Abstract
Objectives
The objective of this study was to assess the ocular complications and visual loss among patients with severe vernal keratoconjunctivitis (VKC).
Methods
Four hundred and thirty-one patients with VKC seen at Ibn Al-Haitham Eye Center were the study group. This is a retrospective non-comparative observational study between 01 January 2002 and 31 December 2002. Visual acuity was measured with the standard Snellen visual acuity chart and for children under 5 years of age Kay pictures were used. Visual impairment was assessed by means of the World Health Organization criteria for visual disabilities. Cases with severe VKC that developed ocular complications leading to blindness and severe visual impairment were analyzed.
Results
The majority of VKC patients were males (75.9%) with a male:female ratio of 3.1:1. A total of 68 (15.7%) patients (54 boys and 14 girls) had severe VKC. The ocular findings among 20 patients with severe VKC that led to blindness and severe visual impairment included keratoconus (7); steroid-induced cataract (5), central corneal scars (5) and steroid-induced glaucoma (3). Two of the keratoconus cases developed acute hydrops.
Conclusion
Severe VKC in developing countries including Yemen is a potentially blinding disease. Visual loss may be due to keratoconus and corneal scars, as well as complications of the unsupervised use of topically administered corticosteroids.
Keywords: Cataract, Glaucoma, Keratoconus, Visual impairment, Vernal keratoconjunctivitis
1. Introduction
The eye is a frequent target of inflammation in both local and systemic allergic reactions. The vast majority of ocular allergy affects the conjunctiva. Vernal keratoconjunctivitis (VKC) is a severe form of ocular allergic conjunctivitis causing disturbance of normal activities at school or work due to severe itching, grittiness, foreign body sensation, difficulty in opening the eyelids, photophobia and copious mucous discharge (Kosrirukvongs et al., 2001).
VKC is a potentially severe, chronic, allergic condition, causing bilateral recurrent inflammatory disorder of the conjunctiva and cornea. Typically occurs in males before the age of 10 in 80% of cases, it lasts 2–10 years, and it usually resolves during puberty. Males have an earlier presentation of symptoms than females and the male to female ratio decreases with age (Bonini et al., 2004).
VKC is more prevalent in hot and dry areas (Mediterranean basin, the Middle East, Africa and the Indian subcontinent). It is relatively unusual in most of North America and Western Europe (Bremond-Gignac et al., 2008). Risk factors include age, underlying atopic predisposition, extent of allergen exposure and individual immune response to antigenic stimulation. There is a significant history of other atopic manifestations such as eczema or asthma in patients with VKC (Ajaiyeoba, 2003). A family history of atopy is found in these patients.
Three types of VKC are recognized. Limbal type (fine papillae with circumferential gelatinous limbal infiltration and Horner-Trantas dots); the palpebral type (giant papillae of >1 mm in diameter on the superior tarsal conjunctiva) and a mixed type. These features leave no doubt as to the diagnosis of VKC. The reasons underlying the development of the various types of VKC in these patients are not understood (Kumar, 2009).
The main symptoms are itching; redness and foreign body sensation. Lacrimation; photophobia; blepharospasm and pseudo-ptosis due to palpebral thickening are highly specific symptoms of VKC. These symptoms if not treated appropriately can persist for weeks (Leonardi and Secchi, 2003). Seasonal exacerbation is common, but patients may have symptoms year-round especially those living in subtropical or desert climates. More than 60% of patients have repeated recurrences all year round (seasonal variation) and this led to the widely accepted hypothesis that VKC is an immunologically mediated hypersensitivity reaction to environmental antigens (Leonardi, 2002).
The signs include papillary response of the conjunctiva, principally of the limbus or upper tarsus; thick, abundant and ropy mucus; Trantas dots and “cobblestone papillae”. Keratitis (which occurs in up to 50% of cases) (Iqbal et al., 2003) and shield ulcers are sight-threatening complications. There is an association of Keratoconus in VKC patients (Totan et al., 2001). Other risks are of cataract and glaucoma due to the steroids. VKC may cause significant complications and lead to loss of vision (Bremond-Gignac et al., 2008; Bonini et al., 2003).
Although several studies have been published on this disease, only few dealt with the visual problems and ocular complications of VKC. The purpose of this study was to assess the ocular complications and visual loss among patients with severe VKC in Yemen.
2. Patients and methods
This is a retrospective non-comparative observational study done in Ibn Al-Haitham Eye Center which is affiliated to the University of Science and Technology in Sana’a, Yemen. The study was done between 01 January 2002 and 31 December 2002.
Four hundred and thirty-one consecutive patients (431) with VKC were the study group during the study period. Inclusion criteria were recurrent bilateral symptomatic VKC with conjunctival giant papillae formation at the superior tarsus and/or limbus as the hallmark of the disease and the presence of persistent and recurrent symptoms of conjunctivitis. A simple clinical score by Bonini et al. (2004) was used for disease severity classification. Patients were deemed to have severe VKC if they had had persistent symptoms and signs (diffuse palpebral conjunctival edema; thickening with papillary hypertrophy (P3); giant papillary conjunctivitis and had limbal infiltration of 180° or more). Cases with allergic conjunctivitis other than VKC were excluded from the study.
Data collected included best spectacle corrected visual acuity (BSCVA) to each eye using Snellen’s projection chart. For children under the age of 5 years Kay pictures were used. The World Health Organization (WHO) criteria for visual disabilities were used to assess visual impairment. The WHO recommended definitions of visual impairment and blindness, where normal vision, mild visual impairment, severe visual impairment and blindness were defined as visual acuity (VA) of 6/6–6/18; 6/24–6/60; <6/60 to 3/60 and <3/60, respectively. Best corrected visual acuity used was in the better eye.
Haag Streit slitlamp bio-microscopy for anterior segment examination, +90 Dioptre Volk lens for fundal examination and Zeiss Goldmann applanation tonometry information were retrieved from the case notes. Case notes of patients with visual acuity (VA) 3/60–6/60 in the better eye (severe visual impairment) or <3/60 (blind) were selected for more extensive chart review to establish the main cause of severe visual impairment or blindness.
Descriptive analysis was performed on the data collected using Microsoft Excel® spreadsheet 2003 (Microsoft Corporation, Seattle, USA).
The study was approved by the Research and Ethics Committee of Ibn Al-Haitham Eye Center and the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975, as revised in 2000.
3. Results
The majority of VKC patients (n = 431) were males (n = 327) and females (n = 104) with a male:female ratio of 3.1:1. Table 1 shows the age distribution of the patients with VKC. Gender distribution, clinical forms and age of onset are shown in Table 2. All cases had bilateral disease.
Table 1.
Age distribution of 431 vernal keratoconjunctivitis patients in Yemen study.
| Age (years) | Male | Female | Total |
|---|---|---|---|
| 0–4 | 25 | 7 | 32 |
| 5–9 | 131 | 49 | 180 |
| 10–14 | 110 | 34 | 144 |
| 15–19 | 56 | 11 | 67 |
| >20 | 5 | 3 | 8 |
| Total | 327 | 104 | 431 |
Table 2.
Details of 431 vernal keratoconjunctivitis patients in Yemen study.
| Total (%) | Palpebral form (%) | Limbal form (%) | Mixed form (%) | Age at onset (years) | |
|---|---|---|---|---|---|
| Male | 327 (75.9) | 128 (39.1) | 146 (44.7) | 53 (16.2) | 6.5 ± 5 (1–24) |
| Female | 104 (24.1) | 19 (18.3) | 61 (58.7) | 24 (23.0) | 7.5 ± 5 (1–25) |
| Total | 431 (100) | 147 (34.1) | 207 (48.0) | 77 (17.9) | 7.0 ± 5 (1–25) |
A total of 68 (15.7%) patients (54 boys and 14 girls) had severe VKC. Fig. 1 shows the visual acuity among the 68 patients with severe VKC. Visual loss among 20 patients with blindness and severe visual impairment included keratoconus (7), steroid-induced cataract (5), central corneal scars (5) and steroid-induced glaucoma (3) (Fig. 2). Keratoconus was progressive in many patients, resulting in visual loss, often not correctable with glasses or contact lenses. Two of the keratoconus cases developed acute hydrops.
Figure 1.
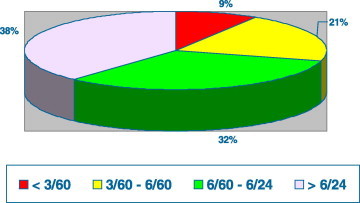
Visual acuity in the 68 patients with severe vernal keratoconjunctivitis.
Figure 2.
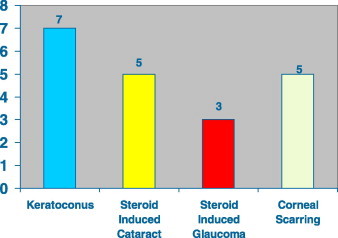
Causes of poor vision in patients with severe vernal keratoconjunctivitis (20 patients).
4. Discussion
Allergic eye diseases including vernal keratoconjunctivitis (VKC) are common diseases in the Mediterranean region including Yemen (Lambiase et al., 2009). In Nigeria, VKC was identified as the most common conjunctival disease in children seen in hospital (Ukponmwan, 2003). A study done by Farouk et al. (2005) in Kuwait University Hospital in Sana’a, Yemen showed that allergic eye diseases are the second most common diagnosis in the eye clinics after refractive errors.
Majority of cases with VKC examined in this study were younger than 10 years. (49.2%) while still the disease persists to the adult age in a number of patients after the age of 20 years (1.9%).
Majority of cases were males (75.9%) with male to female ratio of 3.1:1 and that is similar to studies from Italy and Thailand (Lambiase et al., 2009; Leonardi et al., 2006; Kosrirukvongs et al., 2003).
Diagnosis of VKC was based on the patient’s history and the presence of typical clinical signs and symptoms. The most common clinical type of VKC in this study was the limbal type followed by the palpebral type and is the same as reported in other studies (Lambiase et al., 2009; Kosrirukvongs et al., 2003).
Fortunately majority of cases have the mild and moderate form of VKC. Severe VKC accounted for 15.7% of the studied group and that is slightly more than the case series published by Leonardi et al. (2006) (7.8%) and Lambiase et al. (2009) (10%).
The ocular complications leading to blindness and severe visual impairment affected 20 patients (4.6%) and that is similar compared to studies (Bremond-Gignac et al., 2008). In a study by Tabbara in Saudi Arabia (Tabbara, 1999), 21% had a BSCVA of 20/200 or less due to steroid-induced cataract, steroid-induced glaucoma, central corneal scars, irregular astigmatism and keratoconus.
Therapy for mild VKC includes preservative-free artificial tears, cold compresses and antihistamines. Treatment in severe cases of VKC is still problematic due to frequent exacerbations. Topical corticosteroids are usually given only for a short period of time to avoid the risk of side effects including glaucoma, cataract and infection (Kumar, 2009). Unfortunately patients and their relatives keep seeing more than one doctor looking for cure from the disease and this leads to the prolonged unsupervised use of steroid drops and increasing the risk of complications.
Cyclosporine drops have good effect in VKC cases with fewer side effects (Gupta and Sahu, 2001) compared to corticosteroids but unfortunately it is not available in Yemen. A close ophthalmological supervision with controlled discontinuous treatment according to the disease severity is essential to avoid self-prescription and misuse of steroid drops.
Limitations of this study are: there were no immunological diagnostic tests done to this group of patients because of limitations in the facilities available in Yemen. History of other associated allergic diseases and family history of allergic diseases were not documented in this study. Also patients were not grouped to those who come from desert areas or the mountains to study the differences in presentations and ocular complications.
5. Conclusions
VKC is not a rare disease in Yemen, and the severe form of VKC is a potentially blinding disease and carries significant societal costs. There is a need to a disease severity grading in order to develop standardized therapeutic guidelines based on the stage of VKC. Visual loss may be due to keratoconus and corneal scars, as well as complications of the unsupervised use of topically administered steroids.
Acknowledgments
We thank administrators and staff of Ibn Al-Haitham Eye Center for permitting us to conduct this study. They assisted and contributed in the patient’s care in our study. Lastly we appreciate the efforts and cooperation of all patients they extended to us in this study.
Footnotes
Presented in the First Yemeni-Saudi conference of Clinical Immunology, Sana’a, 25–26 May 2005.
References
- Ajaiyeoba A.I. Prevalence of atopic diseases in Nigerian children with vernal kerato-conjunctivitis. West Afr. J. Med. 2003;22(1):15–17. doi: 10.4314/wajm.v22i1.27971. [DOI] [PubMed] [Google Scholar]
- Bonini S., Lamiase A., Sgrutella R., Bonini S. Allergic chronic inflammation of the ocular surface in vernal keratoconjunctivitis. Curr. Opin. Allergy Clin. Immunol. 2003;3(5):381–387. doi: 10.1097/00130832-200310000-00011. [DOI] [PubMed] [Google Scholar]
- Bonini S., Coassin M., Aronni S., Lambiase A. Vernal keratoconjunctivitis. Eye. 2004;18:345–351. doi: 10.1038/sj.eye.6700675. [DOI] [PubMed] [Google Scholar]
- Bremond-Gignac D., Donadieu A., Leonardi A., Pouliquen P., Doan S., Chiambarretta F. Prevalence of vernal keratoconjunctivitis: a rare disease? Br. J. Ophthalmol. 2008;92:1097–1102. doi: 10.1136/bjo.2007.117812. [DOI] [PubMed] [Google Scholar]
- Farouk, S., Al-Tal, A., Bamashmus, M., Haider, T., 2005. Prevalence of allergic eye diseases in Kuwait University Hospital, Sana’a, Yemen. Presented in the First Yemeni-Saudi Conference of Clinical Immunology, Sana’a, May 25–26, 2005.
- Gupta V., Sahu P.K. Topical cyclosporine A in the management of vernal conjunctivitis. Eye. 2001;15:39–41. doi: 10.1038/eye.2001.10. [DOI] [PubMed] [Google Scholar]
- Iqbal A., Jan S., Babar T.F., Khan M.D. Corneal complications of vernal catarrh. J. Coll. Physicians Surg. Pak. 2003;13(7):394–397. [PubMed] [Google Scholar]
- Kosrirukvongs P., Visitsunthorn N., Vichyanond P., Bunnag C. Allergic conjunctivitis. Asian Pac. J. Allergy Immunol. 2001;19:237–244. [PubMed] [Google Scholar]
- Kosrirukvongs P., Vichyanond P., Wongsawad W. Vernal keratoconjunctivitis in Thailand. Asian Pac. J. Allergy Immunol. 2003;21:25–30. [PubMed] [Google Scholar]
- Kumar S. Vernal keratoconjunctivitis: a major review. Acta Ophthalmol. Scand. 2009;87:133–147. doi: 10.1111/j.1755-3768.2008.01347.x. [DOI] [PubMed] [Google Scholar]
- Lambiase A., Minchiotti S., Leonardi A., Secchi A.G., Rolando M., Calabria G. Prospective, multicenter demographic and epidemiological study on vernal keratoconjunctivitis: a glimpse of ocular surface in Italian population. Ophthalmic Epidemiol. 2009;16:38–41. doi: 10.1080/09286580802573177. [DOI] [PubMed] [Google Scholar]
- Leonardi A. Vernal keratoconjunctivitis: pathogenesis and treatment. Prog. Ret. Eye Res. 2002;21:319–339. doi: 10.1016/s1350-9462(02)00006-x. [DOI] [PubMed] [Google Scholar]
- Leonardi A., Secchi A.G. Vernal keratoconjunctivits. Int. Ophthalmol. Clin. 2003;43:41–58. doi: 10.1097/00004397-200343010-00007. [DOI] [PubMed] [Google Scholar]
- Leonardi A., Busca F., Motterle L., Cavarzeran F., Fregona I.A., Plebani M. Case series of 406 vernal keratoconjunctivitis patients: a demographic and epidemiological study. Acta Ophthalmol. Scand. 2006;84:406–410. doi: 10.1111/j.1600-0420.2005.00622.x. [DOI] [PubMed] [Google Scholar]
- Tabbara K.F. Ocular complications of vernal keratoconjunctivitis. Can. J. Ophthalmol. 1999;34(2):88–92. [PubMed] [Google Scholar]
- Totan Y., Hepsen I.F., Cekic O., Gunduz A., Aydin E. Incidence of keratoconus in subjects with vernal keratoconjunctivitis: a videokeratographic study. Ophthalmology. 2001;108:824–827. doi: 10.1016/s0161-6420(00)00664-3. [DOI] [PubMed] [Google Scholar]
- Ukponmwan C.U. Vernal keratoconjunctivitis in Nigerians: 109 consecutive cases. Trop. Doct. 2003;33:242–245. doi: 10.1177/004947550303300419. [DOI] [PubMed] [Google Scholar]


