Abstract
We are reporting a 34-year-old Arabic white female patient who presented with a white mass covering her left cornea following multiple ocular surgeries and healed corneal ulcer. The lesion obscured further view of the iris, pupil and lens. The patient underwent penetrating keratoplasty and the histopathologic study of the left corneal button showed epithelial hyperplasia, absent Bowman’s layer and subepithelial fibrovascular proliferation. The histopathologic appearance was suggestive of a corneal keloid which was supported by further ultrastructural study. The corneal graft remained clear 6 months after surgery and the patient was satisfied with the visual outcome. Penetrating keratoplasty may be an effective surgical option for corneal keloids in young adult patients.
Keywords: Corneal mass, Histopathology, Keloid, Penetrating keratoplasty
Introduction
Keloids and hypertrophic scars are fibrous tissue outgrowths that result from a deviation from normal wound-healing process and were first described in 1865.1 Clinically, corneal keloids appear as gray–white elevated masses diffusely involving the entire stroma or as localized solitary nodules.2–4 There have been approximately 76 documented cases of corneal keloid previously reported.5 Most reported cases have occurred secondary to penetrating injury or surgery, and it is thought that inflammation causes a fibroblastic reaction resulting in the keloid,4 others have developed after small pox, rubella6 or can arise de-novo.5
Keloids are usually unilateral, but can be bilateral if associated with genetic syndromes. Surgical results of congenital corneal keloids have been very poor and in spite of repeated penetrating keratoplasties, the reconstruction of the anterior segment has been often unsuccessful.7 In extreme cases, the eyes were eventually enucleated due to spontaneous corneal perforation or buphthalmos.8 We describe a case of corneal keloid after healed corneal ulcer which was successfully managed by penetrating keratoplasty. The clinical, histopathologic, and ultrastructural findings are all presented.
Case report
A white 34 year old female patient presented with a white mass covering her left cornea, and was unable to close her left eye (Fig. 1a and b). Her ophthalmic history revealed complicated cataract surgery (phacoemulsification with intraocular lens implantation) 9 years prior to her presentation. Her cataract was secondary to Fuchs uveitis. This was followed one year later, by intractable glaucoma which was controlled by Trabeculectomy with Mitomycin C followed by fixation of a pars plana Ahmed valve. The patient then developed paracentral sterile corneal ulcer measuring 3.00 × 2.50 mm involving the mid-stroma. The corneal ulcer healed after using conservative measures with residual left dense corneal scar.
Figure 1.
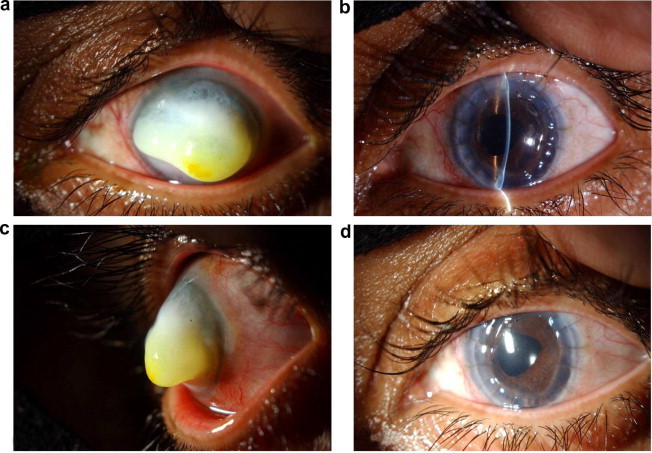
(a) The clinical appearance of the whitish lesion covering most of the left cornea. (b) Side view of the left corneal keloid. (c) Slitlamp photography showing clear corneal graft with no recurrence 6 months after surgery. (d) The post-operative clinical appearance showing peaked pupil secondary to peripheral anterior synechiae at 2 o’clock.
At the time of presentation her uncorrected visual acuity was hand motion in left eye and 20/30 in right eye. Slitlamp biomicroscopy showed large, dome shaped white non-vascularized mass measuring 8.00 × 7.50 mm, protruding from the lids with exposure keratopathy overlying the mass (fluorescein positive staining). Applanation tonometry, anterior segment examination, and dilated fundus examination were precluded by the corneal mass.
The patient underwent penetrating keratoplasty in her left eye. In order to excise the entire corneal mass, large sized trephination was performed (with 9.00 mm corneal trephine) and the donor corneal graft measured 9.25 mm. This graft was secured with interrupted 10/0 sutures. The postoperative best spectacle corrected visual acuity in left eye was 20/60 with clear graft for a follow up period of 6 months (Fig. 1c). However, pupil distortion was noted due to peripheral anterior synechia (Fig. 1d). Half of the corneal button obtained during surgery was fixed in 3% formalin solution for light microscopy and the other half was fixed in 0.25 glutaraldehyde for Electron Microscopy. Examination of the histologic sections showed acanthotic epithelial layer and localized subepithelial fibroblastic and myofibroblastic proliferation. At the base of the lesion, Bowman’s membrane was fragmented and the stroma showed extensive neovascularization and scarring (Fig. 2a). An irregular thick Descemet’s membrane with normal endothelium was present. The proliferating spindle cells representing fibroblasts were positively stained with Vimentin (Fig. 2b). Few myofibroblasts showing positive staining with smooth muscle actin (SMA) were also present.
Figure 2.
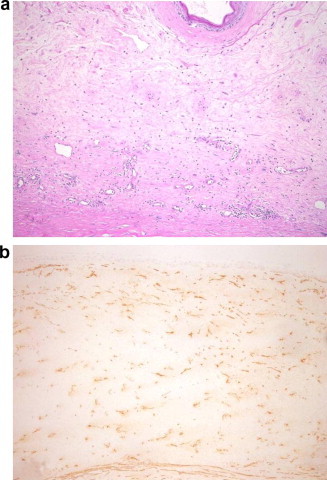
(a) Stromal neovascularization at the base of the lesion (periodic acid schiff, original magnification × 100). (b) Vimentin stain showing reactive fibroblasts (original magnification × 100).
The Electron Microscopy showed mature myofibroblasts (Fig. 3a) displaying thin actin-like filaments under the plasmalemma, scattered fusiform densities and focal basement membrane (Fig. 2b). In addition, fibroblasts and inactive fibrocytes were also identified (Fig. 3c). The fibroblasts showed fibrillar cytoplasm (Fig. 3d).
Figure 3.
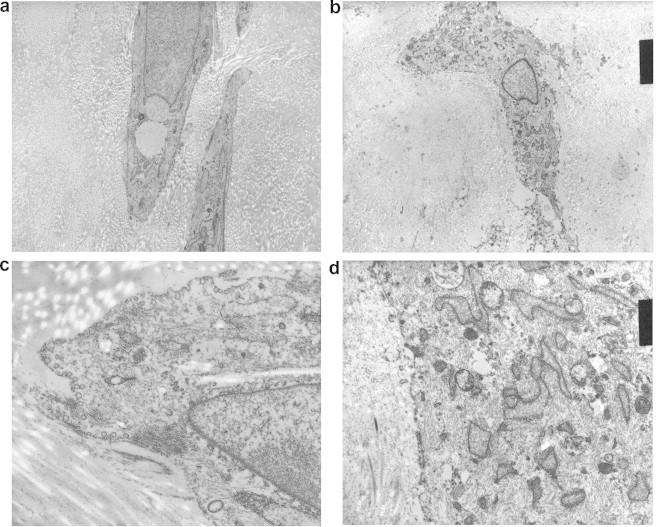
(a) Electron Micrograph of mature myofibroblasts (original magnification × 4900). (b) Electron Micrograph of myofibroblast showing numerous thin filaments and fusiform densities (original magnification × 24,000). (c) Electron Micrograph of inactive fibrocyte (original magnification × 3300). (d) Electron Micrograph of fibroblast with fibrillar cytoplasm (original magnification × 18,500).
Discussion
Corneal keloids and hypertrophic scars are relatively uncommon. They tend to occur more in children and young adults.2,3,7,9
The clinical differential diagnosis includes sclerocornea, dermoid, myxoma, Salzmann nodular degeneration, Peter anomaly, congenital hereditary endothelial dystrophy, congenital glaucoma, familial band-shaped keratopathy, spheroidal degeneration, squamous cell carcinoma, juvenile xanthogranuloma, fibrous histiocytoma, birth trauma, infection, and metabolic disease.5,9
The origin and nature of such tumors remain obscure. Most cases reported in the literature are described along with fibrous tumors of cornea. Some researchers attributed them to undifferentiated hyperplasia of opaque corneal and scleral tissue of developmental origin.6
Smith1 ascribed it to inflammation, injury or surgery proceeding such overgrowths of the cornea. From his observation of 27 cases, he concluded that although lesions resembling true tumors did originate from corneal tissue, it was very difficult to determine histologically whether these lesions were to be classified as true fibrous tumors or merely as keloids. Others6 gave the term Juvenile fibromatosis’ to different varieties’ of fibrous tissue proliferation, which was found in patients below 15 years of age. In their view such tumors arose in sub-epithelial soft parts and at sites where scar tissue was known to have existed and they could hardly be differentiated from keloids.
In our case, peripheral anterior synechiae at 2 o’clock was seen at time of penetrating keratoplasty (Fig. 1d), which can be explained by the occurence of silent sealed corneal perforation with iris prolapse complicating the corneal ulcer. This goes in line with the hypothesis by Pansons in 1904 and Fuchs in 1918 that corneal hypertrophic scars originate from the stromal cells of the iris tissue.10,11
However, others reported similar cases with no histologic evidence of iris involvement thus suggesting that the pathogenesis of such lesions is related to primary corneal reparative activity and that it is actually derived from the corneal stroma.12
Most reported cases have occurred secondary to penetrating injury or surgery, and it is thought that inflammation causes a fibroblastic reaction resulting in the keloid.4 Mejia in 2001 reported a patient with no history of previous trauma, but had exacerbation in the growth of bilateral corneal keloid after superficial keratectomy.8 The present case reported here seems to have developed corneal keloid in relation to the healing of a pre-existing corneal ulcer rather than to surgery because it was not even close to the site of neither Trabeculectomy nor Ahmed valve.
Most of the cases in the literature were diagnosed by histopathologic examination rather than clinical diagnosis,13 which was also true in our case. The histopathology and the ultrastructure of corneal keloid in the current report were similar to the ones previously described in literature.8,9,14,15
It is believed that in keloids, myofibroblasts, which are transformed fibroblasts that express smooth muscle phenotypes persist and often dominate the cell population. Eventually, they deposit material that slowly collagenizes in the center but does not retract. This produces a bulky slow growth of active fibroblasts and myofibroblasts.14,16
The neovascularization can be located anteriorly or in a diffuse pattern within the keloid, however in our case as well as other cases reported in the same position, the blood vessels were mainly observed deeper in the stroma or at the base of the lesion.14–16 The origin of these blood vessels can either be from the peripheral cornea or from incarcerated iris tissue, which can be the cause for bloody discharge.5
The management of such lesions includes; simple excision, lamellar keratoplasty and penetrating keratoplasty. Penetrating keratoplasty might be preferred because of the reported recurrence of keloids in cases of subtotal simple excision.17 Treatment goals are directed at removing the existing lesion and preventing recurrence by inhibiting fibroblastic proliferation and collagen synthesis. In the ultrastructural analysis of corneal keloids, it was noted that the persistence of mast cells promotes recurrent inflammation and utilizing topical steroids or mast cell stabilizers may be useful.2,18 Other options that have been proposed include topical cyclosporine3 or the use of physical forms of treatment such as ultrasound, cryotherapy, pressure therapy, and laser.18 The utilization of amniotic membrane after superficial keratectomy has been successful in preventing recurrence and epithelial breakdown because of its antifibroblastic and anti-inflammatory properties.9
Conclusion
The etiology of the development of corneal keloids as a healing response remains obscured. Genetic analysis to understand the reason why such patients have higher susceptibility for an abnormal reparative process is helpful to predict future cases, since some studies have shown the association between some genetic disorders and development of corneal keloids e.g. Lowe syndrome2,3,18 and Rubinstein–Taybi syndrome.7
One of the drawbacks in the management of our case, is that we did not evaluate by ultrasonic biomicroscopy (UBM) the anterior chamber depth behind the corneal keloid prior to its removal in order to take into consideration the extensive anterior synechia at the time of corneal trephination. Also, a longer follow up after penetrating keratoplasty is required to exclude the possibility of recurrence in corneal keloid cases.
Conflict of interest statement
The authors have no conflict of interest related to this paper.
References
- 1.Smith H.C. Keloid of the cornea. Trans Am Ophthalmol Soc. 1940;38:519–538. [PMC free article] [PubMed] [Google Scholar]
- 2.Cibis G.W., Tripathi R.C., Tripathi B.J., Harris D.J. Corneal keloid in Lowe’s syndrome. Arch Ophthalmol. 1982;100:1795–1799. doi: 10.1001/archopht.1982.01030040775013. [DOI] [PubMed] [Google Scholar]
- 3.Esquenazi S., Eustis H.S., Bazan H.E., Leon A., He J. Corneal keloid in Lowe syndrome. J Pediatr Ophthalmol Strabismus. 2005;42:308–310. doi: 10.3928/0191-3913-20050901-16. [DOI] [PubMed] [Google Scholar]
- 4.Shoukrey N.M., Tabbara K.F. Ultrastructural study of a corneal keloid. Eye (Lond) 1993;7(Pt 3):379–387. doi: 10.1038/eye.1993.76. [DOI] [PubMed] [Google Scholar]
- 5.Jung J.J., Wojno T.H., Grossniklaus H.E. Giant corneal keloid: case report and review of the literature. Cornea. 2010 doi: 10.1097/ICO.0b013e3181d83858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shukla I.M., Arora N.P., Arora M.M. Corneal keloid. Indian J Ophthalmol. 1975;23:18–19. [PubMed] [Google Scholar]
- 7.Rao S.K., Fan D.S., Pang C.P., Li W.W., Ng J.S., Good W.V. Bilateral congenital corneal keloids and anterior segment mesenchymal dysgenesis in a case of Rubinstein–Taybi syndrome. Cornea. 2002;21:126–130. doi: 10.1097/00003226-200201000-00028. [DOI] [PubMed] [Google Scholar]
- 8.Mejia L.F., Acosta C., Santamaria J.P. Clinical, surgical, and histopathologic characteristics of corneal keloid. Cornea. 2001;20:421–424. doi: 10.1097/00003226-200105000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Chawla B., Agarwal A., Kashyap S., Tandon R. Diagnosis and management of corneal keloid. Clin Exp Ophthalmol. 2007;35:855–857. doi: 10.1111/j.1442-9071.2007.01608.x. [DOI] [PubMed] [Google Scholar]
- 10.Farkas T.G., Znajda J.P. Keloid of the cornea. Am J Ophthalmol. 1968 August:319–323. doi: 10.1016/0002-9394(68)92081-3. [DOI] [PubMed] [Google Scholar]
- 11.Vanathi M., Sen S., Panda A., Dada T., Behera G., Khokhar S. Unilateral congenital corneal keloid with anterior segment mesenchymal dysgenesis and subluxated lens: case report and review of literature. Cornea. 2007;26:111–113. doi: 10.1097/01.ico.0000243952.84224.fe. [DOI] [PubMed] [Google Scholar]
- 12.O’Grady R.B., Kirk H.Q. Corneal keloids. Am J Ophthalmol. 1972;73(2):206–213. doi: 10.1016/0002-9394(72)90134-1. [DOI] [PubMed] [Google Scholar]
- 13.Mendez E.A., Daza M.T. Sclerokeratoplasty in a case of corneal keloid. Cornea. 1972;10:183–184. doi: 10.1097/00003226-199103000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Bourcier T., Baudrimont M., Boutboul S., Thomas F., Borderie V., Laroche L. Corneal keloid: clinical, ultrasonographic, and ultrastructural characteristics. J Cataract Refract Surg. 2004;30:921–924. doi: 10.1016/j.jcrs.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 15.Al-Katan HM, Al-Arfaj KM. Corneal keloid: case report and literature review. Saudi J Ophthalmol 2007; 21(2):130–136.
- 16.Risco J.M., Huaman A., Antonios S.R. A case of corneal keloid: clinical, surgical, pathological, and ultrastructural characteristics. Br J Ophthalmol. 1994;78:568–571. doi: 10.1136/bjo.78.7.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaviria J.G., Johnson D.A., Scribbick F., III Corneal keloid mimicking a recurrent limbal dermoid. J Pediatr Ophthalmol Strabismus. 2005;42:189–190. doi: 10.3928/01913913-20050501-10. [DOI] [PubMed] [Google Scholar]
- 18.McElvanney A.M., Adhikary H.P. Corneal keloid: aetiology and management in Lowe’s syndrome. Eye (Lond) 1995;9(Pt 3):375–376. doi: 10.1038/eye.1995.75. [DOI] [PubMed] [Google Scholar]


