Abstract
The primary cilium is an immotile sensory and signaling organelle found on the majority of mammalian cell types. Of the multitude of roles that the primary cilium performs, perhaps some of the most important include maintenance of differentiation, quiescence, and cellular polarity. Given that the progression of cancer requires disruption of all of these processes, we have investigated the effects of several carcinogens on the primary cilium of the RPTEC/TERT1 human proximal tubular epithelial cell line. Using both scanning electron microscopy and immunofluorescent labeling of the ciliary markers acetylated tubulin and Arl13b, we confirmed that RPTEC/TERT1 cells express primary cilium upon reaching confluence. Treatment with the carcinogens ochratoxin A (OTA) and potassium bromate (KBrO3) caused a significant reduction in the number of ciliated cells, while exposure to nifedipine, a noncarcinogenic renal toxin, had no effect on primary cilium expression. Flow cytometric analysis of the effects of all three compounds on the cell cycle revealed that only KBrO3 resulted in an increase in the proportion of cells entering the cell cycle. Microarray analysis revealed dysregulation of multiple pathways affecting ciliogenesis and ciliary maintenance following OTA and KBrO3 exposure, which were unaffected by nifedipine exposure. The primary cilium represents a unique physical checkpoint with relevance to carcinogenesis. We have shown that the renal carcinogens OTA and KBrO3 cause significant deciliation in a model of the proximal tubule. With KBrO3, this was followed by reentry into the cell cycle; however, deciliation was not found to be associated with reentry into the cell cycle following OTA exposure. Transcriptomic analysis identified dysregulation of Wnt signaling and ciliary trafficking in response to OTA and KBrO3 exposure.
Keywords: deciliation, carcinogenesis, proximal tubule, cilia
the primary cilium (also referred to as the central cilium) is an immotile, microtubule-based organelle found on the majority of mammalian cell types. Until relatively recently, the primary cilium was considered a vestigial organelle from our unicellular evolutionary lineage. However, it is now widely acknowledged that cilia play a central role in an increasing number of diverse cellular processes, including chemo- and mechanosensation, signal transduction, phototransduction, and developmental patterning (4, 5, 32, 42).
The ciliary body (or axoneme) is composed of nine microtubule doublets enveloped in a ciliary membrane. These doublets are composed of α-tubulin and β-tubulin and are heavily posttranslationally modified through acetylation, glutamylation, or glycylation to increase microtubule stability and functionality (15, 29, 31). The ciliary membrane forms a distinct region of the cell membrane as lateral diffusion between it and the wider cell membrane does not readily occur (47, 33). This results in localization of a distinct pool of proteins in the ciliary membrane facilitating concentration of particular receptors and signaling proteins within the cilium (34). Therefore, the primary cilium represents a highly specialized sensory and signal transduction hub. Numerous receptor signaling components are highly enriched in the cilium, including Sonic Hedgehog signaling proteins, canonical and noncanonical Wnt signaling components, the platelet derived growth factor (PDGF) receptor (12, 26, 42), and receptors involved in phototransduction and olfaction (3, 24). The renal primary cilium extends into the lumen of the renal tubule and is proposed to play a major sensory role (46).The ability of this organelle to sense fluid flow and initiate calcium-based signaling is thought to contribute to the maintenance of normal epithelial phenotype and function throughout the renal tubule (39, 40).
The physiological importance of the central cilium is highlighted by the growing list of disorders where the primary etiology is rooted in abnormal ciliary function, termed “ciliopathies” (18). Autosomal dominant polycystic kidney disease (ADPKD), the first disease identified as a ciliopathy, is the result of a single mutation in either polycystin-1 or polycystin-2. ADPKD is characterized by epithelial dedifferentiation and overproliferation, leading to progressive cyst formation, gross architectural disruption of the kidney, end-stage renal disease, and increased risk of renal cancer (17). Other ciliopathies include Bardet-Beidl syndrome, situs inversus, Meckel-Grüber syndrome, and nephronophthisis (2, 6, 9, 36, 48).
More recently, there has been focus on the potential importance of the primary cilium in carcinogenesis. Several studies have observed that primary cilia are suspiciously absent in a variety of cancer cell types, including mammary and pancreatic cancers (43, 50). In addition, several cilia-related genes are dysregulated in cancer. For example, somatic mutations in the tumor suppressor von Hippel-Lindau, which is necessary for ciliogenesis (13), occur in the majority of clear cell renal cell carcinomas (41). In ovarian cancer cells, inhibition of aurora A kinase, which regulates cilia assembly, induced cell cycle arrest (7). However, the suggested link between the primary cilium and tumorigenesis lies in the fact that cilium formation is closely linked to the cell cycle. In proliferating cells, the centrosomes coordinate spindle pole formation during mitosis. In quiescent or interphase (G1 phase) cells, the centrosomes migrate to the cell surface to form the mother centriole and subsequently the basal body, which nucleates primary cilium formation. Therefore, an inverse relationship exists between primary cilium formation and cellular proliferation. Since the primary cilium is now known to play a role in maintaining these cell features (14, 22), there is significant potential for the involvement of ciliary dysfunction in carcinogenesis.
In this study, the effects of known renal carcinogens ochratoxin A (OTA) and potassium bromate (KBrO3) and a noncarcinogen, nifedipine, were examined in a novel proximal tubular epithelial cell line (RPTEC/TERT1). Given the association between ciliary disruption and cancer, we examined the effects of these renal chemical carcinogens on cilia formation in proximal tubular epithelial cells.
MATERIALS AND METHODS
Cell culture and treatment.
The human RPTEC/TERT1 renal proximal tubular epithelial cell line, purchased from Evercyte, was used throughout this study. The RPTEC/TERT1 cell line was established via the introduction of human telomerase (hTERT) into primary human proximal tubular epithelial cells (49). RPTEC/TERT1 cells were maintained at 37°C in a 5% CO2 humidified atmosphere in low-glucose (5 mM) DMEM/nutrient mix F-12 supplemented with ITS, EGF, hydrocortisone, l-glutamine, and penicillin/streptomycin. Cells were maintained for at least 10 days upon reaching confluence before treatment to allow stabilization of the monolayer (27). Nifedipine, OTA, and KBrO3 were obtained from Sigma-Aldrich. In all cases, DMSO to a final concentration of 0.1% (vol/vol) was used as a vehicle. In all experiments, control cells were also exposed to 0.1% DMSO.
Scanning electron microscopy.
Cells were cultured to confluence on Thermanox coverslips (Nunc, Rochester, NY). Monolayers were fixed for 45 min with Karnovsky's fixative (2% formaldehyde, 0.5% glutaraldehyde) and postfixed for 45 min with 1% OsO4 in 0.1 M sodium cacodylate buffer (pH 7.4). Specimens were dehydrated with methanol followed by critical point drying. Specimens were finally sputter-coated with a 30- to 50-nm gold/palladium layer for observation with a scanning electron microscope (JEOL jsm-25s). Images were processed using Adobe Photoshop.
Resazurin assay.
Differentiated RPTEC/TERT1 cells were treated with increasing concentrations of OTA, KBrO3, and nifedipine for 72 h in a 96-well format. Following exposure, culture medium was removed and 100 μl of 1 mg/ml resazurin in serum-free medium was added to each well and incubated for 120 min at 37°C. Fluorescence was then measured using a Victor Wallac plate reader using 530-nm excitation wavelength and 590-nm emission wavelength. Data are expressed as a percentage of vehicle control (0.1% DMSO).
Phase-contrast microscopy.
Cellular morphology was observed by phase-contrast microscopy using a JVC high-resolution digital camera (KY-F55BE) attached to a Nikon TMS phase-contrast microscope. Micrographs were processed using Adobe Photoshop.
Immunofluorescent labeling.
RPTEC/TERT1 cells were cultured on eight-well chamber slides (Nunc LabTekII) for 10 days following confluence and then treated as indicated for 72 h. Cells were fixed with 3.7% paraformaldehyde for 20 min at room temperature and permeabilized with 0.2% Triton X-100 (vol/vol). Nonspecific binding was reduced by blocking in 0.5% (wt/vol) BSA/PBS. Acetylated α-tubulin was labeled using a mouse anti-human antibody (1:400, Sigma-Aldrich). Zonula occludens-1 (ZO-1) was labeled using a rabbit anti-human antibody (1:300, Zymed). Arl13b was labeled using a rabbit anti-human antibody (1:200, provided by Oliver Blaque). Nuclei were stained with 4,6-diamidino-2-phenylindole (1:1,000). Slides were imaged using a Zeiss LSM510 confocal microscope, and images were processed using ImageJ.
Flow cytometry.
RPTEC/TERT1 cells were seeded on six-well culture plates. Ten days following confluence, cells were treated with 300 nM OTA, 1 mM KBrO3, or 10 μM nifedipine for 72 h. The supernatant was removed and stored on ice. Cells were then washed with ice-cold PBS, and trypsinized with 5% trypsin-EDTA, which was then neutralized with DMEM/F-12 containing 10% (vol/vol) FCS. Supernatants were added once again to the corresponding samples and centrifuged at 1,000 rpm for 3 min to pellet the cells. Cells were resuspended to a density of 1 × 106 cells/ml in ice-cold PBS and pelleted by centrifugation at 1,000 rpm for 3 min. Cells were then fixed with 70% ice-cold EtOH. EtOH was removed by washing with ice cold PBS. Cells were spun down at 3,000 rpm for 5 min and resuspended in 50 ng/ml propidium iodide in EDTA/Triton X-100 buffer for at least 15 min. The effects of all three compounds on cycle were analyzed using an Accuri C6 flow cytometer and FCS Express 4.
RNA isolation and microarray hybridization.
PTEC/TERT1 cells were grown to confluence in six-well plates. Ten days postconfluence, cells were treated with 300 nM OTA, 1 mM KBrO3 or 10 μM nifedipine for 72 h. Total RNA was extracted (n = 3 for each condition) using TRIzol according to the manufacturer's instructions and purified using RNeasy Mini Kits (Qiagen). RNA purification and quality were assessed using the Agilent 2100 Bioanalyzer to determine the 28S:18S rRNA ratio. Sample preparation, hybridization, washing, staining, and scanning of the Affymetrix Human Genome U133 Plus 2.0 GeneChip arrays were performed at the Netherlands Toxicogenomics Centre (Maastricht, The Netherlands) according to the manufacturer's instructions as previously described (20).
Microarray data analysis.
All analyses were based on normalized expression values generated with Genedata Expressionist 6.2, which after a quality assessment step were normalized with GeneChip RMA (GC-RMA). Normalized data were further analyzed with Genedata Expressionist 6.2 and GeneSpring (GX 10.0.1), respectively. To identify differentially regulated genes for each treatment, at each time point comparisons were made against the time-matched vehicle control. Differentially expressed genes were selected based on the fold-change (2-fold change or greater) and one-way ANOVA (P < 0.05).
Bioinformatic analysis.
Network analysis was conducted with MetaCore Network Tools (GeneGo, San Diego, CA). Gene Set Enrichment Analysis (GSEA) was performed to determine whether the cilia-related gene set was enriched within chemical datasets. GSEA was performed following the developer's protocol (45) (http://www.broad.mit.edu/gsea/).
Statistical analysis.
Loss of the primary cilium following carcinogen exposure was quantified by determining the ratio of cilia to nuclei using multiple independent fields of view (n = 3). The ratio of cilia to nuclei was then expressed as a percentage of the control for each time point. Statistical analysis was performed using one-way ANOVA and a Bonferroni posttest. Statistical significance was assumed for P < 0.05. Data are shown ±SE (***P < 0.001).
RESULTS
Differentiated human renal proximal tubular cells express a primary cilium.
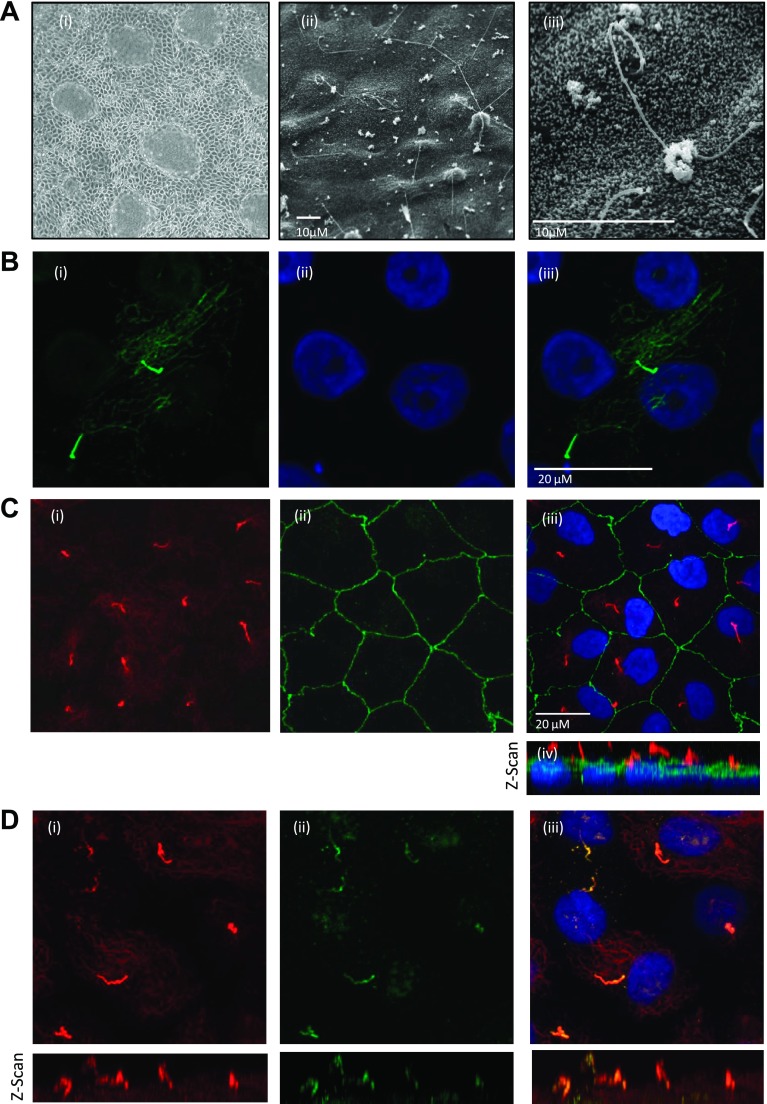
Human renal proximal tubular cells (RPTEC/TERT1) were differentiated on six-well plates. RPTEC/TERT1 cells formed fluid-filled domes which were distributed across the monolayer, indicating that the epithelial monolayer had formed a functional barrier (Fig. 1Ai). The presence of cilia on RPTEC/TERT1 cells was examined by both scanning electron microscopy (Fig. 1A, ii and iii) and indirect immunofluorescent staining for acetylated α-tubulin (Fig. 1B). Primary cilia extended above the apical surface of the RPTEC/TERT1 and were observed in >90% of cells. A three-dimensional (3D) projection of the collapsed Z-stack confocal images in Fig. 1B shows the spatial distribution of acetylated α-tubulin in the cell. Cytoplasmic microtubules were also visualized. However, this cytoplasmic network was located in a different plane from the primary cilium [for 3D projection, see Supplementary Data File 1 (video); supplemental material for this article is available on the journal website]. Using ZO-1 staining to identify cellular borders in conjunction with acetylated α-tubulin staining to visualize the primary cilium, it was observed that each cell expressed a single primary cilium (Fig. 1C, i–iii). The length of the primary cilium varied from cell to cell, ranging from 10 to 20 μm (Fig. 1C, iv). The small GTPase, ADP-ribosylation factor-like protein 13b (Arl13b) is a specific marker for the primary cilium and was seen to colocalize with acetylated α-tubulin in the primary cilium in confluent RPTEC/TERT1 cells (Fig. 1D, i–iii).
Fig. 1.
Confluent human proximal tubular epithelial RPTEC/TERT1 cells express primary cilia. Ai: phase-contrast micrograph showing 10-day confluent RPTEC/TERT1 cells (×100). ii and iii: Micrographs of confluent RPTEC/TERT1 cells at different magnifications. B: stills from 3-dimensional projection (see Supplementary Data File 1; video). i: High-magnification image of immunofluorescently labeled acetylated α-tubulin. ii: Genetic material stained with 4,6-diamidino-2-phenylindole (DAPI). iii: Merge. Ci: immunofluorescently labeled acetylated α-tubulin shows the presence of multiple primary cilia. ii: Cellular borders are demarcated by immunofluorescent labeling of the tight junction component zonula occludens (ZO)-1. iii: Merge. iv: Composite image of collapsed Z-scans shows spatial distribution of the cilia in relation to the cellular surface. Di: immunofluorescently labeled acetylated α-tubulin. ii: Immunofluorescently labeled Arl13b. iii: Composite images showing colocalization of acetylated α-tubulin and Arl13b to the primary cilium.
Effects of nifedipine, OTA, and KBrO3 on RPTEC/TERT1 viability.
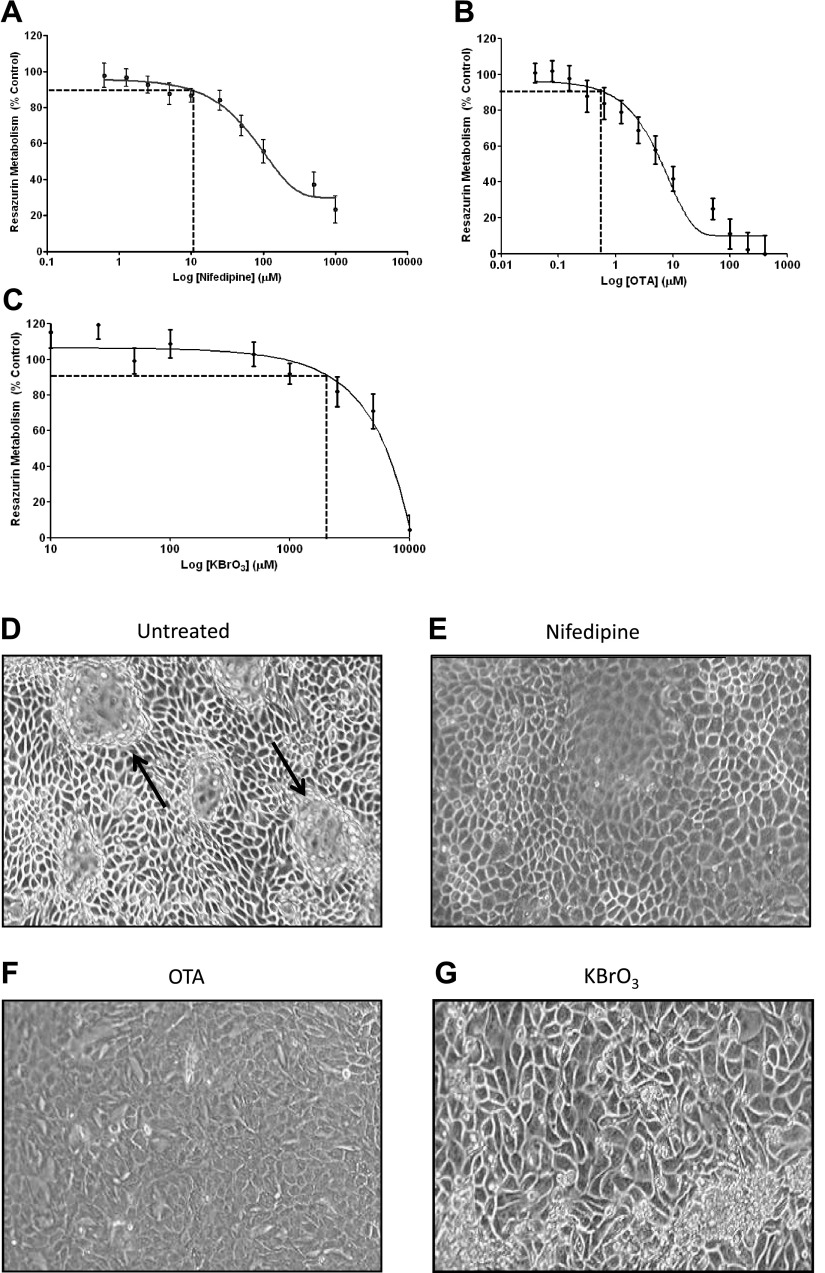
The effects of OTA, KBrO3 (which are classified as IARC class IIb renal carcinogens), and nifedipine (a noncarcinogenic compound) on the viability of the RPTEC/TERT1 cell line were examined using a resazurin cell viability assay to determine the concentration of each compound to be used for transcriptomic profiling. The aim was to induce a limited amount of measurable toxicity across all three compounds. Therefore, a 10% decrease in resazurin conversion was considered an appropriate concentration for all further experiments. Working concentrations were initially determined in a 96-well format (Fig. 2, A–C) and then refined for a 6-well format. The final concentrations to be used for further studies were 300 nM OTA, 1 mM KBrO3, and 10 μM nifedipine. By phase-contrast microscopy, it was observed that while these concentrations induced significant alterations in cell morphology (such as loss of fluid-filled domes as indicated by arrows), no dramatic reduction in cell numbers was observed (Fig. 3, D–G). Subtle alterations in the shape and size of cells were also observed following treatment with OTA, KBrO3, and nifedipine compared with vehicle controls.
Fig. 2.
Effects of nifedipine, ochratoxin A (OTA), and potassium bromate (KBrO3) on RPTEC/TERT1 viability and morphology. The effects of nifedipine (A), OTA (B), and KBrO3 (C) on RTPEC/TERT1 viability as assessed by resazurin metabolism. Results are expressed as percentage of control ± SE; n = 3. Also shown is a morphological analysis of untreated RPTEC/TERT1 cells (D) compared with the effects of 10 μM nifedipine (E), 300 nM OTA (F), and 1 mM KBrO3 (G) assessed by phase-contrast microscopy.
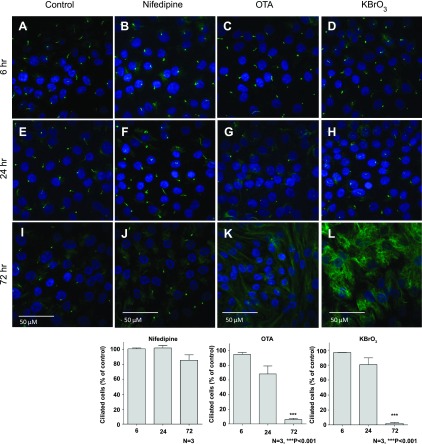
Fig. 3.
Exposure to carcinogens induced loss of the primary cilium. RPTEC/TERT1 cells were grown in 8-well chamber slides (Nunc) and treated 10 days postconfluence. A–C: control RPTEC/TERT1 cells stained for acetylated α-tubulin and DAPI at 6, 24, and 72 h. D–F: 10 μM nifedipine-treated RPTEC/TERT1 cells at 6, 24, and 72 h. G–I: 300 nM OTA-treated RPTEC/TERT1 cells at 6, 24, and 72 h. J–L: 1 mM KBrO3-treated RPTEC/TERT1 cells at 6, 24, and 72 h. Bottom: graph showing the number of ciliated cells obtained by determining the ratio of cilia to nuclei, expressed as a percentage of its time-matched control for nifedipine-, OTA-, and KBrO3-treated cells at 6, 24, and 72 h; n = 3. ***P < 0.001.
Renal carcinogens induced loss of the primary cilium in proximal tubular epithelial cells.
The effects of nifedipine, OTA, and KBrO3 exposure on the primary cilium of the RPTEC/TERT1 cell line was assessed using immunofluorescent labeling of acetylated α-tubulin. This was carried out at three time points (6, 24, and 72 h). Exposure to nifedipine, OTA, and KBrO3 for 6 h had no effect on the proportion of ciliated cells compared with vehicle controls (Fig. 3, A–D). By 24 h, however, cells exposed to either OTA or KBrO3 displayed a definite trend toward a reduction in the proportion of cells expressing a primary cilium (although these did not reach statistical significance) (Fig. 3, E–H). By 72 h, RPTEC/TERT1 cells exposed to either OTA or KBrO3 showed a statistically significant and nearly complete loss of cilia (n = 3, ***P < 0.001) (Fig. 3, I–L). While a small proportion of cells did retain cilia, these appeared dramatically shortened compared with those of vehicle controls. In contrast, exposure to nifedipine had no statistically significant effect on the proportion of ciliated cells even after 72 h (Fig. 3, I–L). After 72-h exposure, there appeared to be more acetylated α-tubulin in the cytosol following exposure to OTA and particularly KBrO3 compared with vehicle controls and nifedipine-treated cells.
Effects of OTA and KBrO3 on the microtubule network.
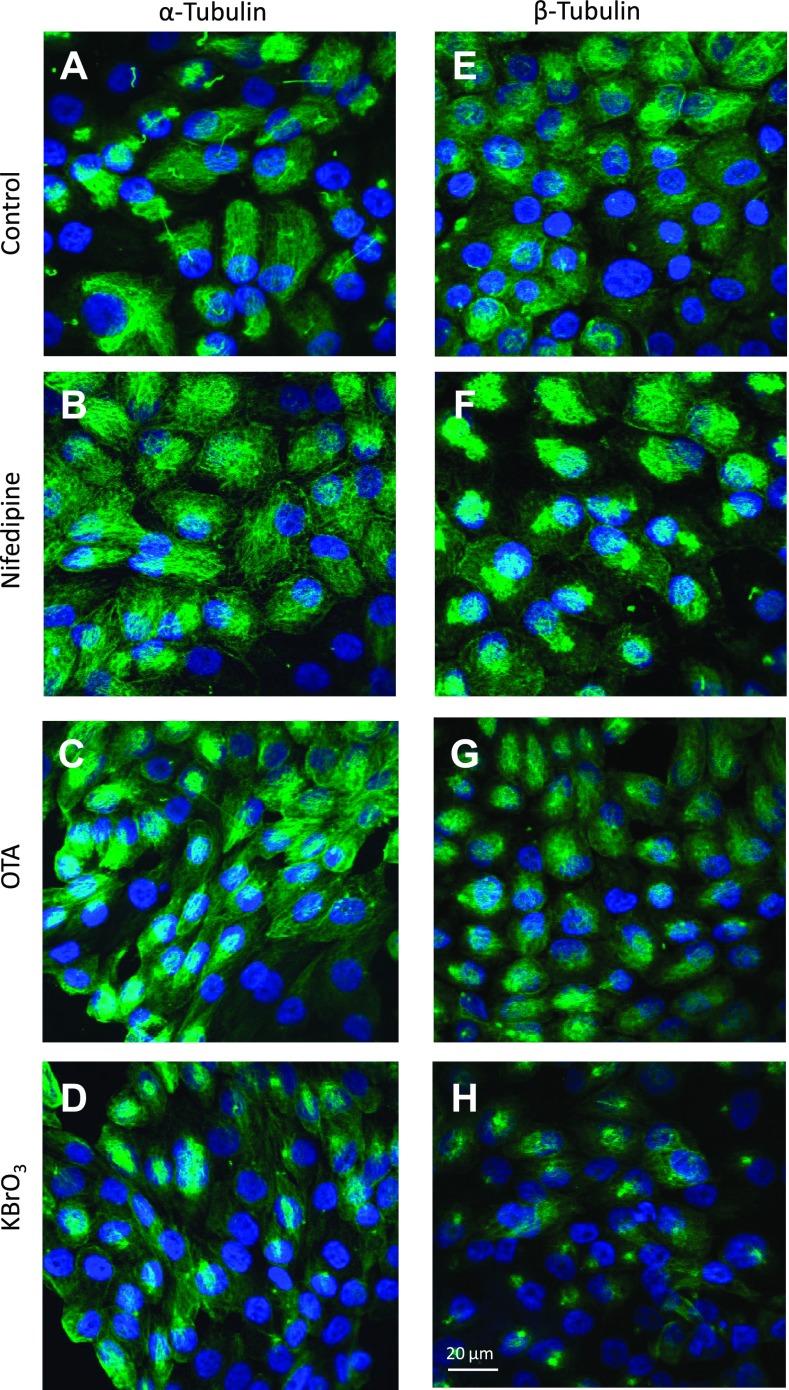
To further explore the finding of increased acetylated α-tubulin in the cytosol following OTA and KBrO3 exposure, we decided to investigate potential effects on microtubule organization by examining total α-tubulin and β-tubulin using immunofluorescence. Primary cilia were observed in both control and nifedipine-treated cells following 72-h exposure stained with α-tubulin (Fig. 4, A and B) and β-tubulin (Fig. 4, E and F), although the primary cilia were less distinguishable from cytosolic staining of microtubules than in those cells stained with acetylated α-tubulin (Fig. 3, I and J). While some minor alterations in α-tubulin were observed following nifedipine treatment (Fig. 4B), there were no gross changes in the overall microtubule network, which was indicated by staining with either α-tubulin (Fig. 4, C and D) or β-tubulin (Fig. 4, G and H) following treatment with either OTA or KBrO3. This would indicate that the changes detected in Fig. 3 following KBrO3 and OTA were confined to acetylated α-tubulin.
Fig. 4.
Effects of OTA and KBrO3 on the tubulin microtubule network. RPTEC/TERT1 cells were grown in 8-well chamber slides (Nunc) and treated 10 days postconfluence. Shown are control RPTEC/TERT1 cells (A) and cells stained for α-tubulin following 72 h exposure to 10 μM nifedipine (B), 300 nM OTA (C), and 1 mM KBrO3 (D). Also shown are control RPTEC/TERT1 cells (E) and cells stained for β-tubulin at 72 h, 10 μM nifedipine (F), 300 nM OTA (G), and 1 mM KBrO3 (H).
Effects on the cell cycle.
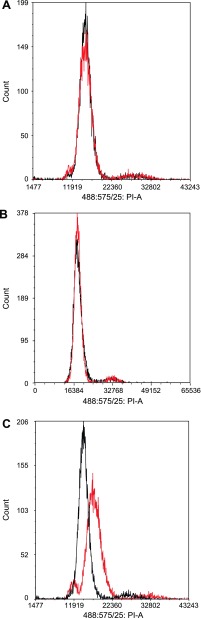
The effects OTA, KBrO3, and nifedipine on the cell cycle of differentiated RPTEC/TERT1 cells were analyzed by propidium iodide staining using flow cytometry. Nifedipine had no significant effect on the proportion of cells in the G0/G1, S, and G2/M phases of the cell cycle (Fig. 5A). Surprisingly, OTA did not significantly affect the proportions of cells in the different phases of the cell cycle compared with vehicle control after 72-h exposure: G0/G1 (85.48 vs. 80.56%), S (2.56 vs. 2.66%), and G2/M (11.97 vs. 16.78%). These results suggested that 300 nM OTA had no significant effect on the RPTEC/TERT1 cell cycle (Fig. 5B). In contrast, KBrO3 caused a significant increase in the proportion of aneuploid cells. The remaining diploid cells showed a decrease in the proportion of cells in the G0/G1 phase of the cell cycle (from 85.48 to 61.15%; see Table 1), with a corresponding increase in the proportion of cells in G2 phase of the cell cycle (from 11.97 to 38.5%; see Table 1). These results indicate that the exposure to KBrO3 resulted in gross chromosomal abnormalities with a significant proportion of diploid cells reentering the cell cycle (Fig. 5C).
Fig. 5.
Alteration of the cell cycle of confluent RPTEC/TERT1 cells. RPTEC/TERT1 cells were treated 10 days postconfluence with 300 nM OTA or 1 mM KBrO3 for 72 h. The effects on the cell cycle as measured by flow cytometric analysis of propidium iodide staining are shown for 10 μM nifedipine (A), 300 nM OTA (B), and 1 mM KBrO3 (C) treatment (n = 3). Vehicle control results are shown in black, and treated cell results are shown in red.
Table 1.
Distribution of cell populations residing in G1, G2, and S phase of the cell cycle
| G1 Mean | G1 CV | %G1 | G2 Mean | G2 CV | %G2 | %S | G2/G1 | |
|---|---|---|---|---|---|---|---|---|
| Control | 15,640.63 | 9.12 | 85.48 | 30,551.4 | 8.87 | 11.97 | 2.56 | 1.95 |
| Nifedipine | 18,353.33 | 7.48 | 93.83 | 33,320.14 | 6.19 | 4.32 | 1.85 | 1.82 |
| OTA | 15,622.12 | 10.11 | 80.56 | 30,787.1 | 9.03 | 16.78 | 2.66 | 1.97 |
| KBrO3 | 11,876.28 | 6.85 | 61.15 | 21,456.56 | 6.88 | 38.85 | 1.81 | 1.81 |
Percentage of the cell populations residing within each phase of the cell cycle following nifedipine, ochratoxin A (OTA), and potassium bromate (KBrO3) treatment for 72 h.
Effects of nifedipine, OTA, and KBrO3 on the RPTEC/TERT1 gene expression profile.
RNA was isolated from cells exposed to 10 μM nifedipine, 300 nM OTA, and 1 mM KBrO3 at 6, 24, and 72 h. Gene expression profiles at each time point were compared with time-matched vehicle controls to identify differentially regulated genes. At 6 h, 31 genes were significantly affected by nifedipine (24 downregulated and 7 upregulated), 7,564 genes were significantly affected by OTA (5,260 downregulated and 2,304 upregulated), and 148 genes were significantly affected by KBrO3 (90 downregulated and 58 upregulated). At 24 h, 375 genes were significantly affected by nifedipine (365 downregulated and 10 upregulated), 10,802 genes were significantly affected by OTA (6,902 downregulated and 3,900 upregulated), and 1,736 genes were significantly affected by KBrO3 (1,347 downregulated and 389 upregulated). By 72 h, 136 genes were significantly affected by nifedipine (63 downregulated and 73 upregulated), 6,944 genes were significantly affected by OTA (3,802 downregulated and 3,142 upregulated), and 3,149 genes were significantly affected by KBrO3 (1,951 downregulated and 1,198 upregulated).
Cilia gene set.
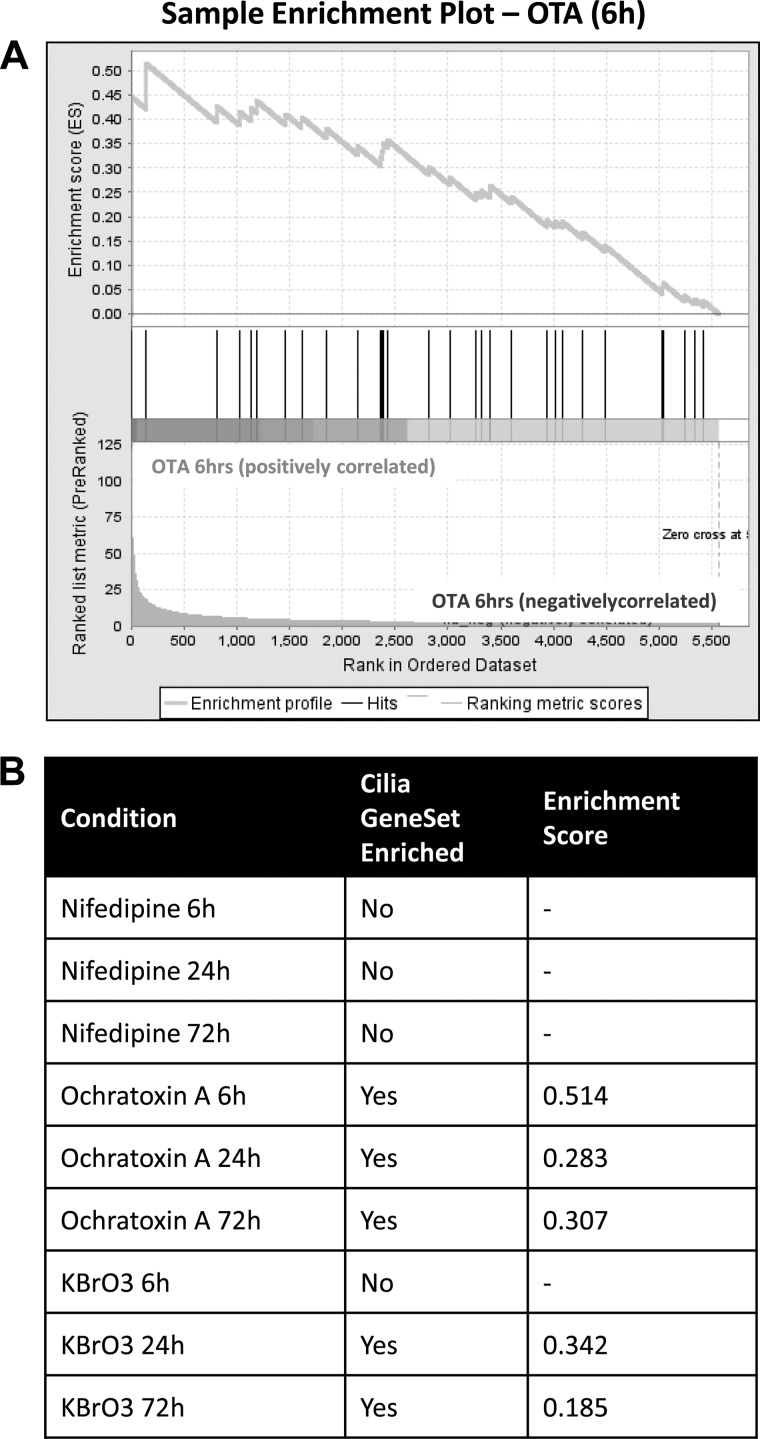
Using a number of publicly available sources including the Ciliome database (http://www.sfu.ca/∼leroux/ciliome_database.htm) and published literature, a list of genes which are known to be involved in ciliogenesis and maintenance of the cilium was compiled to form a cilia gene set (see Supplementary Table S3 for the full cilia gene set). GSEA was performed on the differentially regulated data sets for each compound to determine whether the cilia gene set was enriched in each condition (Fig. 6). The cilia gene set was not found to be enriched at any time point in cells exposed to nifedipine. In contrast, the cilia gene set was enriched after OTA treatment at all three time points with the highest enrichment score observed after 6 h. The cilia gene set was also enriched by exposure to KBrO3 at the 24- and 72-h time points, but not at 6 h. This analysis suggests that significant numbers of genes related to cilia formation and maintenance were dysregulated by exposure to OTA and KBrO3. To further characterize the nature of these transcriptomic changes, a detailed characterization of the individual genes affected was performed and is summarized in Table 2. Nifedipine did not result in dysregulation of any ciliary genes at any time point in the RPTEC/TERT1 cell line. Of the genes found to be significantly dysregulated by OTA and KBrO3, genes involved in ciliary targeting such as KIF3A, KIF3B, ARL6, and several of the BBS gene family members were dysregulated. Of note was the observation that the vast majority of the genes which were dysregulated by OTA or KBrO3 were downregulated.
Fig. 6.
Effects of carcinogens on cilia-related genes. Gene Set Enrichment Analysis (GSEA) was performed to determine if the cilia gene set was significantly enriched in nifedipine-, OTA-, and KBrO3-treated cells. A sample GSEA enrichment plot is depicted.
Table 2.
Effects of carcinogens on cilia-related genes
| 6 h |
24 h |
72 h |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene Symbol | Gene Name | OTA | KBrO3 | Nif | OTA | KBrO3 | Nif | OTA | KBrO3 | Nif |
| ADCY7 | Adenylate cyclase 7 | −2.13 | ||||||||
| ALMS1 | Alstrom syndrome 1 | −24.96 | −4.19 | −2.11 | ||||||
| AHI1 | Abelson helper integration site 1 | −2.16 | ||||||||
| ARL3 | ADP-ribosylation factor-like 3 | 3.44 | 2.66 | 7.67 | ||||||
| ARL6 | ADP-ribosylation factor-like protein 6 | −8.71 | −5.45 | −2.55 | −2.4 | |||||
| ARL13B | ADP-ribosylation factor-like protein 13b | −12.23 | ||||||||
| B2M | β2- Microglobulin | −2.33 | ||||||||
| BBS1 | Bardet-Biedl syndrome 1 | −10.35 | −4.9 | |||||||
| BBS2 | Bardet-Biedl syndrome 2 | −15.03 | −2.02 | −2.89 | −3 | |||||
| BBS4 | Bardet-Biedl syndrome 4 | −2.39 | −6.94 | |||||||
| BBS7 | Bardet-Biedl syndrome 7 | −6.07 | −11.31 | −9.48 | ||||||
| BBS9 | Bardet-Biedl syndrome 9 | −2.38 | −2.57 | −2.27 | ||||||
| CALM1 | Calmodulin 1 | 2.04 | ||||||||
| CDC42 | Cell division control 42 | 3.64 | ||||||||
| CETN2 | Centrin 2 | −2.23 | ||||||||
| CEP290 | Centrosomal protein of 290 kDa | −2.23 | ||||||||
| CLUAP1 | Clusterin-associated protein 1 | −9.34 | −15.56 | −4.03 | −2.58 | |||||
| DNAJB13 | DNAJB13 | 2.23 | −2.04 | −2.01 | −2.04 | −2.01 | ||||
| DYNC2H1 | Dynein 2 heavy chain 1 | −2.25 | −3.48 | |||||||
| DYNC2L1 | Dynein 2 light intermediate chain 1 | −3.69 | −4.43 | −2.73 | −4.78 | −3.36 | ||||
| FAT4 | FAT tumor suppressor homolog 4 | −2.5 | ||||||||
| ICQB1 | IQ calmodulin-binding motif-containing 1 | −5.26 | ||||||||
| IFT57 | Intraflagellar transport 57 | 2.47 | 2.47 | 6.76 | −3 | |||||
| IFT80 | Intraflagellar transport 80 | −8.01 | −2.77 | −3.46 | ||||||
| IFT81 | Intraflagellar transport 81 | −11.4 | −2.9 | −3.27 | −3.64 | |||||
| IFT88 | Intraflagellar transport 88 | 2.11 | −2.02 | |||||||
| IFT122 | Intraflagellar transport 122 | 2.15 | −2.25 | −2.15 | −2.56 | |||||
| IFT172 | Intraflagellar transport 172 | −4.88 | −2.84 | |||||||
| INVS | Inversin | −2.12 | −2.27 | |||||||
| INTU | Inturned | −3.95 | ||||||||
| KIFAP3 | Kinesin-associated protein 3 | −2.99 | ||||||||
| KIF3A | Kinesin family member 3A | −5.34 | −2.48 | |||||||
| KIF3B | Kinesin family member 3B | −2.19 | ||||||||
| MAPK1 | Mitogen- activated protein kinase 1 | −2.26 | 4.67 | −2.14 | −2.29 | 2.13 | −3.51 | |||
| MAPRE1 | Microtubule-associated protein RP/EB family 1 | −2.71 | ||||||||
| MKKS | McKusick-Kaufman syndrome | −5.11 | ||||||||
| NPHP1 | Nephronophthisis 1 | 2.07 | −3.2 | −2.17 | −3.2 | −2.17 | ||||
| OFD1 | Oral-facial-digital syndrome 1 | −15.06 | ||||||||
| PKD1 | Polycystin-1 | −2.74 | −4.81 | |||||||
| PTCH1 | Patched 1 | −5.85 | – | |||||||
| RPL13A | Ribosomal protein L13a | −2.23 | ||||||||
| RSPH1 | Radial spoke head 1 | 3.26 | ||||||||
| RSPH10b | Radial spoke head 10b | 2.16 | ||||||||
| SHH | Sonic Hedgehog | 2.3 | ||||||||
| STAT6 | Signal transduction and transcription 6 | −2.64 | ||||||||
| TSC1 | Tuberous sclerosis 1 | −2.42 | ||||||||
| TSC2 | Tuberous sclerosis 2 | 2.34 | −2.27 | |||||||
| TUBA1A | α-Tubulin 1a | −5.65 | −3.87 | |||||||
Fold-change of cilia-related genes found to be significantly dysregulated by 300 nM OTA or 1 mM KBrO3. Nif, nifedipine. P < 0.05, fold-change ≥2 at 6-, 24-, and 72-h time points.
To further characterize the effects of OTA and KBrO3 on canonical signaling pathways in RPTEC/TERT1 cells, a global pathway analysis of the transcriptomic data was performed using MetaCore (Table 3). This analysis identified the most significantly dysregulated pathways in OTA- and KBrO3-treated cells. A number of pathways with relevance to cancer development were identified, and several of these such as Wnt, transforming growth factor (TGF)-β related cytoskeletal remodeling, cell cycle, epithelial-mesenchymal transition, and cell adhesion were common to both OTA and KBrO3 (Table 3). However, in some cases, OTA and KBrO3 resulted in opposing effects on these common pathways. For example, the cell cycle influence of Ras and Rho proteins on the G1/S transition network was negatively regulated by OTA, but this gene network was positively regulated by KBrO3.
Table 3.
Pathway analysis of significantly dysregulated pathways following OTA and KBrO3 treatment
| Rank | OTA Maps | P Value | KBrO3 Maps | P Value |
|---|---|---|---|---|
| 1 | Cytoskeleton remodeling_TGF, WNT and cytoskeletal remodeling | 6.718E-11 | Cytoskeleton remodeling_TGF, WNT and cytoskeletal remodeling | 6.718E-11 |
| 2 | Cytoskeleton remodeling_Cytoskeleton remodeling | 2.512E-10 | Cytoskeleton remodeling_Cytoskeleton remodeling | 2.512E-10 |
| 3 | Translation_Non-genomic (rapid) action of Androgen Receptor | 1.276E-08 | Cell adhesion_Chemokines and adhesion | 9.296E-08 |
| 4 | Development_TGF-β-dependent induction of EMT via MAPK | 6.784E-08 | Development_ERBB-family signaling | 2.456E-07 |
| 5 | Cell adhesion_Chemokines and adhesion | 9.296E-08 | Development_EGFR signaling pathway | 6.862E-07 |
| 6 | Cell cycle_Influence of Ras and Rho proteins on G1/S Transition | 8.018E-07 | Cell cycle_Influence of Ras and Rho proteins on G1/S Transition | 8.018E-07 |
| 7 | Neurophysiological process_Receptor-mediated axon growth repulsion | 8.424E-07 | Development_TGF-β-dependent induction of EMT via RhoA, PI3K and ILK. | 2.076E-06 |
| 8 | Development_Growth hormone signaling via PI3K/AKT and MAPK cascades | 1.258E-06 | Translation_Non-genomic (rapid) action of Androgen Receptor | 1.276E-08 |
| 9 | Signal transduction_PTEN pathway | 1.265E-06 | Development_TGF-β-dependent induction of EMT via MAPK | 6.784E-08 |
| 10 | G-protein signaling_Regulation of p38 and JNK signaling mediated by G-proteins | 1.866E-06 | PGE2 pathways in cancer | 3.327E-06 |
| 11 | Development_EGFR signaling pathway | 6.862E-07 | Neurophysiological process_Receptor-mediated axon growth repulsion | 8.424E-07 |
| 12 | Development_TGF-β-dependent induction of EMT via RhoA, PI3K and ILK. | 2.076E-06 | Signal transduction_PTEN pathway | 1.265E-06 |
| 13 | Development_Regulation of epithelial-to-mesenchymal transition (EMT) | 7.100E-06 | Transcription_P53 signaling pathway | 1.305E-05 |
| 14 | Development_IGF-1 receptor signaling | 7.844E-06 | Development_Regulation of epithelial-to-mesenchymal transition (EMT) | 7.100E-06 |
| 15 | Cell adhesion_Plasmin signaling | 9.750E-06 | Signal transduction_AKT signaling | 3.587E-05 |
| 16 | Cell adhesion_Integrin-mediated cell adhesion and migration | 1.221E-05 | Reproduction_GnRH signaling | 1.761E-05 |
| 17 | SCAP/SREBP Transcriptional Control of Cholesterol and FA Biosynthesis | 1.430E-05 | Cytoskeleton remodeling_Integrin outside-in signaling | 1.303E-04 |
| 18 | Reproduction_GnRH signaling | 1.761E-05 | Development_Growth hormone signaling via PI3K/AKT and MAPK cascades | 1.258E-06 |
| 19 | Proteolysis_Putative SUMO-1 pathway | 2.139E-05 | Development_TGF-β-dependent induction of EMT via SMADs | 2.091E-05 |
| 20 | Muscle contraction_Regulation of eNOS activity in endothelial cells | 2.572E-05 | Development_TGF-β receptor signaling | 1.581E-04 |
| 21 | Development_Endothelin-1/EDNRA transactivation of EGFR | 2.656E-05 | Development_IGF-1 receptor signaling | 7.844E-06 |
| 22 | Signal transduction_Erk Interactions: Inhibition of Erk | 3.342E-05 | Immune response_Oncostatin M signaling via MAPK in human cells | 1.011E-04 |
| 23 | Signal transduction_AKT signaling | 3.587E-05 | Stellate cells activation and liver fibrosis | 1.415E-04 |
| 24 | Development_ERBB-family signaling | 2.456E-07 | Regulation of lipid metabolism_Regulation of lipid metabolism via LXR, NF-Y and SREBP | 3.472E-04 |
| 25 | Development_TGF-β-dependent induction of EMT via SMADs | 2.091E-05 | Cytoskeleton remodeling_Fibronectin-binding integrins in cell motility | 2.461E-04 |
The most significantly dysregulated canonical pathways as determined by MetaCore pathway analysis for both OTA and KBrO3 are listed. Those pathways which are unique to either OTA or KBrO3 treatment are shown in bold, while the remaining pathways are common to both treatment groups.
DISCUSSION
The primary cilium is essential for a wide variety of cellular functions (8, 44). The ubiquitous presence of this organelle indicates that the primary cilium likely plays an important role across many cell types and species. In this study, we have shown that differentiated human proximal tubular epithelial cells express a single primary cilium. Furthermore, we have shown that after exposure to two renal carcinogens these cells lose their primary cilium. This deciliation was not a generalized response to nephrotoxin exposure, and perhaps more significantly, loss of the primary cilium did not necessarily coincide with reentry into the cell cycle. While KBrO3 induced significant chromosomal abnormalities and limited reentry into the cell cycle, OTA had no effect on the cell cycle, suggesting that there are likely diverse mechanisms underlying the effects of these two carcinogens. Gene expression analysis demonstrated that loss of the cilium was correlated with significant dysregulation of cilia-related genes and further underlined the divergent mechanisms of OTA- and KBrO3-induced deciliation.
Loss of the primary cilium may occur through two distinct mechanisms. The cilium may be reabsorbed into the cell body, or the entire organelle may be shed from the surface of the cell. Removal of the primary cilium through reabsorption occurs as cells progress through the cell cycle. Indeed, removal of the cilium is a necessary event for mitosis to occur as the mother centrioles which nucleate the primary cilia also contribute to formation of the centrosome, the microtubule-organizing center responsible for arranging the mitotic spindles during mitosis (23). Ciliary shedding, on the other hand, may be a response to exposure to toxic stimuli (37). In this study, we have shown that loss of the primary cilium occurred following exposure of human renal proximal tubular cells to the renal carcinogens KBrO3 and OTA as early as 24 h following exposure, and that by 72 h deciliation was almost complete. The loss of the cilia was also associated with increased levels of acetylated α-tubulin in the cytosol, especially following treatment with KBrO3. These findings are in agreement with a study on chemical deciliation in the Madin-Darby canine kidney tubular cell line, which reported an increase in the cytosolic staining of acetylated α-tubulin (37). The authors of that study interpreted their findings as indicating that cells continue to acetylate α-tubulin molecules, even during stress-induced deciliation, but that this pool of acetylated tubulin remains cytosolic as there is no ciliary axoneme to incorporate it. In our present study, the primary cilia were unaffected by the noncarcinogenic nifedipine, so this was not a generalized response to the presence of a chemical stressor. However, a wider panel of chemical carcinogens should be examined in future studies to determine whether this effect is an important event during chemical carcinogenesis or a compound-specific observation.
To further characterize the mechanisms governing loss of the primary cilium following exposure to both OTA and KBrO3, we analyzed the effects of both compounds on the gene expression profile of the RPTEC/TERT1 cells at early (6 h), medium (24 h), and late (72 h) time points. A gene set with known ciliary function was compiled to facilitate a targeted approach to this analysis. Both OTA and KBrO3 were found to cause significant dysregulation of many genes involved in ciliogenesis and cilia maintenance in RPTEC/TERT1 cells. Pathway analysis of the RPTEC/TERT1 transcriptomic data using MetaCore pathway analysis revealed that Wnt signaling and cytoskeletal remodeling were ranked the most significantly altered pathway following both OTA and KBrO3 treatment. Previous studies have shown that loss of the primary cilium results in enhanced canonical Wnt signaling in cells (16). Recent evidence has suggested that β-catenin is sequestered in the cilium, suggesting that an intact cilium acts as a negative regulator of Wnt signaling (25). Since canonical Wnt signaling is implicated in the progression of malignant tumors, this is a potential mechanistic link between deciliation and carcinogenesis. Investigation of individual cilia-related genes revealed a number of potentially important transcriptional alterations. Kinesin II is a heterotrimeric motor complex composed of Kif3a, Kif3b, and KAP-3, which is responsible for anterograde transport to the ciliary tip. Mice lacking correct kinesin II function fail to form primary cilia (28). In the current study, OTA caused downregulation KIF3A as early as 6 h, and this downregulation was maintained until the 24-h time point when both of the remaining components of kinesin II, KIF3B and KIFAP3, were also downregulated, suggesting significant disruption of anterograde transport following OTA exposure. At the medium and late time points, numerous components of the intraflagellar transport or IFT pathway and BBS genes were dysregulated. Given that BBS and IFT pathways are known to be involved in maintenance of the primary cilium, this finding supports the time-dependent effects on deciliation observed following OTA and KBrO3 exposure at the medium and late time points. Anterograde IFT is responsible for transport of intracellular cargo, including receptors, signaling components, and tubulin, to the cilium. The BBSome is believed to play a role in targeting membrane proteins to the primary cilium (21), and knockdown of individual components in either pathway has been shown to hinder construction of the primary cilium (28, 35, 38). Our data indicate that OTA and KBrO3 may inhibit anterograde transport toward the cilium, resulting in shortening and ultimately reabsorption of the cilium. The retrograde motor dynein 2, which is responsible for transport from the cilium toward the cell, was also downregulated by OTA at all three time points and by KBrO3 at 24 and 72 h. Studies have shown that dynein 2 depletion can result in shortening of the cilium (1, 19, 30).
Since loss of the primary cilium facilitates progression through the cell cycle, we examined whether OTA or KBrO3 caused alterations in the cell cycle. Nifedipine, which did not cause loss of primary cilia, did not significantly affect the proportion of cells residing in the G0/G1 phase of the cell cycle. Similarly, exposure to OTA did not induce an observable effect on the cell cycle. Previous studies have reported that OTA induces mitotic arrest (11), and downregulation of several cell division cycle proteins including Cdc25 and Cdk1 was seen 24 h following OTA treatment of GES-1 cells (10). In the current study, pathway analysis of transcriptomic data suggested that OTA may negatively regulate factors which promote G2/S progression. In contrast, KBrO3 caused a significant increase in the proportion of cells in the G2/S phase, and this finding was in keeping with our pathway analysis which identified the cell cycle as the sixth most significantly dysregulated pathway following exposure to KBrO3. It is unclear whether loss of the primary cilia is a cause of, or a result of, cells reentering the cell cycle following treatment. However, our observation that OTA-induced deciliation appears to occur independently of cell cycle progression is a significant finding and one that requires further investigation. Taken together, these results suggest that while both OTA and KBrO3 induced deciliation in the RPTEC/TERT1 cells, distinct mechanisms are likely involved, and the divergent effects on cell cycle may be related to the proposed mechanisms of carcinogenicity for each chemical (i.e., OTA is a nongenotoxic, promoting agent, while KBrO3 is directly genotoxic).
The current study has characterized the primary cilium in a novel human renal proximal tubular epithelial cell line, RPTEC/TERT1. We have identified loss of the primary cilium as a potentially important effect of exposure to chemical carcinogens, and further elucidation of the mechanisms underlying these deciliation events will further enhance our understanding of the role of the primary cilium in carcinogenesis.
GRANTS
This work was funded by the EU 6th Framework grant “carcinoGENOMICS”; PL-037712. The Conway Institute of Biomolecular and Biomedical Research is supported under the Program for Research in Third Level Institutions administered by the Higher Education Authority of Ireland. C. Slattery is a Government of Ireland Research Fellow supported by the Irish Research Council for Science, Technology and Engineering.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: R.R., C.S., O.B., M.P.R., and T.M. provided conception and design of research; R.R., C.S., and P.J. performed experiments; R.R., C.S., H.G., J.V.D., and T.M. analyzed data; R.R., C.S., O.B., W.P., and M.P.R. interpreted results of experiments; R.R. and C.S. prepared figures; R.R. and C.S. drafted manuscript; P.J., O.B., W.P., H.G., J.V.D., M.P.R., and T.M. edited and revised manuscript; T.M. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge the assistance of Dr. Alfonso Blanco Fernandez in the Flow Cytometry Core Technology Facility at the UCD Conway Institute.
REFERENCES
- 1. Adhiambo C, Forney JD, Asai DJ, LeBowitz JH. The two cytoplasmic dynein-2 isoforms in Leishmania mexicana perform separate functions. Mol Biochem Parasitol 143: 216– 225, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, Mah AK, Johnsen RC, Cavender JC, Lewis RA, Leroux MR, Beales PL, Katsanis N. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature 425: 628– 633, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol 4: e20, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Besschetnova TY, Kolpakova-Hart E, Guan Y, Zhou J, Olsen BR, Shah JV. Identification of signaling pathways regulating primary cilium length and flow-mediated adaptation. Curr Biol 20: 182– 187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boldt K, Mans DA, Won J, van Reeuwijk J, Vogt A, Kinkl N, Letteboer SJF, Hicks WL, Hurd RE, Naggert JK, Texier Y, den Hollander AI, Koenekoop RK, Bennett J, Cremers FPM, Gloeckner CJ, Nishina PM, Roepman R, Ueffing M. Disruption of intraflagellar protein transport in photoreceptor cilia causes Leber congenital amaurosis in humans and mice. J Clin Invest 121: 2169– 2180, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cardenas-Rodriguez M, Badano JL. Ciliary biology: understanding the cellular and genetic basis of human ciliopathies. Am J Med Genet C Semin Med Genet 151C: 263– 280, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Chefetz I, Holmberg JC, Alvero AB, Visintin I, Mor G. Inhibition of Aurora-A kinase induces cell cycle arrest in epithelial ovarian cancer stem cells by affecting NFκB pathway. Cell Cycle 10: 2206– 2214, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Christensen ST, Pedersen LB, Schneider L, Satir P. Sensory cilia and integration of signal transduction in human health and disease. Traffic 8: 97– 109, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Cui C, Chatterjee B, Francis D, Yu Q, SanAgustin JT, Francis R, Tansey T, Henry C, Wang B, Lemley B, Pazour GJ, Lo CW. Disruption of Mks1 localization to the mother centriole causes cilia defects and developmental malformations in Meckel-Gruber syndrome. Dis Model Mech 4: 43– 56, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cui J, Xing L, Li Z, Wu S, Wang J, Liu J, Wang J, Yan X, Zhang X. Ochratoxin A induces G2 phase arrest in human gastric epithelium GES-1 cells in vitro. Toxicol Lett 193: 152– 158, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Czakai K, Müller K, Mosesso P, Pepe G, Schulze M, Gohla A, Patnaik D, Dekant W, Higgins JMG, Mally A. Perturbation of mitosis through inhibition of histone acetyltransferases: the key to ochratoxin a toxicity and carcinogenicity? Toxicol Sci 122: 317– 329, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol 23: 345– 373, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Esteban MA, Harten SK, Tran MG, Maxwell PH. Formation of primary cilia in the renal epithelium is regulated by the von Hippel-Lindau tumor suppressor protein. J Am Soc Nephrol 17: 1801– 1806, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Ezratty EJ, Stokes N, Chai S, Shah AS, Williams SE, Fuchs E. A role for the primary cilium in Notch signaling and epidermal differentiation during skin development. Cell 145: 1129– 1141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gaertig J, Wloga D. Ciliary tubulin and its post-translational modifications. Curr Top Dev Biol 85: 83– 113, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Gerdes JM, Liu Y, Zaghloul NA, Leitch CC, Lawson SS, Kato M, Beachy PA, Beales PL, DeMartino GN, Fisher S, Badano JL, Katsanis N. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet 39: 1350– 1360, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Hajj P, Ferlicot S, Massoud W, Awad A, Hammoudi Y, Charpentier B, Durrbach A, Droupy S, Benoît G. Prevalence of renal cell carcinoma in patients with autosomal dominant polycystic kidney disease and chronic renal failure. Urology 74: 631– 634, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med 364: 1533– 1543, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci USA 102: 11325– 11330, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jennen DG, Magkoufopoulou C, Ketelslegers HB, van Herwijnen MH, Kleinjans JC, van Delft JH. Comparison of HepG2 and HepaRG by whole-genome gene expression analysis for the purpose of chemical hazard identification. Toxicol Sci 115: 66– 79, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Jin H, White SR, Shida T, Schulz S, Aguiar M, Gygi SP, Bazan JF, Nachury MV. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell 141: 1208– 1219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones C, Roper VC, Foucher I, Qian D, Banizs B, Petit C, Yoder BK, Chen P. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat Genet 40: 69– 77, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Kobayashi T, Dynlacht BD. Regulating the transition from centriole to basal body. J Cell Biol 193: 435– 444, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krieger J, Schleicher S, Strotmann J, Wanner I, Boekhoff I, Raming K, De Geus P, Breer H. Probing olfactory receptors with sequence-specific antibodies. Eur J Biochem 219: 829– 835, 1994 [DOI] [PubMed] [Google Scholar]
- 25. Lancaster MA, Louie CM, Silhavy JL, Sintasath L, Decambre M, Nigam SK, Willert K, Gleeson JG. Impaired Wnt-beta-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy. Nat Med 15: 1046– 1054, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lancaster MA, Schroth J, Gleeson JG. Subcellular spatial regulation of canonical Wnt signalling at the primary cilium. Nat Cell Biol 13: 700– 707, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Limonciel A, Aschauer L, Wilmes A, Prajczer S, Leonard MO, Pfaller W, Jennings P. Lactate is an ideal non-invasive marker for evaluating temporal alterations in cell stress and toxicity in repeat dose testing regimes. Toxicol In Vitro 25: 1855– 1862, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LSB, Somlo S, Igarashi P. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA 100: 5286– 5291, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loktev AV, Zhang Q, Beck JS, Searby CC, Scheetz TE, Bazan JF, Slusarski DC, Sheffield VC, Jackson PK, Nachury MV. A BBSome subunit links ciliogenesis, microtubule stability, and acetylation. Dev Cell 15: 854– 865, 2008 [DOI] [PubMed] [Google Scholar]
- 30. May SR, Ashique AM, Karlen M, Wang B, Shen Y, Zarbalis K, Reiter J, Ericson J, Peterson AS. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev Biol 287: 378– 389, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Million K, Larcher J, Laoukili J, Bourguignon D, Marano F, Tournier F. Polyglutamylation and polyglycylation of alpha- and beta-tubulins during in vitro ciliated cell differentiation of human respiratory epithelial cells. J Cell Sci 112: 4357– 4366, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Murcia NS, Richards WG, Yoder BK, Mucenski ML, Dunlap JR, Woychik RP. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development 127: 2347– 2355, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol 26: 59– 87, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AEH, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129– 137, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Nishimura DY, Fath M, Mullins RF, Searby C, Andrews M, Davis R, Andorf JL, Mykytyn K, Swiderski RE, Yang B, Carmi R, Stone EM, Sheffield VC. Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proc Natl Acad Sci USA 101: 16588– 16593, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Otto EA, Schermer B, Obara T, O'Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D, Foreman JW, Goodship JA, Strachan T, Kispert A, Wolf MT, Gagnadoux MF, Nivet H, Antignac C, Walz G, Drummond IA, Benzing T, Hildebrandt F. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet 34: 413– 420, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Overgaard CE, Sanzone KM, Spiczka KS, Sheff DR, Sandra A, Yeaman C. Deciliation is associated with dramatic remodeling of epithelial cell junctions and surface domains. Mol Biol Cell 20: 102– 113, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, Igarashi P. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet 17: 1578– 1590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol 15: 105– 110, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol 184: 71– 79, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Prowse AH, Webster AR, Richards FM, Richard S, Olschwang S, Resche F, Affara NA, Maher ER. Somatic inactivation of the VHL gene in Von Hippel-Lindau disease tumors. Am J Hum Genet 60: 765– 771, 1997 [PMC free article] [PubMed] [Google Scholar]
- 42. Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol 15: 1861– 1866, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Seeley ES, Carrière C, Goetze T, Longnecker DS, Korc M. Pancreatic cancer and precursor pancreatic intraepithelial neoplasia lesions are devoid of primary cilia. Cancer Res 69: 422– 430, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Singla V, Reiter JF. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science 313: 629– 633, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102: 15545– 15550, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Verghese E, Ricardo SD, Weidenfeld R, Zhuang J, Hill PA, Langham RG, Deane JA. Renal primary cilia lengthen after acute tubular necrosis. J Am Soc Nephrol 20: 2147– 2153, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vieira OV, Gaus K, Verkade P, Fullekrug J, Vaz WLC, Simons K. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc Natl Acad Sci USA 103: 18556– 18561, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vogel P, Read R, Hansen GM, Freay LC, Zambrowicz BP, Sands AT. Situs inversus in Dpcd/Poll−/−, Nme7−/−, and Pkd1l1−/− mice. Vet Pathol 47: 120– 131, 2010 [DOI] [PubMed] [Google Scholar]
- 49. Wieser M, Stadler G, Jennings P, Streubel B, Pfaller W, Ambros P, Riedl C, Katinger H, Grillari J, Grillari-Voglauer R. hTERT alone immortalizes epithelial cells of renal proximal tubules without changing their functional characteristics. Am J Physiol Renal Physiol 295: F1365– F1375, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Yuan K, Frolova N, Xie Y, Wang D, Cook L, Kwon YJ, Steg AD, Serra R, Frost AR. Primary cilia are decreased in breast cancer: analysis of a collection of human breast cancer cell lines and tissues. J Histochem Cytochem 58: 857– 870, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.