Abstract
Purpose
To determine the discriminating ability of retinal nerve fiber layer (RNFL) thickness measured with spectral-domain optical coherence tomography (SD-OCT) in different stages of glaucoma.
Patients and methods
Thirty normal, 150 glaucomatous eyes were included. Glaucomatous eyes were graded into early, moderate and severe stages according to one of the global indices called visual field index (VFI). Complete ophthalmic examination, white on white perimetry and SD-OCT were done for all patients. RNFL thickness of quadrants and average thickness were recorded. Area under receiver operating characteristic curves (AUCs) were used to assess the performance of OCT parameters.
Results
Average, inferior and superior RNFL thickness were the best parameters to discriminate normal from early glaucoma (AUC: 0.91–86), early from moderate (AUC: 0.77–0.70) and moderate from severe (AUC: 0.85–83). Average RNFL loss was 18% in early glaucoma, 28% in moderate glaucoma and 41% in severe glaucoma. Early damage tends to be focal and in the lower quadrant. A significant correlation was detected between mean VFI and mean RNFL loss. Glaucoma was restaged according to average RNFL loss into early: ⩾97.5 μ, moderate: <97.5–72.5 μ and severe: <72.5 μ.
Conclusion
RNFL thickness measured with SD-OCT could discriminate the three stages of glaucoma. RNFL loss can be correlated to visual field loss. Future OCT-based staging of glaucoma, adjunctive to perimetry is possible.
Keywords: Discrimination, Glaucoma, Optical coherence tomography, Perimetry, Stages
Introduction
Glaucoma is a multifactorial optic neuropathy known to cause progressive loss of retinal ganglion cells and their axons.1 Evaluation of the retinal nerve fiber layer (RNFL) and optic nerve head (ONH) is a crucial step in diagnosing and monitoring glaucoma.2 It has been suggested that early detection and treatment of glaucomatous optic neuropathy may reduce the incidence of blindness from glaucoma.3
Staging glaucomatous damage into broad categories of damage such as, mild, moderate, and advanced enhances management. It promotes careful assessment and documentation of clinical damage, thereby facilitating monitoring for stability versus progression and provides a common language for both clinical and research purposes.4
Automated computerized devices can discriminate between normal and glaucomatous eyes, objectively with good results. These include scanning laser ophthalmoscopy, scanning laser polarimetry and optical coherence tomography (OCT).5 Each can analyze and quantify the optic nerve and RNFL thickness allowing broad staging of structural damage. However these expensive and sophisticated technologies are evolving faster than clinical assessment of their utility.4
The recently introduced spectral domain OCT (SD-OCT) system is a rapidly emerging imaging modality in diagnosing glaucomatous damage.6,7 It is particularly valuable in glaucoma detection and monitoring through identification of subtle RNFL or ONH changes over time.8 However the role of OCT remains less certain in eyes with early damage.9 Although several studies addressed the discriminating ability of OCT in glaucomatous eyes,10,11 its role in differentiating glaucomatous stages has not been well-established yet. The aim of this study is to determine the discriminating ability of retinal nerve fiber layer (RNFL) thickness measured with spectral domain optical coherence tomography (SD-OCT) in different stages of glaucoma.
Patients and methods
This was an observational case-control study involving 180 eyes of 180 subjects during the period from May 2010 to May 2011. All eyes underwent complete ophthalmic examination including best corrected visual acuity, dilated fundoscopic examination, slit lamp biomicroscopy, gonioscopy, Goldmann applanation tonometry, standard automated perimetry (SAP) and SD-OCT. Eyes with myopic refraction >−5.0 diopters (D), retinal diseases, uveitis, or non glaucomatous optic neuropathy were excluded from the study.
Participants in the study included 30 normal subjects and 150 eyes with definite glaucomatous damage. Informed consent was obtained from all patients following all the guidelines required by the institutional review board and Ethics Committee.
Normal eyes
All eyes had intraocular pressure <21 mmHg, normal optic disc appearance and normal visual field. Normal visual field was defined as a mean defect and pattern standard deviation within 95% normal limits and a glaucoma hemifield test result within normal limits.
Glaucomatous eyes
Eyes were considered glaucomatous, if they had two consecutive abnormal visual field test results regardless of the optic nerve head and RNFL appearance to avoid potential bias in the evaluation of sensitivity and specificity of RNFL measurement for glaucoma detection.
Visual field testing
This was done with Humphrey field analyzer (Carl Zeiss Meditec, Inc) using Swedish Interactive Thresholding Algorithm (SITA) standard strategy, program 24-2. A visual field defect was defined as having >3 non edge contiguous points demonstrating a threshold sensitivity loss on the pattern deviation plot with p < 5% with at least one of the points depressed at p < 1%, or a > 10 dB difference across the nasal horizontal midline at two or more adjacent locations. In addition, abnormal glaucoma hemifield test (GHT) was required.
Visual field staging
The severity of glaucomatous damage was graded into early, moderate and severe according to the University of São Paulo Glaucoma Visual Field Staging System (USP-GVFSS) proposed by Susanna and Vessani.4 It is a new system in which qualitative and quantitative characteristics of the visual field defect are described. The new system proposed includes the Visual Field Index (VFI), a new parameter recently introduced in the Humphrey Visual Field (Carl Zeiss Meditec Inc, Dublin, CA).12
The mean deviation value (MD) in dB is replaced with% for a full visual field with comprehensible scale ranging from 100% to 0% (normal function to perimetric blindness). The Pattern Deviation probability map is used to identify normal and abnormal points. Points <0 dB are considered to have 0% sensitivity. Normal points have 100% sensitivity. The amount of loss is then calculated using total deviation numerical maps. Defect depth is recalculated in%.
In USP GVFSS, VFI cut-off values are established for each stage as follows: early defect: VFI > 91%, moderate defect: VFI > 78% and < 91%, severe defect: VFI < 78%. In addition location is considered in three categories: VF defect inside the 5 central degrees (5); VF defect inside the 10 central degrees but outside the 5 central degrees (10); VF defect outside the 10 central degrees (10+). One (1) versus both hemifield (2) involvement is included. The relationship to the blind spot is based on points depressed below 0.5% level on the pattern deviation plot and it is characterized as A, if the visual field defect is not connected with the blind spot, or B, if the visual field defect is connected with the blind spot.
Imaging with SD-OCT
A three dimensional, Fourier-domain OCT system which is combined with non mydriatic retinal camera; 3D OCT 2000 (Topcon Corp, Tokyo, Japan) was used in this study. A super luminescent diode with a wavelength of 840 nm and a bandwidth of 50 nm is used as a light source. This system has approximately 5 μ axial resolution, 20 μ horizontal resolution and acquires 18,700 axial scans per second. Three dimensional data were obtained using the raster scanning technique centered on the ONH covering a square area; 6 mm (horizontal) × 6 mm (vertical) × 1.7 mm (axial depth). The raster pattern acquires 128 horizontal scans, each scan is composed of 512 axial scans, thus it provides a comprehensive topographic and cross-sectional image information because full 3D data are available at a large number of transverse points across the ONH. The total acquisition time is about 3.7 s. RNFL thickness maps and 3.4 mm diameter circumpapillary OCT image can be generated from the 3D OCT data. The circumpapillary OCT image can be manually repositioned to provide accurate centration around the ONH. An OCT virtual fundus image (projection image) generated directly from 3D data, was used to enable precise registration of OCT image and RNFL maps with the specific fundus features. Improved registration of OCT images and RNFL maps with fundus features ensure measurement reproducibility. Fixation changes during imaging were identified by observing discontinuities in blood vessels on the virtual image.
Errors in circumpapillary RNFL thickness measurement caused by blood vessels interfering with the segmentation algorithm can be identified and corrected using information from the OCT fundus image. RNFL measurements were expressed as an average over four quadrants, 12 clock hours and mean thickness of the total circumpapillary scan. Based on the instrument’s normative database, any RNFL measurements outside 95% normal limits that were confirmed on at least 2 of 3 repeat scans were highlighted as abnormal thinning in red or yellow according to the severity of thinning. Diffuse RNFL loss was considered if thinning involves one or more quadrants, while focal loss is considered if loss involves 1 or 2 contiguous or >2 non contiguous clock hours.
Statistical analysis
Analysis was done using MedCalc – version 12.1.3.0. Mean values of peripapillary RNFL thickness were compared between normal, whole glaucoma group using t test. Mean peripapillary thickness in different stages of glaucoma was compared using one way analysis of varience followed by Sidac post-Hoc test. Receiver operating characteristic (ROC) curves were used to describe the ability of each parameter to differentiate between different glaucoma stages.
Areas under receiver operating characteristics (AUCs) measure a test’s diagnostic ability (its power to correctly classify those with and without the disease). An AUC of 1 (100% sensitivity and 100% specificity) represents a perfect test, whereas an AUC of 0.5 indicates a completely worthless test. In this study, the AUC was classified as follows13: 0.9–1 = excellent, 0.80–0.89 = good, 0.70–0.79 = fair, 0.60–0.69 = poor and 0.50–0.59 = worthless test.
Values on the ROC curves that have the best sensitivity and specificity were chosen as cut off values that could separate between each two consecutive stages.
Pearson correlation test was used to correlate visual field loss and RNFL loss.
Results
A total of 180 eyes of 180 patients were enrolled in this study. Thirty eyes (30) were normal, 150 were glaucomatous. Glaucomatous eyes were further classified into: early glaucoma (53), moderate glaucoma (45) and severe glaucoma (52). There were ninety-three males and eighty-seven females. Table 1 summarizes the baseline characteristics of the study population. No difference was detected between normal and glaucomatous eyes with regard to age, gender and refractive error. A statistically significant difference of intraocular pressure, mean deviation (MD), pattern standard deviation (PSD), RNFL thickness (all quadrants and average values) existed between the normal and glaucomatous eyes.
Table 1.
Baseline data of the study population.
| Normal | Glaucoma | P | |
|---|---|---|---|
| No | 30 | 150 | |
| Age | 50.5 ± 11.1 | 53.4 ± 13.3 | 0.3a |
| Gender | 40.9% | 67.9% | 0.06b |
| Male/female | |||
| IOP(mmHg) | 14.0 ± 2.6 | 24.0 ± 2.3 | 0.03a |
| Refractive errors (D) | −0.6 ± 1.2 | −0.8 ± 2.1 | 0.6a |
| MD ± SD (dB) | −1.6 ± 1.2 | −14.3 ± 10.3 | 0.001 |
| PSD ± SD (dB) | 1.8 ± 0.9 | 3.2 ± 2.8 | 0.001 |
| RNFL thickness (μ) | |||
| Superior | 126.0 ± 14.6 | 94.2 ± 20.5 | 0.001 |
| Inferior | 135.2 ± 15.3 | 91.3 ± 23.4 | 0.001 |
| Nasal | 94.0 ± 15.3 | 74.5 ± 16.0 | 0.001 |
| Temporal | 82.7 ± 13.2 | 64.0 ± 15.1 | 0.001 |
| Average | 110.2 ± 11.1 | 81.1 ± 14.3 | 0.001 |
IOP – intraocular pressure, D – diopter; MD – mean deviation, PSD – pattern standard deviation, dB – decibel, RNFL – retinal nerve fiber layer.
t test.
Pearson chi-square.
OCT measurements could discriminate all stages of glaucomatous damage. Mean superior, inferior and average RNFL thickness values were significantly different in all stages of glaucoma (p < 0.001). All comparisons were significantly different; normal versus early, early versus moderate and moderate versus severe. No significant difference of mean nasal or temporal RNFL thickness was detected between early and moderate stages. No significant difference of mean temporal RNFL thickness was detected between moderate and severe stages (Table 2).
Table 2.
Comparison between mean RNFL thickness in different stages of glaucoma.
| Quadrant | Normal N = 30 | Early N = 53 | Moderate N = 45 | Severe N = 52 | P | Normal vs early | Early vs moderate | Moderate vs severe |
|---|---|---|---|---|---|---|---|---|
| Superior | 126.0 + 14.6 | 107.5 + 12.0 | 91.9 + 16.7 | 73.3 + 17.2 | 0.00 | 0.000 | 0.006 | 0.001 |
| Inferior | 135.2 + 15.3 | 105.2 + 20.9 | 89.9 + 16.7 | 69.2 + 13.5 | 0.00 | 0.000 | 0.001 | 0.02 |
| Nasal | 94.0 + 15.3 | 81.0 + 12.9 | 75.2 + 18.3 | 60.7 + 10.2 | 0.00 | 0.001 | 0.8 | 0.02 |
| Temporal | 82.7 + 13.2 | 67.5 + 13.3 | 63.6 + 19.0 | 58.2 + 13.2 | 0.00 | 0.000 | 0.8 | 0.9 |
| Average | 110.2 + 11.1 | 90.5 + 10.5 | 79.5 + 11.5 | 65.9 + 7.7 | 0.00 | 0.000 | 0.001 | 0.004 |
One way ANOVA, post Hoc test.
ROC curves in differentiating stages
To detect the most sensitive OCT parameters which can discriminate between different stages, AUCs were calculated (Table 3)(Fig. 1a–c). The best RNFL parameters which could distinguish normal from early stage were: average, inferior and superior RNFL thickness (AUC: 0.91–0.86).
Table 3.
Discriminating ability of RNFL thickness between different glaucoma stages using area under receiver operating characteristic curves.
| Variable | Normal vs early | 95% CI | Early vs moderate | 95% CI | Moderate vs severe | 95% CI |
|---|---|---|---|---|---|---|
| Inferior RNFL thickness | 0.88 | 0.80–0.94 | 0.70 | 0.55–0.83 | 0.86 | 0.67–0.95 |
| Superior RNFL thickness | 0.86 | 0.77–0.92 | 0.77 | 0.62–0.89 | 0.84 | 0.65–0.94 |
| Nasal RNFL thickness | 0.74 | 0.64–0.83 | 0.58 | 0.42–0.72 | 0.72 | 0.58–0.90 |
| Temporal RNFL thickness | 0.80 | 0.70–0.88 | 0.64 | 0.49–0.78 | 0.62 | 0.38–0.75 |
| Average RNFL thickness | 0.91 | 0.83–0.96 | 0.75 | 0.59–0.86 | 0.86 | 0.67–0.95 |
vs = versus.
Figure 1.
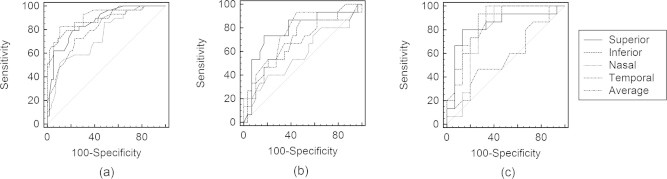
(a) The ROC curve of the best RNFL parameters for discriminating between normal and early stages of glaucoma. (b) The ROC curve of the best RNFL parameters for discriminating between early and moderate stages of glaucoma. (c) The ROC curve of the best RNFL parameters for discriminating between moderate and severe stages of glaucoma.
Cut off values of RNFL thickness were as follows: average: 97.5, inferior: 117.5 and superior: 115.5.
The best RNFL parameters which could distinguish early stage from moderate stage were: superior, average and inferior RNFL thickness (AUC: 0.70–0.77). Cut off values of RNFL thickness were as follows: average: 84, superior: 97.5, and inferior: 101.5.
The best RNFL parameters which distinguished moderate from severe stage were: average, inferior and superior RNFL thickness (AUC: 0.85–0.83). Cut off values of RNFL thickness were as follows: average: 72.5, inferior: 81 and superior: 82.5.
Quantification of RNFL loss in different stages of glaucoma
Mean RNFL thickness of each glaucoma stage was compared to normal eyes in all quadrants to obtain the percentage of RNFL loss (Table 4). In early glaucoma, the average loss was 18% (range: 14–23%). The most affected quadrants were the inferior (23%). In moderate stage, the average loss was 28% (range 21–35%). The most affected quadrants were the inferior (35%). In severe glaucoma, the average loss was 41% (range: 30–49%). The loss was maximal inferiorly (49%).
Table 4.
Quantification of RNFL thickness loss in different stages of glaucoma.
| Early glaucoma/normal | % of loss | Moderate glaucoma/normal | % of loss | Severe glaucoma/normal | % of loss | |
|---|---|---|---|---|---|---|
| Superior | 107.5/126.0 | 16 | 91.9/126.0 | 28 | 73.3/126.0 | 43 |
| Inferior | 105.2/135.2 | 23 | 89.9/135.2 | 35 | 69.2/135.2 | 49 |
| Nasal | 81.0/94.0 | 14 | 75.2/94.0 | 21 | 60.7/94.0 | 37 |
| Temporal | 67.5/82.7 | 19 | 63.6/82.7 | 24 | 58.2/82.7 | 30 |
| Average | 90.5/110.2 | 18 | 79.5/110.2 | 28 | 65.9/110.2 | 41 |
The pattern of RNFL loss in early stage was focal in 60%. In 56% of these eyes, RNFL thickness distribution respects the ISNT (inferior, superior, nasal, temporal) rule, representing a normal order of thickness in disc sectors.
In moderate stage, focal loss was present in 53%. In severe stage diffuse loss was present in 58%, mixed diffuse and focal in 29%. In 83% of eyes with severe damage, RNFL thickness distribution deviated from the ISNT rule.
Correlation between structural and functional loss
A significant correlation (p < 0.005) was detected between mean VFI (indicating total visual field deviation from normal) and mean value of average RNFL thickness loss (R = 0.77). According to cut off values of average RNFL thickness, a simple grading method can be used as follows: early glaucoma >97.5 μ, moderate stage glaucoma: <97.5–72.5 μ and severe stage: <72.5 μ.
Discussion
In the present study, OCT derived measures of RNFL thickness were significantly different between the three stages of glaucoma in all comparisons (normal versus early, early versus moderate and moderate versus severe). Average, inferior and superior RNFL thickness were the best RNFL discriminators between normal versus early stage and moderate versus severe stage (AUC: 0.91–0.86 and 0.85–0.83 respectively). The best RNFL parameters which could distinguish early from moderate stage were: superior, average and inferior RNFL thickness (AUC: 0.70–0.77).
In this study, the ability of RNFL parameters to discriminate between different stages appeared to be less than the reported power of discrimination between normal and glaucomatous eyes in the literature. A similar finding was detected by Mwanza et al.2 who recorded that the AUCs of average and inferior RNFL decreased gradually from discriminating between normal and all glaucomatous eyes (discrimination 1, AUC: 0.95) to discriminating between normal from eyes with mild glaucoma (discrimination 2, AUC: 0.89–0.91) and moderate from severe glaucoma (discrimination 3, AUC: 0.66–0.78). It may be that, the difference between normal and glaucomatous eyes is large than the difference between consecutive stages of glaucoma.
Several studies reported that the average and inferior RNFL thickness are the best discriminators between normal and glaucomatous eyes, but with higher range of AUC areas. Budenz and colleagues14 reported AUCs of 0.97, 0.97, and 0.95 for inferior, average and superior RNFL thickness respectively. Bowd et al.15 reported AUC of 0.91 for inferior RNFL, Badala et al.16 reported AUC of 0.96 and 0.95 for average RNFL and inferior RNFL respectively.
Mwanza and colleagues2 using SD-OCT, reported AUC between 0.92 and 0.95 for all RNFL parameters on discrimination between normal and glaucoma groups (regardless of the glaucoma stage) and AUC between 0.89 and 0.91 for discrimination between normal eyes and eyes with mild glaucoma. The authors found no significant differences between AUCs that best differentiate between normal and whole glaucomatous eyes or between normal and eyes with mild glaucoma. Nouri-Mahdavi and colleagues9 reported that RNFL thickness measurement performed well for discrimination of early perimetric glaucoma from normal eyes while its discriminating power in a glaucoma suspect with suspicious optic disc cupping and normal achromatic visual fields was less adequate.
These contradictory results in eyes with early glaucoma may be due to variable inclusion criteria which depend upon the definition of early glaucoma. Early damage may include ocular hypertensive eyes, eyes with optic disc changes with normal SAP and or normal short wave automated perimetry or mild stage of perimetric glaucoma. In addition, sample size, generation of OCT used and treatment which may or may not be employed are other factors. Therefore, inclusion criteria of population samples may differ across different studies.
Brusini and Johnson17 explained why any visual field staging system has failed to have a widespread acceptance. First, the method is simple and easy, however, it is subjective, with poor reproducibility. Second, the method is accurate and standardized, but is too time-consuming and requires complicated calculations or software which is either rarely used or not easily available.
In the present study, a significant correlation was detected between mean VFI (indicating mean visual field deviation from normal) and mean value of average RNFL thickness loss. It is known that loss of RNFL is responsible for structural alternation of optic nerve head appearance and functional changes in the form of reduction of visual field sensitivity. Quantification of average loss of RNFL is a simple and objective method. It seems possible to use average loss of RNFL as simple staging system in adjunction to perimetry.
In this study, the most common situation of damage was inferior and superior quadrants.
Mok and associates and8 Nouri-Mahdavi et al.,9 reported that eyes with suspicious disc and normal achromatic visual field had a lower RNFL thickness in superotemporal and inferotemporal sectors of the peripapillary area. The earliest detectable perimetric damage corresponded to average loss of 18% of RNFL thickness. The average loss in moderate and severe glaucoma stages was 28% and 41% respectively. In a post mortem study, Quigley and colleagues18 found that 40% axonal loss may occur before any change in visual function is detected with perimetry.
In this study, a high prevalence rate of RNFL focal defects was detected in both early and moderate glaucoma stages (60% and 53%, respectively). This allows for a more homogenous sample in which the two stages are not wide apart. This may explain the lower AUC areas for discrimination between early and moderate stages. The prevalence of focal defects explains that 56% of eyes in early stage respect the ISNT rule. On averaging the data in different quadrants, focal defects at one clock hour can be compensated by adjacent normal sectors.
While in severe glaucoma stage, diffuse defects were more prevalent (58%) in addition to mixed pattern (29%) with larger percentage of eyes that deviate from the ISNT rule (83%). It seems that early glaucoma starts with focal defects which coalesce into diffuse defects in late stages. Leung and associates19 postulated that most RNFL damage begins with localized RNFL defects involving the inferior or superior quadrants. As the disease advances, localized RNFL defects expand in size in the same quadrant before the fellow quadrant becomes affected.
The limitation of this study is that it is a single-center study with small purposive convenient sample. Further research with larger adequate sample is required. Also the predictive value of the cut-off values that discriminate different stages are to be evaluated in further studies. Finally, results of the present study will not be applicable to any machine using software not including VFI.
In conclusion, RNFL thickness measured with SD-OCT can discriminate between the three stages of glaucoma. Structural damage represented by RNFL loss can be correlated to functional damage represented by visual field loss. This finding may be the basis for future OCT-based staging of glaucoma, adjunctive to perimetry.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Sehi M., Pinzon-Plazas M., Feuer W.J., Greenfield D.S. Relationship between pattern electroretinogram standard automated perimetry and optic nerve structural assessments. Glaucoma. 2009;18:608–617. doi: 10.1097/IJG.0b013e31819afb5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mwanza J.C., Oakley J.D., Budenz D.L. Cirrus optical coherence tomography normative database study group. Ability of Cirrus HD-OCT optic nerve head parameters to discriminate normal from glaucomatous eyes. Ophthalmology. 2011;118:241–248. doi: 10.1016/j.ophtha.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagga H., Feuer W.J., Greenfield D.S. Detection of psychophysical and structural injury in eyes with glaucomatous optic neuropathy and normal standard automated perimetry. Arch Ophthalmol. 2006;124:169–176. doi: 10.1001/archopht.124.2.169. [DOI] [PubMed] [Google Scholar]
- 4.Susanna R., Jr., Vessani R.M. Staging glaucoma patient: why and how? Open Ophthalmol J. 2009;3:59–64. doi: 10.2174/1874364100903010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zangwill L.M., Bowd C., Berry C.C. Discriminating between normal and glaucomatous eyes using the Heidelberg retina tomograph, GDx nerve fiber analyzer, and optical coherence tomograph. Am J Ophthalmol. 2001;119:985–993. doi: 10.1001/archopht.119.7.985. [DOI] [PubMed] [Google Scholar]
- 6.Rao H.L., Zangwill L.M., Weinreb R.N. Comparison of different spectral-domain optical coherence tomography scanning areas for glaucoma diagnosis. Ophthalmology. 2010;117:1692–1699. doi: 10.1016/j.ophtha.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 7.Sehi M., Grewal D.S., Sheets C.W. Diagnostic ability of Fourier-domain vs time domain optical coherence tomography for glaucoma detection. Am J Ophthalmol. 2009;148:597–605. doi: 10.1016/j.ajo.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mok K.H., Lee V.W., So K.F. Retinal nerve fiber layer measurement by optical coherence tomography in glaucoma suspects with short-wavelength perimetry abnormalities. J Glaucoma. 2003;12:45–49. doi: 10.1097/00061198-200302000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Nouri-Mahdavi K., Hoffman D., Tannenbaum D.P. Identifying early glaucoma with optical coherence tomography. Am J Ophthalmol. 2004;137:228–235. doi: 10.1016/j.ajo.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Kanamori A., Nakamura M., Escano M.F. Evaluation of the glaucomatous damage on retinal nerve fiber layer thickness measured by optical coherence tomography. Am J Ophthalmol. 2003;135:513–520. doi: 10.1016/s0002-9394(02)02003-2. [DOI] [PubMed] [Google Scholar]
- 11.Manassakorn A., Nouri-Mahdavi K., Caprioli J. Comparison of retinal nerve fiber layer thickness and optic disk algorithms with optical coherence tomography to detect glaucoma. Am J Ophthalmol. 2006;141:105–115. doi: 10.1016/j.ajo.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 12.Bengtsson B., Heijl A. A visual field index for calculation of glaucoma rate of progression. Am J Ophthalmol. 2008;145:343–353. doi: 10.1016/j.ajo.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 13.Tape TG. Interpreting diagnostic tests. Available at: <http://gim.unmc.edu/dxtests/Default.htm> [accessed March 11 2011].
- 14.Budenz D.L., Michael A., Chang R.T. Sensitivity and specificity of the Stratus OCT for perimetric glaucoma. Ophthalmology. 2005;112:3–9. doi: 10.1016/j.ophtha.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 15.Bowd C., Zangwill L.M., Berry C.C. Detecting early glaucoma by assessment of retinal nerve fiber layer thickness and visual function. Invest Ophthalmol Vis Sci. 2001;42:1993–2003. [PubMed] [Google Scholar]
- 16.Badala F., Nouri-Mahdavi K., Raoof D.A. Optic disc and nerve fiber layer imaging to detect glaucoma. Am J Ophthalmol. 2007;144:724–732. doi: 10.1016/j.ajo.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brusini P., Johnson C.A. Staging functional damage in glaucoma: review of different classification methods. Surv Ophthalmol. 2007;52:156–179. doi: 10.1016/j.survophthal.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Quigley H.A., Addicks E.M., Green R.W. Optic nerve damage in human glaucoma, III: quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema and toxic neuropathy. Arch Ophthalmol. 1982;100:135–146. doi: 10.1001/archopht.1982.01030030137016. [DOI] [PubMed] [Google Scholar]
- 19.Leung C.K.S., Lam S., Weinreb R.N. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography. Analysis of the retinal nerve fiber layer map for glaucoma detection. Ophthalmology. 2010;117:2337–2344. doi: 10.1016/j.ophtha.2010.01.026. [DOI] [PubMed] [Google Scholar]



