Abstract
Management of diabetes should involve both systemic and ocular aspects. Control of hyperglycemia, hypertension and dyslipidemia are of major role in the management of diabetic retinopathy. In the ocular part; laser treatment remains the cornerstone of treatment of diabetic macular edema (focal/grid), severe non-proliferative and proliferative diabetic retinopathy (panretinal photocoagulation). There is a strong support to combination therapy. Using one or two intravitreal injections such as anti-VEGF and or steroid to reduce central macular thickness followed by focal or grid laser to give a sustained response may offer an alternative to treatment in diabetic macular edema. Anti-VEGF were found to be effective as an adjunct therapy in proliferative diabetic retinopathy patient who is going to have vitrectomy for vitreous hemorrhage with neovascularization, panretinal photocoagulation, and other ocular surgery such as cases with neovascular glaucoma and cataract with refractory macular edema.
Keywords: Diabetic retinopathy, Pathophysiology, Management, Anti-VEGF, Steroids, Laser
1. Introduction
Diabetes mellitus is a chronic disorder characterized by the impaired metabolism of glucose due to insulin deficiency or its resistance, leading to hyperglycemia and late development of vascular and neuropathic complications. It is of two types: type 1, primarily caused by autoimmune pancreatic β-cell destruction and characterized by absolute insulin deficiency, and type 2 characterized by insulin resistance and relative insulin deficiency.
In general in the USA; it was estimated that nearly 21 million Americans (or nearly 7% of the US population) fulfilled the diagnostic criteria for diabetes mellitus. Diabetic retinopathy at the time of the diagnosis of diabetes is lower with type I being 0.4% in type I while 7.6% in type II (Roy et al., 2004).
In Saudi Arabia the prevalence of diabetes mellitus was 34.1% in males and 27.6% in females and it increases with age (Alqurashi et al., 2011). In the eastern part of Saudi Arabia the prevalence of diabetes mellitus was 17.2% (Al-Baghli et al., 2010). It is the commonest cause of legal blindness in individuals between the age of 20 and 65 years of age. Recently an extensive work had been done in different aspects of diabetic retinopathy worth reviewing.
2. Pathogenesis
Retina is a thin transparent structure constituting of several layers. The cells within the retina fall into one of three groups: (1) neuronal component (photoreceptors, interneurons, and ganglion cells and their interconnections) which give the retina its visual function by converting light to electrical signals. (2) Glial components (Muller cells) are the supporting column in the retina. (3) Vascular components consist of the branches of central retinal artery which, supplies the inner retina while the outer retinal is being supplied by diffusion from choroidal circulation. The retinal vessels maintain blood–retinal barriers due to the single layer of the non-fenestrated endothelial cells with tight junctions between them. The wall of the retinal capillaries is made of endothelial cells, Pericytes (with contractile characteristics) embedded within the endothelium basement membrane. Diabetes will produce its effect on both neuronal and vascular components of the retina. Loss of pericytes, with compensatory synthesis and deposition of extracellular proteins, characterizes early diabetic retinopathy.
Several factors were found to influence diabetic retinopathy including long duration of the disease, age, level of hyperglycemia control, level of blood pressure control, puberty, Pregnancy, hyperlipidemia, hyperviscosity, renal failure and anemia. Hyperviscosity of the blood due to any cause such as dehydration (Alghadyan, 1993) and polycythemia may influence the diabetic retinopathy. More important is the contribution of the biochemical changes associated with hyperglycemia. Knowing these factors will help in a better management; for example in cases of fluid retention cases it will be better first control the underlying causes such as high blood pressure and other systemic causes and the ocular treatment. The need for oxygen differs in different parts of the retina. The thin peripheral retina needs less oxygen and it receives much of its oxygen from the choroid, which may offer relative protection against apoptosis in the face of retinal capillary insufficiency. Perhaps a similar mechanism underlies the apparent protective effect of high myopia and advanced glaucoma on the progression of diabetic retinopathy (Henkind, 1978; McLeod, 2007; Sultanov and Gadzhiev, 1990; Dogru et al., 1998; Klein et al., 1988, 1997).
The exact mechanism by which hyperglycemia causes vascular disruption seen in retinopathy is not clear. Probably the intraocular formation of reactive oxygen species fuels the subsequent pathological, biochemical changes seen in diabetic retinopathy (Fig. 1). These biochemical changes include: (1) protein kinase C is a subclass of the transferases that catalyze the transfer of a high-energy group from a donor (usually ATP) to an acceptor (e.g., protein). It is known that hyperglycemia increases the activity of various Protein kinase C isoforms which were found to play an important role in the pathogenesis of diabetic retinopathy (Fig. 1). Activation of protein kinase C causes cellular changes (Xia et al., 1996; Miller et al., 1997), leading to: (a) enhanced permeability of retinal vasculature and alterations in retinal blood flow, (b) basement membrane thickening causing ischemia and cellular signaling by vascular endothelial growth factors (VEGFs) leading to ocular neovascularization. (2) The non enzymatic binding of glucose to key protein side chains as a result of hyperglycemia causes glycation of these proteins as seen in hemoglobin A1C (Brownlee et al., 1984). Animal studies have demonstrated that accumulation of advanced glycation end products (AGE) is associated with microaneurysm formation and pericyte loss whereas animals treated with AGE formation inhibitor (such as aminoguanidine) showed reduced retinal damage (Wautier and Guillausseau, 2001; Hammes et al., 1991). (3) polyol (such as sorbitol) accumulation: Aldose reductase is the first enzyme in the polyol pathway, has a low affinity for glucose at normal concentrations. Hyperglycemia results in increased conversion of glucose into sorbitol. The increase in intracellular sorbitol concentration has been hypothesized to cause osmotic damage to vasculature of the retina (Gabbay, 1975). In animal experiments; polyol was found to be associated with changes similar to those seen in diabetic retinopathy in humans (Frank et al., 1983; Engerman and Kern, 1984). (4) Oxidative stress caused by formation of free radicals as a result of hyperglycemia and the above mentioned biochemical pathways lead to damage to retinal vasculature. It was found that antioxidants such as vitamin E may prevent some of the vascular dysfunction associated with diabetes (Kunisaki et al., 1995; Bursell and King, 1999; Bursell et al., 1999). (5) Growth factors are diverse group of peptides that affect various cellular processes, including metabolic regulation; tissue differentiation; cell growth and proliferation; maintenance of viability and changes in cell morphology (Bursell and King, 1999; Bursell et al., 1999; Aiello et al., 1994; Pouvlaki et al., 2004). The growth factors are synthesized in a variety of cells and have a spectrum of target cells. The presence of various growth factors in retina, vitreous, aqueous humor, and corneal tissues had been demonstrated. These factors include: epidermal growth factor, fibroblast growth factors, transforming growth factors, vascular endothelial growth factor, and insulin-like growth factors. Vascular endothelial growth factor (VEGF), also known as vasculotropin, deserves special attention due to its role in diabetic retinopathy. It is a heparin-binding polypeptide mitogen and has four isoforms. It is one of many cytokines that plays a prominent role in diabetic retinopathy and it is induced by ischemic neurosensory retina. It is a marker of oxidative stress and induces hyperpermeability of macular capillaries contributing to macular edema. It also induces endothelial proliferation and migration consistent with clinical findings of microaneurysm and neovascular membrane formation. It prevents apoptosis of capillary endothelial cells.
Figure 1.
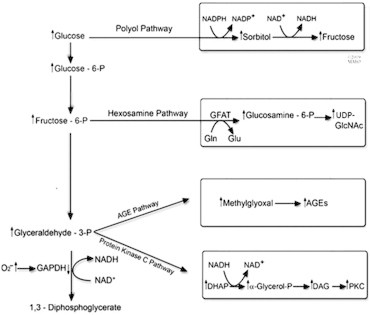
This schematic shows the four biochemical pathways that lead to diabetic retinopathy. DHAP, dihydroxyacetone phosphate; DAG, diacylglycerol; PKC, protein kinase C; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; AGEs, advanced glycation end products, UDP-GlcNAC, N-acetylglucosamine.
3. Presentation of diabetic retinopathy
Evaluating the patients will include: (1) complete history and clinical ocular examination including fundus biomicroscopy; (2) stereoscopic color fundus photography; (3) fluorescein angiography will help to determine the origin of the leakage and identify the ischemic areas; (4) optical coherence tomography (OCT) is helpful in determining the response of macular edema to therapy. The morphology of OCT may alter the prognosis (presence of cystic changes are indicative of chronicity and poorer response to therapy) or alter therapy (presence of vitreomacular traction needing surgery). The retina is particularly vulnerable to microvascular damage in diabetes. Retinal damage is caused by both microvascular leakage from breakdown of the inner blood–retinal barrier and microvascular occlusion. Diabetic retinopathy can be classified into non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR).
Non-proliferative diabetic retinopathy characterized by microaneurysm, exudate, hemorrhages and microinfarcts. This further can be classified into mild, moderate and severe depending on the extent of these changes (Table 1). Microaneurysms are outpouchings of capillaries and are among the first clinically detectable signs of retinopathy. They arise due to ballooning of weakened capillary walls or endothelial buds attempting to revascularize ischemic retina. They appear as tiny red dots, commonly temporal to the macula. Although microaneurysms are not fixed features and may even disappear. Sudden appearance of numerous microaneurysms is an indication of worsening retinal ischemia. Hard exudates consist of lipoproteins and other proteins leaking through abnormal retinal vessels. They appear as yellow lipid deposits with a waxy or shiny appearance and may form a circinate pattern around foci of leaking capillaries and microaneurysms. Hemorrhages occur due to rupture of weakened capillaries. They can be small dots or larger blot hemorrhages present within the densely packed deeper layers of retina. The flame shaped hemorrhages occur in the superficial nerve fiber layer. Microinfarcts in the nerve fiber layer (also known as soft exudates or cotton wool spots) appear in advanced stages of NPDR due to vascular occlusion and they appear as white lesions with vague margins when they heal they might form a depressed area due to tissue loss.
Table 1.
The early treatment diabetic retinopathy study (ETDRS) grading system (Early Treatment Diabetic Retinopathy Study Research Group, 1991c,e).
| Non-proliferative diabetic retinopathy (NPDR) | Mild to moderate NPDR | Microaneurysms, intra-retinal hemorrhages, hard exudate ± macular edema |
| Moderate to severe NPDR | Extensive intra-retinal hemorrhages and/or microaneurysms and/or cotton wool spots, venous beading or intra-retinal microvascular abnormalities (IRMA) | |
| Severe NPDR to very severe NPDR | Plus Cotton wool spots, venous beading, and IRMA, all present in at least two quadrants. This can be simplified by the rule of 4:2:1. Intra-retinal hemorrhages in four quadrants, Venous beading in two quadrants, Severe IRMA in one quadrant | |
| Proliferative retinopathy (PDR) | Neovascularization of the disk and /or Neovascularization elsewhere in the retina | |
| Early PDR | Pre-retinal hemorrhage | |
| PDR with high-risk criteria | High risk = the presence of any of the following:
|
|
| PDR with advanced eye disease | Tractional retinal detachment, Neovascularization of the iris/angle | |
DD = disk diameter, DR = diabetic retinopathy, PDR = proliferative diabetic retinopathy, NPDR = non-proliferative diabetic retinopathy, IRMA = intra-retinal microvascular abnormalities.
The macula is a highly vascularized and its involvement causes a serious impact on visual function. The macula is usually involved with macular edema associated with broken retinal blood barrier or ischemic or both a new vascularization. Macular edema results from leakage from the broken blood–retinal barriers. Movement of fluids both into and out of the body’s capillaries, including those of the retina, is dependent upon (1) hydrostatic pressure which is determined by blood pressure and intra-ocular pressure and (2) oncotic pressure which depends on protein content in the capillaries and in the intertrial fluid. The net force pushing fluid out of capillaries is the difference between hydrostatic pressures and oncotic pressures, any disturbance to this equilibrium will result in retinal edema. When the edema involves the macula and affects vision it is called a clinically significant macular edema which is defined as any one of the following: (1) retinal edema within 500 μm (one third of a disk diameter) of the fovea, (2) hard exudates within 500 μm of the fovea if associated with adjacent retinal thickening, (3) retinal edema that is one disk diameter (1500 μm) or larger, any part of which is within one disk diameter of the fovea (Diabetic Retinopathy Study Research Group, 1976) (Fig. 2).
Figure 2.
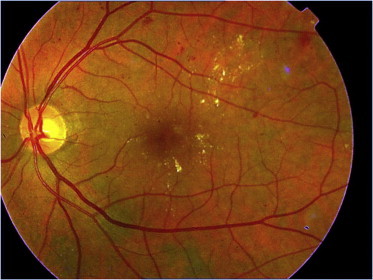
Moderate non-PDR with CSME.
Ischemic maculopathy arises due to extensive microvascular occlusion and may cause severe loss of central vision. Macular ischemia is caused by complex interactions of the cellular and noncellular constituents of the vascular wall. It can be detected early in diabetic retinopathy and becomes increasingly apparent with advancing stages of severity of diabetic retinopathy (Patz and Smith, 1991). In patients with decreased vision; it is suspected clinically by funduscopy as areas of ‘‘featureless’’ retina surrounded by typical diabetic microangiopathy. Fluorescein angiography demonstrates the non-filling of macular capillaries, enlargement and irregularity of the foveal avascular zone (FAZ) (a reliable follow up sign), and increased perifoveal intercapillary area (Fig. 3a and b). Optical coherence tomography reveals neurosensory macular thinning.
Figure 3.
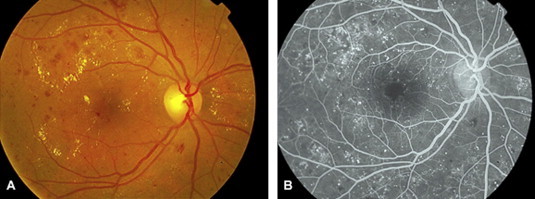
Non PDR with early sign of ischemia of the fovea; (a) clinical photo and (b) fluorescein angiogram.
The optic nerve might be involved in diabetes mellitus (Fig. 4). The vascular supply of the anterior optic nerve is primarily derived from the short posterior ciliary arteries. Due to the effect of diabetes on the blood vessels; diabetes is a risk factor for non arteritic ischemic optic neuropathy (NAION) with high possibility of involvement of the other eye. Other factors may aggravate the diabetic effect. Blood pressure and intra-ocular pressure influence anterior optic nerve perfusion pressure. Diurnal variations in blood pressure and medications may influence optic nerve perfusion; conditions that can be managed.
Figure 4.
Ischemic optic neuropathy (note white swelling of the disk).
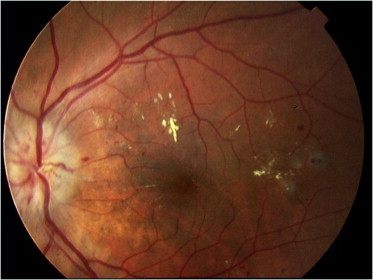
Proliferative diabetic retinopathy (PDR) is the advanced stage of diabetic retinopathy. It is characterized by new vessel formation commonly arising on the optic disk (New vessels on the disk NVD) or arise on other parts of the retina (new vessel elsewhere or NVE) induced by ischemic changes in the retina and an imbalance between angiogenic and antiangiogenic factors (Fig. 5a and b). The NVD carries the worst prognosis due to many factors including attachment of the vitreous to the optic disk. Early stage of PDR starts as neovascularization and pre-retinal hemorrhages (Table 1). This might progress to vitreous hemorrhages and in late stages it may cause tractional retinal detachment and neovascular glaucoma.
Figure 5.
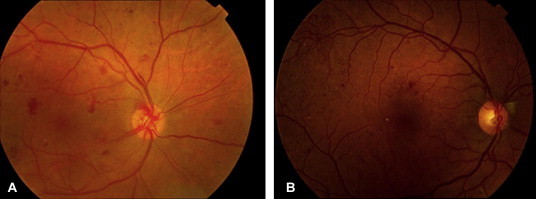
PDR with NVD in photo (a), and NVE supra temporal in photo (b).
4. Management of diabetic retinopathy
Ophthalmologists should not forget the systemic aspect of the disease because management should be directed toward both systemic and ocular aspects of the disease (Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group, 2000; Anon., 1995; Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group, 2002; Diabetes Control and Complications Trial Research Group, 1993, 1998; UK Prospective Diabetes Study Group, 1998a,b; Egger et al., 1997; American Diabetes Association, 2004; Diabetic Retinopathy Study Research Group, 1981, 1985; Early Treatment Diabetic Retinopathy Study Research Group, 1987, 1991a,b,c,d,e; Rand et al., 1985; Kaufman et al., 1987, 1989; Ferris et al., 1987; Aiello et al., 2010; Ferris, 1996; Davis et al., 1998; Braun et al., 1995; Fong et al., 1999; Diabetic Retinopathy Vitrectomy Study Research Group, 1985, 1988a,b; DRVS, 1985; Flynn et al., 1992; Chew et al., 1995). Systemic management should include controlling blood sugar, blood pressure and serum lipids. (a) In glycemic control; there is a direct and consistent relationship between HbA1c (glycated hemoglobin) level and the incidence of diabetic retinopathy. Effective glycemic control has been demonstrated to reduce both the incidence and progression of diabetic retinopathy. It will be nice to have the target of glycemic control HbA1C to be 6% (Table 2). (b) Hypertension is another important risk factor for the development and/or worsening of diabetic retinopathy. High blood pressure causes endothelial stress with release of VEGF altering retinal autoregulation leading to increased perfusion pressure and injury (Suzuma et al., 2001; Matthews et al., 2004; Estacio et al., 2000; Schrier et al., 2002). Fortunately this risk factor can be treated. It will be nice to have the target of high blood pressure treatment equal to or less than 130/80 mmHg. (c) Renin-angiotensin system is involved in blood pressure control and retinal dysfunction and angiogenesis. It had been shown that angiotensin converting enzyme (ACE) is locally produced by endothelial cells of retinal blood vessels and retinal pigment epithelial cells (Danser et al., 1994; Wagner et al., 1996) and found to be in high concentration in aqueous humor in patients with proliferative diabetic retinopathy (Aydin et al., 2010). The use of ACE inhibitors such as lisinopril and candesartan were found to have favorable effect on the progression of diabetic retinopathy (Chaturvedi et al., 1998, 2008; Sjolie et al., 2008), which might be a good choice for diabetic patients with hypertension. (d) There is a positive correlation between dyslipidemia and progression of diabetic retinopathy or macular edema. Dyslipidemia leads to the development of hard exudates (Chew et al., 1996; Lyons et al., 2004). Clinical studies had shown the beneficial effects of lipid lowering agents such as atorvastatin and simvastatin in reducing hard exudates and progression of retinopathy (Harrold et al., 1969; Cullen et al., 1974).
Table 2.
Summary of the important results of some of the studies done on diabetic retinopathy.
| Study | Recommendations |
|---|---|
| DRS |
|
| ETDRS |
|
| DRVS |
|
| DCCT 1993 | Long time result in tight hyperglycemic control showed significantly reduced the progression of diabetic retinopathy |
| UKPDS 1998 | Tight glycemic control showed 34% reduction in progression of DR and 47% in reducing the risk of deterioration of vision |
DRS = diabetic retinopathy study, ETDRS = early treatment diabetic retinopathy study, DRVS = diabetic retinopathy vitrectomy study, DCCT = diabetes control complication trials, UKPDS = United Kingdom prospective diabetes study (Aiello et al., 1994, 2010; Pouvlaki et al., 2004; Diabetic Retinopathy Study Research Group, 1976, 1985; Patz and Smith, 1991; Diabetes Control and Complications Trial Research Group, 1993, 1995, 1998; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group, 2000; Anon., 1995; Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group, 2002; UK Prospective Diabetes Study Group, 1998a,b; Egger et al., 1997; American Diabetes Association, 2004; Rand et al., 1985; Kaufman et al., 1987, 1989; Ferris et al., 1987; Ferris, 1996; Early Treatment Diabetic Retinopathy Study Research Group, 1991a,b,c,d,e; Davis et al., 1998; Braun et al., 1995; Fong et al., 1999; Diabetic Retinopathy Vitrectomy Study Research Group, 1985, 1988a,b; DRVS, 1985; Flynn et al., 1992; Chew et al., 1995; Suzuma et al., 2001; Flynn et al., 1992; Chew et al., 1995), PRP = panretinal photocoagulation, NVD = neovascularization on the disk, NVE = neovascularization elsewhere in the retina, SVL = severe visual loss.
5. Ocular managements of diabetic retinopathy
It may involve any or combination of laser, vitrectomy and/or pharmacological therapy. Laser photocoagulation is accomplished by directing a focused laser (Light Amplification by the Stimulated Emission of Radiation) beam of a discrete wavelength onto specified parts of the retina. Its absorption in a variety of intra-ocular pigmented retinal layers, causes a local rise in temperature which in turn causes denaturation of tissue proteins and coagulative necrosis. Laser treatment is used to treat diabetic macular edema either in the form of focal or grid using small spot size, short duration and low power enough to produce whitening of the retina. Focal treatment is required for focal lesions (e.g., microaneurysms, IRMA) located between 500 and 3000 μm from the center of the macula, which causes the hard exudates and retinal thickening. Photocoagulation may also be used in a form of a grid pattern sparing the fovea and the maculopapillary area to treat diffuse areas of leakage in the macula (Dowler, 2003; Mohamed et al., 2007). Panretinal photocoagulation (PRP) is indicated for the treatment of high-risk proliferative diabetic retinopathy and eyes with severe non-proliferative diabetic retinopathy and early proliferative diabetic retinopathy that are at high risk for progression or for poor outcome. Results of Diabetic Retinopathy study (DRS) (Anon., 1978; Shin et al., 2009; Diabetic Retinopathy Study Research Group, 1981) and the Early Treatment Diabetic Retinopathy Study (ETDRS) (Anon., 1991), have provided the strongest evidence to establish the place of panretinal photocoagulation as a standard technique for treating severe non-proliferative and proliferative diabetic retinopathy. Full PRP as used by DRS and ETDRS included 1200 or more 500 micron burns separated from each other by one half burn width at 0.1 s duration. It also had shown that panretinal photocoagulation reduces the risk of moderate and severe visual loss by 50% in patients with severe non-proliferative and proliferative retinopathy (Table 2). The aim of panretinal photocoagulation is to prevent the onset or induce the regression of neovascularization without vitreous hemorrhage or fibrovascular proliferation. This is done by destroying the ischemic peripheral retina with 1500–3000 burns that spare the disk, the macula and maculopapillary nerve bundle. It is done using enough power to produce a mild-to-moderate white burn, using shorter burn duration for patients comfort. This will result in concentrating the remaining retinal blood flow onto the macula and adjacent important areas. Laser photocoagulation is not without adverse effect. The adverse effects of PRP include visual field constriction, night blindness, color vision changes, accidental laser burn to macula.
6. Ocular pharmacotherapy
Advances in pharmacotherapy had shown encouraging promise in the treatment of diabetic retinopathy. (a) VEGF inhibitors are group of drugs that bind to VEGF receptors without causing its activation thus blocking new vessels formation and enhanced vessels permeability. Examples of these drugs include Pegaptanib, Ranibizumab, bevacizumab and Regeneron. They play an important role in the management of diabetic retinopathy and it was found to be safe in humans. Intravitreal injections of anti-VEGF drugs produce reductions in macular thickening, but on average the magnitudes of the reductions and the durations of responses are less than with intravitreal triamcinolone injections. This might suggest that other biochemical pathways not involving VEGF are important in the pathogenesis of diabetic macular edema (Roh et al., 2008; Arevalo et al., 2007; Do et al., 2009; Ozkiris, 2009; Querques et al., 2009; Adamis et al., 2006). Pegaptanib (Macugen) is a pegylated RNA an anti-VEGF that acts by targeting the 165 isoform of VEGF was approved for the treatment of neovascular age-related macular degeneration (AMD). It had also been shown to improve diabetic macular edema (Hornan et al., 2010; Macugen Diabetic Retinopathy Study Group, 2005) and cause regression of neovascularization in patients with proliferative diabetic retinopathy and help in cases with vitreous hemorrhages. Ranibizumab (Lucentis), is a recombinant humanized monoclonal antibody fragment with specificity for all isoforms of human VEGF-A. It had been approved for the treatment of neovascular age-related macular degeneration (Brown et al., 2006; Rosenfeld et al., 2006; Chun et al., 2006; Massin et al., 2010; Nguyen et al., 2009; Avery, 2006; Avery et al., 2006; Kook et al., 2009). It has also been found to be useful for diabetic macular edema. In the 2 years update READ2 study presented at the AAO Oct 2010; on the effect of ranibizumab for diabetic macular edema it improves visual acuity and reduces retinal thickness but repeated injection may be necessary. Combination with laser reduces the need for repeated injection. Restore study report after 12 months of ranibizumab in the treatment of macular edema revealed that ranibizumab alone is superior to laser monotherapy and combination of laser did not add much (AAO meeting Oct. 2010). Bevacizumab (Avastin) is a full-length humanized monoclonal antibody against all isoform of VEGF-A. It was found to effective for the treatment of neovascular age-related macular degeneration and for diabetic retinopathy (Avery, 2006; Avery et al., 2006; Kook et al., 2009; Scott et al., 2007; Spaide and Fisher, 2006; Rosenfeld, 2006; Karim and Tang, 2010; Arevalo et al., 2010; Gulkilik et al., 2010; di Lauro et al., 2010; Hernández-Da Mota and Nuñez-Solorio, 2010; Abdelhakim et al., 2010). It has been shown to be effective in minimizing the risk for post operative hemorrhage after vitrectomy (Saito et al., 2010; Brouzas et al., 2009; Hattori et al., 2010) and surgery for neovascular glaucoma (Wu et al., 2010; Parravano et al., 2009). Sometimes there is a need for repeating the intravitreal injections. However, the number of repeated injections is not settled. Avastin had been used in combination with triamcinolone at the end of vitrectomy for vitreous hemorrhage in patients with proliferative diabetic retinopathy with encouraging results. It is worth mentioning that intravitreal Avastin at the time of cataract surgery is effective in reducing diabetic macular edema post operatively (Fard et al., 2010). VEGF Trap eye (Regeneron) is S fusion protein specifically designed to bind all forms of VEGF-A. It had been shown that a single intravitreal injection of VEGF Trap-Eye was well tolerated and was effective in patients with diabetic macular edema.
The anti-VEGF drugs have lower side effect profile without the tendency to cause cataract and raise intra-ocular pressure as compared with steroid (Marticorena et al., 2010; Micieli et al., 2010). However; epiretinal membrane was reported after intravitreal Avastin for retinal vein occlusion and some systemic adverse effects were reported. In the review of 12,699 patients who received intravitreal Avastin: high blood pressure (0.46%), cerebrovascular accidents (0.21%) and myocardial infarction (0.19%) were reported. Whether these adverse effects related to the medicine or to the stress associated with the procedure remain unclear.
Corticosteroids are group of compounds which share three six membered carbon rings and one five membered carbon ring. The natural occurring members of this group are the sex hormones and the hormones of the adrenal glands. The synthetic members of this group are wide range of products used for therapeutic purposes with different potency (Table 3). They may produce their effects through multiple mechanisms of actions including their potent anti-inflammatory and VEGF regulating effects. They had been used in the treatment of diabetic retinopathy as peribulbar, sub-tenon and intravitreal injections. Peribulbar triamcinolone or methylprednisolone injections have been used to treat diabetic macular edema either as monotherapy or as adjunctive therapy to laser. Short-term efficacy in thinning the macula and improving visual acuity has been demonstrated but less effective than intravitreal. Intravitreal triamcinolone (IVTA) has shown significant improvements in diabetic macular edema and visual acuity in short term and it was found to be superior to sub-tenon injection (Jonas, 2007; Gillies et al., 2006; Massin et al., 2004; Dehghan et al., 2008; Audren et al., 2006; Avitabile et al., 2005; Wu et al., 2008; Yilmaz et al., 2009; Takata et al., 2010). The short term effect necessitates repeated intravitreal injections which was associated with some complications including steroid-induced elevation of intra-ocular pressure (IOP), crystalline maculopathy and steroid-induced cataract (Bursell et al., 1999; Yilmaz et al., 2009; Sarraf et al., 2010). To minimize the side effects lower dose of triamcinolone were studied, and it was found that intravitreal injection of 4 mg had better effect as compared with 1 mg injection but the complications were more with the higher dose. In a randomized clinical trial comparing serial intravitreal triamcinolone injection therapy using 1 or 4 mg to focal/grid photocoagulation, focal/grid photocoagulation showed superior efficacy and fewer side effects. A single injection of intravitreal of more potent steroid (dexamethasone 0.4 or 0.8 mg) did not have significant beneficial effects on diabetic macular edema within 3 months from injection (Chan et al., 2010). The use of slow release medications had gained increasing interest. Liposomes are microscopic, spherical vesicles that form when hydrated phospholipids arrange themselves in circular sheets with consistent head–tail orientation. These sheets join each other to form a bilayer membrane that encloses some of the water and water-soluble materials (e.g., drugs) in a phospholipid sphere. Liposomes can be custom-designed for almost any need by varying the lipid contents, sizes, surface charges, and method of preparation. Alghadyan et al. had studied the effect and the half life of intravitreal injection of liposome with penicillin and cyclosporin in rabbits and found encouraging results (Alghadyan et al., 1988a–e). Recently intravitreal retinal implants had also been developed, allowing extended drug delivery. Implanted intravitreal fluocinolone acetonide was shown to be associated with improvement in visual acuity in diabetic macular edema (Pearson et al., 2006; Schwartz and Flynn, 2010). A sustained release drug delivery system for dexamethasone inserted trans-sclerally into the vitreous produced statistically significant visual acuity improvement for 90 days after insertion and was well tolerated for 180 days (Kuppermann et al., 2007). In Fame study 24 months report on the use of fluocinolone acetonide insert (Iluvein) in the treatment of diabetic macular edema was found to be of benefit in reducing the macular edema. Cataract and the glaucoma were reported as complications of the treatment (AAO meeting Oct. 2010). Similar results were found with Placid trial. Placid trial report in AAO Oct. 2010, reported the result of the use of Dexamethasone implant (Ozurdex) for the treatment of diabetic macular edema and they found that visual acuity improved with combination of dexamethasone with laser more than with laser alone. Still elevated IOP was one of the complications they faced.
Table 3.
Relative potencies of corticosteroids (Katzung, 2004).
| Types | Potencies |
|---|---|
| Cortisone | 0.8 |
| Cortisol | 1 |
| Prednisone | 4 |
| Methylprednisolone | 5 |
| Triamcinolone | 5 |
| Betamethasone | 25 |
| Dexamethasone | 25 |
| Fluocinolone | 25 |
Other pharmacotherapies in the management of diabetic retinopathy were suggested. Protein Kinase C (PKC) inhibitors such as Ruboxistaurin are expected to play a role in the management of diabetic retinopathy. The oral administration of this medication demonstrated a positive result in reducing macular edema (Aiello et al., 2006; Fabbro et al., 2000). Growth hormone inhibitors (somatostatin analogs) may inhibit angiogenesis directly through somatostatin receptors present on endothelial cells and indirectly through the inhibition of postreceptor signaling events of peptide growth factors such as insulin-like growth factor 1 and VEGF. It was found that Octreotide (growth hormone inhibitor) therapy for severe non-proliferative and early proliferative diabetic retinopathy retard the progression of the diabetic retinopathy (Grant et al., 2000). Short-term high-dose antioxidant therapy with oral vitamin E may help in normalizing retinal hemodynamics in diabetic patients (Kunisaki et al., 1995; Bursell and King, 1999; Bursell et al., 1999). The place of many potential pharmacotherapies in diabetic retinopathy such as Interferon-alpha 2a, acetazolamide, intravitreal injection of tissue plasminogen activator and pigment epithelium-derived factor needs to be evaluated. Intravitreal injections of erythropoietin in eyes with severe, chronic diabetic macular edema showed a short-term positive response (Li et al., 2010). Anti-tumor necrosis factor (TNF) infliximab (monoclonal antibody) also showed some benefit in the management of diabetic macular edema (Sfikakis et al., 2010).
Combination therapy had been used with some encouraging results. The use anti-VEGF therapy as an adjunct to the panretinal photocoagulation was found to be beneficial. Intravitreal bevacizumab or triamcinolone with macular photocoagulation were found to be superior than either one of the modalities alone in diabetic macular edema (Solaiman et al., 2010; Cho et al., 2010; Kang et al., 2006; Lam et al., 2007). There were some evidences suggesting that combined intravitreal and Peribulbar Triamcinolone and Focal Laser Therapy reduces macular thickening somewhat better. Intravitreal bevacizumab and triamcinolone as initial injection followed by two intravitreal bevacizumab injections given at 6-weeks intervals were no more effective in decreasing diabetic macular than three consecutive intravitreal injections of bevacizumab given at 6-week intervals (Soheilian et al., 2007). In Diabetic Retinopathy Clinical Research (DRCR) network reported at the AAO Oct 2010 the result on: (1) intravitreal ranibizumab with or without laser in treatment of diabetic macular edema and they found that the combination is than the laser alone; (2) intravitreal triamcinolone with or without laser and they found that the combination is not superior to laser alone; (3) they recommended that ranibizumab with laser should be considered for the treatment of Diabetic retinopathy.
Surgical management may involve less invasive procedures such as laser, intravitreal injection of medications or gas or more invasive procedures such as vitrectomy. Laser (argon, krypton, or Nd:YAG) may be used to create an opening in the posterior hyaloid face to aid in the breakthrough of subhyaloid hemorrhage into the vitreous cavity to move it away from the fovea. Intravitreal gas (SF6) injection can also resolve subhyaloid hemorrhage through the induction of a posterior vitreous detachment (Chung et al., 2008; Raymond, 1995; Park and Seo, 2004; Nasrallah et al., 1988). The indications for vitrectomy in diabetics include: (1) vitreous hemorrhage (final visual result is dependent on the status of the macula), (2) traction retinal detachment (best results if vitrectomy is performed soon after macular involvement or when macula is threatened), (3) combined traction–rhegmatogenous retinal detachment (vitrectomy with silicone oil tamponade yields fairly good results), (4) severe fibrovascular proliferation (important to apply extensive PRP prior to vitrectomy, if possible), (5) postvitrectomy fibrinoid syndrome (best managed with adjunctive medication), (6) anterior hyaloidal fibrovascular proliferation (poor prognosis, therefore, best prevented with appropriate anterior retinopexy in high-risk eyes). Early vitrectomy has been shown to improve visual recovery in patients with proliferative diabetic retinopathy and severe vitreous hemorrhage. The Diabetic Retinopathy Vitrectomy Study Research Group (1985, 1988a,b) (Table 2) evaluated the risks and the benefits of early surgical intervention versus conventional treatment for vitreous hemorrhage and very severe PDR. The results of DRVS demonstrated that patients who underwent early vitreoretinal surgery had better outcome than those treated conservatively, with 25% of the early vitrectomy group versus 15% of the observation group having 20/40 or greater vision after 2 years’ follow-up. Eyes with diabetic macular edema (DME) have a lower prevalence of posterior vitreous detachment than eyes without DME (Nasrallah et al., 1988). The observation that resolution of DME after posterior vitreous detachment suggested that surgical induction of a vitreomacular separation might improve diabetic macular edema (Hikichi et al., 1997). Intravitreal ovine hyaluronidase injection and autologous plasmin enzyme were found to induce vitreolysis and posterior vitreous detachment and subsequent resolution of diabetic macular edema. Vitrectomy including removal of posterior hyaloid for diabetic macular edema was found to be of benefit (Recchia et al., 2005; Patel et al., 2006a,b; Pendergast et al., 2000; Rosenblatt et al., 2005; Stolba et al., 2005; Harbour et al., 1996; Kumagai et al., 2009) In refractory macular edema vitrectomy can be used for eyes with a stretched posterior hyaloid adherent to the macula, and for eyes with persistent diabetic macular edema despite previous focal laser or intravitreal triamcinolone injection. Vitrectomy has a potential to be a primary therapy in eyes with more severe edema and greater visual acuity loss at presentation.
References
- Abdelhakim M.A., Macky T.A., Mansour K.A., Mortada H.A. Bevacizumab (Avastin) as an adjunct to vitrectomy in the management of severe proliferative diabetic retinopathy: a prospective case series. Ophthalmic Res. 2010;45(1):23–30. doi: 10.1159/000314721. [DOI] [PubMed] [Google Scholar]
- Adamis A.P., Altaweel M., Bressler N.M., Cunningham E.T., Jr., Davis M.D., Goldbaum M., Gonzales C., Guyer D.R., Barrett K., Patel M., Macugen Diabetic Retinopathy Study Group Changes in retinal neovascularization after pegaptanib (Macugen) therapy in diabetic individuals. Ophthalmology. 2006;113(1):23–28. doi: 10.1016/j.ophtha.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Aiello L.P., Avery R.L., Arrigg P.G., Keyt B.A., Jampel H.D., Shah S.T. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- Aiello L.P., Davies M.D., Girach A., Kles K.A., Milton R.C., PKC-DRS2 Group Effect of ruboxistaurin on visual loss in patients with diabetic retinopathy. Ophthalmology. 2006;113:2221–2230. doi: 10.1016/j.ophtha.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Aiello L.P., Edwards A.R., Beck R.W., Bressler N.M., Davis M.D., Ferris F., Glassman A.R., Ip M.S., Miller K.M. Diabetic retinopathy clinical research network factors associated with improvement and worsening of visual acuity 2 years after focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2010;117:946–953. doi: 10.1016/j.ophtha.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Baghli N.A., Al-Ghamdi A.J., Al-Turki K.A., Al Elq A.H., El-Zubaier A.G., Bahnassy A. Prevalence of diabetes mellitus and impaired fasting glucose levels in the Eastern Province of Saudi Arabia: results of a screening campaign. Singapore Med. J. 2010;51(12):923–930. [PubMed] [Google Scholar]
- Alghadyan A.A. Retinal vein occlusion in Saudi Arabia. Possible role of dehydration. Ann. Ophthalmol. 1993;25(10):394–398. [PubMed] [Google Scholar]
- Alghadyan A., Peyman G., Khoobehi B. Intravitreal injection of liposome-encapsulated penicillin for the treatment of bacterial endophthalmitis. Afro-Asian J. Ophthalmol. 1988;7:43–45. [Google Scholar]
- Alghadyan A.A., Peyman G.A., Khoobehi B. Intravitreal injection of liposome-encapsulated penicillin: toxicity and clearance. Afro-Asian J. Ophthalmol. 1988;7:40–42. [Google Scholar]
- Alghadyan A.A., Peyman G.A., Khoobehi B. Liposome bound cyclosporine: aqueous and vitreous level after subconjunctival injection. Int. Ophthalmol. 1988;12:101–104. doi: 10.1007/BF00137133. [DOI] [PubMed] [Google Scholar]
- Alghadyan A.A., Peyman G.A., Khoobehi B. Liposome bound cyclosporine: clearance after intravitreal injection. Int. Ophthalmol. 1988;12:109–112. doi: 10.1007/BF00137135. [DOI] [PubMed] [Google Scholar]
- Alghadyan A.A., Peyman G.A., Khoobehi B., Liu K.-R. Liposome bound cyclosporine: retinal toxicity after intravitreal injection. Int. Ophthalmol. 1988;12:105–107. doi: 10.1007/BF00137134. [DOI] [PubMed] [Google Scholar]
- Alqurashi K.A., Aljabri K.S., Bokhari S.A. Prevalance of diabetes Mellitus in Saudi community. Ann. Saudi Med. 2011;31(1):19–23. doi: 10.4103/0256-4947.75773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association Standards of medical care in diabetes. Diabetes Care. 2004;27:15–35. [Google Scholar]
- Anon. Photocoagulation treatment of proliferative diabetic retinopathy: the second report of diabetic retinopathy study findings. Ophthalmology. 1978;85:82–106. doi: 10.1016/s0161-6420(78)35693-1. [DOI] [PubMed] [Google Scholar]
- Anon. Early treatment diabetic retinopathy study design and baseline patient characteristics: ETDRS report number 7. Ophthalmology. 1991;98:741–756. doi: 10.1016/s0161-6420(13)38009-9. [DOI] [PubMed] [Google Scholar]
- Anon. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44:968–983. [PubMed] [Google Scholar]
- Arevalo J.F., Fromow Guerra J., Quiroz Mercado H., Sanchez J.G., Wu L., Maia M. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema. Results from the Pan American Collaborative Retina Study Group at 6 month follow up. Ophthalmology. 2007;114:743–750. doi: 10.1016/j.ophtha.2006.12.028. [DOI] [PubMed] [Google Scholar]
- Arevalo J.F., Sanchez J.G., Lasave A.F., Pan-American Collaborative Retina Intravitreal Bevacizumab (Avastin(®)) for Diabetic Retinopathy at 24-months: the 2008 Juan Verdaguer-Planas Lecture. Curr. Diabetes Rev. 2010;6(5):313–322. doi: 10.2174/157339910793360842. [DOI] [PubMed] [Google Scholar]
- Audren F., Lecleire Collet A., Erginay A., Haouchine B., Benosman R., Bergmann J.F. Intravitreal triamcinolone acetonide for diffuse diabetic macular edema: phase 2 trial comparing 4 mg vs. 2 mg. Am. J. Ophthalmol. 2006;142:794–799. doi: 10.1016/j.ajo.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Avery R.L. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina. 2006;26:352–354. doi: 10.1097/00006982-200603000-00016. [DOI] [PubMed] [Google Scholar]
- Avery R.L., Pearlman J., Pieramici D.J., Rabena M.D., Castellarin A.A., Nasir M.A. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113(1695):e1–e15. doi: 10.1016/j.ophtha.2006.05.064. [DOI] [PubMed] [Google Scholar]
- Avitabile T., Longo A., Reibaldi A. Intravitreal triamcinolone compared with macular laser grid photocoagulation for the treatment of cystoid macular edema. Am. J. Ophthalmol. 2005;140:695–702. doi: 10.1016/j.ajo.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Aydin E., Demir H.D., Sahin S. Plasma and aqueous humor angiotensin-converting enzyme levels in patients with diabetic retinopathy. Curr. Eye Res. 2010;35(3):230–234. doi: 10.3109/02713680903484242. [DOI] [PubMed] [Google Scholar]
- Braun C.I., Benson W.E., Remaley N.A. Accommodative amplitudes in the early treatment diabetic retinopathy study. Retina. 1995;15:275–281. doi: 10.1097/00006982-199515040-00001. [DOI] [PubMed] [Google Scholar]
- Brouzas D., Charakidas A., Moschos M., Koutsandrea C., Apostolopoulos M., Baltatzis S. Bevacizumab (Avastin) for the management of anterior chamber neovascularization and neovascular glaucoma. Clin. Ophthalmol. 2009;3:685–688. doi: 10.2147/opth.s7698. Epub 2009 Dec 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.M., Kaiser P.K., Michels M., Soubrane G., Heier J.S., Kim R.Y. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N. Engl. J. Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Vlassara H., Cerami A. Nonenzymatic glycosylation and the pathogenesis of diabetic complications. Ann. Intern. Med. 1984;101:527–537. doi: 10.7326/0003-4819-101-4-527. [DOI] [PubMed] [Google Scholar]
- Bursell S.E., King G.L. Can protein kinase C inhibition and vitamin E prevent the development of diabetic vascular complications? Diabetes Res. Clin. Pract. 1999;45:169–182. doi: 10.1016/s0168-8227(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Bursell S.E., Clermont A.C., Aiello L.P., Aiello L.M., Schlossman D.K., Feener E.P. High-dose vitamin E supplementation normalizes retinal blood flow and creatinine clearance in patients with type 1 diabetes. Diabetes Care. 1999;22:1245–1251. doi: 10.2337/diacare.22.8.1245. [DOI] [PubMed] [Google Scholar]
- Chan C.K., Mohamed S., Lee V.Y., Lai T.Y., Shanmugam M.P., Lam D.S. Intravitreal dexamethasone for diabetic macular edema: a pilot study. Ophthalmic Surg. Lasers Imaging. 2010;41(1):26–30. doi: 10.3928/15428877-20091230-05. [DOI] [PubMed] [Google Scholar]
- Chaturvedi N., Sjolie A.K., Stephenson J.M., Abrahamian H., Keipes M., Castellarin A., EURODIAB Controlled Trial of Lisinopril in Insulin-Dependent Diabetes Mellitus Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. Lancet. 1998;351:28–31. doi: 10.1016/s0140-6736(97)06209-0. [DOI] [PubMed] [Google Scholar]
- Chaturvedi N., Porta M., Klein R., Orchard T., Fuller J., Parving H.H. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: randomised, placebo-controlled trials. Lancet. 2008;372:1394–1402. doi: 10.1016/S0140-6736(08)61412-9. [DOI] [PubMed] [Google Scholar]
- Chew E.Y., Klein M.L., Murphy R.P. Effects of aspirin on vitreous/preretinal hemorrhage in patients with diabetes mellitus. Early Treatment Diabetic Retinopathy Study Report No. 20. Arch. Ophthalmol. 1995;113:52–55. doi: 10.1001/archopht.1995.01100010054020. [DOI] [PubMed] [Google Scholar]
- Chew E.Y., Klein M.L., Ferris F.L., 3rd, Remaley N.A., Murphy R.P., Chantry K. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch. Ophthalmol. 1996;114:1079–1084. doi: 10.1001/archopht.1996.01100140281004. [DOI] [PubMed] [Google Scholar]
- Cho W.B., Moon J.W., Kim H.C. Intravitreal triamcinolone and bevacizumab as adjunctive treatments to panretinal photocoagulation in diabetic retinopathy. Br. J. Ophthalmol. 2010;94(7):858–863. doi: 10.1136/bjo.2009.168997. [DOI] [PubMed] [Google Scholar]
- Chun D.W., Heier J.S., Topping T.M., Duker J.S., Bankert J.M. A pilot study of multiple intravitreal injections of ranibizumab in patients with center involving clinically significant diabetic macular edema. Ophthalmology. 2006;113:1706–1712. doi: 10.1016/j.ophtha.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Chung J., Park Y.H., Lee Y.C. The effect of Nd:YAG laser membranotomy and intravitreal tissue plasminogen activator with gas on massive diabetic premacular hemorrhage. Ophthalmic Surg. Lasers Imaging. 2008;39:114–120. doi: 10.3928/15428877-20080301-06. [DOI] [PubMed] [Google Scholar]
- Cullen J.F., Town S.M., Campbell C.J. Double-blind trial of Atromid-S in exudative diabetic retinopathy. Trans. Ophthalmol. Soc. UK. 1974;94:554–562. [PubMed] [Google Scholar]
- Danser A.H., Derkx F.H., Admiraal P.J., Deinum J., Jong P.T., Schalekamp M.A. Angiotensin levels in the eye. Invest. Ophthalmol. Vis. Sci. 1994;35:1008–1018. [PubMed] [Google Scholar]
- Davis M.D., Fisher M.R., Gangnon R.E. Risk factors for high risk proliferative diabetic retinopathy and severe visual loss: early Treatment Diabetic Retinopathy Study Report #18. Invest. Ophthalmol. Vis. Sci. 1998;39:233–252. [PubMed] [Google Scholar]
- Dehghan M.H., Ahmadieh H., Ramezani A., Entezari M., Anisian A. A randomized, placebo controlled clinical trial of intravitreal triamcinolone for refractory diabetic macular edema? Int. Ophthalmol. 2008;28:7–17. doi: 10.1007/s10792-007-9097-y. [DOI] [PubMed] [Google Scholar]
- di Lauro R., De Ruggiero P., di Lauro R., di Lauro M.T., Romano M.R. Intravitreal bevacizumab for surgical treatment of severe proliferative diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2010;248(6):785–791. doi: 10.1007/s00417-010-1303-3. Epub 2010 Feb 5. [DOI] [PubMed] [Google Scholar]
- Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Diabetes Control and Complications Trial Research Group Progression of retinopathy with intensive versus conventional treatment in the diabetes control and complications trial. Ophthalmology. 1995;102:647–661. doi: 10.1016/s0161-6420(95)30973-6. [DOI] [PubMed] [Google Scholar]
- Diabetes Control and Complications Trial Research Group Early worsening of diabetic retinopathy in the diabetes control and complications trial. Arch. Ophthalmol. 1998;116(7):874–886. doi: 10.1001/archopht.116.7.874. [DOI] [PubMed] [Google Scholar]
- Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N. Engl. J. Med. 2000;342:381–389. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetic Retinopathy Study Research Group Preliminary report on effects of photocoagulation therapy. Am. J. Ophthalmol. 1976;81:383–396. doi: 10.1016/0002-9394(76)90292-0. [DOI] [PubMed] [Google Scholar]
- Diabetic Retinopathy Study Research Group Photocoagulation treatment of proliferative diabetic retinopathy: Clinical application of Diabetic Retinopathy Study (DRS) findings: DRS Report Number 8. Ophthalmology. 1981;88:583–600. [PubMed] [Google Scholar]
- Diabetic Retinopathy Study Research Group Photocoagulation for diabetic macular edema. Arch. Ophthalmol. 1985;103:1796–1806. [PubMed] [Google Scholar]
- Diabetic Retinopathy Vitrectomy Study Research Group Early vitrectomy for severe vitreous hemorrhage in diabetic retinopathy. Two year results of a randomized trial. Diabetic Retinopathy Vitrectomy Study report 2. Arch. Ophthalmol. 1985;103:1644–1652. [PubMed] [Google Scholar]
- Diabetic Retinopathy Vitrectomy Study Research Group Early vitrectomy for severe proliferative diabetic retinopathy in eyes with useful vision. Results of a randomized trial Diabetic Retinopathy Vitrectomy Study Report 3. Ophthalmology. 1988;95:1307–1320. doi: 10.1016/s0161-6420(88)33015-0. [DOI] [PubMed] [Google Scholar]
- Diabetic Retinopathy Vitrectomy Study Research Group Early vitrectomy for severe proliferative diabetic retinopathy in eyes with useful vision. Clinical application of results of a randomized trial Diabetic Retinopathy Vitrectomy Study Report 4. Ophthalmology. 1988;95:1321–1334. doi: 10.1016/s0161-6420(88)33014-9. [DOI] [PubMed] [Google Scholar]
- Do D.V., Nguyen Q.D., Shah S.M., Browning D.J., Haller J.A., Chu K. An exploratory study of the safety, tolerability and bioactivity of a single intravitreal injection of vascular endothelial growth factor (VEGF) trap eye in patients with diabetic macular edema. Br. J. Ophthalmol. 2009;93:144–149. doi: 10.1136/bjo.2008.138271. [DOI] [PubMed] [Google Scholar]
- Dogru M., Inoue M., Nakamura M., Yamamoto M. Modifying factors related to asymmetric diabetic retinopathy. Eye. 1998;12(Pt 6):929–933. doi: 10.1038/eye.1998.241. [DOI] [PubMed] [Google Scholar]
- Dowler J.G. Laser management of diabetic retinopathy. J. R. Soc. Med. 2003;96:277–279. doi: 10.1258/jrsm.96.6.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetic Retinopathy Vitrectomy Study (DRVS) report #1 Two year course of visual acuity in severe proliferative diabetic retinopathy with conventional management. Ophthalmology. 1985;92:92–502. doi: 10.1016/s0161-6420(85)34002-2. [DOI] [PubMed] [Google Scholar]
- Early Treatment Diabetic Retinopathy Study Research Group Techniques for scatter and local photocoagulation treatment of diabetic retinopathy. Early Treatment Diabetic Retinopathy Study Report No. 3. Int. Ophthalmol. Clin. 1987;27:254–264. doi: 10.1097/00004397-198702740-00005. [DOI] [PubMed] [Google Scholar]
- Early Treatment Diabetic Retinopathy Study Research Group Effects of aspirin treatment on diabetic retinopathy. ETDRS report Number 8. Ophthalmology. 1991;98:757–765. [PubMed] [Google Scholar]
- Early Treatment Diabetic Retinopathy Study Research Group Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS Report Number 12. Ophthalmology. 1991;98:823–833. [PubMed] [Google Scholar]
- Early Treatment Diabetic Retinopathy Study Research Group Grading diabetic retinopathy from stereoscopic color fundus photographs an extension of the modified Airlie House classification. ETDRS Report Number 10. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- Early Treatment Diabetic Retinopathy Study Research Group Fluorescein angiographic risk factors for progression of diabetic retinopathy. ETDRS Report Number 13. Ophthalmology. 1991;98:834–840. [PubMed] [Google Scholar]
- Early Treatment Diabetic Retinopathy Study Research Group Classification of diabetic retinopathy from fluorescein angiograms. ETDRS Report Number 11. Ophthalmology. 1991;98:807–822. [PubMed] [Google Scholar]
- Egger M., Davey Smith G., Stettler C., Diem P. Risk of adverse effects of intensified treatment in insulin dependent diabetes mellitus: a meta-analysis. Diabetes Med. 1997;14:919–928. doi: 10.1002/(SICI)1096-9136(199711)14:11<919::AID-DIA456>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Engerman R.L., Kern T.S. Experimental galactosemia produces diabetic-like retinopathy. Diabetes. 1984;33:97–100. doi: 10.2337/diab.33.1.97. [DOI] [PubMed] [Google Scholar]
- Estacio R.O., Jeffers B.W., Gifford N., Schrier R.W. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care. 2000;23:54–64. [PubMed] [Google Scholar]
- Fabbro D., Ruetz S., Bodis S., Pruschy M., Csermak K., Man A. PKC412: a protein kinase inhibitor with a broad therapeutic potential. Anticancer Drug Des. 2000;15:17–28. [PubMed] [Google Scholar]
- Fard M.A., Yazdanei Abyane A., Malihi M. Prophylactic intravitreal bevacizumab for diabetic macular edema (thickening) after cataract surgery: prospective randomized study. Eur. J. Ophthalmol. 2010 doi: 10.5301/EJO.2010.1405. [DOI] [PubMed] [Google Scholar]
- Ferris F. Early photocoagulation in patients with either type I or type II diabetes. Trans. Am. Ophthalmol. Soc. 1996;94:503–537. [PMC free article] [PubMed] [Google Scholar]
- Ferris F.L., III, Podgor M.J., Davis M.D. Macular edema in diabetic retinopathy study patients. Diabetic Retinopathy Study Report Number 12. Ophthalmology. 1987;94:754–760. doi: 10.1016/s0161-6420(87)33526-2. [DOI] [PubMed] [Google Scholar]
- Flynn H.W., Jr., Chew E.Y., Simons B.D., The Early Treatment Diabetic Retinopathy Study Research Group Pars plana vitrectomy in the early treatment diabetic retinopathy study. ETDRS Report Number 17. Ophthalmology. 1992;99:1351–1357. doi: 10.1016/s0161-6420(92)31779-8. [DOI] [PubMed] [Google Scholar]
- Fong D.S., Ferris F.L., III, Davis M.D., Chew E.Y., Early Treatment Diabetic Retinopathy Study Research Group Causes of severe visual loss in the early treatment diabetic retinopathy study: ETDRS Report No. 24. Am. J. Ophthalmol. 1999;127:137–141. doi: 10.1016/s0002-9394(98)00309-2. [DOI] [PubMed] [Google Scholar]
- Frank R.N., Keirn R.J., Kennedy R.A., Frank K.W. Galactose induced retinal basement membrane thickening: prevention by sorbinil. Invest. Ophthalmol. Vis. Sci. 1983;24:1519–1524. [PubMed] [Google Scholar]
- Gabbay K.H. Hyperglycemia, polyol metabolism and complications of diabetes mellitus. Annu. Rev. Med. 1975;26:521–536. doi: 10.1146/annurev.me.26.020175.002513. [DOI] [PubMed] [Google Scholar]
- Gillies M.C., Sutter F.K., Simpson J.M., Larsson J., Ali H., Zhu M. Intravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double masked, placebo-controlled, randomized clinical trial. Ophthalmology. 2006;113:1533–1538. doi: 10.1016/j.ophtha.2006.02.065. [DOI] [PubMed] [Google Scholar]
- Grant M.B., Mames R.N., Fitzgerald C., Hazariwala K.M., Cooper-DeHoff R., Caballero S. The efficacy of octreotide in the therapy of severe nonproliferative and early proliferative diabetic retinopathy: a randomized controlled study. Diabetes Care. 2000;23:504–509. doi: 10.2337/diacare.23.4.504. [DOI] [PubMed] [Google Scholar]
- Gulkilik G., Taskapili M., Kocabora S., Muftuoglu G., Demirci G. Intravitreal bevacizumab for persistent macular edema with proliferative diabetic retinopathy. Int. Ophthalmol. 2010 doi: 10.1007/s10792-010-9403-y. [DOI] [PubMed] [Google Scholar]
- Hammes H.P., Martin S., Federlin K., Geisen K., Brownlee M. Aminoguanidine treatment inhibits the development of experimental diabetic retinopathy. Proc. Natl. Acad. Sci. USA. 1991;88:11555–11558. doi: 10.1073/pnas.88.24.11555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour J.W., Smiddy W.E., Flynn H.W., Rubsamen P.E. Vitrectomy for diabetic macular edema associated with a thickened and taut posterior hyaloid membrane. Am. J. Ophthalmol. 1996;121:405–413. doi: 10.1016/s0002-9394(14)70437-4. [DOI] [PubMed] [Google Scholar]
- Harrold B.P., Marmion V.J., Gough K.R. A double-blind controlled trial of clofibrate in the treatment of diabetic retinopathy. Diabetes. 1969;18:285–291. doi: 10.2337/diab.18.5.285. [DOI] [PubMed] [Google Scholar]
- Hattori T., Shimada H., Nakashizuka H., Mizutani Y., Mori R., Yuzawa M. Dose of intravitreal bevacizumab (Avastin) used as preoperative adjunct therapy for proliferative diabetic retinopathy. Retina. 2010;30(5):761–764. doi: 10.1097/IAE.0b013e3181c70168. [DOI] [PubMed] [Google Scholar]
- Henkind P. Ocular neovascularization. The Krill memorial lecture. Am. J. Ophthalmol. 1978;85:287–301. [PubMed] [Google Scholar]
- Hernández-Da Mota S.E., Nuñez-Solorio S.M. Experience with intravitreal bevacizumab as a preoperative adjunct in 23-G vitrectomy for advanced proliferative diabetic retinopathy. Eur. J. Ophthalmol. 2010;20(6):1047–1052. doi: 10.1177/112067211002000604. [DOI] [PubMed] [Google Scholar]
- Hikichi T., Fujio N., Akiba J., Azuma Y., Takahashi M., Yoshida A. Association between the short term natural history of diabetic macular edema and the vitreomacular relationship in type II diabetes mellitus. Ophthalmology. 1997;104:473–478. doi: 10.1016/s0161-6420(97)30289-9. [DOI] [PubMed] [Google Scholar]
- Hornan D., Edmeades N., Krishnan R., Khan J., Lochhead J. Use of pegaptanib for recurrent and non-clearing vitreous haemorrhage in proliferative diabetic retinopathy. Eye (Lond) 2010;24(8):1315–1319. doi: 10.1038/eye.2010.14. [DOI] [PubMed] [Google Scholar]
- Jonas J.B. Intravitreal triamcinolone acetonide for diabetic retinopathy. Dev. Ophthalmol. 2007;39:96–100. doi: 10.1159/000098502. [DOI] [PubMed] [Google Scholar]
- Kang S.W., Sa H.S., Cho H.Y., Kim J.I. Macular grid photocoagulation after intravitreal triamcinolone acetonide for diffuse diabetic macular edema. Arch. Ophthalmol. 2006;124:653–658. doi: 10.1001/archopht.124.5.653. [DOI] [PubMed] [Google Scholar]
- Karim R., Tang B. Use of antivascular endothelial growth factor for diabetic macular edema. Clin. Ophthalmol. 2010;4:493–517. doi: 10.2147/opth.s8980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzung B.G., editor. Basic and Clinical Pharmacology. Mc. Graw Hill; 2004. [Google Scholar]
- Kaufman S.C., Ferris F.L., III, Swartz M. Intraocular pressure following panretinal photocoagulation for diabetic retinopathy. Diabetic Retinopathy Report No. 11. Arch. Ophthalmol. 1987;105:807–809. doi: 10.1001/archopht.1987.01060060093040. [DOI] [PubMed] [Google Scholar]
- Kaufman S.C., Ferris F.L., III, Seigel D.G. Factors associated with visual outcome after photocoagulation for diabetic retinopathy. Diabetic Retinopathy Study Report #13. Invest. Ophthalmol. Vis. Sci. 1989;30:23–28. [PubMed] [Google Scholar]
- Klein R., Klein B.E., Moss S.E., Davis M.D., DeMets D.L. Glycosylated hemoglobin predicts the incidence and progression of diabetic retinopathy. JAMA. 1988;260:2864–2871. [PubMed] [Google Scholar]
- Klein R., Palta M., Allen C., Shen G., Han D.P., D’Alessio D.J. Incidence of retinopathy and associated risk factors from time of diagnosis of insulin-dependent diabetes. Arch. Ophthalmol. 1997;115:351–356. doi: 10.1001/archopht.1997.01100150353007. [DOI] [PubMed] [Google Scholar]
- Kook D., Wolf A., Kreutzer T., Neubauer A., Strauss R., Ulbig M. Long term effect of intravitreal bevacizumab (Avastin) in patients with chronic diffuse diabetic macular edema. Retina. 2009;28:1053–1060. doi: 10.1097/IAE.0b013e318176de48. [DOI] [PubMed] [Google Scholar]
- Kumagai K., Furukawa M., Ogino N., Larson E., Iwaki M., Tachi N. Long term follow up of vitrectomy for diffuse non tractional diabetic macular edema. Retina. 2009;29:464–472. doi: 10.1097/IAE.0b013e31819c632f. [DOI] [PubMed] [Google Scholar]
- Kunisaki M., Bursell S.E., Clermont A.C., Ishii H., Ballas L.M., Jirousek M.R. Vitamin E prevents diabetes-induced abnormal retinal blood flow via the diacylglycerol-protein kinase C pathway. Am. J. Physiol. 1995;269:239–246. doi: 10.1152/ajpendo.1995.269.2.E239. [DOI] [PubMed] [Google Scholar]
- Kuppermann B.D., Blumenkranz M.S., Haller J.A., Williams G.A., Weinberg D.V., Chou C. Randomized controlled study of an intravitreous dexamethasone drug delivery system in patients with persistent macular edema. Arch. Ophthalmol. 2007;125:309–317. doi: 10.1001/archopht.125.3.309. [DOI] [PubMed] [Google Scholar]
- Lam D.S.C., Chan C.K.M., Mohamed S., Lai T.Y.Y., Lee V.Y.W., Liu D.T.L. Intravitreal triamcinolone plus sequential grid laser versus triamcinolone or laser alone for treating diabetic macular edema. Six month outcomes. Ophthalmology. 2007;114:2162–2167. doi: 10.1016/j.ophtha.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Li W., Sinclair S.H., Xu G.T. Effects of intravitreal erythropoietin therapy for patients with chronic and progressive diabetic macular edema. Ophthalmic Surg. Lasers Imaging. 2010;41(1):18–25. doi: 10.3928/15428877-20091230-03. [DOI] [PubMed] [Google Scholar]
- Lyons T.J., Jenkins A.J., Zheng D., Lackland D.T., McGee D., Garvey W.T. Diabetic retinopathy and serum lipoprotein subclasses in the DCCT/EDIC cohort. Investig. Ophthalmol. Visual. Sci. 2004;45:910–918. doi: 10.1167/iovs.02-0648. [DOI] [PubMed] [Google Scholar]
- Macugen Diabetic Retinopathy Study Group A phase II randomized double masked trial of pegaptanib, an anti vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 2005;112:1747–1757. doi: 10.1016/j.ophtha.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Marticorena J., Romano M.R., Heimann H., Stappler T., Gibran K., Groenewald C., Pearce I., Wong D. Intravitreal bevacizumab for retinal vein occlusion and early growth of epiretinal membrane: a possible secondary effect? Br. J. Ophthalmol. 2010 doi: 10.1136/bjo.2009.177287. [DOI] [PubMed] [Google Scholar]
- Massin P., Audren F., Haouchine B., Erginay A., Bergmann J.F., Benosman R. Intravitreal triamcinolone acetonide for diffuse diabetic macular edema; preliminary results of a prospective controlled trial. Ophthalmology. 2004;111:218–225. doi: 10.1016/j.ophtha.2003.05.037. [DOI] [PubMed] [Google Scholar]
- Massin P., Bandello F., Garweg J.G., Hansen L.L., Harding S.P., Larsen M., Mitchell P., Sharp, Wolf-Schnurrbusch U.E., Gekkieva M., Weichselberger A., Wolf S. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33(11):2399–2405. doi: 10.2337/dc10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D.R., Stratton I.M., Aldington S.J., Holman R.R., Kohner E.M., UK Prospective Diabetes Study Group Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch. Ophthalmol. 2004;122:1631–1640. doi: 10.1001/archopht.122.11.1631. [DOI] [PubMed] [Google Scholar]
- McLeod D. A chronic grey matter penumbra, lateral microvascular intussusception and venous peduncular avulsion underlie diabetic vitreous hemorrhage. Br. J. Ophthalmol. 2007;91:677–689. doi: 10.1136/bjo.2006.109199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micieli J.A., Micieli A., Smith A.F. intravitreal bevacizumab: systematic review of the literature and the Canadian Adverse Drug Reaction Database. Can. J. Ophthalmol. 2010;45(3):231–238. doi: 10.3129/i10-027. [DOI] [PubMed] [Google Scholar]
- Miller J.W., Adamis A.P., Aiello L.P. Vascular endothelial growth factor in ocular neovascularization and proliferative diabetic retinopathy. Diabetes Metab. Rev. 1997;13:37–50. doi: 10.1002/(sici)1099-0895(199703)13:1<37::aid-dmr174>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Mohamed Q., Gillies M.C., Wong T.Y. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298:902–916. doi: 10.1001/jama.298.8.902. [DOI] [PubMed] [Google Scholar]
- Nasrallah F.P., Jalkh A.E., Coppenolle F.V., Kado M., Trempe C.L., McMeel J.W. The role of the vitreous in diabetic macular edema. Ophthalmology. 1988;95:1335–1339. doi: 10.1016/s0161-6420(88)33004-6. [DOI] [PubMed] [Google Scholar]
- Nguyen Q.D., Shah S.M., Heier J.S., Do D.V., Lim J., Boyer D., Abraham P., Campochiaro P.A., READ-2 Study Group Primary end point (Six Months) results of the Ranibizumab for edema of the macula in Diabetes (READ-2) study. Ophthalmology. 2009;116(11):2175–2181. doi: 10.1016/j.ophtha.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Ozkiris A. Intravitreal bevacizumab (Avastin) for primary treatment of diabetic macular edema. Eye. 2009;23:616–620. doi: 10.1038/eye.2008.40. [DOI] [PubMed] [Google Scholar]
- Park S.W., Seo M.S. Subhyaloid hemorrhage treated with SF6 gas injection. Ophthalmic Surg. Lasers Imaging. 2004;35:335–337. [PubMed] [Google Scholar]
- Parravano M., Menchini F., Virgili G. Antiangiogenic therapy with anti-vascular endothelial growth factor modalities for diabetic macular edema. Cochrane Database Syst. Rev. 2009;(4) doi: 10.1002/14651858.CD007419.pub2. CD007419. [DOI] [PubMed] [Google Scholar]
- Patel J.I., Hykin P.G., Schadt M., Luong V., Bunce C., Fitzke F. Diabetic macular edema: pilot randomized trial of pars plana vitrectomy vs. macular argon photocoagulation? Eye. 2006;20:873–881. doi: 10.1038/sj.eye.6702012. [DOI] [PubMed] [Google Scholar]
- Patel J.I., Hykin P.G., Schadt M., Luong V., Fitzke F., Gregor Z.J. Pars plana vitrectomy for diabetic macular edema: OCT and functional correlations. Eye. 2006;20:674–680. doi: 10.1038/sj.eye.6701945. [DOI] [PubMed] [Google Scholar]
- Patz A., Smith R.E. The ETDRS and diabetes 2000. Ophthalmology. 1991;98:730–740. doi: 10.1016/s0161-6420(13)38007-5. [DOI] [PubMed] [Google Scholar]
- Pearson, P., Levy, B., Comstock, T., 2006. Fluocinolone acetonide intravitreal implant to treat diabetic macular edema: 3-year results of a multi-center clinical trial: Poster presented at the Association for Research in Vision and Ophthalmology, April 30–May 4, 2006, Fort Lauderdale, Fla., Abstract 5442.
- Pendergast S.D., Hassan T.S., Williams G.A., Cox M.S., Margherio R.R., Ferrone P.J. Vitrectomy for diffuse diabetic macular edema associated with a taut premacular posterior hyaloid. Am. J. Ophthalmol. 2000;130:178–186. doi: 10.1016/s0002-9394(00)00472-4. [DOI] [PubMed] [Google Scholar]
- Pouvlaki V., Joussen A.M., Mitsiades N., Mitsiades C.S., Iliaki E.F., Adamis A.P. Insulin like growth factor I plays a pathogenetic role in diabetic retinopathy. Am. J. Pathol. 2004;165:457–469. doi: 10.1016/S0002-9440(10)63311-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querques G., Bux A.V., Martinelli D., Iaculli C., Noci N.D. Intravitreal pegaptanib sodium (Macugen) for diabetic macular oedema. Acta Ophthalmol. 2009;87(6):623–630. doi: 10.1111/j.1755-3768.2009.01580.x. [DOI] [PubMed] [Google Scholar]
- Rand L.I., Prud’homme G.J., Ederer F., Canner P.L. Factors influencing the development of visual loss in advanced diabetic retinopathy. Diabetic Retinopathy Study (DRS) Report No. 10. Invest. Ophthalmol. Vis. Sci. 1985;26:983–991. [PubMed] [Google Scholar]
- Raymond L.A. Neodymium: YAG laser treatment for hemorrhages under the internal limiting membrane and posterior hyaloid face in the macula. Ophthalmology. 1995;102:406–411. doi: 10.1016/s0161-6420(95)31008-1. [DOI] [PubMed] [Google Scholar]
- Recchia F.M., Ruby A.J., Recchia C.A.C. Pars plana vitrectomy with removal of the internal limiting membrane in the treatment of persistent diabetic macular edema. Am. J. Ophthalmol. 2005;139:447–454. doi: 10.1016/j.ajo.2004.09.076. [DOI] [PubMed] [Google Scholar]
- Roh M.I., Byeon S.H., Kwon O.W. Repeated intravitreal injection of bevacizumab for clinically significant diabetic macular edema. Retina. 2008;28:1314–1318. doi: 10.1097/IAE.0b013e3181853d2a. [DOI] [PubMed] [Google Scholar]
- Rosenblatt B.J., Shah G.K., Sharma S., Bakal J. Pars plana vitrectomy with internal limiting membranectomy for refractory diabetic macular edema without a taut posterior hyaloid. Graefe’s Arch Clin. Exp. Ophthalmol. 2005;243:20–25. doi: 10.1007/s00417-004-0958-z. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P.J. Intravitreal avastin: the low cost alternative to lucentis? Am. J. Ophthalmol. 2006;142:141–143. doi: 10.1016/j.ajo.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P.J., Brown D.M., Heier J.S., Boyer D.S., Kaiser P.K., Chung C.Y. Ranibizumab for neovascular age related macular degeneration. N. Engl. J. Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- Roy M.S., Klein R., O’Colmain B.J. The prevalence of diabetic retinopathy among adult type 1 diabetic persons in the United States. Arch. Ophthalmol. 2004;122:546–551. doi: 10.1001/archopht.122.4.546. [DOI] [PubMed] [Google Scholar]
- Saito Y., Higashide T., Takeda H., Ohkubo S., Sugiyama K. Beneficial effects of preoperative intravitreal bevacizumab on trabeculectomy outcomes in neovascular glaucoma. Acta Ophthalmol. 2010;88(1):96–102. doi: 10.1111/j.1755-3768.2009.01648.x. Epub 2009 Sep 23. [DOI] [PubMed] [Google Scholar]
- Sarraf D., Vyas N., Jai A., Bui A., Kertes P.J., Freund B., Chan C. Triamcinolone – associated maculopathy. Arch. Ophthalmol. 2010;128(6):685–690. doi: 10.1001/archophthalmol.2010.109. [DOI] [PubMed] [Google Scholar]
- Schrier R.W., Estacio R.O., Esler A., Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int. 2002;61:1086–1097. doi: 10.1046/j.1523-1755.2002.00213.x. [DOI] [PubMed] [Google Scholar]
- Schwartz, S.G., Flynn Jr, H.W., 2010. Fluocinolone Acetonide Implantable Device for Diabetic Retinopathy. Curr Pharm Biotechnol. 2010 Nov 12 (epub ahead of print). [DOI] [PubMed]
- Scott I.U., Edwards A.R., Beck R.W., Bressler N.M., Chan C.K., Diabetic Retinopathy Clinical Research Network A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema? Ophthalmology. 2007;114:1860–1867. doi: 10.1016/j.ophtha.2007.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfikakis P.P., Grigoropoulos V., Emfietzoglou I. Infliximab for diabetic macular edema refractory to laser photocoagulation: a randomized, double-blind, placebo-controlled, crossover, 32-week study. Diabetes Care. 2010;33(7):1523–1528. doi: 10.2337/dc09-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y.W., Lee Y.J., Lee B.R., Cho H.Y. Effects of an intravitreal bevacizumab injection combined with panretinal photocoagulation on high-risk proliferative diabetic retinopathy. Korean J. Ophthalmol. 2009;23(4):266–272. doi: 10.3341/kjo.2009.23.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjolie A.K., Klein R., Porta M., Orchard T., Fuller J., Parving H.H. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trial. Lancet. 2008;372:1385–1393. doi: 10.1016/S0140-6736(08)61411-7. [DOI] [PubMed] [Google Scholar]
- Soheilian M., Ramezani A., Bijanzadeh B., Yaseri M., Ahmadieh H., Dehghan M.H. Intravitreal bevacizumab (Avastin) injection alone or combined with triamcinolone versus macular photocoagulation as primary treatment of diabetic macular edema. Retina. 2007;27:1187–1195. doi: 10.1097/IAE.0b013e31815ec261. [DOI] [PubMed] [Google Scholar]
- Solaiman K.A., Diab M.M., Abo-Elenin M. Intravitreal bevacizumab and/or macular photocoagulation as a primary treatment for diffuse diabetic macular edema. Retina. 2010;30(10):1638–1645. doi: 10.1097/IAE.0b013e3181e1ed07. [DOI] [PubMed] [Google Scholar]
- Spaide R.F., Fisher Y.L. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006;26:275–278. doi: 10.1097/00006982-200603000-00004. [DOI] [PubMed] [Google Scholar]
- Stolba U., Binder S., Gruber D., Krebs I., Aggermann T., Neumaier B. Vitrectomy for persistent diffuse diabetic macular edema. Am. J. Ophthalmol. 2005;140:295–301. doi: 10.1016/j.ajo.2005.03.045. [DOI] [PubMed] [Google Scholar]
- Sultanov M.I., Gadzhiev R.V. The characteristics of the course of diabetic retinopathy in myopia. Vestn. oftalmol. 1990;106:49–51. [PubMed] [Google Scholar]
- Suzuma I., Hata Y., Clermont A., Pokras F., Rook S.L., Suzuma K. Cyclic stretch and hypertension induce retinal expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2: potential mechanisms for exacerbation of diabetic retinopathy by hypertension. Diabetes. 2001;50:444–454. doi: 10.2337/diabetes.50.2.444. [DOI] [PubMed] [Google Scholar]
- Takata C., Messias A., Folgosa M.S., Lucena L.R., Lucena D.R., Scott I.U., Jorge R. Intravitreal injection versus subtenon infusion of triamcinolone acetonide during cataract surgery in patients with refractory diabetic macular edema. Retina. 2010;30(4):562–569. doi: 10.1097/IAE.0b013e3181c969b4. [DOI] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonyl-ureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- Wagner J., Danser A.H., Derkx F.H., Jong T.V., Paul M., Mullins J.J. Demonstration of renin mRNA, angiotensinogen mRNA, and angiotensin converting enzyme mRNA expression in the human eye: Evidence for an intraocular renin-angiotensin system. Br. J. Ophthalmol. 1996;80:159–163. doi: 10.1136/bjo.80.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wautier J.L., Guillausseau P.J. Advanced glycation end products, their receptors and diabetic angiopathy. Diabetes Metab. 2001;27:535–542. [PubMed] [Google Scholar]
- Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287:2563–2569. doi: 10.1001/jama.287.19.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Evans T., Arévalo J.F., Berrocal M.H., Rodríguez F.J., Hsu M., Sánchez J.G. Long-term effect of intravitreal triamcinolone in the nonproliferative stage of type II idiopathic parafoveal telangiectasia. Retina. 2008;28(2):314–319. doi: 10.1097/IAE.0b013e31814cf03e. [DOI] [PubMed] [Google Scholar]
- Wu L., Arevalo J.F., Berrocal M.H., Maia M., Roca J.A., Morales-Cantón V., Alezzandrini A.A., Díaz-Llopis M.J. Comparison of two doses of intravitreal bevacizumab as primary treatment for macular edema secondary to central retinal vein occlusion: results of the pan American collaborative retina study group at 24 months. Retina. 2010;30(7):1002–1011. doi: 10.1097/IAE.0b013e3181cea68d. [DOI] [PubMed] [Google Scholar]
- Xia P., Aiello L.P., Ishii H., Jiang Z.Y., Park D.J., Robinson G.S. Characterization of vascular endothelial growth factor’s effect on the activation of protein kinase C, its isoforms, and endothelial cell growth. J. Clin. Invest. 1996;98:2018–2026. doi: 10.1172/JCI119006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz T., Weaver C.D., Gallagher M.J., Cordero-Coma M., Cervantes-Castaneda R.A., Klisovic D., Lavaque A.J., Larson R.J. Intravitreal triamcinolone acetonide injection for treatment of refractory diabetic macular edema: a systematic review. Ophthalmology. 2009;116(5):902–911. doi: 10.1016/j.ophtha.2009.02.002. [DOI] [PubMed] [Google Scholar]


