Abstract
Purpose
To report late onset corneal ectasia following myopic LASIK.
Methods
A retrospective cohort case series. Nineteen patients with late onset corneal ectasia following LASIK procedure were examined at The Eye Center, Riyadh, Saudi Arabia. Patients underwent LASIK for myopia with spherical equivalent ranging from −1.4 to −13.75 diopters. Age and gender, history of systemic or local diseases, and time of onset of corneal ectasia were recorded. Eye examination and corneal topographical analyses were done before and after LASIK surgery.
Results
Nineteen patients (29 eyes) with late onset corneal ectasia were identified from 1998 to 2008 in 13 male and six female patients. The mean follow-up period was 108 ± 23 months (range 72–144 months). No patient had pre-operative identifiable risk factors for corneal ectasia and the mean time of onset was 57 ± 24 months (range 24–120 months after LASIK). The pre-operative values included mean central pachymetry 553 ± 25 μm, mean keratometry reading of 42.9 ± 1.5 diopters, average oblique cylinder of 1.4 ± 1.2 diopters, posterior surface elevation of 26 ± 2.1 diopters, corneal flap thickness of 160 μm, mean spherical equivalent of −5.6 ± 3.6 diopters, and calculated residual corneal stromal bed thickness was 288 ± 35 μm. Three (5 eyes) patients developed ectasia after pregnancy. Three (4 eyes) patients developed corneal ectasia following severe adenoviral keratoconjunctivitis and had positive PCR for adenovirus type 8.
Conclusions
Corneal ectasia may develop many years after LASIK surgery and symptoms could go undetected for some time. Pregnancy and adenoviral keratoconjunctivitis occurred post-operatively in six patients.
Keywords: Corneal ectasia, Corneal topography, Keratoconus, LASIK, Adenovirus, Adenoviral keratoconjunctivitis
1. Introduction
Corneal ectasia is the most feared complication following LASIK (Tabbara and Kotb, 2006; Rad et al., 2004; Randleman et al., 2008a,b; Dawson et al., 2008; PIccoli et al., 2003; Ou et al., 2002; Pallikaris et al., 2001). It is characterized by progressive thinning with central and inferior steepening of the cornea. The incidence of progressive corneal ectasia after LASIK has been estimated to be 0.2% (Rad et al., 2004). Current theories suggest that the two main causes of post-LASIK ectasia are preexisting corneal pathology (keratoconus or forme fruste keratoconus), mechanical instability produced by the weakening of the residual corneal stromal bed induced by the flap creation, the effect of laser ablation, and probably eye rubbing. Careful pre-operative assessment of the cornea can greatly reduce the risk of ectasia, although it may occur in previously healthy and unsuspected corneas. Risk factors for corneal ectasia include a thin cornea at baseline, thick corneal flap, excessive ablation, irregular corneal thickness, diverse ablation rates, preexisting keratoconus or form fruste keratoconus, and high intraocular pressure (IOP). In one study abnormal topography was the most significant factor that discriminated cases from controls, followed by residual corneal stromal bed thickness, age, and pre-operative corneal thickness (Randleman et al., 2008a,b). Topographical analysis parameters such as central keratometry, oblique cylinder, pachymetry, inferior and superior corneal diopteric power, posterior surface elevation, and the ratio of posterior to anterior best sphere fit were used as a single proprietary device for pre-operative prediction of keratectasia (Tabbara and Kotb, 2006; Pallikaris et al., 2001). Other scoring systems proposed addition of age and degree of myopia (Randleman et al., 2008a,b; Dawson et al., 2008; Piccoli et al., 2003; Ou et al., 2002; Pallikaris et al., 2001). Patients presented in this case series however had no recognizable pre-operative underlying corneal risk factors for post-LASIK ectasia.
The main purpose of this study is to report a case series of late onset corneal ectasia following LASIK with otherwise no pre-operative corneal risk factors.
2. Patients and methods
2.1. Patients
The medical records of patients with late onset post-LASIK corneal ectasia were reviewed in patients that had the diagnosis of corneal ectasia two years or more after LASIK procedure for myopia ranging from −1.4 to −13.75 diopters. Pre-operative and post-operative topographical analysis was used to assess the risk factors. Eye examination and corneal topographical analyses (ORBSCAN II) were done before and after surgery. Table 1 shows the patients’ characteristics including age, gender, and time of diagnosis of ectasia. Table 2 includes pre-operative and post-operative risk factor assessments that were evaluated. Parameters such as central keratometry, oblique cylinder, pachymetry, inferior and superior corneal diopteric value, posterior surface elevation, and the ratio of posterior to anterior best sphere fit were determined. Ectasia grading system using the above parameters was used to assess the cumulative risk factors pre-operatively, these were compared to post-operative values, and the p-value was determined. Flap thickness and residual corneal stromal bed thickness were assessed. None of the patients had enhancement. Table 3 illustrates the detailed pre-operative assessment of 19 patients (29 eyes) with post-LASIK corneal ectasia. Three (5 eyes) female patients developed bilateral post-LASIK corneal ectasia following pregnancy. Three (4 eyes) patients developed ectasia after adenoviral keratoconjunctivitis.
Table 1.
Characteristics of patients with late onset corneal ectasia after LASIK surgery.
| Number of patients | 19 (29 eyes) |
| Mean age (years) ± SD | 35 ± 6 years |
| Age range | 27–45 years |
| Mean follow-up period ± SD | 108 ± 23 months |
| Gender | |
| Male | 13 patients |
| Female | 6 patients |
| Mean onset of ectasia after LASIK (months ± SD) | 57 ± 24 months |
| Mean post-operative residual corneal stromal bed thickness | 288 ± 35 μm |
| Range of post-operative residual corneal stromal bed thickness | 260–365 μm |
SD = standard deviation.
Table 2.
Pre- and post-operative risk assessment factors.
| Pre-operative | Post-operative | p-Value | |
|---|---|---|---|
| Spherical equivalent (diopters) | |||
| Average | −6.4 ± 3.3 | −1.1 ± 1 | <0.001 |
| Range | −1.4 to −13.75 | −0.25 to −3.25 | |
| K-reading (diopters) | |||
| Average | 42.9 ± 1.5 | 46 ± 5 | 0.012 |
| Range | 41–47 | 37–55 | |
| Central corneal thickness (μm) | |||
| Average | 553 ± 25 | 402 ± 56 | <0.001 |
| Range | 504–590 | 281–508 | |
| Oblique cylinder | |||
| Average | 1.4 ± 1.2 | 3.8 ± 2.4 | <0.001 |
| Range | 0.2–1.5 | 0.6–9.6 | |
| Difference between inferior and superior corneal diopter | |||
| Average | 1 ± 0.02 | 1.1 ± 0.14 | 0.004 |
| Range | 0.9–1.1 | 0.8–1.5 | |
| Posterior BSF/anterior BSF | |||
| Average | 1.0 ± 0.2 | 1.2 ± 0.3 | <0.001 |
| Range | 1–1.24 | 1.2–1.4 | |
| Posterior surface elevation (μm) | |||
| Average | 26 ± 2.1 | 74 ± 19 | <0.001 |
| Range | 22–29 | 23–100 | |
BSF = best sphere fit.
Table 3.
Pre-operative assessments of 19 patients with post-LASIK late onset corneal ectasia.
| Case no. | Eye | Age & gender | Year of Surgery | Pachymetry (μm) | Keratometry | Spherical equivalent (diopters) | I-S index | Time of onset of ectasia following LASIK (years) | Residual corneal stromal bed (μm) | Oblique cylinder | Postop events |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | OD | 28 M | 2001 | 520 | 44.1 D | −8.00 | 1.00 | 5 | 250 | −0.80 D | None |
| OS | 28 M | 2001 | 520 | 42.1 D | −9.00 | 1.00 | 5 | 260 | −0.80 D | ||
| 2 | OD | 36 M | 1999 | 540 | 46.31 D | −7.25 | 1.05 | 5 | 215 | −0.60 D | None |
| OS | 36 M | 1999 | 590 | 47.25 D | −7.75 | 1.03 | 5 | 217 | −1.50 D | ||
| 3 | OD | 45 M | 1999 | 510 | 40.75 D | −4.00 | 1.03 | 6 | 207 | −1.25 D | None |
| OS | 45 M | 1999 | 520 | 42.00 D | −4.25 | 0.99 | 6 | 224 | −1.50 D | ||
| 4 | OD | 28 F | 2000 | 560 | 43.64 D | −6.75 | 0.99 | 2 | 263 | −1.30 D | Pregnancy |
| OS | 28 F | 2000 | 580 | 43.28 D | −7.25 | 0.99 | 2 | 256 | −1.11 D | ||
| 5 | OD | 35 F | 1997 | 556 | 43.14 D | −12.75 | 0.99 | 5 | 235 | −0.49 D | None |
| OS | 35 F | 1997 | 556 | 42.78 D | −13.75 | 0.99 | 5 | 221 | −0.34 D | ||
| 6 | OD | 42 M | 1998 | 580 | 42.32 D | −8.25 | 0.97 | 2 | 270 | −0.80 D | None |
| OS | 42 M | 1998 | 580 | 42.24 D | −10.75 | 1.00 | 2 | 238 | −0.21 D | ||
| 7 | OD | 32 F | 2002 | 560 | 42.38 D | −8.50 | 0.97 | 5 | 310 | −0.78 D | Pregnancy |
| OS | 32 F | 2002 | 560 | 42.5 D | −9.00 | 0.98 | 5 | 312 | −0.90 D | ||
| 8 | OD | 42 M | 1997 | 550 | 41.64 D | −6.75 | 1.04 | 5 | 281 | −1.50 D | None |
| OS | 42 M | 1997 | 550 | 41.75 D | −6.75 | 0.99 | 11 | 281 | −1.40 D | ||
| 9 | OD | 27 M | 1999 | 560 | 42.31 D | −5.50 | 1.00 | 10 | 249 | −0.26 D | None |
| OS | 27 M | 1999 | 560 | 41.99 D | −5.00 | 1.00 | 10 | 261 | −0.72 D | ||
| 10 | OD | 29 M | 1999 | 590 | 42.4 D | −1.50 | 1.04 | 6 | 367 | −1.28 D | AKC |
| OS | 29 M | 1999 | 560 | 41.44 D | −1.50 | 1.01 | 6 | 349 | −0.15 D | AKC | |
| 11 | OS | 37 F | 1999 | 560 | 44.87 D | −2.50 | 1.02 | 3 | 311 | −1.52 D | AKC |
| 12 | OD | 33 M | 1999 | 560 | 43.95 D | −3.75 | 0.99 | 5 | 269 | −1.38 D | None |
| 13 | OD | 29 M | 2000 | 590 | 43.42 D | −4.50 | 0.98 | 3 | 300 | −1.45 D | None |
| 14 | OD | 27 M | 2003 | 504 | 45.70 D | −1.50 | 0.98 | 5 | 315 | −0.50 D | AKC |
| 15 | OD | 29 M | 2003 | 569 | 42.64 D | −12.00 | 1.01 | 4 | 256 | −0.20 D | None |
| 16 | OS | 44 M | 2003 | 510 | 41.12 D | −4.50 | 0.99 | 5 | 270 | −1.25 D | None |
| 17 | OD | 28 F | 2002 | 560 | 41.61 D | −3.00 | 1.02 | 6 | 318 | −0.71 D | None |
| 18 | OS | 37 F | 2000 | 560 | 44.16 D | −5.00 | 1.00 | 3 | 247 | −1.48 D | Pregnancy |
| 19 | OD | 38 M | 2000 | 510 | 44.9 D | −5.00 | 0.99 | 2 | 275 | −1.50 D | None |
AKC = adenoviral keratoconjunctivitis (Type 8).
2.2. Immunochromatography (IC) test
Using a sterile swab, conjunctival specimens were taken from patients with keratoconjunctivitis. This was followed by a short extraction step using a buffer solution. Using the specimen pipettes provided, four drops of the specimen solution were placed into the specimen well of the kit. Time was allowed for the specimen to filter through the kit to the specimen and the control positions. Appearance of a colored line at each position was observed. The test was considered to be positive if two lines were visible each at the specimen and control positions. The test was considered negative if line was visible only at the control position. If no line was visible, the test was considered invalid and a repeat test was performed for that particular specimen. Readings were finalized not more than thirty minutes from application of specimen to the kit. The IC test (Adeno Test, SA Scientific, San Antonio, Texas, USA) was performed according to the manufacturer’s instructions.
2.3. Polymerase chain reaction (PCR)
Conjunctival swabs were obtained from patients who developed keratoconjunctivitis. Viral DNA was extracted from 100 μl of the conjunctival specimen with a Sumitest EX-R&D kit (Medical & Biological Laboratories Co., Ltd., Nagano, Japan), according to the manufacturer’s instructions. The extracted DNA was suspended in 100 μl of TE buffer (10 mM Tris, 1 mM EDTA, pH 8.0), and subjected to PCR as previously described (Saitoh-Inagawa et al., 1996).
2.4. Statistical analysis
All information about patients were collected and entered into a computer-based standardized data entry form for statistical analysis such as: age, gender, mean follow-up, mean onset of ectasia, initial and follow-up visual acuity with p-value assessed using multiple measures ANOVA. The assessment risk factor score was evaluated statistically pre-operatively and post-operatively using the following parameters: central keratometry, oblique cylinder, spherical equivalence, central corneal thickness, difference between inferior and superior corneal diopteric value, posterior surface elevation, and the ratio of posterior to anterior best sphere fit. The p-value was assessed using the paired t-test. All p-values were two-sided and the significance level was set at 0.05. Data analyses were performed using SPSS for windows version 11.0 (SPSS, Inc, Chicago, Illinois, USA).
3. Results
Nineteen patients (29 eyes) with late onset corneal ectasia were identified. There were 13 male patients and six female patients with a mean age of 35 ± 6 years. No patient had identifiable risk factor using the grading system pre-operatively (Figs. 1–3). The mean follow-up period was 108 ± 23 months (range 72 to 144 months) with a mean time of diagnosis of corneal ectasia of 57 ± 24 months (range 24–120 months). The corneal flap thickness was estimated to be 160 μm. Patients underwent LASIK for myopia ranging from −1.4 to −13.75 diopters. Patients had a mean residual corneal thickness (mean 288 μm) (Table 1). The pre-operative values for the assessment of corneal risk are shown in Tables 2 and 3. The mean central corneal thickness was 553 ± 25 μm, mean pre-operative keratometry reading was 42.9 ± 1.5 diopters, average oblique cylinder of 1.4 ± 1.2 diopters, posterior surface elevation of 26 ± 2.1 diopters, mean difference between inferior and superior corneal diopters value was 1 ± 0.02, mean pre-operative spherical equivalent was −6.4 ± 3.3 diopters, and mean posterior best sphere fit over anterior best sphere fit was 1 ± 0.2 diopters. All p-values were significant when compared to post-operative values. Three (5 eyes) patients had corneal ectasia after pregnancy. Three (4 eyes) had post-operative adenoviral keratoconjunctivitis (with interface keratitis). No patients exhibited abnormally high intraocular pressure. No intraoperative or post-operative complications were encountered.
Figure 1.
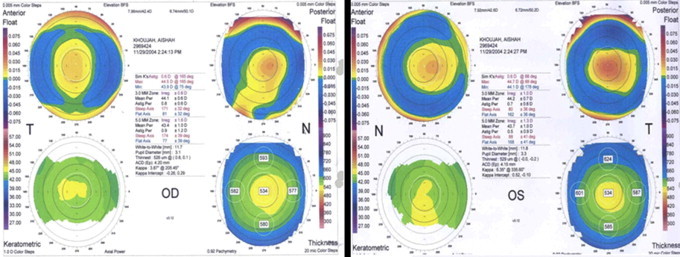
ORBscan of the right and left eyes of a patient before LASIK. Patient had corneal refractive stability for five years after LASIK.
Figure 2.
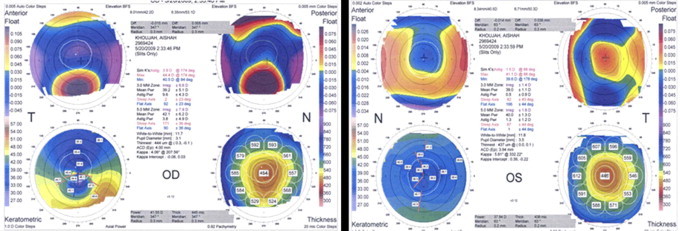
ORBscan of the patient in Fig. 1 five years after LASIK. Patient developed corneal ectasia after delivery.
Figure 3.
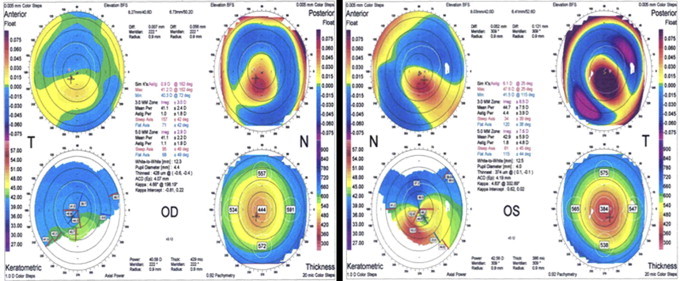
A 28-year-old male who had stable post-LASIK refraction and 20/20 visual acuity for eight years. Patient developed progressive deterioration in vision following adenovirus type 8 infection and bilateral diffuse lamellar keratitis. The ORBscan shows thinning of the cornea with steepening of the anterior surface and posterior surface elevation.
Immunochromatography (IC) (Adeno Test®) tests of conjunctival scrapings were positive for adenovirus and PCR showed all four specimens were adenovirus type 8. All four patients had severe acute adenoviral keratocojunctivitis with preauricular lymphadenopathy, lid swelling, membranous conjunctivitis, follicular conjunctivitis, conjunctival hemorrhage, and chemosis. Two patients had ecchymosis of the eyelids. Patients developed diffuse interface and superficial stromal keratitis which was treated with topical fluorometholone 0.1% eyedrops four times daily which was tapered and discontinued.
4. Discussion
Corneal ectasia is a rare but serious complication of refractive surgery. It is progressive steepening and thinning of the cornea. Many factors have been associated with an increased risk for ectasia including pre-operative topographic abnormality, low pachymetry, elevated posterior surface, low residual corneal stromal bed thickness, young age, and high myopia (Tabbara and Kotb, 2006; Rad et al., 2004; Randleman et al., 2008a,b). Corneal flap creation by mechanical microkeratomes and femtosecond lasers may lead to variable corneal flap thickness and this may influence the residual corneal stromal bed.
The development of corneal ectasia after surgery has grave medical and legal consequences for the patient and the physician. Most cases reported in the literature have been associated with certain risk factors. Certain individuals, however, may develop post-operative corneal ectasia in the absence of known risk factors (Tabbara and Kotb, 2006; Rad et al., 2004; Randleman et al., 2008a,b). The average time of onset of corneal ectasia is 15.3 months (Randleman et al., 2008a,b). The incidence of post-LASIK corneal ectasia has been reduced however by applying careful selection criteria (Randleman et al., 2008a,b). The cases presented, herewith, had late onset post-LASIK ectasia despite “normal” pre-op corneal topography, calculated residual corneal stromal bed thickness of more than 250 μm, normal pachymetry, low myopia, and a mean age of 35 years. Post-operative factors may play a role in the pathogenesis of post-LASIK corneal ectasia. Patients undergoing LASIK should be aware of the fact that there may be a small risk of late onset post-LASIK corneal ectasia several years following successful procedures.
Corneal ectasia may follow corneal refractive surgery because of corneal abnormalities or because of inducing mechanical weakness of the collagen tensile strength. The reduction in the physio-mechanical stability of the cornea appears to result from the permanent weakening of the anterior corneal lamellae and from the thinning of the residual corneal stromal bed (Kerautret et al., 2008; Maguen et al., 2007; Condon et al., 2007; Binder, 2007; Meghpara et al., 2008). The residual stromal thickness following LASIK depends on the thickness of the flap and ablation depth. Flap thickness is not always predictable which may lead to a stromal bed which is thinner than expected (Ou et al., 2002; Pallikaris et al., 2001). Successful cases of LASIK are reported from short term follow-up periods but the incidence of late onset corneal ectasia following LASIK is not known. Histopathologic studies suggest that intermellar and interfibrillar biomechanical slippage occurs when the cornea becomes ectatic after LASIK or PRK in the post-operative stress-bearing regions of the corneal stroma (Dawson et al., 2008). This 2-phase chronic biomechanical failure process is similar to that seen in keratoconus. Composite sciences classify this chronic biomechanical failure process as interfiber fracture (Dawson et al., 2008).
In this study, we report 19 patients with no pre-operative corneal risk factors who developed late onset corneal ectasia after LASIK. Nine eyes of six patients had late onset corneal ectasia with other conditions. Three (5 eyes) patients developed post-LASIK corneal ectasia following pregnancy and three (4 eyes) patients followed keratoconjunctivitis caused by adenovirus type 8. This may be related to physiochemical modulation of the collagen by hormones such as relaxin or by inflammatory mediators.
The role of pregnancy in corneal ectasia may be incidental, circumstantial, or causal. Relaxin hormone increases during pregnancy. Relaxin has been shown to inhibit collagen remodeling of airway disease in mice (Royce et al., 2009). Relaxin may affect the collagen integrity of ligaments and wound healing (Cooney et al., 2009) and can induce matrix degradation of target synovial joints by matrix metalloproteinase (Kapila et al., 2009). The increase in the level of relaxin hormone during pregnancy may contribute to changes in the physiochemical properties of corneal collagen leading to ectasia (Royce et al., 2009; Cooney et al., 2009; Kapila et al., 2009). Adenoviral inflammation of the corneal interface may lead to upregulation of inflammatory cytokines and metalloproteinases leading to collagen loss and further weakening of the corneal stroma.
With time, there is apoptosis of the corneal stromal keratocytes which may contribute to a decrease in corneal collagen synthesis and further weakening of the corneal stroma. Correction of the refractive errors by LASIK weakens the tensile strength of the anterior stroma and depletes the posterior stromal keratocyte (Randleman et al., 2008a,b). The corneal collagen is a dynamic structure with constant remodeling. We tend to diminish permanently the anterior structural integrity of the cornea following LASIK. Delayed onset of corneal ectasia with no risk factors was also reported by Tuli and Iyer (2007). Twa et al (2004) defined the characteristics of corneal ectasia after LASIK for myopia. Furthermore, Rad and associates (2004) reported progressive corneal ectasia after LASIK and found out that ectasia may occur in healthy or diseased eyes. The most important risk factors are residual corneal stromal bed thickness and pre-existing corneal topography. Piccoli et al (2003) reported a case of corneal ectasia that was detected 32 months after LASIK.
In this patient series, pre-operative corneal topography and parameters did not reveal pre-operative underlying risk factors that could predict the development of corneal ectasia. Although pre-operative corneal factors are important predictors of post-LASIK corneal ectasia, ectasia can occur after a successful LASIK procedure even in the absence of apparent pre-operative risk factors (Klein et al., 2006).
The cornea is a dynamic structure. It is a biological system under constant remodeling. Several factors may influence its extracellular matrix homeostasis. Future studies should focus on the corneal keratocytes, corneal collagen, and exogenous and endogenous factors that may influence corneal collagen synthesis and modulation in order to find means of preventing corneal ectasia following LASIK. Late onset corneal ectasia is rare but may occur in the absence of pre-operative corneal risk factors.
Conflict of interest statement
The authors disclose no proprietary or commercial interest in this study. This study was performed as required by the Institutional Review Board of The Eye Center, Riyadh, Saudi Arabia, which all authors are affiliated with.
Acknowledgments
The authors thank the Administrator of The Eye Center, Mrs. Najwa Tabbara for her help and support.
This study was supported in part by a Fund from The Eye Foundation for Research in Ophthalmology and The Eye Center, Riyadh, Saudi Arabia.
References
- Binder P.S. Analysis of ectasia after laser in situ keratomileusis: risk factors. J. Cataract Refract. Surg. 2007;33:1530–1538. doi: 10.1016/j.jcrs.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Condon P.I., O’Keefe M., Binder P.S. Long-term results of laser in situ keratomileusis for high myopia: risk for ectasia. J. Cataract Refract. Surg. 2007;33:583–590. doi: 10.1016/j.jcrs.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Cooney T.E., Schober J.M., Lubahn J.D., Konieczko E.M. Relaxin’s involvement in extracellular matrix homeostatis. Ann. NY Acad. Sci. 2009;1160:329–335. doi: 10.1111/j.1749-6632.2008.03801.x. [DOI] [PubMed] [Google Scholar]
- Dawson D.G., Randleman J.B., Grossniklaus H.E. Corneal ectasia after excimer laser keratorefractive surgery: histopathology, ultrastructure, and pathophysiology. Ophthalmology. 2008;115:2181–2191. doi: 10.1016/j.ophtha.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Kapila S., Wang W., Uston K. Matrix metalloproteinase induction by relaxin causes cartilage matrix degradation in target synovial joints. Ann. NY Acad. Sci. 2009;1160:332–338. doi: 10.1111/j.1749-6632.2009.03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerautret J., Colin J., Touboul D., Roberts C. Biochemical characteristics of the ectatic cornea. J. Cataract Refract. Surg. 2008;34:510–513. doi: 10.1016/j.jcrs.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Klein S.R., Epstein R.J., Randleman J.B., Stulting R.D. Corneal ectasia after laser in situ keratomileusis in patients without apparent preoperative risk factors. Cornea. 2006;25:388–403. doi: 10.1097/01.ico.0000222479.68242.77. [DOI] [PubMed] [Google Scholar]
- Maguen E., Maguen B., Regev L., Ljubimov A.V. Immunohistochemical evaluation of two corneal buttons with post-LASIK keratectasia. Cornea. 2007;26:983–991. doi: 10.1097/ICO.0b013e3180de1d91. [DOI] [PubMed] [Google Scholar]
- Meghpara B., Nakamura H., Macsai M. Keratectasia after laser in situ keratomileusis: a histopathologic and immunohistochemical study. Arch. Ophthalmol. 2008;126:1655–1663. doi: 10.1001/archophthalmol.2008.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou R.J., Shaw E.L., Glasgow B.J. Keratectasia after laser in situ keratomileusis (LASIK): evaluation of the calculated residual stromal bed thickness. Am. J. Ophthalmol. 2002;134:771–773. doi: 10.1016/s0002-9394(02)01656-2. [DOI] [PubMed] [Google Scholar]
- Pallikaris I.G., Kymionis G.D., Astyrakakis N.I. Corneal ectasia induced by laser in situ keratomileusis. J. Cataract Refract. Surg. 2001;27:1796–1802. doi: 10.1016/s0886-3350(01)01090-2. [DOI] [PubMed] [Google Scholar]
- Piccoli P.M., Gomes A.A., Piccoli F.V. Corneal ectasia detected 32 months after LASIK for correction of myopia and asymmetric astigmatism. J. Cataract Refract. Surg. 2003;29:1222–1225. doi: 10.1016/s0886-3350(02)01914-4. [DOI] [PubMed] [Google Scholar]
- Rad A.S., Jabbarvand M., Saifi N. Progressive keratectasia after laser in situ keratomileusis. J. Refract. Surg. 2004;20:S718–S722. doi: 10.3928/1081-597X-20040903-18. [DOI] [PubMed] [Google Scholar]
- Randleman J.B., Woodward M., Lynn M.J., Stulting R.D. Risk assessment for ectasia after corneal refractive surgery. Ophthalmology. 2008;115:37–50. doi: 10.1016/j.ophtha.2007.03.073. [DOI] [PubMed] [Google Scholar]
- Randleman J.B., Trattler W.B., Stulting R.D. Validation of the ectasia risk score system for preoperative Laser in situ keratomileusis screening. Am. J. Ophthalmol. 2008;145:813–818. doi: 10.1016/j.ajo.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royce S.G., Miao Y.R., Lee M., Samuel C.S., Tregear C.W., Tang M.L. Relaxin reverses airway remodeling and airway dysfunction in allergic airway disease. Endocrinology. 2009;150:2692–2699. doi: 10.1210/en.2008-1457. [DOI] [PubMed] [Google Scholar]
- Saitoh-Inagawa W., Oshima A., Aoki A. Rapid diagnosis of adenoviral conjunctivitis by PCR and restriction fragment length polymorphism analysis. J. Clin. Microbiol. 1996;34:2113–2116. doi: 10.1128/jcm.34.9.2113-2116.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabbara K.F., Kotb A.A. Risk factors for corneal ectasia after LASIK. Ophthalmology. 2006;113:1618–1622. doi: 10.1016/j.ophtha.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Tuli S.S., Iyer S. Delayed ectasia following LASIK with no risk factors: is a 300-micron stromal bed enough? J. Refract. Surg. 2007;23:620–622. doi: 10.3928/1081-597X-20070601-14. [DOI] [PubMed] [Google Scholar]
- Twa M.D., Nichols J.J., Joslin C.E. Characteristics of corneal ectasia after LASIK for myopia. Cornea. 2004;23:447–457. doi: 10.1097/01.ico.0000122702.49054.12. [DOI] [PubMed] [Google Scholar]


