Abstract
General principles provide the framework for eyelid and periorbital reconstruction following tumor excision. Eyelid tumors involving the medial canthus region and/or lacrimal system add to the complexity of reconstructive planning. The nature of the tumor, patient and tissue factors, and surgeon preference guide repair design choices. Reconstructive considerations and options following medial canthal tumor resection are described.
Keywords: Medial canthus, Tumor, Reconstruction, Graft, Flap
1. Introduction
Reconstructive surgical planning is tempered by several factors including the nature of the defect, the health and age of the patient, the availability and integrity of surrounding tissues, and the surgeon’s experience and preferences. In many cases there are multiple choices of repair design, making surgical planning more complex. This article discusses eyelid and periorbital reconstruction options following medial canthal tumor resection.
In general, the following principles provide a guide to successful eyelid and periorbital reconstruction:
-
(1)
If both anterior and posterior eyelid lamellae are to be reconstructed, only one can consist of a graft, the other must be composed of a vascularized flap. Graft on graft reconstruction has a high failure probability.
-
(2)
Provide adequate canthal fixation.
-
(3)
Minimize vertical traction on the eyelids.
-
(4)
Perform any appropriate direct closure of the defect prior to sizing a graft.
-
(5)
Match tissue characteristics as best as possible for grafts and flaps.
-
(6)
Balance complexity of technique(s) with improvement(s) in outcome.
In reconstruction of defects involving the eyelid margin, addressing the following criteria is imperative for a successful outcome:
-
(1)
Development of an aligned and stable eyelid margin.
-
(2)
Maintenance or creation of a “conjunctiva-like” posterior eyelid surface.
-
(3)
Provide sufficient horizontal and vertical eyelid dimensions for maximal function.
-
(4)
Eyelid closure adequate to avoid exposure sequelae.
-
(5)
Optimal eyelid symmetry and cosmesis (tissue of appropriate color, texture, and thickness).
Tumors involving the medial canthal region and/or lacrimal system add to the complexity of reconstructive planning. The literature is replete with reports of various types of skin–muscle flaps to repair medial canthal defects (Harris and Logani, 1998; Chao et al., 2009; Meadows and Manners, 2003; Motomura et al., 2006; Onishi et al., 2007). However, many of these techniques fail to address the issues of eyelid function and stability or recreation of lacrimal drainage. In order to properly design a comprehensive surgical reconstruction plan, an intimate knowledge of the region’s anatomy and function is crucial.
2. Clinical anatomy
2.1. Eyelids
The average distance between the upper and lower eyelids, the vertical palpebral fissure (VPF), is 8–10 mm (mm) in primary (straight) gaze (Read et al., 2006). The upper eyelid margin rests approximately 2 mm below the superior corneal limbus. The lower eyelid margin should be at or 1 mm above the inferior corneal limbus so that no sclera is visible between the limbus and eyelid. The horizontal width of the palpebral fissure (HPF) from medial to lateral canthus is approximately 28–30 mm (Whitnall, 1932). The lateral canthal angle is positioned approximately 2 mm higher than the horizontal meridian of the medial canthal angle. The normal upper eyelid has a travel distance of 13–17 mm from downgaze to upgaze (Nerad, 2001a). The motion of the eyelids along with orbicularis muscle contraction provides the impetus for the lacrimal pump mechanism. Disruption of the position of the eyelid or motion of the blink may result in epiphora.
3. Surgical anatomy
3.1. Medial canthus
The structure of the palpebral fissure is maintained by the tarsal plates suspended by the medial and lateral canthal tendons (Fig. 1A). The medial canthal tendon is formed by the merging of two tendinous arms originating from the anterior and posterior lacrimal crests. The arms fuse temporal to the lacrimal sac and then re-divide into two different arms that attach to the upper and lower eyelid tarsal plates. The tendinous attachment at the anterior lacrimal crest is robust, whereas that of the posterior lacrimal crest is delicate but crucial in maintaining a posterior vector that holds the eyelids in apposition to the globe (Holds, 2007–2008). Reconstruction of the medial canthus must include provisions for a stable replacement of the medial canthal tendon, and an attachment site that is sufficiently posterior to recreate the appropriate contour of the eyelid.
Figure 1.
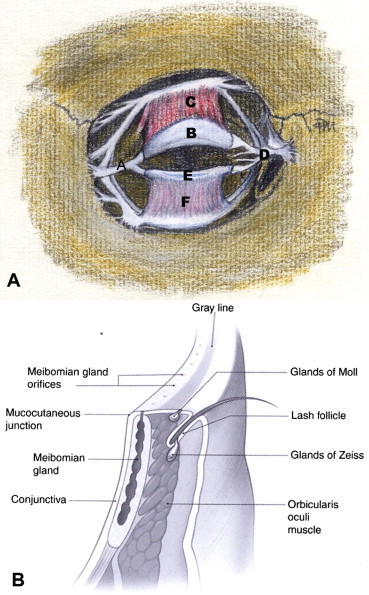
(A) Anterior view of deeper eyelid and periorbital structures: (A) lateral canthal tendon; (B) upper eyelid tarsus; (C) levator aponeurosis; (D) medial canthal tendon; (E) lower eyelid tarsus; (F) lower eyelid retractors (illustration by Trish Michels). (B) Cross-section of the eyelid margin anatomy.
(Reproduced, with permission, from Holds, 2007–2008.)
3.2. Eyelids
The upper eyelid consists of seven distinct anatomic layers (Fig. 1B). From anterior to posterior the layers are (1) skin and subcutaneous tissue, (2) orbicularis oculi muscle, (3) orbital septum, (4) orbital fat, (5) levator and Müller’s muscles (eyelid retractors), (6) tarsus, (7) conjunctiva. The anatomy of the lower eyelid is similar to that of the upper eyelid with two distinct differences. In the lower eyelid, the capsulopalpebral fascia and inferior tarsal muscle are analogous to the upper eyelid’s levator and Müller’s muscles, respectively. These lower eyelid structures are less well defined and developed than their upper lid counterparts. For reconstructive purposes, the eyelids may be divided into surgical units of anterior and posterior lamella. The anterior lamella is composed of skin and orbicularis oculi muscle and the posterior of the tarsus and conjunctiva. The middle lamella is a conceptual space consisting of everything between the anterior and posterior lamella (Collin, 2006a). Any reconstructive design must incorporate replacement of the anterior and posterior lamella.
The tarsi are firm, dense plates of specialized fibrous connective tissue that serve as the structural support of the eyelids (Milz et al., 2005). At its center, the upper eyelid tarsal plate has a greater vertical dimension (10–12 mm) than the lower eyelid tarsal plate (4 mm) (Doxanas and Anderson, 1984). Both upper and lower tarsal plates measure approximately 1 mm in thickness and taper toward their attachments (Holds, 2007–2008). The upper and lower eyelid tarsal plates have sturdy attachments to the periosteum via the canthal tendons medially and laterally. Mechanical disruption or involutional change of either supporting tendon can result in displacement of the tarsi. The shape and rigidity of the tarsi are uniquely important to the final shape of the lid, and replacement with similar shape and texture tissue should be incorporated in the reconstructive plan. Rotated or grafted tarsus and a modified ear cartilage graft are ideal autogenous materials for reconstruction.
The eyelid margin has a rectangular profile (Fig. 1B). The cutaneous epithelium on the anterior surface and the conjunctiva of the posterior surface are separated by non-keratinized epithelium. The gray line is visible along the center of the eyelid margin. The gray color results from the darker color of the superficial portion of the orbicularis oculi muscle anterior to the tarsus, known as the muscle of Riolan lying just deep to the epithelium (Wulc et al., 1987). The gray line is often confused with the mucocutaneous junction of the eyelid margin, which is actually located posterior to the meibomian gland orifices along the eyelid margin. The gray line is a surgically important landmark in reconstruction, canthoplasty and other surgical procedures involving the eyelids. Aligning the gray line of two separated lid margins is one of the cardinal tenets of eyelid reapproximation.
The palpebral conjunctiva lines the posterior surface of the upper and lower eyelids (Figs. 1B and 2). The conjunctiva that envelops the globe is the bulbar conjunctiva. The fornices are the areas of transition between bulbar and palpebral conjunctiva. The function of the conjunctiva is to lubricate, support, and protect the ocular surfaces (Doxanas and Anderson, 1984). In reconstructive surgery, conjunctiva can be rotated or advanced as a flap, grafted from the ipsilateral or contralateral eye, or simulated with split-thickness or full-thickness oral mucosa.
Figure 2.
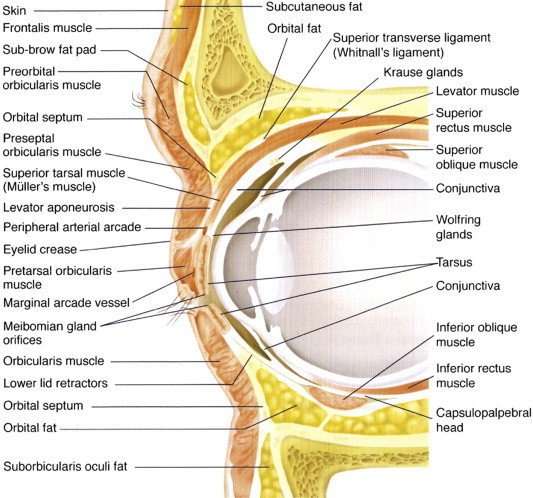
Cross-section of the orbital and periorbital anatomy.
(Reproduced, with permission, from Holds, 2007–2008.)
The upper and lower eyelids are highly vascularized by an anastomosing network of vessels from the internal and external carotid arteries (Erdogmus and Govsa, 2007; Lopez et al., 2008). The exact formation of the network seems to have mild anatomical variation between individuals. This rich blood supply contributes to the high survival rate of grafts and flaps in periocular reconstruction. It also reduces the need to design flap construction based upon specific vessels.
3.3. Lacrimal system
The lacrimal system is comprised of the puncta, canaliculi, common canaliculus, lacrimal sac, and nasal lacrimal duct. The puncta is located at the eyelid margin in both the upper and lower eyelids and connects to the canaliculi. The canaliculi travel 2 mm vertically from the puncta and then turn medially to parallel the eyelid margin for a total length of 8–10 mm. The upper and lower canaliculi merge into a common canaliculus prior to entering the lacrimal sac in approximately 90% of patients, with the remainder entering separately (Orhan et al., 2009). The valve of Rosenmuller lies at this junction preventing regurgitation of fluid within the lacrimal sac into the common canaliculus. This is not a true valve but an orientation of the tissues that acts similar to a valve. The lacrimal sac is 12–15 mm in length and lies within the lacrimal sac fossa which is comprised of the maxilla anteriorly and the lacrimal bone posteriorly. The superior one-third of the lacrimal sac is above the medial canthal tendon while the remainder is below the tendon (Nerad, 2001b). The lacrimal sac fossa is adjacent to the middle meatus within the nose usually at or just anterior to the tip of the middle turbinate. The lacrimal sac drains into the nasal lacrimal duct that is approximately 12–18 mm in length. The nasal lacrimal duct empties into the nasal cavity under the inferior turbinate with flow controlled by the valve of Hasner. Disruption of the lacrimal outflow system results in some degree of epiphora for patients. Repair of existing tissue and recreation or replacement of lacrimal drainage is an important consideration in medial canthal reconstruction.
4. Reconstructive design
4.1. Medial canthus
One of the primary tenets of cancer surgery is complete removal of the lesion and obtaining margins cleared of cancer without regard to the challenges it may present in performing repair. This is especially critical in the medial canthal region where posterior spread of the primary tumor may result in extension to the brain and ultimately death. Tumor margins should be microscopically evaluated and determined “free” of atypia before reconstruction is undertaken.
Reconstructive planning in the medial canthal region is complex due to the variety of structures that can be involved, the unique contours, and the multitude of techniques available. If the defect is restricted to the anterior lamella, spontaneous granulation or FTSG can be employed, with FTSG being a more common and faster healing technique (Lowry et al., 1997). For larger anterior defects, or those lacking a vascular bed for FTSG survival, various transposition or advancement flaps can be employed, such as a glabellar forehead flap or finger flap (Harris and Logani, 1998; Chao et al., 2009; Meadows and Manners, 2003; Motomura et al., 2006; Onishi et al., 2007). When the defect involves full-thickness medial eyelid(s) or sacrifice of the medial canthal tendon the remaining eyelid must be reapproximated and fixated to periosteum or bone. Full-thickness eyelid defects involving more than 50% of the lid rarely can be closed and reapproximated to the canthal tendon primarily. In these instances both anterior and posterior lamellar components must be addressed via lid sharing techniques, free grafts, and flaps. Defects involving the lacrimal system add a degree of complexity and may require either primary microsurgical reconstruction or delayed repair depending on the amount of disruption and the nature of the excised tumor.
Facial plastic surgeons are familiar with skin grafting techniques and the various flaps utilized in medial canthal reconstruction. However, when eyelid reconstruction is incorporated into the repair, modifications to standard flap and grafting techniques may be required. Full-thickness skin grafts to the eyelids and canthus must be thinner than those used on other areas of the face. Consequently, pre and post auricular grafts debrided of their subcutaneous tissues work best. Advancement and rotational flaps of thicker forehead and cheek tissue must also be thinned. This can decrease their vascular perfusion, their ability to support a posterior lamellar graft, and even their own survival. If necessary a flap can be thinned as a secondary procedure.
When the medial canthal tendon is interrupted or completely lost, the remaining eyelid portion of the tendon must be fixated to the area of the tendinous origin to ensure proper alignment and support of the eyelid(s). If only the anterior limb of the tendon is disrupted, it may not cause malposition of the medial canthus if the posterior limb remains intact (Collin, 2006b). If any tendon remains on the anterior or posterior lacrimal crest, direct reattachment with polyglactin or polypropylene suture is possible. When the tendon is absent and the periosteum remains intact, suture can be used to fixate to the periosteum of the posterior lacrimal crest. In cases where periosteum is absent or of insufficient quality, a titanium miniplate can be fixated to bone and the tendon sutured to the plate (Howard et al., 1992). Alternatively or when the bony anatomy precludes use of a miniplate, transnasal wiring may be employed. One must also take into account the nature of the tumor as metallic based implants or wires that can produce artifact or limitations on surveillance scans of the resected area.
4.2. Eyelids
4.2.1. Small defects of the upper and lower eyelid
Small eyelid defects (<33% involvement) can be repaired by direct primary closure (Fig. 3A and B). An additional 2–5 mm of medial advancement of the lateral portion of the lid can be accomplished via a canthotomy and cantholysis of the superior or inferior arms of the lateral canthal tendon. In these cases, anterior and posterior eyelid margin alignment and sturdy tarsal reapproximation are the keys to satisfactory reconstruction.
Figure 3.
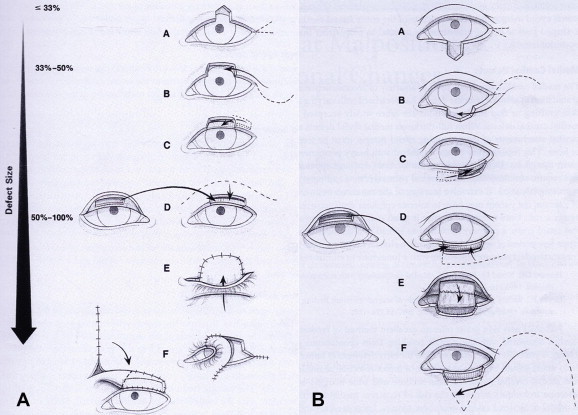
(A) Reconstructive options for upper eyelid defects: (A) direct primary closure with or without canthotomy and cantholysis; (B) direct primary closure with canthotomy/cantholysis and semicircular flap; (C) adjacent tarsoconjunctival flap with FTSG (FTSG not drawn); (D) free tarsoconjuctival graft and skin–muscle flap; (E) Cutler–Beard full-thickness lower eyelid advancement flap; (F) lower eyelid switch flap; (G) glabellar/median forehead flap with free tarsoconjunctival graft. (B) Reconstructive options for lower eyelid defects: (A) direct primary closure with or without canthotomy and cantholysis; (B) direct primary closure with canthotomy/cantholysis and semicircular flap; (C) adjacent tarsoconjunctival flap with FTSG; (D) free tarsoconjunctival graft and skin–muscle flap; (E) Hughes upper eyelid tarsoconjunctival flap and FTSG (FTSG not drawn); (F) Mustardè flap with free tarsoconjunctival graft.
(Adapted from and reproduced, with permission, from Holds, 2007–2008.)
4.2.2. Moderate defects of the upper and lower eyelid
Moderate eyelid defects (33–50% involvement) can potentially be reconstructed by direct closure with canthotomy and cantholysis. When sufficient horizontal lid mobilization or tissue is not provided with canthotomy/cantholysis, a semicircular rotational flap (Tenzel) can be constructed with an arching incision above (for lower eyelid) or below (for upper eyelid) the lateral canthal angle (Fig. 3A and B and B-B). The flap is then advanced medially allowing the lid to be closed in a direct fashion. A “new” lateral canthus is created by aligning the rotated skin and orbicularis with the gray line of the intact eyelid and securing the rotated lateral canthal subcutaneous tissue to the intact arm of the lateral canthal tendon.
In cases where eyelid structural support is lacking tarsal sharing procedures can be utilized. An adjacent tarsoconjunctival flap can be formed lateral to the defect and advanced medially reforming the posterior lamella (Fig. 3A–C and B and C). This is more difficult in the lower eyelid since the modest vertical height of the tarsus allows only horizontal sharing if sufficient tarsus for lid stabilization is to remain. Alternatively a free tarsoconjunctival graft from the contralateral upper lid or tarsal substitutes can be used. The anterior lamella is then reconstructed with an adjacent advancement or transposition flap should the eyelid skin display sufficient laxity. Midface elevation may also be required to prevent lower eyelid retraction and ectropion.
4.2.3. Large defects of the upper and lower eyelid
Large eyelid defects (>50% involvement) are the most challenging reconstructions to design and perform. The amount of tissue loss usually requires both grafting and advancement of adjacent tissues. In defects of this size it is paramount that the structural support of the posterior lamella be addressed for the resulting eyelid reconstruction. A free tarsoconjunctival graft can be harvested from the contralateral upper lid to repair the posterior lamellar aspect of the defect. The anterior portion can be composed of a sliding skin–muscle flap of adjacent eyelid tissues if there is sufficient laxity. Alternatively a tarsoconjunctival flap can be combined with a FTSG harvested from the contralateral upper eyelid or other preferred sources (pre/post auricular, clavicular). A slight “over-estimation” of tissues should be grafted as there is a tendency for the reconstructed areas to retract (Collin, 2006c).
When sufficient tarsus or conjunctiva is not available, a hard palate graft is a viable alternative that provides both support and a posterior mucosal surface (Leibovitch et al., 2006). This method is primarily used in the lower eyelid. Hard palate in the upper lid causes increased risk for corneal irritation. Alternatively, a nasal septal cartilage graft with intact mucoperichondrium can be utilized (Collin, 2006a). If sufficient tarsus remains for lid margin stability, oral mucosa or AlloDerm® (Life Cell, Branchburg, NJ) can be grafted as a conjunctival substitute in fornix reconstruction. When tarsus is deficient, ear cartilage covered posteriorly by vascularized conjunctiva can replace the posterior lamella. As previously noted, a vascularized flap must be in apposition with any free graft to improve viability.
4.2.4. Large defects of the lower eyelid
The modified Hughes technique is often the procedure of choice for large lower eyelid defects (Fig. 3B–E). In the Hughes technique, a vascularized flap of upper lid conjunctiva and tarsus is created and advanced into the lower lid defect. It is sutured into place to remaining tarsus, conjunctival cul-de-sac, or to the canthal angle reconstruction. The anterior lamella is reconstructed with a FTSG taken from contralateral eyelid or pre/post auricular area. This reconstructive design results in a conjunctival bridge from the upper eyelid across the pupil into the lower defect for several weeks. Once the lower eyelid graft is revascularized in approximately 4–8 weeks, a second procedure divides the flap. Amblyopia should be considered when planning reconstruction involving eyelid closure in young children.
The Mustardè cheek flap works well for a large anterior lamellar defect (Fig. 3B–F). It can be considered a progression of the smaller Tenzel rotational flap and utilized with any separate posterior lamellar repair technique. Consideration of this procedure should be made in any large lower eyelid defect when eyelid closure is a concern such as a monocular state or children with amblyopia risk. This reconstructive design also has the advantage of being able to repair defects with extensive vertical involvement that extends down the lower lid into the cheek.
4.2.5. Large defects of the upper eyelid
The Cutler–Beard procedure consists of advancing a full-thickness eyelid flap from the ipsilateral lower eyelid (Fig. 3A–E). The counterpart to the Hughes flap for lower eyelid defects, the Cutler–Beard differs in that it supplies tissues for both posterior and anterior reconstruction. A tarsal substitute of cartilage, tarSys™ (IOP, Costa Mesa, CA), or thick AlloDerm® should be inserted between the anterior and posterior lamella. While convenient, lower lid deformity and upper lid entropion are common complications. Alternatively, the “Bucket–Beard” procedure utilizes the same lower eyelid flap and interposed tarsal replacement, with skin and orbicularis brought down in a bucket handle flap from below the brow to replace the margin anterior lamellae (Foster, 2007). A FTSG is then placed below the brow. Less shifting of the lower eyelid skin is required, and this diminishes postoperative lower eyelid complications. A secondary procedure is required by both techniques for flap division.
A lower eyelid switch flap is another type of full-thickness lower eyelid pedicle graft (Fig. 3A–F). Unlike the Cutler–Beard, the lid switch flap is advanced and rotated into position. While there is the benefit of the lower eyelid lashes being in proper position when rotated into the upper lid, there is a significant disadvantage in the form of the large lower lid defect that is created. This defect must be repaired in a secondary procedure with a posterior lamellar graft and a cheek rotation flap to cover the anterior portion of the defect. There is added risk of 7th nerve palsy from flap dissection as well as lower lid retraction from the weight and subcutaneous tissue contraction of the cheek flap (Collin, 2006c). Additionally, a second procedure is required to sever the conjunctival bridge approximately 3–6 weeks after placement.
A glabellar (median forehead) flap can be rotated to provide tissues for repair of the anterior lamella of the upper lid and medial canthus if it is also involved (Fig. 3A–G). The flap requires primary thinning if used for upper lid repair and may require secondary thinning procedures. Daily digital massage over a period of time may also be required to reduce the incidence of scarring in the medial canthal region (Collin, 2006c). The skin type match is not as good as some of the alternatives and care needs to be taken not to include hair follicles if possible.
In large upper lid defects, if the levator aponeurosis is disrupted or partially excised in the tumor resection, upper eyelid ptosis may result. Even if this is recognized at the time of the reconstruction, it is hard to primarily gauge the resultant effect on eyelid position. Typically, when the primary reconstruction of the tissue is stable, a secondary levator advancement or frontalis suspension procedure is performed for correction.
4.3. Combined upper and lower eyelid defects
Defects of any size involving both upper and lower eyelids are reconstructed following the same principles as isolated lid defects. Similarly there is no alteration in the decision making process if medial canthal structures or the lacrimal system is also involved. There exists no singular flap that can address all full-thickness upper and lower eyelid and medial canthus defects. Thus, a minimum of two flaps is required for these types of combined defects (Motomura et al., 2006).
4.4. Lacrimal system involvement
Microsurgical repair of the lacrimal system can be combined with various eyelid and medical canthal reconstructions. However, until tumor recurrence can be ruled out, lacrimal surgery that creates a pathway for tumor spread into the sinuses or nasal cavities is avoided. Length of clinical surveillance without signs of recurrence prior to lacrimal repair varies depending upon lesion type, with melanoma and sebaceous cell requiring the longest interval.
Partial interruptions of the canaliculus of the upper or lower eyelid can be repaired primarily with silicone intubation and surgical reanastomosis of the canalicular ends. Crawford (JEDMED, St. Louis, MO) stents or Infant Bika (FCI Ophthalmics, Marshfield Hills) intubation are utilized for bicanalicular and Mini Monoka (FCI) or Mono-Crawford (FCI) stents for monocanalicular stenting. Using a pigtail probe, silicone tubing can also be used to stent the upper and lower canaliculi without the need to intubate the nasolacrimal duct. The silicone stents are generally left in place three months and then removed. Silicone intubation as part of the primary repair demonstrates a high success rate in maintaining a patent, functional drainage system in the absence of tumor of the lacrimal system (Spinelli et al., 2005).
In instances where tumor resection involves the complete loss of one canaliculus and primary reconstruction is not possible, follow-up evaluation is used to determine whether the patient subsequently develops epiphora. Some patients remain asymptomatic if there is one normal functioning canaliculus and good position of both lids. If both canaliculi have been sacrificed options are tempered by the nature of the lesion and the remaining tissues.
Following primary reconstruction and sufficient tumor surveillance, in cases where the patient has symptomatic epiphora and primary lacrimal repair is unsuccessful or the nature of the tumor does not allow for it, then a conjunctivodacryocystorhinostomy (CDCR) with Jones tube placement can be offered. If the patient is asymptomatic regarding tearing, further intervention to re-establish a lacrimal drainage system is not required. Older patients are less symptomatic with epiphora due to the age-related decrease in tear function (Mathers et al., 1996).
5. Summary
It is nearly an impossible task to cover every permutation of defects and available reconstructive choices for this highly complex facial region. The goal of this article is to provide a summary of the pertinent anatomy, fundamental reconstructive principles, and “workhorse” techniques available for successful functional and cosmetic outcomes. Figs. 4 and 5 depict a common medial canthal lesion with subsequent reconstruction and postoperative results. Fig. 6 illustrates a “decision tree” outlining the reconstructive options discussed.
Figure 4.
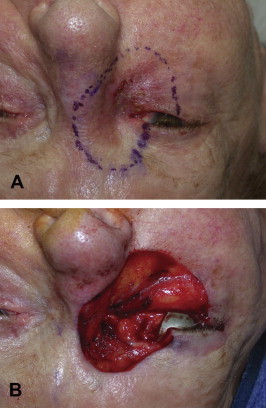
(A) Preoperative photograph of an 88-year-old female with biopsy confirmed melanoma of the right medial canthus. The excision margins have been marked with a 5 mm border surrounding the lesion. The margins include the entire medial canthus, greater than 50% of the lower lid, 33–50% of the upper lid, and lacrimal systems of both upper and lower eyelids. Portions of the brow, nose, and cheek are also encompassed within the resection margins. (B) Intraoperative photograph of the resulting defect immediately following excision of the tumor detailed in (A). The depth of the resection was to bare sclera and continued medially on the surface of the medial canthal tendon to the lateral nasal wall. Dissection was continued superiorly and inferiorly to the included portions of the brow and cheek, respectively. All tissues anterior to periosteum were removed.
Figure 5.
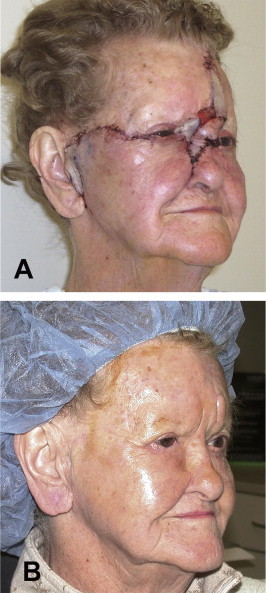
(A) Postoperative Day 1. The lower eyelid was reconstructed with a free tarsoconjunctival graft (posterior lamella) from the contralateral upper lid and a Mustardè flap (anterior lamella). The upper eyelid was reapproximated to the medial canthal tendon following a lateral canthotomy/cantholysis. A glabellar flap was rotated inferior to the lateral nasal wall and a portion of the medial canthus. Telfa gauze is placed under the flap bridge in anticipation for subsequent reversal. A FTSG was harvested from the patient’s left clavicular area and portioned to supply graft material to the preauricular, medial canthal, and forehead regions. The lacrimal system was not addressed secondary to the nature of the tumor. (B) Postoperative Day 136. All original flaps and grafts remain viable and no tumor recurrence is noted. The glabellar flap has previously been reversed. The raised island of tissue above the left brow was thinned at this visit. Lid margins are in good alignment and there was no indication of ocular exposure.
Figure 6.
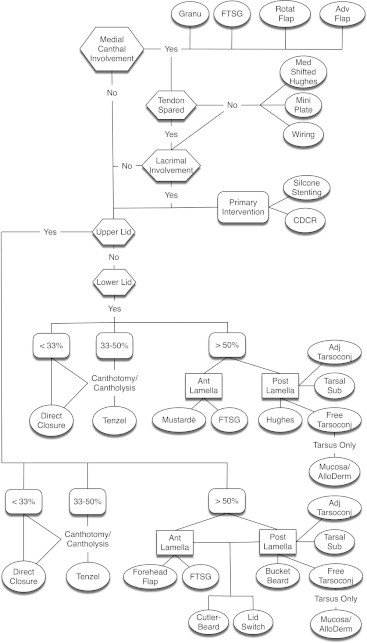
Decision tree outlining surgical design options for upper and lower lid reconstruction with or without medical canthal, tendinous, and/or lacrimal involvement.
Acknowledgment
The authors thank Trish Michels for her medical artwork.
References
- Chao, Y., Xin, X., Jiangping, C., 2009. Medial canthal reconstruction with combined glabellar and orbicularis oculi myocutaneous advancement flaps. J. Plast. Reconstr. Aesthet. Surg. (published online ahead of print December 1, 2009). [DOI] [PubMed]
- Collin J.R.O. third ed. Butterworth–Heinemann; Oxford: 2006. A Manual of Systematic Eyelid Surgery. pp. 16–23. [Google Scholar]
- Collin J.R.O. third ed. Butterworth–Heinemann; Oxford: 2006. A Manual of Systematic Eyelid Surgery. p. 149. [Google Scholar]
- Collin J.R.O. third ed. Butterworth–Heinemann; Oxford: 2006. A Manual of Systematic Eyelid Surgery. pp. 115–145. [Google Scholar]
- Doxanas M.T., Anderson R.L. Clinical Orbital Anatomy. Williams & Wilkins; Baltimore: 1984. Eyebrows, eyelids, and anterior orbit; pp. 57–88. [Google Scholar]
- Erdogmus S., Govsa F. The arterial anatomy of the eyelid: importance for reconstructive and aesthetic surgery. J. Plast. Reconstr. Aesthet. Surg. 2007;60(3):241–245. doi: 10.1016/j.bjps.2006.01.056. [DOI] [PubMed] [Google Scholar]
- Foster, J.A., 2007. The Bucket–Beard: a new reconstruction technique for large upper eyelid defects. In: Facial Reconstructive and Aesthetic Surgery Meeting, January 31, 2007, Salt Lake City, UT.
- Harris G.J., Logani S.C. Multiple aesthetic unit flaps for medial canthal reconstruction. Ophthalmol. Plast. Reconstr. Surg. 1998;14(5):352–359. doi: 10.1097/00002341-199809000-00010. [DOI] [PubMed] [Google Scholar]
- Holds J.B., editor. Basic and Clinical Science Course Section 7: Orbit, Eyelids, and Lacrimal System. American Academy of Ophthalmology; San Francisco: 2007–2008. pp. 141–149. [Google Scholar]
- Howard G.R., Nerad J.A., Kersten R.C. Medial canthoplasty with microplate fixation. Arch. Ophthalmol. 1992;110(12):1793–1797. doi: 10.1001/archopht.1992.01080240133046. [DOI] [PubMed] [Google Scholar]
- Leibovitch I., Malhotra R., Selva D. Hard palate and free tarsal grafts as posterior lamella substitutes in upper lid surgery. Ophthalmology. 2006;113(3):489–496. doi: 10.1016/j.ophtha.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Lopez R., Lauwers F., Paoli J.R., Boutault F., Guitard J. The vascular system of the upper eyelid. Anatomical study and clinical interest. Surg. Radiol. Anat. 2008;30(3):265–269. doi: 10.1007/s00276-008-0323-8. [DOI] [PubMed] [Google Scholar]
- Lowry J.C., Bartley G.B., Garrity J.A. The role of second-intention healing in periocular reconstruction. Ophthalmol. Plast. Reconstr. Surg. 1997;13(3):174–188. doi: 10.1097/00002341-199709000-00004. [DOI] [PubMed] [Google Scholar]
- Mathers W.D., Lane J.A., Zimmerman M.B. Tear film changes associated with normal aging. Cornea. 1996;15:229–234. doi: 10.1097/00003226-199605000-00001. [DOI] [PubMed] [Google Scholar]
- Meadows A.E., Manners R.M. A simple modification of the glabellar flap in medial canthal reconstruction. Ophthalmol. Plast. Reconstr. Surg. 2003;19(4):313–315. doi: 10.1097/01.IOP.0000075796.48708.4D. [DOI] [PubMed] [Google Scholar]
- Milz S., Neufang J., Higashiyama I. An immunohistochemical study of the extracellular matrix of the tarsal plate in the upper eyelid in human beings. J. Anat. 2005;206(1):37–45. doi: 10.1111/j.0021-8782.2005.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motomura H., Taniguchi T., Harada T., Muraoka M. A combined flap reconstruction for full-thickness defects of the medial canthal region. J. Plast. Reconstr. Aesthet. Surg. 2006;59(7):747–751. doi: 10.1016/j.bjps.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Nerad J.A. Mosby; St. Louis: 2001. Oculoplastic Surgery. p. 171. [Google Scholar]
- Nerad J.A. Mosby; St. Louis: 2001. Oculoplastic Surgery. p. 50. [Google Scholar]
- Onishi K., Maruyama Y., Okada E., Ogino A. Medial canthal reconstruction with glabellar combined Rintala flaps. Plast. Reconstr. Surg. 2007;119(2):537–541. doi: 10.1097/01.prs.0000246381.45284.a1. [DOI] [PubMed] [Google Scholar]
- Orhan, M., Govsa, F., Saylam, C., 2009. Anatomical details used in the surgical reconstruction of the lacrimal canaliculus: cadaveric study. Surg. Radiol. Anat. (published online ahead of print May 29, 2009). [DOI] [PubMed]
- Read S.A., Collins M.J., Carney L.G., Iskander R.D. The morphology of the palpebral fissure in different directions of vertical gaze. Optom. Vis. Sci. 2006;83(10):715–722. doi: 10.1097/01.opx.0000236811.78177.97. [DOI] [PubMed] [Google Scholar]
- Spinelli H.M., Shapiro M.D., Wei L.L., Elahi E., Hirmand H. The role of lacrimal intubation in the management of facial trauma and tumor resection. Plast. Reconstr. Surg. 2005;115(7):1871–1876. doi: 10.1097/01.prs.0000164687.41948.62. [DOI] [PubMed] [Google Scholar]
- Whitnall S.E. second ed. Oxford Medical Publishers; London: 1932. The Anatomy of the Human Orbit and Accessory Organs of Vision. pp. 115–123. [Google Scholar]
- Wulc A.E., Dryden R.M., Khatchaturian T. Where is the gray line? Arch. Ophthalmol. 1987;105:1092–1098. doi: 10.1001/archopht.1987.01060080094035. [DOI] [PubMed] [Google Scholar]


