Abstract
Thyroid-associated orbitopathy (TAO) is a diverse spectrum of signs and symptoms that appears to have immunologic and pathologic causative factors as diverse as its clinical presentations. Lymphocytes, hormones, and cytokines affect orbital fibroblasts and other similar cells, which exert their effects on orbital tissues, including the extraocular muscles, orbital fat, and optic nerve. This complicated inflammatory cascade and the myriad of clinical findings that result contributes to the active phase of TAO. The distinction between the active and inactive phases of TAO is an important one, as the proper treatment will depend on the disease phase and degree thereof. Several clinical grading scales and scores have been established to help qualify and quantify the disease severity. Aiding clinical exam and acumen, proper and reproducible imaging of the orbit and ocular adnexa is incredibly important to the management of TAO. Orbital ultrasound, computed tomography, magnetic resonance imaging, and scintigraphy each have unique abilities, including quantifying orbital changes, assessing disease activity, correlating orbital findings with clinical changes, guiding appropriate treatment, and monitoring therapeutic responses. Further, study ease, accessibility, cost, sensitivity, specificity, reproducibility, and risks are all important considerations in picking the right test with which to diagnose and follow TAO. This analysis will provide a review of orbital imaging for TAO, including the mechanism of each imaging technique as well as their rationales, advantages, disadvantages, and utilities.
Keywords: Thyroid-associated orbitopathy, Graves orbitopathy, Magnetic resonance imaging, Computed tomography, Orbital ultrasound, Scintigraphy
Introduction
Thyroid-associated orbitopathy (TAO), also referred to as Graves’ ophthalmopathy, Graves’ orbitopathy, and thyroid eye disease, is a constellation of signs and symptoms resulting from chronic autoimmune-related orbital inflammation. Through extensive investigations into its etiology, many immunologic and pathologic causative factors have been revealed. Genetic and environmental factors have been related both to TAO susceptibility as well as to prognosis and response to treatment.1 On an immunologic level, the extraocular muscles and orbital fat have been found targeted by T lymphocytes and mast cells in association with interferon, tumor necrosis factor, somatostatin, and interleukin activity.1–4 At a cellular level, orbital fibroblasts proliferate in response to the resultant inflammatory cascades and release abnormal amounts of prostaglandins and hyaluronan, which may increase the orbital volume.1,2,5–7 While the accumulation of this high-molecular-weight compound may be responsible for the orbital changes characteristic of TAO, gangliosides have also been shown to potentially contribute.8 Even the role of autoantibodies targeting thyrotropin receptors remains unclear,1,2,9–11 with other autoimmune targets such as calequestrin and collagen XIII being researched.9,12 As a result, the correlation between TAO and Graves’ disease is not a straightforward one.
As many as 50% of patients with Graves’ disease, a form of hyperthyroidism in which autoantibodies stimulate the thyrotropin receptor, will develop orbital changes.1,8,13–15 Interestingly, while TAO remains the most common extra-thyroidal complication of Graves’ disease, it occurs in euthyroid and hypothyroid patients up to 10% of the time.1,13,15 Except for these comparatively rare cases, the diagnosis of hyperthyroidism and TAO usually occurs within 18 months of each other.16,17 The incidence of TAO tends to show a bimodal peak, with the first peak between 40 and 50 years of age and the second occurring between 60 and 70 years of age, and occurs in women about 10 times more frequently than in men.16–19
The signs and symptoms of TAO are as varied as the pathophysiology and epidemiology of the disease. Symptoms range from tearing to eye pain to double vision, and signs extend from conjunctival injection and chemosis to lid retraction and exposure keratopathy to strabismus patterns (Fig. 1). While approximately half of patients with Graves’ disease are asymptomatic from an ophthalmic standpoint, 20–30% of patients with orbital and ocular involvement are so affected they require targeted therapies.20,21 And while Rundle’s curve first introduced the accepted course of TAO as one of an active, inflammatory phase followed by a static, fibrotic phase, the exact rate and duration of disease phases may be as varied and unpredictable as the disease itself.1,20 The distinction is important, though, as one would not want to operate on actively inflamed muscles, nor would radiation be indicated in fibrotic disease.
Figure 1.
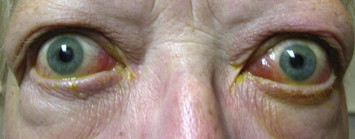
External photograph of TAO patient with exophthalmos and ocular misalignment.
Ultimately, up to 5% of patients with TAO will suffer severe, vision-threatening forms of the disease, including compressive optic neuropathies.16,17 Compounding these disfiguring, disabling, and painful potential manifestations of TAO, extensive research has documented the profound effects this disease has on the mental health and quality of life of those that endure it.22–24
Several different classification systems have been developed to assess the varied effects of Graves’ disease on the eye and orbit. Werner, in 1969, first introduced the well-known NOSPECS classification; a summary of signs and symptoms further modified in 1977, this system categorizes TAO patients in six different classes.25,26 This classification system is still used today, although minor changes in clinical disease, as well as active and quiescent phases of TAO, are difficult to distinguish with NOSPECS criteria. In 1989 Mourits et al. developed a clinical activity score (CAS), a 10-point system that combines symptoms and signs of TAO in a way that is perhaps more useful in following prognosis and response to treatment, as well as being theoretically easier to perform in a primary-care or endocrinologist’s office setting.27–29
More recently, the European Group on Graves’ Orbitopathy (EUGOGO) classified TAO itself in terms of severity, establishing mild, moderate-to-severe, and sight-threatening groups,30 a classification system that gave way to referral indications and treatment protocols.20,30 Whether a wait-and-see approach is established for a mild case of TAO, glucocorticoids used in moderate disease, or orbital decompression used for severe cases, TAO patients must be followed and treated based on disease severity and activity.20,29,30 This is particularly true since the establishment of a euthyroid state is important in the course of most patients with Graves’ disease and TAO, and treatment with radioiodine therapy may develop worsening orbitopathy up to 15% of the time.30,31
As a result of the vast spectrum of etiologies, associations, signs, symptoms, treatments, and clinical courses presented by TAO, proper and reproducible imaging of the orbit and ocular adnexa is incredibly important to its management. And, from a diagnostic standpoint, disorders such as idiopathic orbital inflammatory syndrome (IOIS), metastases, sinus inflammations or tumors, carotid-cavernous sinus fistulas, orbital sarcoid, and lymphoma must be ruled out. In 1998 members of the European Thyroid Association reported that most centers used orbital ultrasound (US) in combination with computed tomography (CT) or magnetic resonance imaging (MRI) to this capacity, while smaller percentages used CT and MRI together or octreotide scanning with either CT or MRI.32,33 Ease, accessibility, cost, sensitivity, specificity, reproducibility, and risks are all important considerations in picking the right test with which to diagnose and/or follow TAO patients. Those matters are discussed in this review.
Ultrasound
Standardized orbital echography is an old and widely-used imaging modality that uses reflected high-frequency (8 MHz) sound waves to form A-scans, supplement them with B-scans, and incorporates Doppler sonography to measure, differentiate, and elucidate the vascularity of a wide array of orbital lesions.33–36 A basic orbital US screens the orbital fat, evaluates and measures the extraocular muscles, and assesses the optic nerves.35 Targeted exams include topographic echography in which a mass may be isolated on the B-scan and then its dimensions were measured using the A-scan, 30° tests to evaluate the subarachnoid optic nerve fluid versus inflammation, quantitative evaluation in which the A-scan uses sonar reflectivity to evaluate a lesion’s tissue properties, and kinetic echography during which the physical pressure of the probe is used to characterize the compressibility of a lesion in question.35,37 As a result, the transocular and orbital use of A-scan, B-scan, and Doppler US has persevered as a fast, inexpensive, and valuable tool in the evaluation of TAO.34,38,39
Studies investigating US in TAO demonstrated that extraocular muscle thickness, as measured via A- and B-scans, increases with increasing disease severity.39 There is also a high degree of correlation between the right and left eyes, the symmetry of which is valuable in distinguishing TAO from other similar but often unilateral diagnostic entities, such as IOIS and lymphoma.38,39 Extraocular muscle thickness demonstrated on US has been shown to correlate with the degree of proptosis as well.40 In fact, Werner et al. demonstrated that extraocular muscle enlargement is detected by US with more sensitivity than by clinical exam.38 Additionally, other sonographic studies of TAO contributed that a significant association between proptosis and the volume of extraocular muscle and orbital fat exists.41
The clinical phase of TAO and disease course may be revealed by echography as well; A-scan measurements have shown significantly lower extraocular muscle reflectivity in patients that respond to TAO therapy, indicating that tissue reflectivity is a marker of edema, inflammation, and disease activity.42 Studies supportive of US cite this as an advantage over CT, which provides an excellent anatomic perspective but cannot evaluate disease activity.43
Prummel et al. followed reflectivity in the extraocular muscles with the lowest echogenecity and found that, after a cut-off value (40%) was assigned, reflectivity under this value had a positive predictive value (PPV) for response to TAO treatment of 73%. The negative predictive value (NPV) of tissue reflectivity over 40% was 100%.42 Contrastingly, a more recent publication by Prummel et al. shows significantly lower predictive values.44 However, even though extraocular muscle reflectivity may not correlate with CAS, when a CAS over 4/10 is combined with US reflectivity the PPV and NPV for response to treatment are 74% and 72% respectively, which rise to 79% and 89% when the duration of eye symptoms is added to these clinical parameters.45 Given-Wilson et al. reported that the medial rectus muscle width itself correlated with clinical score, as well as with CT measurements for the same muscle.46 Similarly, several studies support orbital US as not only an accurate means of diagnosing TAO pathology and predicting its clinical course, but as a way to follow the course of the disease and the response to treatment as well.47,48
The addition of color Doppler imaging to US in TAO also aids its diagnosis, as blood flow measurements in the ophthalmic artery, central retinal artery, and central retinal vein have all been correlated with extraocular muscle diameter and TAO.49 Further, these blood flow parameters measured sonographically correlate with CAS levels, and may contribute to the distinction of active versus inactive disease.50 Reversal and reduction of blood flow through the superior ophthalmic vein may independently be a sign of severe orbitopathy and progression to optic neuropathy.51,52
Ultrasound use in the diagnosis, prognosis, and monitoring of TAO is not without its shortcomings, however. The orbital apex may be poorly visualized.39 US alone may be inadequately diagnostic, and may not differentiate TAO from other causes of large muscles.38 Additionally, although many of the above references showed positive and encouraging results using US in TAO, they have also reported a wide range of average muscle widths and volumes with little consistency from study to study.39,40,42,47 In several studies looking at US in TAO the superior rectus is the largest muscle group40,42; Shammas et al. explain that the superior rectus and superior oblique tendons may be measured as one on US, further confusing orbital measurements.53 Moreover, the user-dependent nature of sonographic imaging may be detrimental to study consistency and reproducibility.39 Extraocular muscle cross-sections are oval rather than round, with their maximum coronal volume occurring over a fixed area, making it difficult for a radiologist or ultrasound technician to reproduce the same angle and location along a muscle for serial measurements.33
As such, average extraocular muscle sizes in many of the aforementioned studies also differ from those taken via CT and MRI.54,55 In the end, while a fast, inexpensive study with no risks of contrast or radiation, US must be interpreted with caution given the variability and inconsistency with which it measures extraocular muscle sizes, particularly compared to MRI.56,57 The larger the muscle, the greater the possibility that US will underestimate its size compared to MRI.55 And, with regard to CT, US is comparably ineffective in imaging the posterior orbit, bony anatomy thereof, and optic nerve.33,34,56
Computed tomography
Computed tomography (CT) is an invaluable imaging modality in the evaluation of TAO, using X-rays and their variable absorptions within the many orbital structures to quantify and qualify orbital pathologies. Orbital fat and water, for example, absorb less X-rays than higher-density structures such as the optic nerve and orbital bone. As a result, fat and water appear hypodense, or darker, than bone and nerve. Additionally, orbital fat is less dense than water; resultant differences in orbital tissue densities allow for high-resolution imaging even without intravenous contrast administration. Contrast is oftentimes reserved, then, for cases of optic nerve pathology or vision loss.33,34,58–60
The resolution and diagnostic utility of CT have consistently evolved with the ability of modern scans to apply spiral techniques and evaluate thin, often less than 1.5 mm, cuts of the orbital space. The ability to correlate extraocular muscle changes with the pathology of TAO became a useful utility of CT, demonstrating swollen muscle bellies with tendon sparing radiographically61 (Fig. 2). Early studies supporting CT in TAO used larger cuts and found that, while the medial and lateral rectus muscles were identifiable and quantifiable at the mid-orbital level, oblique muscles could not be isolated and, unless markedly enlarged, the vertical rectus muscles could not be accurately evaluated.62 Still, although superior orbital and apical CT cuts were indistinct in earlier studies, there was high intra-observer consistency on mid-orbital views with a 66% diagnostic accuracy for TAO.62 Up to 80% of TAO patients were found to have lateral rectus enlargement in studies using early imaging, while 77% had medial rectus enlargement; these data are inconsistent with reproducible percentages based on more modern CT and MRI values, many of which show inferior rectus enlargement more commonly than medial rectus enlargement, followed by superior pathology and then lateral rectus enlargement55,63–65 (Fig. 3).
Figure 2.
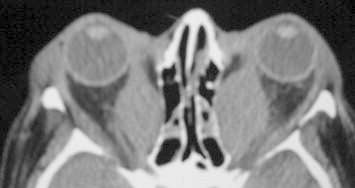
Axial orbital CT of TAO with severe fusiform medial rectus enlargement.
Figure 3.
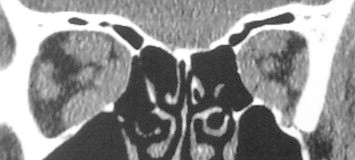
Coronal orbital CT of TAO showing extraocular muscle enlargement with relative sparing of the lateral rectus muscles.
Investigating this, Nugent et al. reviewed orbital CTs of non-TAO patients and found the average extraocular muscle sizes, in descending order, to be inferior rectus > medial rectus > superior muscle group (superior rectus and levator complex) > lateral rectus.54 The muscle enlargement ratios, calculated by dividing the average muscle diameters of TAO orbits from those of normal orbits, found that the superior muscle group was most often involved by TAO, followed by the medial rectus, the inferior rectus, and finally the lateral rectus. However, the average diameters for TAO patients with and without optic neuropathies, while significantly larger than those taken from normal orbits, maintained inferior > medial > superior > lateral relationships. Nugent et al. theorize that the inferior rectus is often the most commonly clinically and radiographically involved extraocular muscle because it is the largest at baseline, whereas the superior muscle group is actually most involved in dysthyroid orbits.54 Studies suggest that while there is an increase in total extraocular muscle volume with increasing clinical ophthalmopathy and increased inferior rectus and orbital fat volumes in all TAO, the superior rectus size increases only in advanced disease.66 Medial and lateral rectus muscles may also correlate directly with clinical disease.
CT as a tool to quantify muscle enlargement, similar to A-scan US previously described, is therefore a valuable aid to the clinical exam. Studies suggest parallels between total extraocular muscle volumes and medial rectus muscle width at the mid-orbital level, and medial rectus muscle width with clinical extraocular muscle restriction, periorbital edema, and optic neuropathy in those with TAO.67–69 In further correlating simple CT measurements with optic neuropathy in TAO, coronal images taken at the mid-point of the orbit were used by Barrett et al. to create a muscular index (MI).65 The sum of the vertical rectus muscle width divided by the orbital height quantifies the vertical MI, and the sum of the horizontal rectus muscle width divided by the orbital width is the horizontal MI; the larger MI was correlated with clinical findings. MI cutoffs may be tailored to accommodate clinical goals, as lower percentages have higher sensitivities and higher percentages have increased specificities for optic neuropathy. Barrett et al. found no neuropathy subjects had an MI less than 50%, and no negative cases had an MI over 67%. Giaconi et al. found an MI over 50% has a sensitivity of 100% for TAO optic neuropathy, but only 47% specificity.70 A study by Monteiro et al. revealed sensitivities of 100% for MI values of 40–50% with decreasing specificities in that range, and a sensitivity of 32% with a specificity of 100% for an MI of 70%. An MI of 60% was 79% sensitive and 72% specific for TAO optic neuropathy, and is therefore the best data point for clinical correlation.71
In looking at changes to and around the optic nerve as markers of optic neuropathy, grading scales from 0 to 3 were created by Nugent et al. to describe the percentage of effacement of perineural fat planes caused by extraocular muscles as markers of nerve crowding.54 Grade 3 crowding, or over 50% effacement, correlated with TAO optic neuropathy over 66% of the time.54,70 Anterior displacement of the lacrimal gland has been associated with optic neuropathy, and posterior herniation of the orbital fat is 94% sensitive and 91% specific for TAO optic neuropathy, with a PPV of 69% and a NPV of 98%.54,70,72
Unlike US and MRI, CT cannot assess disease activity and involves radiation. CT, however, remains a fast, available, relatively inexpensive test that is highly reproducible.33,34 Although its assessment of the optic nerve itself is less specific than that of MRI, orbital findings seen on CT are highly diagnostic and may be directly correlated with optic neuropathy and a myriad of other clinical changes. And while CT’s ability to assess bony anatomy is distinctly advantageous in correlating clinical changes with imaging characteristics, its utility in the pre-operative setting, in preparation for orbital decompression, is invaluable.
Magnetic resonance imaging
In magnetic resonance imaging (MRI), a set of superconducting coils polarizes a given field by magnetizing certain nuclei.73 That magnetization depends on protons, positively charged particles that are constantly rotating, producing an electric current that gives rise to a magnetic field. Protons and electrons are normally atomically balanced, and no net magnetic field is generated. However, several nuclei form magnetic isotopes containing an odd number of nucleons; the hydrogen proton is the most common biologic example, and these unpaired protons generate the signal read on MRI.33,34,73 Still, random rotation of protons normally exerts no net magnetic charge; when MRI polarizes the field those axes align, and an electromagnetic radio-frequency wave acts on the protons, raising them to an even higher energy level. T1 and T2 relaxation times refer to the longitudinal relaxation times and transverse relaxation times, respectively, of these charged particles after the excitatory wave is discontinued.33,34,73 Variations of those signals provide information on tissue structure and composition and, as a result, are uniquely helpful in the diagnosis and monitoring of TAO.
Similar to US, MRI aids in the diagnosis of TAO activity. MRI is more sensitive and specific in detecting extraocular muscle enlargement than US, however, particularly when muscles are markedly enlarged55 (Fig. 4). And while contrast-enhancement of the extraocular muscles on MRI may or may not be of prognostic value compared to clinical assessment,74,75 longer T2 relaxation times are indicative of edema and inflammation independent of muscle size55 (Figs. 5 and 6). Nagy et al. found that T2 relaxation times on MRI provided more diagnostic and prognostic information than 11 clinical and laboratory factors.55
Figure 4.
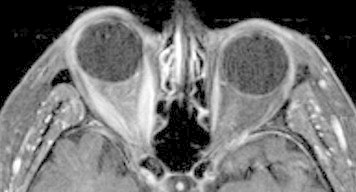
Axial post-contrast T1 MRI of TAO demonstrating horizontal rectus muscle enlargement.
Figure 5.
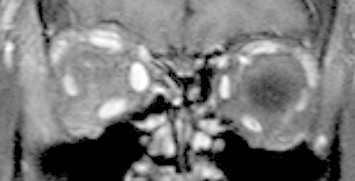
Coronal post-contrast T1 MRI of TAO showing marked enlargement of extraocular muscles on the right side.
Figure 6.
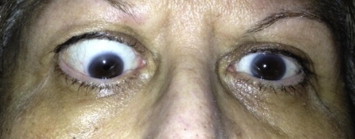
External photograph of TAO demonstrated radiographically in Fig. 5.
T2 relaxation times as markers of TAO active phases are valuable tools, both in the selection of proper therapy as well as for the prediction and monitoring of treatment response.76 Just et al. report decreasing T2 relaxation times in the extraocular muscles of active TAO patients in response to orbital radiation, and Hosten et al. showed similar signal intensity changes after immunosuppression.76,77 Hiromatsu et al. correlated increased signal intensity ratio on short T1 inversion recovery sequencing to be indicative of activity and a marker of response to steroid treatment well.78 Lessening T2 relaxation times of the extraocular muscles in response to cyclosporine and corticosteroid therapy occur even in spite of unchanged laboratory markers during and after treatment.79 A lack of correlation between muscle changes on MRI and thyroid function testing is confirmed elsewhere.80
For further clinical implications, just as CT can effectively aid the diagnosis of TAO-related optic neuropathy, MRI is a useful tool in examining pressure on the optic nerve. MRI examines the optic nerve dimensions itself, though, and does so with greater accuracy than US.81 MRI has demonstrated a reproducible decrease in mean nerve diameter as it extends posterior from the globe, and some report this decrease up to the orbital apex81,82 while others report a minimum diameter 15 mm posterior to the globe with gradual enlargement thereafter.83 That ability to quantify the nerve itself has translated to optic neuropathy in TAO, in which the optic nerve diameter at the orbital apex has been found significantly reduced on MRI.83 This is true even in optic neuropathy orbits without increased muscle indices.
Although its use is limited by long exam time, expense, availability, and magnetism contraindicated in patients with certain implants, MRI remains a valuable tool in the care of TAO. It provides remarkable images of orbital anatomy, except for bony structures, particularly at the orbital apex where resonance may degrade CT images.34,73 Its ability to quantify muscle enlargement supersedes that of US and probably CT, and MRI’s comparatively precise and reproducible capability to qualify disease stage and activity makes it a unique tool in determining proper treatment and monitoring therapeutic response.
Somatostatin receptor scintigraphy
Somatostatin, a neuroendocrine hormone, has receptors on many human cells, including fibroblasts, myoblasts, and lymphocytes which, we have seen, contribute to the pathology of active TAO. Octreotide, and several peptide analogs thereof, has been developed as a longer-acting somatostatin analog labeled with indium.84 While the intravenously administered dose and time interval are non-standardized33, this radionuclide has been shown to accumulate within the orbit of active TAO patients, is detected via single-photon emission CT (SPECT), and may serve as a unique marker of disease activity as a result.84–93
In this way, orbital scintigraphy is quite similar to T2 relaxation times on MRI in assessing disease activity, with significant correlations being documented radiographically.85 Orbital increase of somatostatin analogs has been found to correlate well with CAS and clinical disease severity.4,84,86–88 In contrast, the NOSPECS classification has been shown in some studies to correlate with somatostatin receptor tracer uptake, while not in others.4,86
Nevertheless, as a result of its clinical associations, scintigraphy in TAO may guide treatment by identifying and grading disease activity.84,85 Gerding et al. specify that octreotide uptake measured 4 h after injection can predict the response to radiation treatment with a PPV of 92% and a NPV of 70% for a given cut-off orbital uptake ratio, whereas imaging 24 h after octreotide administration is less useful.89 Octreotide uptake has also served as a marker of treatment effectiveness, decreasing in those undergoing corticosteroid and somatostatin analog therapy.84,87
Thus, the main utility of orbital octreotide scintigraphy in TAO seems to be the prediction of those who will benefit from immunosuppressive or hormonal therapy.33,90–92 Further studies have shown PPVs of 87–90% and NPVs of up to 100% for octreoscan in the prediction of response to somatostatin analog therapy,90,92,93 with sensitivities and specificities of 94% and 100%, respectively, after certain uptake cutoffs on imaging were established.84 It is limited, however, by its high cost, inter-observer variance, radiation, lack of diagnostic specificity for the inflammatory cells it targets or the surrounding orbital structures, and poor availability.33,90
Conclusion
The available imaging modalities in the evaluation and management of TAO are varied, each one having advantages, disadvantages, and particular utilities. Orbital US is a widely used technique that may quantify extraocular muscle enlargement and inflammation with the added benefits of ease, low cost, high accessibility, short exam time, and lack of radiation. The disadvantages of orbital US include poor visualization of the posterior orbit, inaccuracy in measurements, and investigator dependence. CT is a fast, easy, inexpensive, and reproducible study that allows for defined imaging of the orbital bones, sinuses, and muscles with shortcomings including exposure to radiation, lack of correlation with disease activity and treatment guidance, and poor qualification of the optic nerve compared to MRI. MRI is an excellent TAO-imaging modality allowing precise measurements of the extraocular muscle size, qualification of inflammation, assessment of optic nerve changes, and evaluation of surrounding orbital and adnexal soft tissue changes without using radiation. However, its high cost, longer exam time, lower availability, and comparative lack of bony resolution compared to CT may limit its use. Octreoscan, while a very sensitive test for inflammation and activity in TAO with an ability to guide treatment as a result, remains an expensive test suffering from radiation burden, lack of availability at many institutions, and inter-observer inconsistency.
The author’s clinical preference in evaluating TAO at the Wills Eye Institute is CT scanning to assess extraocular muscle size and shape, apical compression, sinus pathology, and orbital bone anatomy both as a baseline exam and in the planning of orbital decompressions or surgeries. We do not rely on imaging to assess the activity of TAO, but rather use clinical judgment and serial exams. However, with advances in MRI and somatostatin receptor scintigraphy, active disease may be confirmed through imaging, and this may play an increasing role in our care of TAO patients in the future.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Naik V.M., Naik M.N., Goldberg R.A., Smith T.J., Douglas R.S. Immunopathogenesis of thyroid eye disease: emerging paradigms. Surv Ophthalmol. 2010;55(3):215–226. doi: 10.1016/j.survophthal.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Douglas R.S., Afifiyan N.F., Hwang C.J., Chong K., Haider U., Richards P. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2010;95(1):430–438. doi: 10.1210/jc.2009-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahaly G.J., Shimony O., Gellman Y.N., Lytton S.D., Eshkar-Sebban L., Rosenblum N. Regulatory T-cells in Graves’ orbitopathy: baseline findings and immunomodulation by anti-T lymphocyte globulin. J Clin Endocrinol Metab. 2011;96(2):422–429. doi: 10.1210/jc.2010-1424. [DOI] [PubMed] [Google Scholar]
- 4.Burggasser G., Hurtl I., Hauff W., Lukas J., Greifeneder M., Heydari B. Orbital scintigraphy with the somatostatin receptor tracer 99mTc-P829 in patients with Graves’ disease. J Nucl Med. 2003;44(10):1547–1555. [PubMed] [Google Scholar]
- 5.Cao H.J., Smith T.J. Leukoregulin upregulation of prostaglandin endoperoxide H synthase-2 expression in human orbital fibroblasts. Am J Physiol. 1999;277(6):1075–1085. doi: 10.1152/ajpcell.1999.277.6.C1075. [DOI] [PubMed] [Google Scholar]
- 6.Han R., Tsui S., Smith T.J. Up-regulation of prostaglandin E2 synthesis by interleukin-1beta in human orbital fibroblasts involves coordinate induction of prostaglandin-endoperoxide H synthase-2 and glutathione-dependent prostaglandin E2 synthase expression. J Biol Chem. 2002;277(19):16355–16364. doi: 10.1074/jbc.M111246200. [DOI] [PubMed] [Google Scholar]
- 7.Kaback L.A., Smith T.J. Expression of hyaluronan synthase messenger ribonucleic acids and their induction by Interleukin-1{beta} in human orbital fibroblasts: potential insight into the molecular pathogenesis of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 1999;84(11):4079–4084. doi: 10.1210/jcem.84.11.6111. [DOI] [PubMed] [Google Scholar]
- 8.Kook K.H., Choi Y.H., Kim Y.R., Park S.J., Jou I., Kim S.J. Altered ganglioside expression modulates the pathogenic mechanism of thyroid-associated ophthalmopathy by increase in hyaluronic acid. Invest Ophthalmol Vis Sci. 2011;52(1):264–273. doi: 10.1167/iovs.10-5276. [DOI] [PubMed] [Google Scholar]
- 9.Lahooti H., Parmar K.R., Wall J.R. Pathogenesis of thyroid-associated ophthalmopathy: does autoimmunity against calsequestrin and collagen XIII play a role? Clin Ophthalmol. 2010;4:417–425. doi: 10.2147/opth.s6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsui S., Naik V., Hoa N., Hwang C.J., Afifiyan N.F., Sinha Hikim A. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves’ disease. J Immunol. 2008;181(6):4397–4405. doi: 10.4049/jimmunol.181.6.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wall J.R., Lahooti H. Pathogenesis of thyroid eye disease-does autoimmunity against the TSH receptor explain all cases? Endokrynol Pol. 2010;61(2):222–227. [PubMed] [Google Scholar]
- 12.Gopinath B., Wescombe L., Nguyen B., Wall J.R. Can autoimmunity against calsequestrin explain the eye and eyelid muscle inflammation of thyroid eye disease? Orbit. 2009;28(4):256–261. [PubMed] [Google Scholar]
- 13.Bahn R.S., Heufelder A.E. Pathogenesis of Graves’ ophthalmopathy. New Engl J Med. 1993;329(20):1468–1475. doi: 10.1056/NEJM199311113292007. [DOI] [PubMed] [Google Scholar]
- 14.Prabhakar B.S., Bahn R.S., Smith T.J. Current perspective on the pathogenesis of Graves’ disease and ophthalmopathy. Endocr Rev. 2003;24(6):802–835. doi: 10.1210/er.2002-0020. [DOI] [PubMed] [Google Scholar]
- 15.Garrity J.A., Bahn R.S. Pathogenesis of graves ophthalmopathy: implications for prediction, prevention, and treatment. Am J Ophthalmol. 2006;142(1):147–153. doi: 10.1016/j.ajo.2006.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiersinga W.M., Bartalena L. Epidemiology and prevention of Graves’ ophthalmopathy. Thyroid. 2002;12(10):855–860. doi: 10.1089/105072502761016476. [DOI] [PubMed] [Google Scholar]
- 17.Burch H.B., Wartofsky L. Graves’ ophthalmopathy: current concepts regarding pathogenesis and management. Endocr Rev. 1993;14(6):747–793. doi: 10.1210/edrv-14-6-747. [DOI] [PubMed] [Google Scholar]
- 18.Larsen P.R., Davies T.F., Schlumberger M.J. Thyrotoxicosis. In: Larsen P.R., Kronenberg H.M., Melmed S., editors. Williams textbook of endocrinology. W.B. Saunders Co.; Philadelphia: 2003. pp. 374–421. [Google Scholar]
- 19.Bartley G.B. The epidemiologic characteristics and clinical course of ophthalmopathy associated with autoimmune thyroid disease in Olmsted County, Minnesota. Trans Am Ophthalmol Soc. 1994;92:477–588. [PMC free article] [PubMed] [Google Scholar]
- 20.Bartalena L. The dilemma of how to manage Graves’ hyperthyroidism in patients with associated orbitopathy. J Clin Endocrinol Metab. 2011;96(3):592–599. doi: 10.1210/jc.2010-2329. [DOI] [PubMed] [Google Scholar]
- 21.Bartalena L., Tanda M.L. Clinical practice-Graves’ ophthalmopathy. New Engl J Med. 2009;360(10):994–1001. doi: 10.1056/NEJMcp0806317. [DOI] [PubMed] [Google Scholar]
- 22.Ghanemm A.A., Amr M.A., Araafa L.F. Graves opthalmopathy and psychoendocrinopathies. Middle East Afr J Ophthalmol. 2010;17(2):169–174. doi: 10.4103/0974-9233.63079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Estcourt S., Quinn A.G., Vaidya B. Quality of life in thyroid eye disease: impact of quality of care. Eur J Endocrinol. 2011;164(5):649–655. doi: 10.1530/EJE-11-0055. [DOI] [PubMed] [Google Scholar]
- 24.Yeatts R.P. Quality of life in patients with Graves ophthalmopathy. Trans Am Ophthalmol Soc. 2005;103:368–411. [PMC free article] [PubMed] [Google Scholar]
- 25.Werner S.C. Classification of the eye changes of Graves’ disease. J Clin Endocrinol Metab. 1969;29(7):982–984. doi: 10.1210/jcem-29-7-982. [DOI] [PubMed] [Google Scholar]
- 26.Werner S.C. Modification of the classification of the eye changes of Graves’ disease. Am J Ophthalmol. 1977;83(5):725–727. doi: 10.1016/0002-9394(77)90140-4. [DOI] [PubMed] [Google Scholar]
- 27.Mourits M.P., Koornneef L., Wiersinga W.M., Prummel M.F., Berghout A., van der Gaag R. Clinical criteria for the assessment of disease activity in Graves’ ophthalmopathy: a novel approach. Br J Ophthalmol. 1989;73(8):639–644. doi: 10.1136/bjo.73.8.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mourits M.P., Prummel M.F., Wiersinga W.M., Koornneef L. Clinical activity score as a guide in the management of patients with Graves’ ophthalmopathy. Clin Endocrinol (Oxf) 1997;47(1):9–14. doi: 10.1046/j.1365-2265.1997.2331047.x. [DOI] [PubMed] [Google Scholar]
- 29.Soeters M.R., van Zeijl C.J., Boelen A., Kloos R., Saeed P., Vriesendorp T.M. Optimal management of Graves orbitopathy: a multidisciplinary approach. Neth J Med. 2011;69(7):302–308. [PubMed] [Google Scholar]
- 30.Bartalena L., Baldeschi L., Dickinson A., Eckstein A., Kendall-Taylor P., Marcocci C. European Group on Graves’ Orbitopathy (EUGOGO). Consensus statement of the European Group on Graves’ orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol. 2008;158(3):273–285. doi: 10.1530/EJE-07-0666. [DOI] [PubMed] [Google Scholar]
- 31.Wiersinga W.M. Management of Graves’ ophthalmopathy. Nat Clin Pract Endocrinol Metab. 2007;3(5):396–404. doi: 10.1038/ncpendmet0497. [DOI] [PubMed] [Google Scholar]
- 32.Weetman A.P., Wiersinga W.M. Current management of thyroid-associated ophthalmopathy in Europe. Results of an international survey. Clin Endocrinol (Oxf) 1998;49(1):21–28. doi: 10.1046/j.1365-2265.1998.00487.x. [DOI] [PubMed] [Google Scholar]
- 33.Kahaly G.J. Imaging in thyroid-associated orbitopathy. Eur J Endocrinol. 2001;145(2):107–118. doi: 10.1530/eje.0.1450107. [DOI] [PubMed] [Google Scholar]
- 34.Kirsch E., Hammer B., von Arx G. Graves’ orbitopathy: current imaging procedures. Swiss Med Wkly. 2009;139(43–44):618–623. doi: 10.4414/smw.2009.12741. [DOI] [PubMed] [Google Scholar]
- 35.Byrne S.F. Standardized echography of the eye and orbit. Neuroradiology. 1986;28(5–6):618–640. doi: 10.1007/BF00344110. [DOI] [PubMed] [Google Scholar]
- 36.Ossoinig K.C. The role of standardized echography in Graves’ disease. Acta Ophthalmol Suppl. 1992;204:81. doi: 10.1111/j.1755-3768.1992.tb04932.x. [DOI] [PubMed] [Google Scholar]
- 37.Hasenfratz G. Orbital tumours-the importance of standardized echography. Acta Ophthalmol Suppl. 1992;204:82–86. doi: 10.1111/j.1755-3768.1992.tb04933.x. [DOI] [PubMed] [Google Scholar]
- 38.Werner S.C., Coleman D.J., Franzen L.A. Ultrasonographic evidence of a consistent orbital involvement in Graves’ disease. New Engl J Med. 1974;290(26):1447–1450. doi: 10.1056/NEJM197406272902602. [DOI] [PubMed] [Google Scholar]
- 39.Willinsky R.A., Arenson A.A.M., Hurwitz J.J., Szalai J. Ultrasonic B-scan measurement of the extraocular muscles in Graves’ orbitopathy. J Can Assoc Radiol. 1984;35(2):171–173. [PubMed] [Google Scholar]
- 40.Imbrasienė D., Jankauskienė J., Stanislovaitienė D. Ultrasonic measurement of ocular rectus muscle thickness in patients with Graves’ ophthalmopathy. Medicina (Kaunas) 2010;46(7):472–476. [PubMed] [Google Scholar]
- 41.Yamamoto K., Itoh K., Yoshida S., Saito K., Sakamoto Y., Matsuda A. A quantitative analysis of orbital soft tissue in Graves’ disease based on B-mode ultrasonography. Endocrinol Jpn. 1979;26(2):255–261. doi: 10.1507/endocrj1954.26.255. [DOI] [PubMed] [Google Scholar]
- 42.Prummel M.F., Suttorp-Schulten M.S.A., Wiersinga W.M., Verbeek A.M., Mourits M.P., Koornneef L. A new ultrasonographic method to detect disease activity and predict response to immunosuppressive treatment in Graves’ ophthalmopathy. Ophthalmology. 1993;100(4):556–561. doi: 10.1016/s0161-6420(93)31607-6. [DOI] [PubMed] [Google Scholar]
- 43.Delint P.J., Mourits M.P., Kerlen C.H., Scheenloop J.J., Wittebol-Post D. B-scan ultrasonography in Graves’ orbitopathy. Doc Ophthalmol. 1993;85(1):1–4. doi: 10.1007/BF01268094. [DOI] [PubMed] [Google Scholar]
- 44.Prummel M.F., Wiersinga W.M., Mourits M.P. Assessment of disease activity of Graves’ ophthalmopathy. In: Prummel M.F., editor. vol. 1(4) Kluwer Publishers; Boston/Dordrecht/London: 2000. pp. 59–79. (Recent developments in Graves’ ophthalmopathy). [Google Scholar]
- 45.Gerding M.N., Prummel M.F., Wiersinga W.M. Assessment of disease activity in Graves’ ophthalmopathy by orbital ultrasonography and clinical parameters. Clin Endocrinol (Oxf) 2000;52(5):641–646. doi: 10.1046/j.1365-2265.2000.00973.x. [DOI] [PubMed] [Google Scholar]
- 46.Given-Wilson R., Pope R.M., Michell M.J., Cannon R., McGregor A.M. The use of real/time orbital ultrasound in Graves’ ophthalmopathy: a comparison with computer tomography. Br J Radiol. 1989;62(740):705–709. doi: 10.1259/0007-1285-62-740-705. [DOI] [PubMed] [Google Scholar]
- 47.Erickson B.A., Harris G.J., Lewandowski M.F., Murray K.J., Massaro B.M. Echographic monitoring of response of extraocular muscles to irradiation in Graves’ ophthalmopathy. Int J Radiat Oncol Biol Phys. 1995;31(3):651–660. doi: 10.1016/0360-3016(94)00364-Q. [DOI] [PubMed] [Google Scholar]
- 48.Holt J.E., O’Connor P.S., Douglas J.P., Byrne B. Extraocular muscle size comparison using standardized A-scan echography and computerized tomography scan measurements. Ophthalmology. 1985;92(10):1351–1355. doi: 10.1016/s0161-6420(85)33858-7. [DOI] [PubMed] [Google Scholar]
- 49.Alp M.A., Ozgen A., Can I., Cakar P., Gunalp I. Colour Doppler imaging of the orbital vasculature in Graves’ disease with computed tomographic correlation. Br J Ophthalmol. 2000;84(9):1027–1030. doi: 10.1136/bjo.84.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yanik B., Conkbayir I., Acaroglu G., Hekimoglu B. Graves’ ophthalmopathy: comparison of the Doppler sonography parameters with the clinical activity score. J Clin Ultrasound. 2005;33(8):375–380. doi: 10.1002/jcu.20154. [DOI] [PubMed] [Google Scholar]
- 51.Monteiro M.L., Moritz R.B., Angotti Neto H., Benabou J.E. Color Doppler imaging of the superior ophthalmic vein in patients with Graves’ orbitopathy before and after treatment of congestive disease. Clinics (Sao Paulo) 2011;66(8):1329–1334. doi: 10.1590/S1807-59322011000800004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakase Y., Osanai T., Yoshikawa K., Inoue Y. Color Doppler imaging of orbital venous flow in dysthyroid optic neuropathy. Jpn J Ophthalmol. 1994;38(1):80–86. [PubMed] [Google Scholar]
- 53.Shammas H.J.F., Minckler D.S., Ogden C. Ultrasound in early thyroid orbitopathy. Arch Ophthalmol. 1980;98(2):277–279. doi: 10.1001/archopht.1980.01020030273005. [DOI] [PubMed] [Google Scholar]
- 54.Nugent R.A., Belkin R.I., Neigel J.M., Rootman J., Robertson W.D., Spinelli J. Graves orbitopathy: correlation of CT and clinical findings. Radiology. 1990;177(3):675–682. doi: 10.1148/radiology.177.3.2243967. [DOI] [PubMed] [Google Scholar]
- 55.Nagy E.V., Toth J., Kaldi I., Damjanovich J., Mezosi E., Lenkey A. Graves’ ophthalmopathy: eye muscle involvement in patients with diplopia. Eur J Endocrinol. 2000;142(6):591–597. doi: 10.1530/eje.0.1420591. [DOI] [PubMed] [Google Scholar]
- 56.Demer J.L., Kerman B.M. Comparison of standardized echography with magnetic resonance imaging to measure extraocular muscle size. Am J Ophthalmol. 1994;118(3):351–361. doi: 10.1016/s0002-9394(14)72960-5. [DOI] [PubMed] [Google Scholar]
- 57.Vlainich A.R., Romaldini J.H., Pedro A.B., Farah C.S., Sinisgalli C.A., Jr. Ultrasonography compared to magnetic resonance imaging in thyroid-associated Graves’ ophthalmopathy. Arq Bras Endocrinol Metabol. 2001;55(3):184–188. doi: 10.1590/s0004-27302011000300002. [DOI] [PubMed] [Google Scholar]
- 58.Casper D.S., Chi T.L., Trokel S.L. 1st ed. Thieme; Stuttgart: 1993. Orbital disease, imaging and analysis. [Google Scholar]
- 59.Peyster R.G., Hoover E.D. Computerized tomography in orbital diseases and neuroopthalmology. 1 ed. Year Book Medical Publisher; Chicago: 1984. Graves’ orbitopathy; pp. 97–114. [chapter 3] [Google Scholar]
- 60.MuÈller-Forell W., Pitz S., Mann W., Kahaly G.J. Neuroradiological diagnosis of thyroid-associated orbitopathy. Exp Clin Endocrinol Diabetes. 1999;107:177–183. doi: 10.1055/s-0029-1212180. [DOI] [PubMed] [Google Scholar]
- 61.Trokel S.L., Jakobiec F.A. Correlation of CT scanning and pathologic features of ophthalmic Graves’ disease. Ophthalmology. 1981;88(6):553–564. doi: 10.1016/s0161-6420(81)34993-8. [DOI] [PubMed] [Google Scholar]
- 62.Enzmann D., Marshal W.H., Jr, Rosenthal A.R., Kriss J.P. Computed tomography in Graves’ ophthalmopathy. Radiology. 1976;118(3):615–620. doi: 10.1148/118.3.615. [DOI] [PubMed] [Google Scholar]
- 63.Enzmann D.R., Donaldson S.S., Kriss J.P. Appearance of Graves’ disease on orbital computed tomography. J Comput Assist Tomogr. 1979;3(6):815–819. [PubMed] [Google Scholar]
- 64.Yoshikawa K., Higashide T., Nakase Y., Inoue T., Inoue Y., Shiga H. Role of rectus muscle enlargement in clinical profile of dysthyroid ophthalmopathy. Jpn J Ophthalmol. 1991;35(2):175–181. [PubMed] [Google Scholar]
- 65.Barrett L., Glatt H.J., Burde R.M., Gado M.H. Optic nerve dysfunction in thyroid eye disease: CT. Radiology. 1988;167(2):503–507. doi: 10.1148/radiology.167.2.3357962. [DOI] [PubMed] [Google Scholar]
- 66.Feldon S.E., Weiner J.M. Clinical significance of extraocular muscle volumes in Graves’ ophthalmopathy: a quantitative computed tomography study. Arch Ophthalmol. 1982;100(8):1266–1269. doi: 10.1001/archopht.1982.01030040244006. [DOI] [PubMed] [Google Scholar]
- 67.Feldon S.E., Lee C.P., Muramatsu S.K., Weiner J.M. Quantitative computed tomography of Graves’ ophthalmopathy. Extraocular muscle and orbital fat in development of optic neuropathy. Arch Ophthalmol. 1985;103(2):213–215. doi: 10.1001/archopht.1985.01050020065021. [DOI] [PubMed] [Google Scholar]
- 68.Hallin E.S., Feldon S.E. Graves’ ophthalmopathy: I. Simple CT estimates of extraocular muscle volume. Br J Ophthalmol. 1988;72(9):674–677. doi: 10.1136/bjo.72.9.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hallin E.S., Feldon S.E. Graves’ ophthalmopathy: II. Correlation of clinical signs with measures derived from computed tomography. Br J Ophthalmol. 1988;72(9):678–682. doi: 10.1136/bjo.72.9.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giaconi J.A., Kazim M., Rho T., Pfaff C. CT scan evidence of dysthyroid optic neuropathy. Ophthal Plast Reconstr Surg. 2002;18(3):177–182. doi: 10.1097/00002341-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 71.Monteiro M.L., Gonçalves A.C., Silva C.T., Moura J.P., Ribeiro C.S., Gebrim E.M. Diagnostic ability of Barrett’s index to detect dysthyroid optic neuropathy using multidetector computed tomography. Clinics (Sao Paulo) 2008;63(3):301–306. doi: 10.1590/S1807-59322008000300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Birchall D., Goodall K.L., Noble J.L., Jackson A. Graves ophthalmopathy: intracranial fat prolapse on CT images as an indicator of optic nerve compression. Radiology. 1996;200(1):123–127. doi: 10.1148/radiology.200.1.8657899. [DOI] [PubMed] [Google Scholar]
- 73.Dutton J.J. Elsevier Incl.; China: 2010. Radiology of the orbit and visual pathways. [Google Scholar]
- 74.Ott M., Breiter N., Albrecht C.F., Pradier O., Hess C.F., Schmidberger H. Can contrast enhanced MRI predict the response of Graves’ ophthalmopathy to orbital radiotherapy? Br J Radiol. 2002;75(894):514–517. doi: 10.1259/bjr.75.894.750514. [DOI] [PubMed] [Google Scholar]
- 75.Jiang H., Wang Z., Xian J., Li J., Chen Q., Ai L. Evaluation of rectus extraocular muscles using dynamic contrast-enhanced MR imaging in patients with Graves’ ophthalmopathy for assessment of disease activity. Acta Radiol. 2012;53(1):87–94. doi: 10.1258/ar.2011.110431. [DOI] [PubMed] [Google Scholar]
- 76.Hosten N., Sander B., Cordes M., Schubert C., Schorner, Felix R. Graves’ ophthalmopathy: MR imaging of the orbits. Radiology. 1989;172(3):759–762. doi: 10.1148/radiology.172.3.2772184. [DOI] [PubMed] [Google Scholar]
- 77.Just M., Kahaly G., Higer H.P., Rosler H.P., Kutzner J., Beyer J. Graves’ ophthalmopathy: role of MR imaging in radiation therapy. Radiology. 1991;179(1):187–190. doi: 10.1148/radiology.179.1.2006276. [DOI] [PubMed] [Google Scholar]
- 78.Hiromatsu Y., Kojima K., Ishisaka N., Tanaka K., Sato M., Nonaka K. Role of magnetic resonance imaging in thyroid-associated ophthalmopathy: its predictive value for therapeutic outcome of immunosuppressive therapy. Thyroid. 1992;2(4):299–305. doi: 10.1089/thy.1992.2.299. [DOI] [PubMed] [Google Scholar]
- 79.Utech C.I., Khatibnia U., Winter P.F., Wulle K.G. MR T2 relaxation time for the assessment of retrobulbar inflammation in Graves’ ophthalmopathy. Thyroid. 1995;5(3):185–193. doi: 10.1089/thy.1995.5.185. [DOI] [PubMed] [Google Scholar]
- 80.Nishikawa M., Yoshimura M., Toyoda N., Masaki H., Yonemoto T., Gondou A. Correlation of orbital muscle changes evaluated by magnetic resonance imaging and thyroid stimulating antibody in patients with Graves’ ophthalmopathy. Acta Endocrinol (Copenh) 1993;129(3):213–219. doi: 10.1530/acta.0.1290213. [DOI] [PubMed] [Google Scholar]
- 81.Karim S., Clark R.A., Poukens V., Demer J.L. Demonstration of systematic variation in human intraorbital optic nerve size by quantitative magnetic resonance imaging and histology. Invest Ophthalmol Vis Sci. 2004;45(4):1047–1051. doi: 10.1167/iovs.03-1246. [DOI] [PubMed] [Google Scholar]
- 82.Lam B.L., Glasier C.M., Feuer W.J. Subarachnoid fluid of the optic nerve in normal adults. Ophthalmology. 1997;104(10):1629–1633. doi: 10.1016/s0161-6420(97)30085-2. [DOI] [PubMed] [Google Scholar]
- 83.Dodds N.I., Atcha A.W., Birchall D., Jackson A. Use of high-resolution MRI of the optic nerve in Graves’ ophthalmopathy. Br J Radiol. 2009;82(979):541–544. doi: 10.1259/bjr/56958444. [DOI] [PubMed] [Google Scholar]
- 84.Kahaly G., Diaz M., Hahn K., Beyer J., Bockisch A. Indium-111-pentetreotide scintigraphy in Graves’ ophthalmopathy. J Nucl Med. 1995;36(4):550–554. [PubMed] [Google Scholar]
- 85.Kahaly G., Diaz M., Just M., Beyer J., Lieb W. Role of octreoscan and correlation with MR imaging in Graves’ ophthalmopathy. Thyroid. 1995;5(2):107–111. doi: 10.1089/thy.1995.5.107. [DOI] [PubMed] [Google Scholar]
- 86.Krassas G.E., Doumas A., Kaltsas T., Halkias A., Pontikides N. Somatostatin receptor scintigraphy before and after treatment with somatostatin analogues in patients with thyroid eye disease. Thyroid. 1999;9(1):47–52. doi: 10.1089/thy.1999.9.47. [DOI] [PubMed] [Google Scholar]
- 87.Colao A., Lastoria S., Ferone D., Pivonello R., Macchia P.E., Vassallo P. Orbital scintigraphy with 111In-diethylenetriamine pentaacetic acid-d-phe1-octreotide predicts the clinical response to corticosteroid therapy in patients with Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1998;83(11):3790–3794. doi: 10.1210/jcem.83.11.5274. [DOI] [PubMed] [Google Scholar]
- 88.Postema P.T., Krenning E.P., Wijngaarde R., Kooy P.P., Oei H.Y., van den Bosch W.A. 111In-DTPA-D-Phe1 octreotide scintigraphy in thyroidal and orbital Graves’ disease: a parameter for disease activity? J Clin Endocrinol Metab. 1994;79(6):1845–1851. doi: 10.1210/jcem.79.6.7989493. [DOI] [PubMed] [Google Scholar]
- 89.Gerding M.N., van der Zant F.M., van Royen E.A., Koornneef L., Krenning E.P., Wiersinga W.M. Octreotide-scintigraphy is a disease-activity parameter in Graves’ ophthalmopathy. Clin Endocrinol (Oxf) 1999;50(3):373–379. doi: 10.1046/j.1365-2265.1999.00681.x. [DOI] [PubMed] [Google Scholar]
- 90.Krassas G.E., Kahaly G.J. The role of octreoscan in thyroid eye disease. Eur J Endocrinol. 1999;140(5):373–375. doi: 10.1530/eje.0.1400373. [DOI] [PubMed] [Google Scholar]
- 91.Krassas G.E. Octreoscan in thyroid-associated ophthalmopathy. Thyroid. 2002;12(3):229–231. doi: 10.1089/105072502753600188. [DOI] [PubMed] [Google Scholar]
- 92.Krassas GEDumas A., Pontikides N., Kaltsas T. Somatostatin receptor scintigraphy and octreotide treatment in patients with thyroid eye disease. Clin Endocrinol (Oxf) 1995;42(6):571–580. doi: 10.1111/j.1365-2265.1995.tb02682.x. [DOI] [PubMed] [Google Scholar]
- 93.Krassas G.E., Kaltsas T., Dumas A., Pontikides N., Tolis G. Lanreotide in the treatment of patients with thyroid eye disease. Eur J Endocrinol. 1997;136(4):416–422. doi: 10.1530/eje.0.1360416. [DOI] [PubMed] [Google Scholar]



