular enhancement is typical of cavernous hemangioma, though also occasionally seen in schwannoma.
MR classically demonstrates nodular enhancement on early post-contrast images, and progressive accumulation of contrast on delayed post-contrast images.31 The same findings are seen with multiphase CT, though concerns related to the increased radiation exposure of multiphase CT make this technique relatively less desirable. Small lesions often show early, uniform enhancement. MR angiography and CT angiography are generally not able to identify the feeding vessels of a cavernous hemangioma, likely due to their small caliber.
Differential diagnosis
Venous varix, schwannoma, optic nerve sheath meningioma, and lymphoma.
Key imaging features
Intraconal location; very bright T2 signal with hypointense pseudocapsule; and an early nodular enhancement with progressive accumulation of contrast on later phase images.
Lymphangioma (venous lymphatic malformation)
Clinicopathologic features
‘Lymphangiomas’ are benign vascular malformations (venous lymphatic malformations), commonly affecting children and rarely involving the orbit. They are unencapsulated and composed of fibrous material including endothelial lacunae filled with blood or serious fluid.45 Lymphangiomas tend to enlarge suddenly due to intralesional bleeding, but may also enlarge with upper respiratory infection.46 Symptoms include: swelling, intraorbital hemorrhage, ocular proptosis, blepharoptosis and cellulitis. Ocular complications include: astigmatism, corneal exposure, hyperopia secondary to pressure on posterior globe, strabismus, glaucoma and compressive optic neuropathy.47–49
Imaging findings
Despite belonging to the same pathological category as ‘cavernous hemangioma’ (i.e. low flow vascular malformation without endothelial proliferation), lymphangioma has a different appearance and growth pattern. In keeping with their unencapsulated nature, lymphangiomas exhibit an infiltrative, trans-spatial growth pattern, often involving both the intraconal and extraconal compartments and pre- and post-septal compartments, violating fascial planes. Both microcysts and macrocysts may be identified.50 CT may demonstrate phleboliths in the venous component of a lesion, and may demonstrate bony abnormalities.46 Venous or solid components of the tumor are hyperdense compared to brain tissue on unenhanced CT scans.50–52
MR is accurate for delineating the anatomic location and vascular components, and fluid filled levels or menisci can be seen.46 The mass is usually isointense to slightly hyperintense relative to normal brain tissue on T1 weighted imaging and very hyperintense relative to the brain on T2 weighted imaging46, with internal septations (Fig. 3). T1 and T2 signal intensity vary depending on the presence and age of internal blood products. The venous component of a lesion will typically enhance, whereas the lymphatic component will only show fine enhancement of septations. No flow voids or enlarged feeder vessels are usually found, in keeping with the low flow nature of lymphangioma and differentiating it from high flow lesions, including high-flow vascular malformations and true neoplasms such as capillary hemangioma.50,53,54
Figure 3.
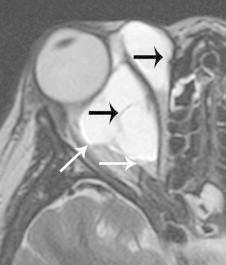
Lymphatic malformation (lymphangioma). Axial T2 shows a hyperintense, trans-spatial mass involving the intra- and extra-conal compartments. Hypointense septations (black arrows) are typical of this lesion. Layering blood products (white arrows) are variably present, and indicate recent intralesional bleeding.
Differential diagnosis
Cavernous hemangioma, capillary hemangioma, dermoid, and rhabdomyosarcoma.
Key imaging features
Trans-spatial, markedly T2 bright non-enhancing mass with internal septations, with or without layering blood products and solidly enhancing components.
Benign neoplasms
Optic nerve sheath meningioma
Clinicopathologic features
The typical clinical presentation of optic nerve sheath meningioma is painless, gradual vision loss and proptosis in a woman between ages 30 and 50. Optic nerve sheath meningioma is typically unilateral but can be bilateral, particularly in the setting of type II neurofibromatosis. Primary meningioma arises from capillary cells of the arachnoid around the intraorbital or intracanalicular portions of the optic nerve. Secondary meningioma arises intracranially from the sphenoid ridge, tuberculum sellae, or olfactory groove and invades the optic canal and orbit by extension between the dura and arachnoid of the optic nerve.55,56 Visual disturbance in the affected eye is common. While some patients only suffer transient visual loss lasting a few seconds, others experience visual loss in a particular field of gaze.57,58 Optic neuropathy, proptosis and strabismus occur later.
Imaging findings
The key imaging finding of optic nerve sheath meningioma is a homogeneously enhancing mass that surrounds the optic nerve. CT may show calcification. MRI typically shows homogeneous, intermediate T1 and T2 signals. The optic nerve may be in the center of the lesion, or may be eccentrically positioned. In some cases, the optic nerve will show abnormal T2 hyperintense signal, which is presumably related to chronic venous insufficiency.
Optic nerve sheath meningioma may produce an expansile mass (Fig. 4). Alternatively, it may only form a thin sheet around the optic nerve, termed “en plaque meningioma”, producing the classically described ‘tram track’ sign.59 In these cases, fat-suppressed post-contrast T1-weighted images in the coronal plane are essential in making the diagnosis (Fig. 5). Any part of the nerve may be involved, and radiologic diagnosis can be challenging when only the intracanalicular portion of the nerve is affected. Fat suppression is helpful in these cases, but can also be degraded by blooming artifact around a well-pneumatized sphenoid sinus. For this reason, coronal post-contrast images without fat suppression should also be considered.
Figure 4.
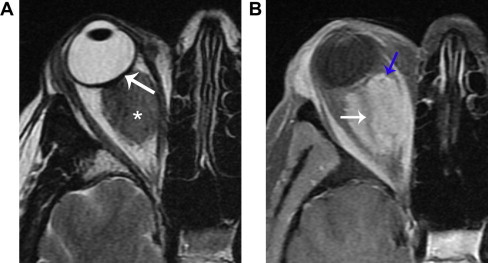
Optic nerve sheath meningioma. A. Axial T2 shows a homogeneous, hypointense mass (*) that mildly distorts the globe (arrow). B. Axial fat suppressed T1 with contrast shows homogeneous enhancement with a well-defined margin (blue arrow), and encasement of the optic nerve (white arrow). Globe distortion helps distinguish this lesion from lymphoma, as does the clinical history of slowly progressive, painless vision loss.
Figure 5.
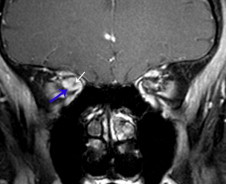
En plaque optic nerve sheath meningioma. Coronal fat suppressed T1 with contrast shows an enhancing mass (blue arrow) surrounding and constricting the optic nerve (white arrow). Fat suppressed post contrast coronal images are essential in identifying meningiomas with this growth pattern.
Optic nerve sheath meningioma is distinguished from optic glioma by the fact that the optic glioma expands the optic nerve. Orbital lymphoma can sometimes surround the optic nerve and simulate optic nerve sheath meningioma by imaging (Fig. 6). In such cases, lymphoma’s tendency to mold around structures helps differentiate it from meningioma, which tends to distort structures. Lymphoma also typically shows less intense enhancement than meningioma. Meningioma frequently shows calcification on CT, whereas untreated lymphoma almost never calcifies. Perineuritic inflammatory disease can produce a similar appearance to en plaque meningioma, but is clinically distinguished by the presence of pain. Demyelinating optic neuritis is distinguished from en plaque meningioma by its typical homogeneous enhancement of the substance of the nerve, as opposed to the target-like enhancement of meningioma.
Figure 6.
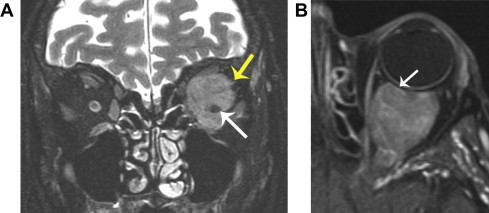
Lymphoma. A. Coronal fat suppressed T2 shows a homogeneous, somewhat hypointense mass that surrounds the optic nerve (white arrow). Although the mass is mostly well-defined, there is a slightly ill-defined lateral margin (yellow arrow). B. Axial fat suppressed T1 shows molding around the globe without globe distortion (arrow). Although optic nerve encasement is typical of meningioma, molding around the globe and poor margin definition are features that suggest lymphoma. Lymphoma also commonly shows slightly less intense enhancement than meningioma, as demonstrated in this case compared to Fig. 4.
Differential diagnosis
Sarcoidosis, pseudotumors, lymphoma, and optic neuritis.
Key imaging features
Mass surrounding and constricting the optic nerve; calcification on CT; extension of tumor into the optic canal; “tram track” or “target” like enhancement.
Nerve sheath tumors
Clinicopathologic features
Nerve sheath tumors can be divided into schwannomas and neurofibromas, which are less common and typically associated with NF-1. Schwannomas constitute 1% of orbital tumors, typically occurring in young and middle-aged adults.30,60 They typically arise from intraorbital branches of V1,60 and produce symptoms related to local mass effect. Schwannomas are associated with NF-1 in 2–18% of patients.61 Neurofibromas are more strongly associated with NF-1, and also produce symptoms related to mass effect.
Imaging findings
Schwannoma appears as a smooth, ovoid, orbital retrobulbar mass, found in the intra- or extra-conal space with the long axis in the anterior–posterior direction (Fig. 7).39,62 CT shows isodensity or hypodensity compared to gray matter. MR shows isointense T1 signal, and T2 hyperintensity. Although some schwannomas may be cystic and non-enhancing,63 most schwannomas enhance with contrast. Although schwannoma enhancement is typically more homogeneous than cavernous hemangioma, in some cases schwannoma can show the same type of early nodular enhancement with progressive fill-in that is commonly seen with cavernous hemangioma. The MR and CT appearance of schwannoma can overlap with optic nerve sheath meningioma. In challenging cases, one should look carefully for extension into the superior orbital fissure, which would favor schwannoma, or for extension into the optic canal, which would favor meningioma. Neurofibromas often have a similar appearance to schwannomas, though plexiform neurofibromas typically show a more infiltrative growth pattern (Fig. 8), and are frequently associated with other stigmata of NF-1.
Figure 7.
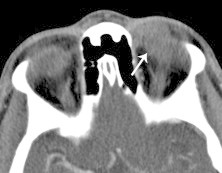
Schwannoma. Axial CT with contrast shows a hypodense, well-defined, extraconal mass with only weak enhancement. This appearance is nonspecific, and is often seen with schwannoma in addition to other benign and malignant masses.
Figure 8.
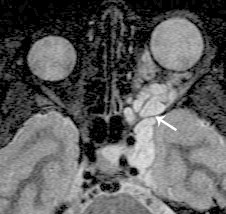
Plexiform neurofibroma. Axial fat suppressed T2 shows a hyperintense mass in the left orbit, with extension through the superior orbital fissure (arrow) into the parasellar region.
Differential diagnosis
Cavernous hemangioma, meningioma, and lymphoma.
Key imaging features
Smooth, homogeneous spherical mass. Extension into superior orbital fissure. Enhancement is typically more heterogeneous than lymphoma or meningioma, but less nodular than cavernous hemangioma.
Malignant neoplasms
Orbital lymphoid tumors (including malignant lymphoma)
Clinicopathologic features
Reactive lymphoid lesions overlap histologically with malignant lymphoma, and are sometimes grouped as ‘orbital lymphoid lesions’.64 The typical presentation is low-grade proptosis with minimal pain.1 Lymphoid masses comprise 10–15% of all orbital tumors65 and up to 55% of malignant orbital masses.66 Malignant lymphoma of the orbit is typically B cell lymphoma of a non-Hodgkin type, arising from mucosa-associated lymphoid tissue of ocular adnexa (MALTOMA).67 Systemic lymphoma may involve the orbit in 1.5–5% of patients.68,69 Up to 75% of patients with orbital lymphoma may later develop systemic disease.70 Suspicion of malignant lymphoma is increased in the presence of systemic disease, lacrimal duct or gland disease, and bilateral disease.71
Image appearance
Lymphoid tumors typically appear as homogeneous, lobulated masses on CT and MRI. They mold around normal structures without deforming them, and typically do not erode adjacent bone. They may be well defined, or infiltrative in appearance. CT shows density similar to skeletal muscle. MRI demonstrates homogenous, intermediate T1 and T2 signals, and homogeneous contrast enhancement.64,72,73
Lymphomas most commonly involve the superolateral aspect of the orbit,72 and bilateral disease is common.1 When multiple sites of disease are present, the intraconal space is most affected (85%). Lymphoma can involve virtually any part of the orbit, including the lacrimal gland, extraocular muscles, lacrimal sac, periorbital fat, and retrobulbar fat (Fig. 9).
Figure 9.
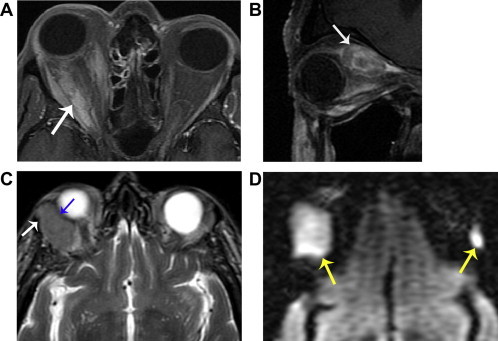
Lymphoma (multiple cases). A. Axial fat suppressed T1 with contrast shows ill-defined intraconal enhancement (arrow), representing infiltrative tumor. B. Parasagittal oblique T1 with contrast in a different patient shows an enhancing mass expanding the superior rectus muscle (arrow). C. Axial T2 in a third patient shows diffuse expansion of the right lacrimal gland, with slightly lobular contour. The mass respects the cortex of the zygoma (white arrow) and molds around the globe without distorting it (blue arrow). D. Axial DWI (same patient as ‘C’) shows very bright signal in both lacrimal glands (yellow arrows), reflecting involvement by hypercellular tumor. Left lacrimal gland disease is better detected with DWI than with conventional images, due to the relatively normal size of the gland and homogeneous appearance.
Lymphoma can be very difficult to distinguish from orbital inflammatory disease (“pseudotumor”). However, diffusion-weighted imaging (DWI) is an MRI technique that has recently been proven effective in distinguishing these entities. Lymphoma typically shows brighter DWI signal and lower apparent diffusion coefficient (ADC) than normal orbital structures, due to its hypercellular nature. Inflammatory lesions, on the other hand, tend to show intermediate DWI and ADC signals, similar to normal lacrimal gland.27 Very low T2 signal (darker than normal white matter) should also suggest an inflammatory lesion.5 DWI is also helpful for identifying lacrimal gland lymphoma.
Differential diagnosis
Orbital inflammatory syndrome, optic nerve sheath meningioma, and orbital metastasis.
Key imaging features (lymphoma)
Homogeneous intermediate T2 signal, lobulated margins with molding around normal structures, homogeneous enhancement, brighter DWI signal and lower ADC than surrounding normal orbital tissues.
Metastatic tumors of the orbit
Clinicopathologic features
In adults orbital metastases usually arise from carcinomatous origin, in children sarcomatous or neural embryonal tumors are more common.74 The spread of tumor to the orbit and ocular adnexa is usually due to hematogenous spread of primary cancer, and is associated with a poor prognosis. Primary metastatic orbital tumors are estimated to account for 1–13% of all orbital tumors, and in cancer patients are prevalent from 2% to 4.7%.75 Improved cancer therapy, improved diagnostic imaging and increased awareness in medical literature have allowed for better diagnosis of these tumors.
Metastatic lesions are found more in the anterior orbit than the posterior orbit.76 The most frequent metastases to the orbit are from: breast, lung, prostate, melanoma, carcinoid, GI, renal cell, neuroblastomas and rhabdomyosarcomas. Rapidly progressive symptoms occur including: diplopia, proptosis, decreased vision, pain, ptosis, and palpable mass.75
Imaging findings
CT is usually performed for orbital imaging, although MR has superior soft tissue contrast. Tumor pattern can vary from diffusely infiltrative with obscured anatomical landmarks to a focal well-defined mass. Orbital metastases from breast cancer are often diffuse and irregular growing along the rectus muscles and fascial planes.76 Scirrhous (fibrotic) breast cancers are unique in their ability to produce enophthalmos and ophthalmoplegia. In these cases, the metastatic lesion is typically very T2 dark, reflecting its fibrotic nature (Fig. 10). Metastases from carcinoid (Fig. 11), renal cell carcinoma and melanoma tend to be circumscribed. All orbital metastases show some degree of MR enhancement.77 Metastases may involve any structure in the orbit, including the intraconal or extraconal space, globe, extraocular muscles and bone (Fig. 12).
Figure 10.
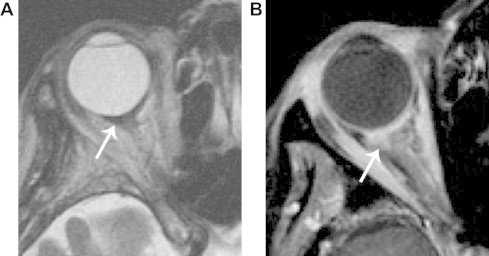
Breast cancer metastasis. A. Axial T2 shows a hypointense retrobulbar mass (arrow) that produces slight posterior tenting of the globe. B. Axial fat suppressed T1 with contrast shows ill-defined enhancement. Enophthalmos and a T2 dark mass are highly suggestive of breast cancer metastasis.
Figure 11.
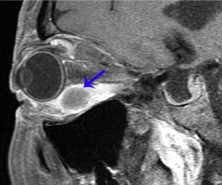
Carcinoid tumor metastasis. Parasagittal oblique fat suppressed T1 with contrast shows a peripherally enhancing mass centered in the distal portion of the inferior rectus muscle (arrow).
Figure 12.
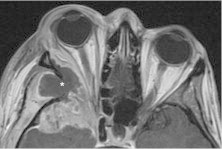
Colonic adenocarcinoma metastasis. Axial T1 with contrast shows a necrotic mass centered in the right sphenoid triangle (*), with enhancing tumor and surrounding inflammation along the margins extending into the middle cranial fossa and extraconal orbit.
Differential diagnosis
Lymphoma, infection, dysthyroid orbitopathy, orbital inflammatory disease, carotid cavernous fistula, and cavernous sinus thrombosis.
Key imaging findings
Imaging findings vary widely. Globe retraction is highly suspicious for scirrhous breast cancer metastasis.
Rhabdomyosarcoma
Clinicopathologic features
This is the most common soft tissue malignancy of childhood and most common primary orbital malignancy.78,79 There is a bimodal distribution of affected ages, commonly occurring between 2 and 5 years80 but is reported in all age groups.81 The embryonal subtype occurs in childhood (mean age 8 years; male to female ratio 5:3); the pleomorphic subtype occurs in adults; the alveolar subtype occurs in young children and adults and is associated with a poor prognosis.79
Primary rhabdomyosarcoma can occur from the conjunctiva, iris, ciliary body, or extension of primary orbital rhabdomyosarcoma.82 Secondary orbital rhabdomyosarcoma occurs from direct extension to orbits from the paranasal sinus, pterygopalatine fossa, infratemporal fossa or nasopharynx. Orbital metastases can also occur from other head and neck rhabdomyosarcomas and carries a poor prognosis. The orbital location varies: extraconal (37%), intraconal (17%), both (47%). The upper outer quadrant is involved in 67%. Presenting complaints include: unilateral proptosis (80–100%), globe displacement (80%), ptosis (30–50%), conjunctival and eyelid swelling (60%), palpable mass (25%), and pain (10%).83,84
Imaging findings
CT shows moderately well defined to ill-defined margins, irregular shape, and mild-moderate contrast enhancement. Adjacent bony destruction occurs in 40% (Fig. 13). Globe distortion85 and extension to the paranasal sinuses82,86 may also be seen. Regional lymph nodes are involved later in the disease course. Calcification is rare in untreated tumors.
Figure 13.
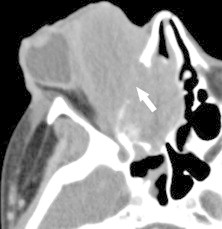
A 26 year-old woman with rhabdomyosarcoma. CT shows an aggressive mass that deforms the globe and grossly destroys bone (arrow), invading the ethmoid sinus through the lamina papyracea.
MR typically shows bright T2 signal, distinguishing rhabdomyosarcoma from other tumors such as chloroma (granulocytic sarcoma), lymphoma and metastatic neuroblastoma (Fig. 14). MR may also better delineate the true extent of disease if surgical resection is planned.87 On occasion, a pyogenic abscess may have a subacute presentation that mimics a necrotic rhabdomyosarcoma clinically and by imaging.88 In such cases, MRI with DWI is critical in distinguishing these entities, through demonstration of restricted diffusion of pus in an abscess cavity,89 as opposed to elevated diffusion in the necrotic portion of a tumor.90
Figure 14.
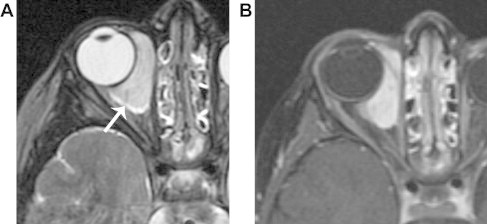
A 3 year-old boy with embryonal rhabdomyosarcoma. A. Axial T2 shows a well-defined, hyperintense mass involving the retrobulbar and extraconal soft tissues, without destruction of the bone or distortion of adjacent structures. B. Axial fat-suppressed T1 with contrast shows intense, homogeneous enhancement. This mass has certain features that are common to capillary hemangioma. However, continued growth of the mass after the first year of life, absence of flow voids, and absence of discrete lobules with T2 hypointense septations, are features that suggest an alternative diagnosis.
Differential diagnosis
Leukemic metastatic deposits; Langerhans cell histiocytosis; aggressive fibromatosis; ruptured dermoid cyst; subperiosteal hematoma; plexiform neurofibromatosis, capillary hemangioma.
Key imaging features
CT: variable definition, bony destruction; MR: bright T2 signal.
Optic nerve glioma
Clinicopathologic features
Optic nerve glioma comprises 1.5–3.0% of orbital tumors, 0.6–7.0% of all intracranial tumors, 1.7–7.0% of gliomas, and 2–5% of gliomas in the pediatric age group.91,92 The peak incidence is in 2–8 year old but can occur at any age and has been reported at up to 79 years of age.93,94 Females are more affected than males. There is a high association (12–37%) with type I neurofibromatosis type 195, which frequently shows bilateral lesions. Notably, optic gliomas associated with NF-1 typically have more indolent behavior than isolated optic nerve gliomas. Symptoms include: proptosis, decreased visual acuity,96 nystagmus, strabismus, central/paracentral field defects, dyschromatopsia, and afferent pupillary defect.92 Disk edema and atrophy may be evident by 8 weeks. The tumors are slow growing with periods of growth and dormancy,97,98 some may regress completely.99,100 In an analysis of affected orbital locations, 48% involved the orbital optic nerve, 24% the orbital and intracranial optic nerve, 10% intracranial optic nerve and chiasm, and just chiasm in up to 5%.101 Surgery is typically reserved for cases where there is significant mass effect.
Imaging findings
MR is optimal for imaging optic nerve glioma.102 The classic finding of optic nerve glioma is sharply circumscribed fusiform thickening and tortuosity of the optic nerve. Optic gliomas are typically T2 hyperintense, and usually show some enhancement, though a wide range of signal intensities and enhancement patterns may be encountered.103 Diffuse involvement of the substance of the nerve differentiates optic nerve glioma from optic nerve sheath meningioma, which surrounds the optic nerve. Any part of the optic nerve may be involved, from the globe to the optic chiasm. Peripheral enhancement of chiasmatic gliomas represents extraneural growth of tumor into the subarachnoid space, mixed with gliomatous tissue and non enhancing central tumor. Optic nerve gliomas that extend to the chiasm through the optic canal form a dumbbell shape (Fig. 15).
Figure 15.
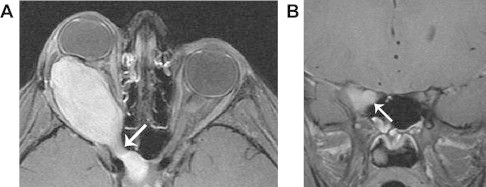
Optic glioma. A. Axial fat-suppressed T1 with contrast shows a solidly enhancing dumbbell-shaped mass extending through the right optic canal (arrow). B. Coronal fat-suppressed T1 with contrast shows diffuse enhancement of the substance of the right optic nerve (arrow), a finding that confirms optic glioma rather than optic nerve sheath meningioma. Meningioma forms an enhancing mass that surrounds and constricts the optic nerve.
Differential diagnosis
Optic nerve sheath meningioma and medulloepithelioma.
Key imaging features
Diffuse enlargement of the substance of the optic nerve, with thickening and tortuosity.
Conclusion
A wide variety of lesions may be encountered in the orbit. Knowledge of the most common lesions, their typical clinical presentation, typical imaging appearance, and key features that distinguish from similar lesions should allow the reader to make the best use of imaging.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Kapur R., Sepahdari A.R., Mafee M.F. MR imaging of orbital inflammatory syndrome, orbital cellulitis, and orbital lymphoid lesions: the role of diffusion-weighted imaging. AJNR Am J Neuroradiol. 2009;30(1):64–70. doi: 10.3174/ajnr.A1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uehara F., Ohba N. Diagnostic imaging in patients with orbital cellulitis and inflammatory pseudotumor. Int Ophthalmol Clin. 2002;42(1):133–142. doi: 10.1097/00004397-200201000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Som P, Curtin H. Head and neck imaging; 2011.
- 4.Yuen S.J., Rubin P.A. Idiopathic orbital inflammation: distribution, clinical features, and treatment outcome. Archiv Ophthalmol. 2003;121(4):491–499. doi: 10.1001/archopht.121.4.491. [DOI] [PubMed] [Google Scholar]
- 5.Cytryn A.S., Putterman A.M., Schneck G.L., Beckman E., Valvassori G.E. Predictability of magnetic resonance imaging in differentiation of orbital lymphoma from orbital inflammatory syndrome. Ophthalmic Plast Reconstr Surg. 1997;13(2):129–134. doi: 10.1097/00002341-199706000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Yan J., Wu Z., Li Y. The differentiation of idiopathic inflammatory pseudotumor from lymphoid tumors of orbit: analysis of 319 cases. Orbit. 2004;23(4):245–254. doi: 10.1080/01676830490487980. [DOI] [PubMed] [Google Scholar]
- 7.Shields J.A., Shields C.L., Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions: The 2002 Montgomery Lecture. Part 1. Ophthalmology. 2004;111(5):997–1008. doi: 10.1016/j.ophtha.2003.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Smoker W.R., Gentry L.R., Yee N.K., Reede D.L., Nerad J.A. Vascular lesions of the orbit: more than meets the eye. Radiograph: A Rev Publ Radiol Soc North America, Inc. 2008;28(1):185–204. doi: 10.1148/rg.281075040. [quiz 325] [DOI] [PubMed] [Google Scholar]
- 9.Bilaniuk L.T. Vascular lesions of the orbit in children. Neuroimag Clin North America. 2005;15(1):107–120. doi: 10.1016/j.nic.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Goawalla A., Mansell N., Pearson A. Septic cavernous sinus thrombosis with bilateral secondary orbital infection. Orbit. 2007;26(2):113–116. doi: 10.1080/01676830600675418. [DOI] [PubMed] [Google Scholar]
- 11.Patel R., Mukherjee B. Mesenchymal chondrosarcoma of the orbit. Orbit. 2012;31(2):126–128. doi: 10.3109/01676830.2011.638097. [DOI] [PubMed] [Google Scholar]
- 12.Yang B.T., Wang Y.Z., Wang X.Y., Wang Z.C. Mesenchymal chondrosarcoma of the orbit: CT and MRI findings. Clin Radiol. 2012;67(4):346–351. doi: 10.1016/j.crad.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Kousoubris P.D., Rosman D.A. Radiologic evaluation of lacrimal and orbital disease. Otolaryng Clin North America. 2006;39(5):865–893. doi: 10.1016/j.otc.2006.07.005. [vi] [DOI] [PubMed] [Google Scholar]
- 14.Mafee M.F., Edward D.P., Koeller K.K., Dorodi S. Lacrimal gland tumors and simulating lesions. Clinicopathologic and MR imaging features. Radiol Clin North America. 1999;37(1):219–239. doi: 10.1016/s0033-8389(05)70089-0. [xii] [DOI] [PubMed] [Google Scholar]
- 15.Vajaranant T.S., Mafee M.F., Kapur R., Rapoport M., Edward D.P. Medulloepithelioma of the ciliary body and optic nerve: clinicopathologic, CT, and MR imaging features. Neuroimag Clin North America. 2005;15(1):69–83. doi: 10.1016/j.nic.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Eijpe A.A., Koornneef L., Bras J., Verbeeten B., Jr., Peeters F.L., Zonneveld F.W. Dermolipoma: characteristic CT appearance. Doc ophthalmol. Adv Ophthalmol. 1990;74(4):321–328. doi: 10.1007/BF00145816. [DOI] [PubMed] [Google Scholar]
- 17.Kim E., Kim H.J., Kim Y.D., Woo K.I., Lee H., Kim S.T. Subconjunctival fat prolapse and dermolipoma of the orbit: differentiation on CT and MR imaging. AJNR Am J Neuroradiol. 2011;32(3):465–467. doi: 10.3174/ajnr.A2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pham N.S., Dublin A.B., Strong E.B. Dermoid cyst of the orbit and frontal sinus: a case report. Skull Base: Off J North American Skull Base Soc.....[et al.] 2010;20(4):275–278. doi: 10.1055/s-0030-1247631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeola M., Joharapurkar S.R., Bhole A.M., Chawla M., Chopra S., Paliwal A. Orbital floor dermoid: an unusual presentation. Indian J Ophthalmol. 2009;57(1):51–52. doi: 10.4103/0301-4738.44486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung W.S., Ahn K.J., Park M.R. The radiological spectrum of orbital pathologies that involve the lacrimal gland and the lacrimal fossa. Korean J Radiol: Off J Korean Radiol Soc. 2007;8(4):336–342. doi: 10.3348/kjr.2007.8.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulliken J.B., Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69(3):412–422. doi: 10.1097/00006534-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Bilaniuk L.T. Orbital vascular lesions. Role of imaging. Radiol Clin North America. 1999;37(1):169–183. doi: 10.1016/s0033-8389(05)70085-3. [xi] [DOI] [PubMed] [Google Scholar]
- 23.Haik B.G., Karcioglu Z.A., Gordon R.A., Pechous B.P. Capillary hemangioma (infantile periocular hemangioma) Surv Ophthalmol. 1994;38(5):399–426. doi: 10.1016/0039-6257(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 24.Dubois J., Garel L. Imaging and therapeutic approach of hemangiomas and vascular malformations in the pediatric age group. Pediat Radiol. 1999;29(12):879–893. doi: 10.1007/s002470050718. [DOI] [PubMed] [Google Scholar]
- 25.Burrows P.E., Laor T., Paltiel H., Robertson R.L. Diagnostic imaging in the evaluation of vascular birthmarks. Dermatol Clin. 1998;16(3):455–488. doi: 10.1016/s0733-8635(05)70246-1. [DOI] [PubMed] [Google Scholar]
- 26.Haik B.G., Jakobiec F.A., Ellsworth R.M., Jones I.S. Capillary hemangioma of the lids and orbit: an analysis of the clinical features and therapeutic results in 101 cases. Ophthalmology. 1979;86(5):760–792. doi: 10.1016/s0161-6420(79)35452-5. [DOI] [PubMed] [Google Scholar]
- 27.Sepahdari A.R., Aakalu V.K., Setabutr P., Shiehmorteza M., Naheedy J.H., Mafee M.F. Indeterminate orbital masses: restricted diffusion at MR imaging with echo-planar diffusion-weighted imaging predicts malignancy. Radiology. 2010;256(2):554–564. doi: 10.1148/radiol.10091956. [DOI] [PubMed] [Google Scholar]
- 28.Politi L.S., Forghani R., Godi C. Ocular adnexal lymphoma: diffusion-weighted mr imaging for differential diagnosis and therapeutic monitoring. Radiology. 2010;256(2):565–574. doi: 10.1148/radiol.10100086. [DOI] [PubMed] [Google Scholar]
- 29.Razek A.A., Elkhamary S., Mousa A. Differentiation between benign and malignant orbital tumors at 3-T diffusion MR-imaging. Neuroradiology. 2011;53(7):517–522. doi: 10.1007/s00234-011-0838-2. [DOI] [PubMed] [Google Scholar]
- 30.Shields J.A., Bakewell B., Augsburger J.J., Flanagan J.C. Classification and incidence of space-occupying lesions of the orbit. A survey of 645 biopsies. Archiv Ophthalmol. 1984;102(11):1606–1611. doi: 10.1001/archopht.1984.01040031296011. [DOI] [PubMed] [Google Scholar]
- 31.Ansari S.A., Mafee M.F. Orbital cavernous hemangioma: role of imaging. Neuroimag Clin North America. 2005;15(1):137–158. doi: 10.1016/j.nic.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Harris G.J., Jakobiec F.A. Cavernous hemangioma of the orbit. J Neurosurg. 1979;51(2):219–228. doi: 10.3171/jns.1979.51.2.0219. [DOI] [PubMed] [Google Scholar]
- 33.Zenobii M., Galzio R.J., Lucantoni D., Caffagni E., Magliani V. Spontaneous intraorbital hemorrhage caused by cavernous angioma of the orbit. J Neurosurg Sci. 1984;28(1):37–40. [PubMed] [Google Scholar]
- 34.Thorn-Kany M., Arrue P., Delisle M.B., Lacroix F., Lagarrigue J., Manelfe C. Cavernous hemangiomas of the orbit: MR imaging. J Neuroradiol. J Neuroradiol. 1999;26(2):79–86. [PubMed] [Google Scholar]
- 35.Ruchman M.C., Flanagan J. Cavernous hemangiomas of the orbit. Ophthalmology. 1983;90(11):1328–1336. doi: 10.1016/s0161-6420(83)34385-2. [DOI] [PubMed] [Google Scholar]
- 36.Wolin M.J., Holds J.B., Anderson R.L., Mamalis N. Multiple orbital tumors were cavernous hemangiomas. Ann Ophthalmol. 1990;22(11):426–428. [PubMed] [Google Scholar]
- 37.Sullivan T.J., Aylward G.W., Wright J.E., Moseley I.F., Garner A. Bilateral multiple cavernous haemangiomas of the orbit. The Brit J Ophthalmol. 1992;76(10):627–629. doi: 10.1136/bjo.76.10.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shields J.A., Hogan R.N., Shields C.L., Eagle R.C., Jr, Kennedy R.H., Singh A.D. Bilateral cavernous haemangiomas of the orbit. The Brit J Ophthalmol. 2000;84(8):928. doi: 10.1136/bjo.84.8.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mafee M.F., Putterman A., Valvassori G.E., Campos M., Capek V. Orbital space-occupying lesions: role of computed tomography and magnetic resonance imaging. An analysis of 145 cases. Radiol Clin North America. 1987;25(3):529–559. [PubMed] [Google Scholar]
- 40.Gyldensted C., Lester J., Fledelius H. Computed tomography of orbital lesions. A radiological study of 144 cases. Neuroradiology. 1977;13(3):141–150. doi: 10.1007/BF00626276. [DOI] [PubMed] [Google Scholar]
- 41.Savoiardo M., Strada L., Passerini A. Cavernous hemangiomas of the orbit: value of CT, angiography, and phlebography. AJNR Am J Neuroradiol. 1983;4(3):741–744. [PMC free article] [PubMed] [Google Scholar]
- 42.Forbes G.S., Sheedy P.F., 2nd, Waller R.R. Orbital tumors evaluated by computed tomography. Radiology. 1980;136(1):101–111. doi: 10.1148/radiology.136.1.7384483. [DOI] [PubMed] [Google Scholar]
- 43.Bilaniuk L.T., Rapoport R.J. Magnetic resonance imaging of the orbit. Top Magn Reson Imag: TMRI. 1994;6(3):167–181. [PubMed] [Google Scholar]
- 44.Fries P.D., Char D.H., Norman D. MR imaging of orbital cavernous hemangioma. J Comput Assist Tomogr. 1987;11(3):418–421. doi: 10.1097/00004728-198705000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Lemke A.J., Kazi I., Felix R. Magnetic resonance imaging of orbital tumors. Eur Radiol. 2006;16(10):2207–2219. doi: 10.1007/s00330-006-0227-0. [DOI] [PubMed] [Google Scholar]
- 46.Chung E.M., Smirniotopoulos J.G., Specht C.S., Schroeder J.W., Cube R. From the archives of the AFIP: Pediatric orbit tumors and tumorlike lesions: nonosseous lesions of the extraocular orbit. Radiograph: A Rev Publ Radiol Soc North America, Inc. 2007;27(6):1777–1799. doi: 10.1148/rg.276075138. [DOI] [PubMed] [Google Scholar]
- 47.Greene A.K., Burrows P.E., Smith L., Mulliken J.B. Periorbital lymphatic malformation: clinical course and management in 42 patients. Plast Reconstr Surg. 2005;115(1):22–30. [PubMed] [Google Scholar]
- 48.Coll G.E., Goldberg R.A., Krauss H., Bateman B.J. Concomitant lymphangioma and arteriovenous malformation of the orbit. Am J Ophthalmol. 1991;112(2):200–205. doi: 10.1016/s0002-9394(14)76702-9. [DOI] [PubMed] [Google Scholar]
- 49.Eiferman R.A., Gushard R.H. Chocolate cysts of the orbit. Ann Ophthalmol. 1986;18(4):156–157. [PubMed] [Google Scholar]
- 50.Bisdorff A., Mulliken J.B., Carrico J., Robertson R.L., Burrows P.E. Intracranial vascular anomalies in patients with periorbital lymphatic and lymphaticovenous malformations. AJNR Am J Neuroradiol. 2007;28(2):335–341. [PMC free article] [PubMed] [Google Scholar]
- 51.Baker L.L., Dillon W.P., Hieshima G.B., Dowd C.F., Frieden I.J. Hemangiomas and vascular malformations of the head and neck: MR characterization. AJNR Am J Neuroradiol. 1993;14(2):307–314. [PMC free article] [PubMed] [Google Scholar]
- 52.Wright J.E., Sullivan T.J., Garner A., Wulc A.E., Moseley I.F. Orbital venous anomalies. Ophthalmology. 1997;104(6):905–913. doi: 10.1016/s0161-6420(97)30208-5. [DOI] [PubMed] [Google Scholar]
- 53.Forbes G. Vascular lesions in the orbit. Neuroimag Clin North America. 1996;6(1):113–122. [vii] [PubMed] [Google Scholar]
- 54.Castillo M., Mukherji S.K., Wagle N.S. Imaging of the pediatric orbit. Neuroimag Clin North America. 2000;10(1):95–116. [viii] [PubMed] [Google Scholar]
- 55.Miller N.R. Primary tumours of the optic nerve and its sheath. Eye (Lond) 2004;18(11):1026–1037. doi: 10.1038/sj.eye.6701592. [DOI] [PubMed] [Google Scholar]
- 56.Dutton J.J. Optic nerve gliomas and meningiomas. Neurol Clin. 1991;9(1):163–177. [PubMed] [Google Scholar]
- 57.Bradbury P.G., Levy I.S., McDonald W.I. Transient uniocular visual loss on deviation of the eye in association with intraorbital tumours. J Neurol, Neurosurg, Psychiatr. 1987;50(5):615–619. doi: 10.1136/jnnp.50.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oohira A., Kubo R. Ocular blood flow defect in gaze-evoked amaurosis. Nippon Ganka Gakkai Zasshi. 1999;103(1):56–60. [PubMed] [Google Scholar]
- 59.Mafee M.F., Goodwin J., Dorodi S. Optic nerve sheath meningiomas. Role of MR imaging. Radiol Clin North America. 1999;37(1):37–58. doi: 10.1016/s0033-8389(05)70077-4. [ix] [DOI] [PubMed] [Google Scholar]
- 60.Albert D. 2nd ed. WB Saunders; Philadelphia: 2000. Principles and practice of ophthalmology. [Google Scholar]
- 61.Gunduz K., Shields C.L., Gunalp I., Erden E., Shields J.A. Orbital schwannoma: correlation of magnetic resonance imaging and pathologic findings. Graefe’s Archiv Clin Exp Ophthalmol = Albrecht von Graefes Archiv klin exp Ophthalmol. 2003;241(7):593–597. doi: 10.1007/s00417-003-0681-1. [DOI] [PubMed] [Google Scholar]
- 62.Dervin J.E., Beaconsfield M., Wright J.E., Moseley I.F. CT findings in orbital tumours of nerve sheath origin. Clin Radiol. 1989;40(5):475–479. doi: 10.1016/s0009-9260(89)80250-8. [DOI] [PubMed] [Google Scholar]
- 63.Lam D.S., Ng J.S., To K.F., Abdulah V., Liew C.T., Tso M.O. Cystic schwannoma of the orbit. Eye (Lond) 1997;11(Pt 6):798–800. doi: 10.1038/eye.1997.208. [DOI] [PubMed] [Google Scholar]
- 64.Westacott S., Garner A., Moseley I.F., Wright J.E. Orbital lymphoma versus reactive lymphoid hyperplasia: an analysis of the use of computed tomography in differential diagnosis. The Brit J Ophthalmol. 1991;75(12):722–725. doi: 10.1136/bjo.75.12.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peyster R.G., Shapiro M.D., Haik B.G. Orbital metastasis: role of magnetic resonance imaging and computed tomography. Radiol Clin North America. 1987;25(3):647–662. [PubMed] [Google Scholar]
- 66.Margo C.E., Mulla Z.D. Malignant tumors of the orbit. Analysis of the florida cancer registry. Ophthalmology. 1998;105(1):185–190. doi: 10.1016/s0161-6420(98)92107-8. [DOI] [PubMed] [Google Scholar]
- 67.Valvassori G.E., Sabnis S.S., Mafee R.F., Brown M.S., Putterman A. Imaging of orbital lymphoproliferative disorders. Radiol Clin North America. 1999;37(1):135–150. doi: 10.1016/s0033-8389(05)70083-x. [x–xi] [DOI] [PubMed] [Google Scholar]
- 68.Rosenberg S.A., Diamond H.D., Jaslowitz B., Craver L.F. Lymphosarcoma: a review of 1269 cases. Medicine. 1961;40:31–84. doi: 10.1097/00005792-196102000-00002. [DOI] [PubMed] [Google Scholar]
- 69.Bairey O., Kremer I., Rakowsky E., Hadar H., Shaklai M. Orbital and adnexal involvement in systemic non-Hodgkin’s lymphoma. Cancer. 1994;73(9):2395–2399. doi: 10.1002/1097-0142(19940501)73:9<2395::aid-cncr2820730924>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 70.Flanders A.E., Espinosa G.A., Markiewicz D.A., Howell D.D. Orbital lymphoma. Role of CT and MRI. Radiol Clin North America. 1987;25(3):601–613. [PubMed] [Google Scholar]
- 71.Akansel G., Hendrix L., Erickson B.A. MRI patterns in orbital malignant lymphoma and atypical lymphocytic infiltrates. Eur J Radiol. 2005;53(2):175–181. doi: 10.1016/j.ejrad.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 72.Hosten N., Schorner W., Zwicker C. [Lymphocytic infiltrations of the orbit in MRT and CT. Lymphoma, pseudolymphoma and inflammatory pseudotumor] RoFo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin. 1991;155(5):445–451. doi: 10.1055/s-2008-1033294. [DOI] [PubMed] [Google Scholar]
- 73.Issing P.R., Ruh S., Kloss A., Kuske M., Lenarz T. Diagnosis and therapy of lymphoid tumors of the orbits. Hno. 1997;45(7):545–550. doi: 10.1007/s001060050129. [DOI] [PubMed] [Google Scholar]
- 74.Garrity J.A. Henderson’s orbital tumors. Raven Press; New York: 2007. Metastatic carcinomas; pp. 313–326. [Google Scholar]
- 75.Ahmad S.M., Esmaeli B. Metastatic tumors of the orbit and ocular adnexa. Curr Opin Ophthalmol. 2007;18(5):405–413. doi: 10.1097/ICU.0b013e3282c5077c. [DOI] [PubMed] [Google Scholar]
- 76.Shields J.A., Shields C.L., Brotman H.K., Carvalho C., Perez N., Eagle R.C., Jr Cancer metastatic to the orbit: the 2000 Robert M. Curts lecture. Ophthal Plast Reconstr Surg. 2001;17(5):346–354. doi: 10.1097/00002341-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 77.DePotter P.D.C., Shields J.A. JB Lippincott Co.; Philadelphia: 1995. MRI of the eye and orbit. pp. 237–43. [Google Scholar]
- 78.MacArthur C.J., McGill T.J., Healy G.B. Pediatric head and neck rhabdomyosarcoma. Clin Pediatr. 1992;31(2):66–70. doi: 10.1177/000992289203100201. [DOI] [PubMed] [Google Scholar]
- 79.Volpe N.J., Jakobiec F.A. Pediatric orbital tumors. Int Ophthalmol Clin. 1992;32(1):201–221. doi: 10.1097/00004397-199203210-00016. [DOI] [PubMed] [Google Scholar]
- 80.Walton R.C., Ellis G.S., Jr., Haik B.G. Rhabdomyosarcoma presumed metastatic to the orbit. Ophthalmology. 1996;103(9):1512–1516. doi: 10.1016/s0161-6420(96)30474-0. [DOI] [PubMed] [Google Scholar]
- 81.Shields J.A., Shields C.L. Rhabdomyosarcoma of the orbit. Int Ophthalmol Clin. 1993;33(3):203–210. doi: 10.1097/00004397-199303330-00027. [DOI] [PubMed] [Google Scholar]
- 82.Shields J.A., Shields C.L. Rhabdomyosarcoma: review for the ophthalmologist. Surv Ophthalmol. 2003;48(1):39–57. doi: 10.1016/s0039-6257(02)00415-0. [DOI] [PubMed] [Google Scholar]
- 83.Jones I.S., Reese A.B., Kraut J. Orbital rhabdomyosarcoma. An analysis of 62 cases. Am J Ophthalmol. 1966;61(4):721–736. [PubMed] [Google Scholar]
- 84.Jones I.S., Reese A.B., Krout J. Orbital rhabdomyosarcoma: an analysis of sixty-two cases. Trans Am Ophthalmol Soc. 1965;63:223–255. [PMC free article] [PubMed] [Google Scholar]
- 85.Sohaib S.A., Moseley I., Wright J.E. Orbital rhabdomyosarcoma – the radiological characteristics. Clin Radiol. 1998;53(5):357–362. doi: 10.1016/s0009-9260(98)80009-3. [DOI] [PubMed] [Google Scholar]
- 86.Folpe A.L., McKenney J.K., Bridge J.A., Weiss S.W. Sclerosing rhabdomyosarcoma in adults: report of four cases of a hyalinizing, matrix-rich variant of rhabdomyosarcoma that may be confused with osteosarcoma, chondrosarcoma, or angiosarcoma. Am J Surg Pathol. 2002;26(9):1175–1183. doi: 10.1097/00000478-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 87.Mafee M.F., Pai E., Philip B. Rhabdomyosarcoma of the orbit. Evaluation with MR imaging and CT. Radiol Clin North America. 1998;36(6):1215–1227. doi: 10.1016/s0033-8389(05)70241-4. [xii] [DOI] [PubMed] [Google Scholar]
- 88.Cota N., Chandna A., Abernethy L.J. Orbital abscess masquerading as a rhabdomyosarcoma. J AAPOS: The Off Publ Am Assoc Pediatr Ophthalmol Strab/Am Assoc Pediatr Ophthalmol Strab. 2000;4(5):318–320. doi: 10.1067/mpa.2000.107897. [DOI] [PubMed] [Google Scholar]
- 89.Sepahdari A.R., Aakalu V.K., Kapur R. MRI of orbital cellulitis and orbital abscess: the role of diffusion-weighted imaging. AJR Am J Roentgenol. 2009;193(3):W244–W250. doi: 10.2214/AJR.08.1838. [DOI] [PubMed] [Google Scholar]
- 90.Koc O., Paksoy Y., Erayman I., Kivrak A.S., Arbag H. Role of diffusion weighted MR in the discrimination diagnosis of the cystic and/or necrotic head and neck lesions. Eur J Radiol. 2007;62(2):205–213. doi: 10.1016/j.ejrad.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 91.Dutton J.J. Gliomas of the anterior visual pathway. Surv Ophthalmol. 1994;38(5):427–452. doi: 10.1016/0039-6257(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 92.Thompson C.R., Lessell S. Anterior visual pathway gliomas. Int Ophthalmol Clin. 1997;37(4):261–279. doi: 10.1097/00004397-199703740-00021. [DOI] [PubMed] [Google Scholar]
- 93.Lertchavanakul A., Baimai C., Siwanuwatn R., Nuchprayoon I., Phudhichareonrat S. Optic nerve glioma in infancy: a case report of the youngest patient in Thailand. J Med Assoc Thailand = Chotmaihet Thangphaet. 2001;84(Suppl 1):S137–S141. [PubMed] [Google Scholar]
- 94.Wulc A.E., Bergin D.J., Barnes D., Scaravilli F., Wright J.E., McDonald W.I. Orbital optic nerve glioma in adult life. Archiv Ophthalmol. 1989;107(7):1013–1016. doi: 10.1001/archopht.1989.01070020075033. [DOI] [PubMed] [Google Scholar]
- 95.Spencer W. Optic nerve. In: Spencer W.H., editor. vol. 3. WB Saunders; Philadelphia7: 1996. pp. 513–622. (Ophthalmic pathology). [Google Scholar]
- 96.Cirak B. Optic nerve glioma. J Neurosurg. 2003;99(2 Suppl):246. doi: 10.3171/spi.2003.99.2.0246. [Author reply 246] [DOI] [PubMed] [Google Scholar]
- 97.Borit A., Richardson E.P., Jr. The biological and clinical behaviour of pilocytic astrocytomas of the optic pathways. Brain: A J Neurol. 1982;105(Pt 1):161–187. doi: 10.1093/brain/105.1.161. [DOI] [PubMed] [Google Scholar]
- 98.Thiagalingam S., Flaherty M., Billson F., North K. Neurofibromatosis type 1 and optic pathway gliomas: follow-up of 54 patients. Ophthalmology. 2004;111(3):568–577. doi: 10.1016/j.ophtha.2003.06.008. [DOI] [PubMed] [Google Scholar]
- 99.Parazzini C., Triulzi F., Bianchini E. Spontaneous involution of optic pathway lesions in neurofibromatosis type 1: serial contrast MR evaluation. AJNR Am J Neuroradiol. 1995;16(8):1711–1718. [PMC free article] [PubMed] [Google Scholar]
- 100.Parsa C.F., Hoyt C.S., Lesser R.L. Spontaneous regression of optic gliomas: thirteen cases documented by serial neuroimaging. Archiv Ophthalmol. 2001;119(4):516–529. doi: 10.1001/archopht.119.4.516. [DOI] [PubMed] [Google Scholar]
- 101.Davis R. Juvenile pilocytic astrocytoma of the optic nerve: clinico-pathologic study of sixty three cases. In: Jakobiec F.A., editor. Ocular and adnexal tumors. Birmingham7 Yanoff Aesculapius; 1978. pp. 685–707. [Google Scholar]
- 102.Holman R.E., Grimson B.S., Drayer B.P., Buckley E.G., Brennan M.W. Magnetic resonance imaging of optic gliomas. Am J Ophthalmol. 1985;100(4):596–601. doi: 10.1016/0002-9394(85)90689-0. [DOI] [PubMed] [Google Scholar]
- 103.Weber A.L., Klufas R., Pless M. Imaging evaluation of the optic nerve and visual pathway including cranial nerves affecting the visual pathway. Neuroimag Clin North America. 1996;6(1):143–177. [PubMed] [Google Scholar]



