Abstract
The emergence and re-emergence of bacterial strains that are resistant to current antibiotics reveals the clinical need for new agents that possess broad-spectrum antibacterial activity. Furthermore, bacteriophobic coatings that repel bacteria are important for medical devices, as the lifetime, reliability, and performance of implant devices are hindered by bacterial adhesion and infection. Dendrimers, a specific class of monodisperse macromolecules, have recently shown potential to function as both antibacterial agents as well as antimicrobial surface coatings. This review discusses the limitations with currently used antibacterial agents and describes how various classes of dendrimers, including glycodendrimers, cationic dendrimers, anionic dendrimers, and peptide dendrimers, have the potential to improve upon or replace certain antibiotics. Furthermore, the unexplored areas in this field of research will be mentioned to present opportunities for additional studies regarding the use of dendrimers as antimicrobial agents.
Keywords: Dendrimer, dendritic polymers, bacteria, antimicrobial, infection, antibacterial agents, coatings, carriers, drug delivery
Introduction
A disturbing trend in recent years is the growth of resistant strains of bacteria with the simultaneous dearth of new antimicrobial agents.1, 2 Few new antimicrobial agents have been developed in the recent past, and those that have been approved, including Cubicin® (injectable daptomycin), Zyvox® (linezolid) and Dificid™ (fidaxomicin), specifically target Gram-positive bacteria. Therefore, there is a need for both new classes of antibiotics that are effective against current resistant strains and new classes that possess broad-spectrum antibacterial activity. Furthermore, bacteriophobic coatings that repel bacteria are highly desirable for medical devices because the lifetime, reliability, and performance of many medical implants are hindered by bacterial adhesion and infection.3- 6 This review will discuss how dendrimers, a specific class of macromolecules, have recently shown potential to function as both antibacterial agents as well as antimicrobial surface coatings.
Dendrimers are well-defined, single molecular weight, globular structures 3 to 7 nanometers in diameter containing a central core, branching layers, and numerous end groups.7, 8 Generally either divergent9-12 or convergent13, 14 routes are used to synthesize dendrimer structures, but recent improvements to synthetic methodology have made large-scale production of many of these structures more viable.15 Since the branching structure of dendrimers was first conceptualized in the early 1970s16 and synthesized in the mid-1980s,9, 11 dendrimers, especially poly(amido amine) (PAMAM),11 polypropylenimine (PPI),16, 17 and dendritic polylysine structures,18 have been actively investigated for a wide range of industrial19-22 and biomedical applications.23-29 The potential for using dendrimers as antimicrobial agents, both as a drug and as a surface coating, has been recognized over the last decade. In this review, the major concerns with current antimicrobial agents will be discussed, and the various classes of dendrimers that have shown potential to overcome emerging and/or re-emerging infectious diseases will be summarized. While a similar review describing several dendrimer skeletons used as antimicrobial agents has recently been published,30 this review will instead focus on how dendrimer surface groups affect antimicrobial activity and how these structures can inhibit biofilm formation.
Limitations of current antimicrobial agents
Prior to the development of antibiotics, infection was the leading cause of hospitalization and mortality in the US. However from 1937 to 1953, the rate of infectious diseaserelated mortality decreased, a trend accredited to the first clinical uses of antibiotics, including sulfonamides (1935), β-lactams (1941), and aminoglycosides (1943).31 Alarmingly, however, this trend was reversed beginning in the 1980s, and for the next 15 years the number of deaths due to infectious diseases increased. While much of this increase was related to acquired immunodeficiency syndrome (AIDS) mortality, the incidence of re-emerging infectious diseases, particularly tuberculosis, increased by more than 20% as well.32 Moreover, infectious disease is responsible for the greatest number of deaths in developing nations. This spike in emerging and re-emerging bacteria-related deaths emphasizes the continuous evolution of infectious diseases and stresses the limitations of current antimicrobial agents.
Nearly all currently used antibacterial agents can be classified into one of the following categories: (1) agents that target bacterial cell wall biosynthesis, which include β-lactams and vancomycin; (2) agents that target bacterial protein synthesis, which include erythromycin, tetracyclines, aminoglycosides, and oxazolidinones; and (3) agents that target bacterial DNA replication and repair, which include fluoroquinolones.33 Despite the wide variety of bacterial drug targets, clinically significant resistance is usually observed within months to several years of introduction into the clinic.34 Furthermore, the ease with which bacteria can collect and exchange antibiotic-resistant genes between other cells and even other species makes the emergence of drug resistance even more troubling.35 The resistance strategies that bacteria have developed include: (1) effluxing the antibiotic from the bacterial cell; (2) acquiring enzymes capable of deactivating the antibiotic; and (3) altering the target structure of the antibiotic on the bacteria cell surface.33 Finally, the traditional developers of antimicrobial agents, large pharmaceutical companies, have largely left this space. Consequently, the development of new agents that can target bacteria through alternate strategies is vital. Fortunately, various classes of dendrimers that can target or inhibit virulence factors critical for establishment of microbial pathogens in the host have been designed. Thus, dendrimers have the ability to prevent infection. Furthermore, dendrimers have been described which can act either as antimicrobial agents, drug-delivery devices, or bacteriophobic coatings, thus these compounds have the potential to serve multiple, distinct roles in the development of novel antimicrobial strategies.
Types of antimicrobial dendrimers
Glycodendrimers—Interfering with adhesion to eukaryotic cells
The earliest events in host-pathogen interactions involve the microbe securing itself to the host. Bacteria can attach to eukaryotic cells via specific carbohydrate-protein interactions that primarily involve noncovalent binding between bacterial cell surface proteins and eukaryotic glycoproteins or glycolipids.36 Furthermore, certain bacteria produce secreted virulence factors known as “AB5 toxins”. The A component of the toxin is the catalytic domain, while the B component presents carbohydrate recognition sites to interact with sugars on the surface of eukaryotic cells and facilitate toxin transport into the host cell.37 By blocking this interaction, bacterial cellular uptake into eukaryotic cells is inhibited (Figure 1). However, because carbohydrate-protein interactions are weak, multivalency is necessary to achieve efficient recognition and binding. By appending carbohydrates on the surface of dendrimers to afford glycodendrimer structures,38 such multivalency can be achieved to improve antimicrobial activity.39, 40
Figure 1.
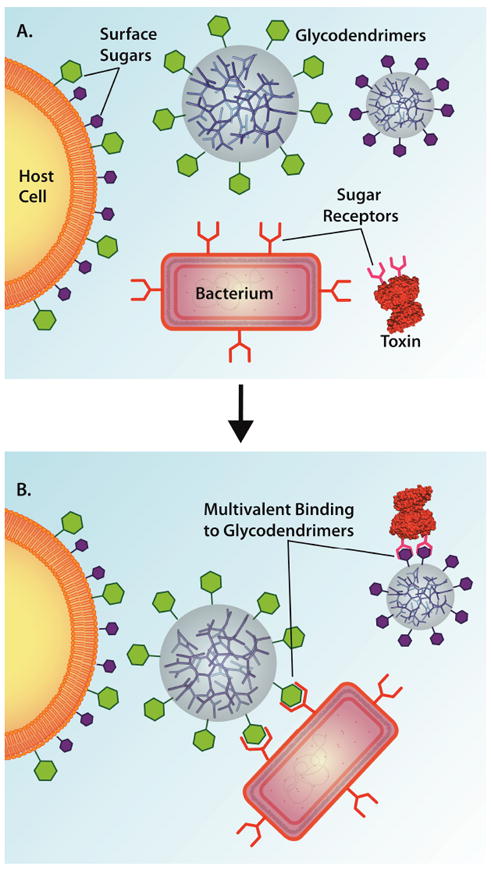
Multivalent binding of a bacterium or a bacterial toxin to a glycodendrimer. (A) Depiction of a host eukaryotic cell with surface sugar groups, two glycodendrimers with surface sugars groups, a bacterial toxin with receptors for the purple sugar groups, and a generic bacterium with receptors for the green sugar groups. (B) Binding of the bacterium and the toxin to the glycodendrimer through multivalent interactions to prevent infection of the host cell. Structures not draw to scale.
Thompson and Schengrund designed three glycodendrimers based on first- and second-generation polypropylenimine (PPI) and first-generation polyamidoamine (PAMAM) dendrimers to inhibit the activity of cholera toxin and the heat-labile enterotoxin of E. coli, both members of the AB5 toxin family described above (Figure 2).41 Cholera toxin is a protein secreted by Vibrio cholerae that, upon entry into epithelial cells lining the intestine, causes diarrhea, massive dehydration, and possible death of the affected individual if left untreated.42 The heat-labile enterotoxin of E. coli is a structurally similar protein to cholera toxin that, upon exposure to cells in the intestine, causes traveler’s diarrhea.43 The binding site for both toxins is GM1, a ganglioside present on the surface of eukaryotic cells.
Figure 2.
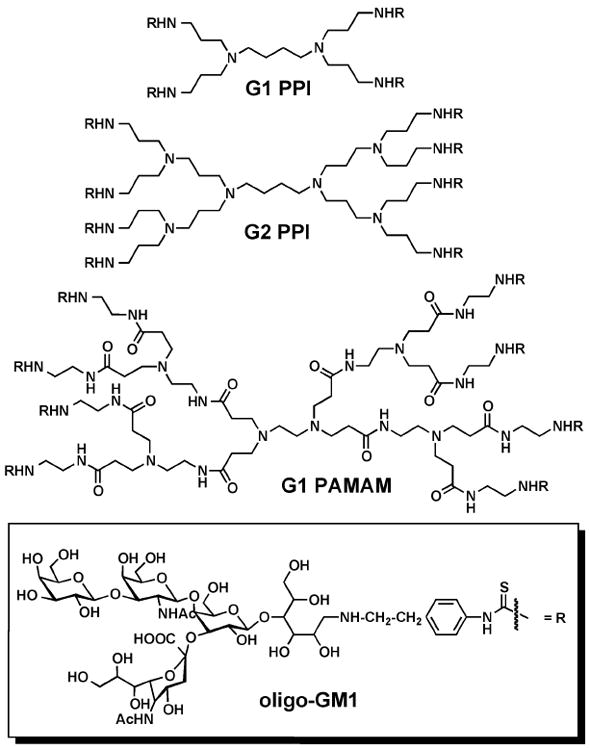
Structures of oligosaccharide dendrimers based on PAMAM and PPI cores with oligo-GM1 sugar appended to the surface.
Three different dendrimers were conjugated with phenylisothiocyanate derivatized (PITC) galβ1-3galNAcβ1-4[sialic acid α2-3]-galβ1-4glc (oligo-GM1) carbohydrate moieties (Figure 2). The ability of these glycodendrimers to inhibit the binding of both 125I-labeled cholera toxin B subunit and 125I-labeled heat-labile enterotoxin to GM1-coated wells was measured. The results showed that the glycodendrimers were able to inhibit the binding of both proteins to the GM1-coated wells at concentrations 5- to 15-fold lower than was achieved with pre-incubation of the toxins with native GM1. Furthermore, the glycodendrimers inhibited binding at concentrations over 1000-fold lower than was achieved with free oligo-GM1, stressing the importance of dendrimer multivalency.41
The following year, Thompson and Schengrund evaluated the activity of the second-generation, oligo-GM1-linked PPI dendrimer to inhibit the binding of cholera toxin and heat-labile enterotoxin to a GM1-treated murine fibroblast cell line (NCTC-2071).44 The results showed a significant reduction in the adherence of 125I-labeled cholera toxin and 125I-labeled heat-labile enterotoxin to the GM1-treated cells, regardless of whether the toxins were exposed to the cells after the addition of the glycodendrimers or were pre-incubated with glycodendrimer prior to addition to cells. Furthermore, the oligo-GM1-linked dendrimer had no effect on cell viability, suggesting that these glycodendrimer structures can function as effective ligands to inhibit the binding of bacterial toxins.
More recently, Pieters, et al. have investigated the affinity of carbohydrateconjugated dendrimers based on the 3,5-di-(2-aminoethoxy)benzoic acid repeat unit for the cholera toxin B subunit. Initially the group conjugated first-, second-, and third-generation dendrimers with 2, 4, or 8 lactose groups, respectively, using thiourea linkages (Figure 3).45 Using a direct fluorescence binding assay, the group observed apparent dissociation constants (Kd values) of 235, 99, and 33 μM for the divalent, tetravalent, and octavalent glycodendrimers, respectively. The modest multivalency effect in this study was related to the better ability of the higher generation glycodendrimers to bridge the binding sites between toxin molecules compared to the first-generation analogue.
Figure 3.
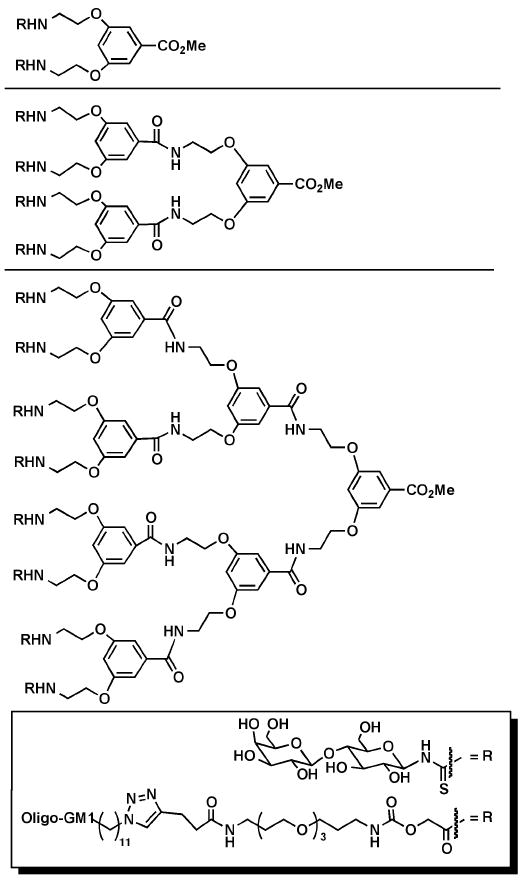
Dendrimers based on the 3,5-di(2-aminoethoxy)benzoic acid repeat units with 2, 4, or 8 oligo-GM1 or lactose groups appended to the surface.
To further improve the inhibitory activity of their glycodendrimer against cholera toxin, Visser, Zuilhof, Pieters, et al. synthesized similar structural analogues optimized by two changes: (1) lactose was replaced with the authentic GM1 oligosaccharide ligand, which was linked to the dendrimer using “click” chemistry; and (2) elongated spacer arms with optimal lengths were used to append the carbohydrate moieties to the dendrimers (Figure 3).46 The ability of these oligo-GM1-linked dendrimers to inhibit the binding of a horseradish peroxidase (HRP)-conjugated cholera toxin B subunit to GM1 ganglioside coated on a 96-well plate was measured using an ELISA assay. The oligo-GM1-linked dendrimers showed unprecedented binding that was up to 380,000-fold stronger than what was observed using the monovalent analogue. Recently, this same trend was reported by Zuilhof and Turnbull, et al., who used the same oligo-GM1-linked dendrimers to inhibit the binding of the B-pentamer of E. coli heat-labile enterotoxin.47 The IC50 values for the heat-labile enterotoxin were higher than those measured for cholera toxin since the values were detected using a less-sensitive indirect ELISA assay. However, the relative inhibitory activities of the glycodendrimers were similar against both toxins.
Despite the high activity of the oligo-GM1-linked dendrimer structures, Pieters et al. pointed out that the ligand is complex and difficult to synthesize on a large scale. Consequently, the group investigated whether binding inhibition of cholera toxin could be achieved using dendrimers linked to simple galactose residues modified with poly(ethylene glycol), thus mimicking the additional sugars of the GM1 oligosaccharide structure, and a lipophilic chain to more closely resemble the structure of the oligo-GM1-linked dendrimers.48 Inhibitory potencies against cholera toxin of 130, 25, and 12 μM were measured using the divalent, tetravalent, and octavalent galactose-conjugated dendrimers. While these IC50 values are only as high as the monovalent oligo-GM1 analogue, the results stress the possibility of designing lower cost ligands (using simple galactose rather than more complicated oligo-GM1) for cholera toxin that still maintain high inhibitory activity.
In addition to using glycodendrimers to target and block the binding of bacterial toxins to eukaryotic cells, groups have also investigated the use of glycodendrimers to inhibit the binding of bacterial cells to human erythrocytes (Figure 1). Pieters, et al. synthesized mono-, di-, and tetravalent galabiose (Galα1-4Gal) glycodendrimers based on 3,5-di-(2-aminoethoxy)benzoic acid, as well as an octavalent galabiose glycodendrimer based on G1 PAMAM.49 The ability of these compounds to inhibit the binding of Streptococcus suis, one of the causative agent of meningitis in humans,50 to human erythrocytes was evaluated in a hemagglutination assay. Results showed that the minimum inhibitory concentration (MIC) for binding of the monovalent glycodendrimer was 900 and 1600 nM against S. suis 628 and S. suis 836, respectively. By increasing valency to di-, tetra-, and octavalent analogues, the MIC was reduced to 6, 2, and 0.3 nM, respectively. Changes in length, rigidity, or orientation of the dendritic arms did not significantly impact the inhibitory efficacy of the glycodendrimers. These same glycodendrimers, as well as an octavalent 3,5-di-(2-aminoethoxy)benzoic acid dendritic analogue, were also evaluated as agents for blocking the binding of E. coli expressing PapGJ96 to human erythrocytes.51 PapG adhesin is involved in the establishment of E. coli urinary tract infections. The relative potency per carbohydrate residue increased slightly as valency of the glycodendrimer was increased. Conversely, when the carbohydrates on these glycodendrimers, as well as a G2 PAMAM analogue, were replaced with mannose and evaluated for their ability to inhibit the binding of recombinant type I (mannose-specific) fimbriated E. coli to a human urinary bladder epithelim (T24) cell line, the multivalency effect was not always observed.52 While the mono-, di-, and tetravalent glycodendrimers showed reduced IC50 values with increased multivalency (IC50 = 337, 204, and 51 μM, respectively), the octavalent analogue showed decreased activity (IC50 = 72 μM). A similar trend was previously observed for thiourea-linked mannose glycodendrimers based on PAMAM. In an earlier study conducted by Lindhorst et al., no improvements in binding affinity for type I fimbriated E. coli was observed for valencies higher than three, possibly indicating that for this particular binding domain, larger glycoclusters are not accommodated by the binding site.53
In addition to using glycodendrimers to target and block the binding of bacteria to eukaryotic cells, groups have also investigated the use of glycodendrimers to inhibit the formation of biofilms. Reymond, et al. synthesized a library of 15,625 glycopeptides dendrimers conjugated at the surface with α-C-fucosyl residues.54 Fucose groups were chosen as ligands because they were previously identified as the target carbohydrate moieties for the lectin LecB, a protein on the surface of Pseudomonas aeruginosa that plays a role in bacterial recognition and attachment to eukaryotic cells.55 P. aeruginosa can cause mortality-related infections, particularly in immunocompromised and cystic fibrosis patients, often exhibiting antibiotic resistance that is partially due to biofilm formation.56 Because P. aeruginosa mutants that lack LecB show impaired biofilm formation,57 blocking this protein with fucose residues should reduce biofilm-associated antibiotic resistance.
Based on binding studies using the initial combinatorial library and LecB, Reymond et al. identified a tetravalent glycopeptide dendrimer FD2 (fuc-α-CH2CO-Lys-Pro-Leu)4(Lys-Phe-Lys-Ile)2Lys-His-Ile-NH2 that showed high potency (IC50 = 0.14 μM) (Figure 4).54 The group later evaluated the ability of FD2 to inhibit or disperse P. aeruginosa biofilms using a steel coupon assay.58 FD2 showed complete inhibition of biofilm formation at a concentration of 50 μM (IC50 = 10 μM) and caused complete dispersion of established biofilms for both the wild-type strain and clinical isolates. Furthermore, the glycopeptides dendrimers showed no cytotoxicity against human kidney-embryonic cells (293T), suggesting that these structures may be relevant in the treatment of P. aeruginosa biofilm-based infections in humans. More recently, Reymond et al. altered the structure of FD2 to contain D- instead of L-amino acids; while the binding of this compound (D-FD2) to LecB was lower than that observed with the original glycopeptides dendrimer, its ability to inhibit P. aeruginosa biofilm formation was retained, and the new analogue showed complete resistance to proteolysis, indicating higher stability.59
Figure 4.
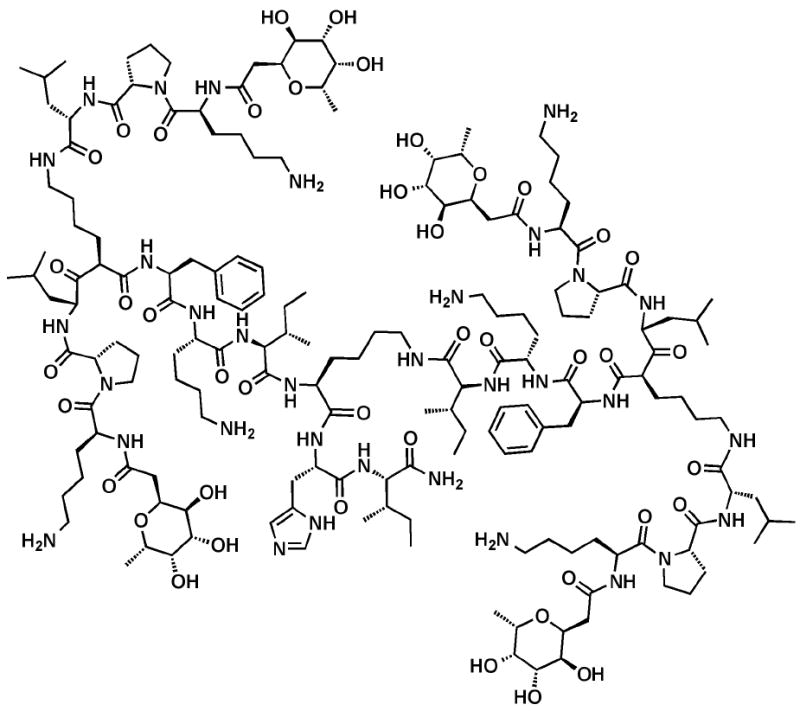
Structure of glycopeptide dendrimer FD2.
Cationic dendrimers: Targeting bacterial membranes
In general, the antimicrobial action of many agents can be attributed to cell membrane disruption and permeation; polycationic compounds, and more specifically dendrimers, are particularly adept at promoting such cell permeation (Figure 5).60, 61 In 1999, Cooper et al. quaternized the surface amines of polypropylenimine (PPI) dendrimers by reacting the amines with 2-chloroethyl isocyanate and then treating the intermediate with dimethyldodecylamine to yield the quaternized analogue.62 The antimicrobial activity of the amphiphilic quaternized dendrimer against the recombinant E. coli strain TV 1048 was measured using a bioluminescence assay and compared to the activity of n-dodecyltrimethylammonium chloride (DTAC), the small molecule analogue. The concentration of the compound that causes 50% reduction in bioluminescence (EC50 value) of the dendrimer was 12 μg/mL while that of the small molecule counterpart was 2000 μg/mL, making the dendrimer over 100-fold more active against E. coli, stressing the influence of multivalency.
Figure 5.
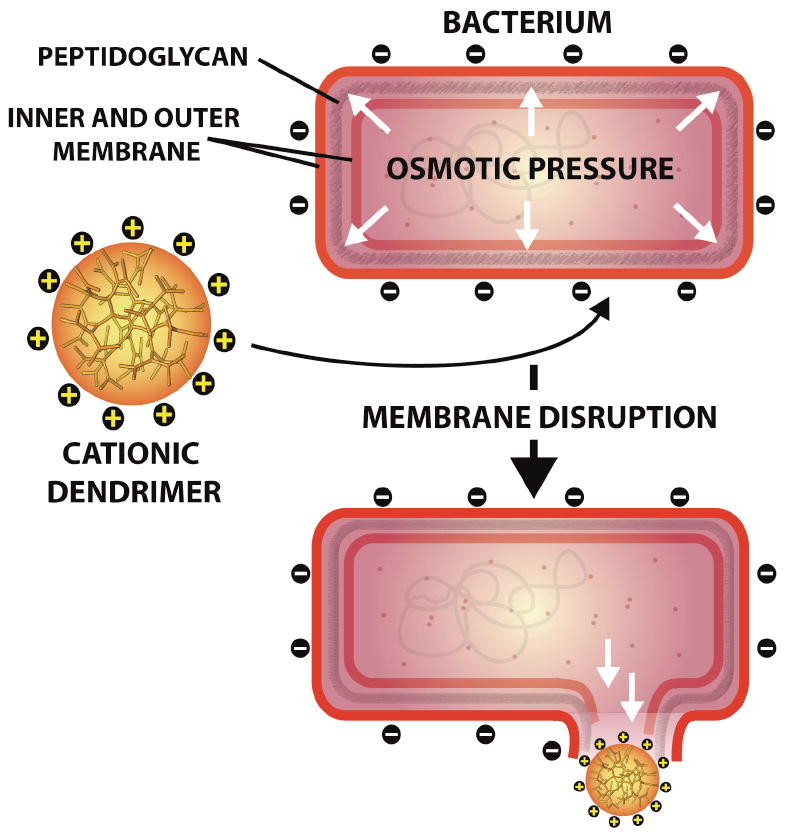
Proposed mechanism of action of cationic dendrimers via initial electrostatic attraction to the negatively charged bacterium followed by membrane and peptidoglycan disruption. Structures not drawn to scale.
To further probe the antimicrobial activity of PPI dendrimers, Cooper et al. modified various aspects of the dendrimer, including the generation number and the hydrophobic chain length, and evaluated the effect of these changes on the antimicrobial activity against E. coli.63 The effect of generation number on antimicrobial activity showed that G5 > G4 > G1 > G2 > G3. This effect was attributed to the balance between the higher potency achieved with a greater number of quaternary amines and the decreased permeability of the larger dendrimer analogues. The effect of the hydrophobic chain length on antimicrobial activity showed that C10 > C8 > C12 > C14 ≈ C16. This trend was attributed to the dual binding site theory, which suggests that on the bacterial cell surface, dual binding sites exist for which the relative binding activities of long and short hydrophobic ligands differ. When the antimicrobial activity of one of the dendrimers was compared to that of a hyperbranched polymer with the same number of functional groups, the dendrimer was significantly more potent.
However, it should be noted that the higher activity of dendrimers as compared to hyperbranched analogues is not always observed. For instance, Gomez and de la Mata et al. have synthesized first- and second-generation ammonium-functionalized carbosilane dendrimers by reacting chloromethylene-terminated dendrimers with 3-dimethylaminophenol followed by treatment with methyl iodide (Figure 6A); the resulting ammonium-functionalized dendrimers were evaluated against S. aureus and E. coli.64 Both dendrimers had lower MIC values than the monofunctional counterpart, although the first-generation carbosilane dendrimer was more active than the second-generation structure, particularly against E. coli (MIC = 64 mg/L and 4 mg/mL for G2 and G1 dendrimers, respectively). These dendrimers necessitated the use of DMSO as a solubilizing agent, thus the groups later modified the synthesis to involve hydrosilylation of N,N-dimethyl-N’-allyl-N’-ethyl-ethylenediamine with hydride-terminated carbosilane dendrimers (G1-G3) followed by quaternization with HCl or MeI (Figure 6B).65 Based on measurements of minimum bactericidal concentration (MBC), these water-soluble dendrimers showed increasing activity with higher generation number against both E. coli (MBC = 1.65, 1.70, and 3.65 for G3, G2, and G1, respectively) and S. aureus (MBC = 0.82, 0.85, and 1.82 for G3, G2, and G1, respectively) strains.
Figure 6.
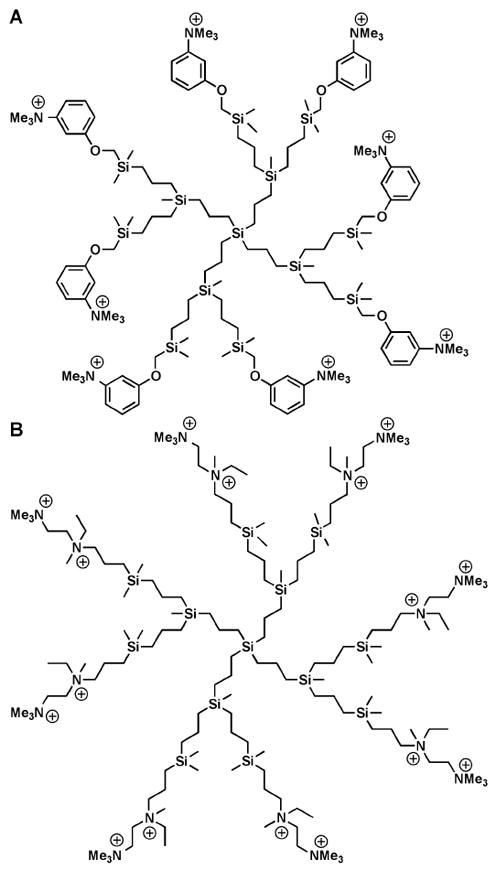
(A) Structure of initial carbosilane used for antimicrobial studies. (B) Structure of modified carbosilane dendrimer with improved solubility in aqueous conditions.
Recently, however, the groups compared the antimicrobial activity of cationic hyperbranched polycarbosilane (PCS 6) with the antimicrobial activity of ammonium-functionalized second- and third-generation carbosilane dendrimers containing quaternized 3-dimethylaminophenol at the periphery.66 PCS 6 showed higher activity than the third-generation carbosilane dendrimer, which had similar molecular weight and ζ potential, and similar activity to the second-generation structure. Consequently, the hyperbranched structures, which were synthesized in less time and at lower cost than the dendrimer analogues, may offer an appealing alternative that can still achieve suitable antimicrobial activity.
Surprisingly, despite the fact that PAMAM dendrimers were one of the earliest of this class of structures to be synthesized and extensively studied for various biomedical applications, their activity as a cationic antimicrobial agent has only recently been evaluated. In some of the earliest studies, PAMAM was not evaluated as the actual antimicrobial agent but rather as an antimicrobial carrier. Yang and Lopina described the covalent surface conjugation of Penicillin V to G2.5- and G3-PEG-PAMAM star polymers using amide and ester linkages.67 While the ester-linked conjugate had activity against S. aureas, confirming that the drug was bioavailable through ester hydrolysis, the dendrimer-drug conjugate did not show higher activity than the drug alone, indicating no synergistic effect. The delivery of antibiotics through noncovalent encapsulation in dendrimers has also been described. Cheng et al. reported the use of G4-PAMAM dendrimers to solublize the quinolone antimicrobials nadifloxacin and prulifloxacin.68 The poor solubility of these quinolones prevents their formulation in liquid and topical dosage forms. The authors observed similar or increased potency against E. coli for both quinolone-encapsulating dendrimers.
In addition to functioning as a drug carrier, several studies have evaluated PAMAM dendrimers as the actual antimicrobial agent. Cai et al. investigated the antimicrobial activity of G5 PAMAM dendrimers with or without partial poly(ethylene glycol) (PEG) coatings against standard laboratory strains of P. aeruginosa and S. aureus, as well as a clinical strain of P. aeruginosa obtained from a patient with keratitis.69 Results showed that PAMAM and PEG-PAMAM were inactive against S. aureus, but these structures afforded EC50 values of 1.5 and 0.9 μg/mL, respectively, against P. aeruginosa. While the clinical strain PA2219 showed increased resistance against PEG-PAMAM (EC50 = 1.4 μg/mL), the activity of the dendrimer was equally as effective as the antimicrobial peptide cathelicidin (LL-37; EC50 = 1.9 μg/mL). Furthermore, both PAMAM and PEG-PAMAM showed lower cytotoxicity against human corneal epithelial cells (HCEC) compared to P. aeruginosa.
Cai et al. later discovered that by using a G3 PAMAM dendrimer (rather than G5) in which 6% of the surface amines were coated with PEG, the activity against P. aeruginosa could be maintained (MIC = 25 μg/mL) while the cytotoxic effects to HCEC could be significantly reduced.70 To address the problem of implant-associated infection, the authors explored the use of the G5 analog. The G5 PEG-PAMAM structures prevented the colonization of P. aeruginosa and S. aureus on titanium-based substrates, even after storage of the dendrimer-titanium samples in PBS for 30 days. Furthermore, these G5 PEG-PAMAM-coated titanium substrates did not inhibit cell adhesion or cause cytotoxicity to human bone mesenchymal stem cells (hMSCs), indicating the potential importance of these compounds in preventing implant-associated infections.71
Recently, Kannan and Kannan, et al. have investigated the inhibitory activity of PAMAM dendrimers in a guinea pig model of chorioamnionitis, an inflammatory response to intrauterine infection that can cause fetal brain injury.72 For the in vivo portion of this study, hydroxyl-terminated G4 PAMAM dendrimers were injected into the cervix of pregnant guinea pigs after E. coli inoculation; forty-eight hours later the animals were euthanized, and the amniotic fluid was evaluated for the presence of bacteria.73 Of the guinea pigs that were inoculated with E. coli and that received no treatment, bacterial growth was observed in 57% of the amniotic fluid samples. However, of the animals that were treated with G4 PAMAM dendrimer, no bacterial growth was observed in any of the amniotic samples, indicating that the hydroxyl-terminated G4 PAMAM dendrimer prevented E. coli from ascending into the uterus. Furthermore, cytokine levels (tumor necrosis factor [TNFα], IL-6 and IL-1β) in the treated animals were similar to animals with no infection, indicating minimal immune response to treatment, while the animals that were infected and left untreated showed significantly higher levels of both groups of cytokines.
In a similar study, Kannan et al. also demonstrated that G4-PAMAM dendrimers encaspsulating amoxicillin with peripheral thiopyridyl groups could be crosslinked via disulfide bonds with thiol-terminated 8-arm PEG polymers to form injectable hydrogels.74 These gels have the potential to afford antimicrobial activity resulting from the sustained release of amoxicillin from the hydrogel. In vivo studies carried out in a pregnant guinea pig model confirmed that hydrogels applied to the cervicovaginal region had a residence time greater than 72 h and did not show toxicity to either the guinea pigs or the fetus (Figure 7). While the in vitro studies showed sustained drug release for up to 240 h, further evaluation of both the antimicrobial activity as well as the in vivo drug release are underway.
Figure 7.
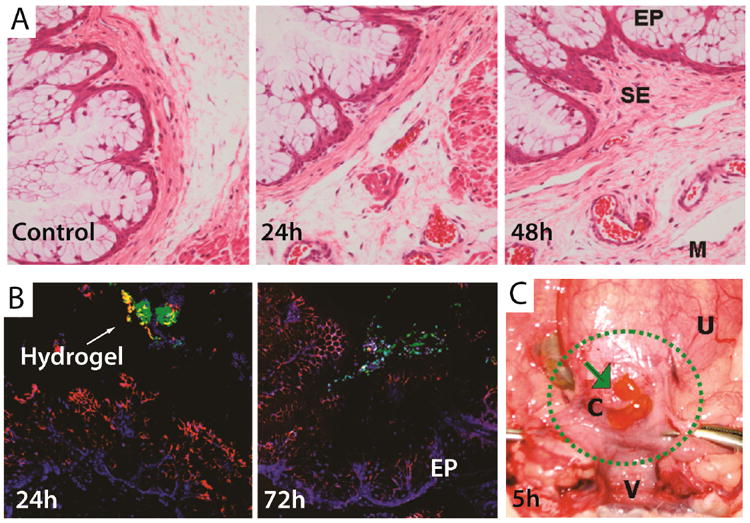
(A) Histological sections of the cervix of pregnant guinea pigs not treated (control) and treated with hydrogel after 24 and 72 hours. The epithelial cells do not show signs of damage or inflammation and are comparable to the control. (B) Confocal image of the guinea pig cervix treated with the hydrogel (green) after 24 and 72 hours confirming that the gel remains on the mucosal layer (red) after 3 days. (C) Hydrogel (green arrow) 5 hours after being placed on the guinea pig cervix. EP = epithelial cells, SE = subepithelium, M = muscular layer, C = cervix, V = vaginal cavity, U = uterus with pups. Adapted from Ref. 73.
Similar to the encapsulation study conducted by Kannan et al., Hammond and coworkers have used a G4-PPO-PAMAM dendrimer as the cationic component, along with negatively charged poly(acrylic acid), of a layer-by-layer device for the slow release of triclosan.75 The group found that the dendrimer was more effective at encapsulating triclosan as compared to a nondendritic surfactant with similar properties. The release of triclosan could be extended over 20 days, and a zone of inhibition assay showed killing of S. aureus. Consequently, the use of dendrimers as antimicrobial drug carrying devices warrants additional research.
Anionic dendrimers: Imitating detergent activity
Grinstaff and group reported that dendritic amphiphiles synthesized from succinic acid, glycerol, myristal alcohol, and myristic acid inhibited the growth of B. subtilis in culture at mM concentrations.76 An amphiphile with a single hydrophobic strand showed toxicity towards cultured HUVEC cells, but an amphiphile with two hydrophobic chains was relatively non-cytotoxic. Recently variations have been made to the length of the hydrophobic chains to see if improvements to the antimicrobial activity of these dendritic amphiphiles could be realized. Unfortunately, increasing the length of the hydrophobic chains did not improve the dendrimers’ antimicrobial activities against clinically relevant bacterial strains.77 In a similar study starting from a poly(oxypropylene) triamine (Jeffamine®) core, Tulu et al. synthesized anionic and cationic PAMAM dendrimers that displayed antimicrobial activity against both Gram-positive and Gram-negative bacteria and a selection of fungi.78 The anionic dendrimers showed comparable activity to gentamycin (antibiotic) and nystatin (antifungal), but in general the cationic dendrimers were more effective against both bacteria and fungi (Table 1).
Table 1.
In vitro minimum inhibitory concentration (MIC, μg mL-1) of dendrimers78
| Microbe | Dendrimer Surface Groups
|
Gentamycin | |||||
|---|---|---|---|---|---|---|---|
| (NH2)3 | (CO2H)6 | (NH2)6 | (CO2H)12 | (NH2)12 | (CO2H)24 | ||
| E. coli | 3.1 | 25.0 | 6.3 | 12.5 | 12.5 | 12.5 | 6.3 |
| S. aureus | 1.6 | 12.5 | 6.3 | 12.5 | 6.3 | 12.5 | 25.0 |
| K. pneumoniae | 6.1 | 12.5 | 12.5 | 12.5 | 12.5 | 25.0 | 6.3 |
| B. cereus | 6.3 | 12.5 | 12.5 | 25.0 | 25.0 | 12.5 | 6.3 |
| M. luteus | 1.6 | 6.3 | 6.3 | 6.3 | 12.5 | 12.5 | 25.0 |
| P. vulgaris | 1.6 | 12.5 | 3.1 | 6.3 | 6.3 | 12.5 | 6.3 |
| M. smegmatis | 12.5 | 12.5 | 12.5 | 25.0 | 12.5 | 12.5 | 12.5 |
| L. monocytogenes | 6.3 | 6.3 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 |
| P. aeruginosa | 6.3 | 12.5 | 6.3 | 25.0 | 25.0 | 12.5 | 6.3 |
Peptide-based dendrimers: Mimicking antimicrobial peptides
Antimicrobial peptides, generally composed of short sequences of cationic and hydrophobic amino acids, participate in innate immune response by killing bacteria through binding to and permeabilizing the bacteria cell membrane.79 As a specific class of dendrimers first synthesized in the 1980s,80 dendrimeric peptides offer a potential structural scaffold to mimic the activity of antimicrobial peptides. Tam et al. synthesized tetravalent (D4) and octavalent (D8) polylysine dendrimer cores and decorated the surface of the cores with either R4 tetrapeptides (Arg-Leu-Tyr-Arg) or R8 octapeptides (Arg-Leu-Tyr-Arg-Lys- Val-Tyr-Gly) (Figure 8).81 These structures were evaluated for antimicrobial activity against several Gram-negative and Gram-positive bacteria strains. Regardless of the peptide sequence length (R4 versus R8), the tetravalent dendrimers showed improved broad-spectrum antimicrobial activity as compared to the divalent analogues. While increasing the dendrimer branching to the octavalent analogue only improved activity slightly compared to the tetravalent structures, the R8 octavalent dendrimer showed significantly better activity under high-salt conditions (i.e. 100 mM NaCl). Furthermore, peptide dendrimers displaying either the R4 or R8 peptides showed low hemolytic activity against human erythrocytes, indicating low cytotoxicity.
Figure 8.
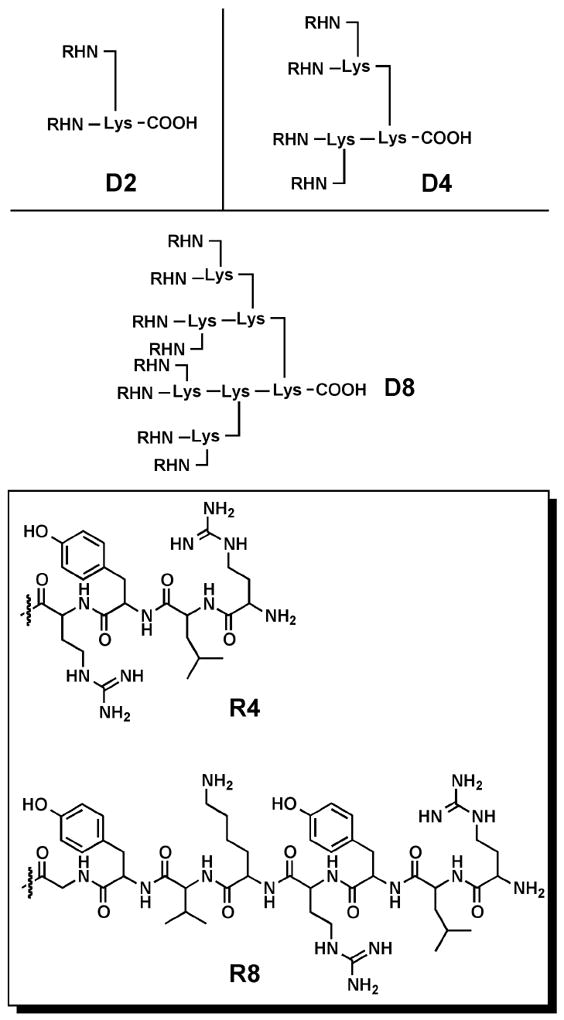
Schematic representation of three types of peptide dendrimer cores (D2, D4, and D8) and the structures of two short peptide sequences (R4 and R8) appended to the surface of the cores.
More recently, Pini et al. used phage display library selection to identify a peptide sequence (Gln-Lys-Lys-Ile-Arg-Val-Arg-Leu-Ser-Ala) that was subsequently appended to a tetravalent polylysine dendrimer core.82 The peptide dendrimer structure showed high activity against a range of bacterial strains (particularly Gram-negative), low cytotoxicity towards human keratinocyte (HaCaT) cells, low hemolytic activity towards human erythrocytes, and good stability in human plasma and serum (Table 2). More recently, the structure of the peptide dendrimer was modified to include a lipophilic amino valeric acid chain to enhance membrane affinity and pyroglutamic acid (rather than glutamine) at the N-terminus to avoid cyclization.83 The new peptide dendrimer showed higher antimicrobial activity against Gram-negative strains and better stability in solution when compared to the original peptide dendrimer.
Table 2.
In vitro minimum inhibitory concentration of antimicrobial peptide dendrimer against gram-positive and gram-negative bacteria82
| Microbe | Relevant features | MIC (μg mL-1) |
|---|---|---|
| Escherichia coli ATTCC 25922 | Reference strain | 8 |
| Escherichia coli W99FI0077 | FQ, ESC (ESBL/SHV type) | 8 |
| Escherichia coli W03BG0025 | FQ, AG, ESC (ESBL/CTX-M-15) | 8 |
| Escherichia coli W03NO0013 | FQ, ESC (ESBL/CTX-M-1) | 8 |
| Pseudomonas aeruginosa ATCC 27853 | Reference strain | 4 |
| Pseudomonas aeruginosa 885149 | FQ, AG, ESC, CP (MBL/IMP-13) | 8 |
| Pseudomonas aeruginosa 891 | FQ, AG, ESC, CP (MBL/VIM-2) | 8 |
| Pseudomonas aeruginosa VA463/98 | FQ, AG, ESC (ESBL/PER-1) | 4 |
| Klebsiella pneumoniae W99FI0057 | ESC (ESBL/SHV type) | 4 |
| Klebsiella pneumoniae W03NO0078 | ESC (ESBL/CTX-M-1) | 16 |
| Klebsiella pneumoniae W03BG0019 | AG, ESC (ESBL/CTX-M-15) | 8 |
| Klebsiella oxytoca W99FI00049 | ESC (ESBL/SHV-12) | 64 |
| Proteus mirabilis W99FI0089 | FQ | >128 |
| Proteus mirabilis W03VA1144 | FQ, AG, ESC (ESBL/PER-1) | 64 |
| Enterobacter aerogenes W03BG0067 | AG, ESC (ESBL/SHV-5) | 8 |
| Enterobacter cloacae W03AN0041 | ESC (ESBL/SHV-12) | 4 |
| Morganella morganii W03VA1342 | FQ, ESC (ESBL/CTX-M-1) | >128 |
| Acinetobacter baumannii AB1MG | FQ, AG, ESC (ESBL/TEM-92) | 16 |
| Acinetobacter baumannii AB7MG | FQ, AG, ESC | 32 |
| Citrobacter freundii W99FI00007 | ESC (ESBL/SHV-12) | 16 |
| Chryseobacterium meningosepticum CCUG4310 | Reference strain | >128 |
| Burkholderia cepacia SMC71 | FQ, AG, ESC | 64 |
| Serratia marcescens W99FI0111 | FQ, AG, ESC (ESBL/SHV-5) | >128 |
| Stenotrophomonas maltophilia PT4/99 | Wild-type profile | >128 |
| Providencia stuartii W03FI0001 | AG, ESC (ESBL/PER-1) | >128 |
| Staphylococcus aureus ATCC 25923 | Reference strain | >128 |
| Staphylococcus aureus MIU-68A | MS | 128 |
Relevant resistance phenotypes and resistance mechanisms are indicated by FQ (resistance to fluoroquinolines), AG (resistance to aminoglycosides), ESC (resistance to extended-spectrum cephalosporins), CP (resistance to carbapenems), ESBL (extended-spectrum β-lactamase), MBL (metallo-β-lactamase), and MS (methicillin susceptible).
With high activity shown for a number of different peptide dendrimers, it is reasonable to postulate if structures with shorter peptide lengths can still exhibit antibacterial properties. Kallenbach et al. synthesized a tetravalent dendrimeric peptide using a lysine dendrimer core and appending Trp-Arg dipeptides to the surface.84 The peptide dendrimer displayed activity against both ampicillin- and streptomycin-resistant E. coli (MIC50 = 4.5 μg/mL) and multidrug resistant S. aureus (MIC50 = 16 μg/mL), while showing low hemolytic activity. Furthermore, the tetravalent peptide dendrimer was more effective as an antimicrobial agent and less cytotoxic than linear brush-like peptide or polymeric structures conjugated to the Trp-Arg peptide sequence. The dendrimer also elicited lower levels of resistance compared to traditional antibiotics, including ciprofloxacin, vancomycin, chlorohexidine, and gentamicin.85
Kallenbach et al. also assessed the tetravalent peptide dendrimer for antimicrobial activity against E. coli RP437 biofilms.86 The initial results showed that at a concentration of 40 μM, the peptide dendrimer reduced the planktonic growth rate of E. coli by 33.5% and inhibited biofilm formation in 96-well plates by 93.5%. Ren et al. showed that, at low concentrations, the same peptide dendrimer is effective in killing E. coli persister cells, strains of bacteria that are tolerant to antibiotic stress even over long treatment periods.87 Furthermore, at a concentration of 80 μM, the tetravalent peptide dendrimer reduced the number of viable E. coli biofilm cells by 99.3%, and caused the persister cells of the biofilm to become highly sensitive to ofloxacin. Consequently, the synergistic effect between peptide dendrimers and antibiotics is a topic that warrants further investigation.
Surface Adsorbed Dendrimers: Coating metal implants to inhibit biofilm formation
In an alternative route to combating biofilm formation, Grinstaff et al. appended titanium-binding peptides (TBP; SKHKGGKHKGGKHKGSSG) to the surface of mono-, di-, and tetravalent lysine dendrimer cores with a single PEG chain appended to a cysteine residue at the C-terminus of the lysine dendrimer (Figure 9).88 These studies built off of an earlier report with a coating composed of a mono-TBP-PEG that exhibited inhibition of bacteria biofilms on surfaces, but the coating possessed limited stability.89 The tetravalent analogue showed high coating performance on titanium beads, with 90% of the coating remaining after the beads were shaken in serum media for 2 weeks compared to 55% and 5% for the di- and mono analogs. Next, the three coatings were evaluated in a series of in vitro S. aureus biofilm assays to determine the effect of multivalency on coating performance. S. aureus is one of the most common causes of hospital-acquired infections and post-surgical wound infections and is often transferred to the implanted devices during handling.90-92 For these studies, Ti-coated slides were used as model substratum and a pathogenic strain of S. aureus (MZ100) was added to the wells at a concentration of ~5×107 CFUs/well, which is ≈ 10,000 times greater than that present on human skin. The tetravalent TBP-PEG dendrimer formed a serum-resistant layer that out performed the mono- and di- analogs, and afforded a 90% reduction in S. aureus biofilm formation as compared to the uncoated titanium control beads (Figure 9). These results indicate the potential for these macromolecule structures to inhibit in vivo orthopedic implant infections.
Figure 9.
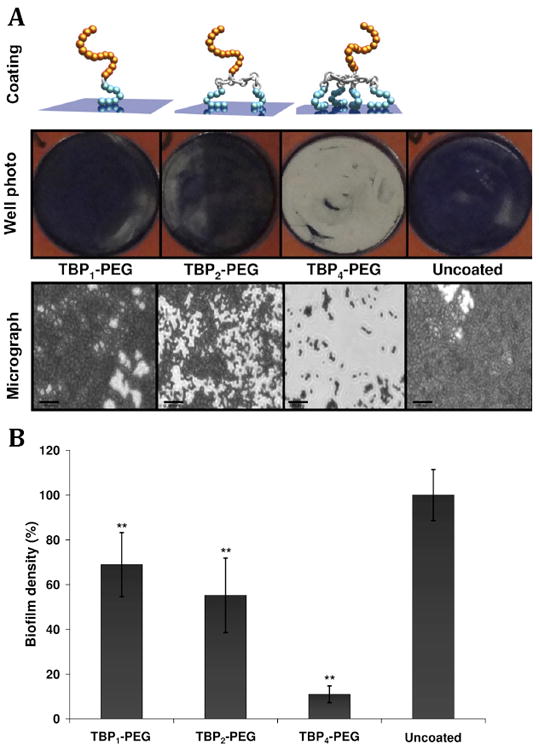
(Top) Illustration of the bacteriophobic coatings possess one or more bottom TBPs (blue), a peptide linker (silver), and a top PEG domain (gold). Schematic of three bacteriophobic coatings under investigation where one (left), two (middle), or four (right) titanium-binding peptides (TBPs) are covalently attached to a polyethylene glycol (PEG). Digital photographs and (bottom) phase-contrast micrographs (Magnification = 630X) of coated and uncoated Ti wells following a 5 h exposure to S. aureus cultures (starting inoculum of ~5×107 CFUs/mL). Bacteria were stained with 0.1% crystal violet to aid visualization. Scale bars = 20 μm. (Bottom) Quantification of biofilm formation on coated and uncoated Ti surfaces following a 5 h exposure to S. aureus (starting inoculum of ~5×107 CFUs/mL) cultures. (N=3, ** P <0.01). Adapted from Ref. 87.
Dendrimer-metal conjugates: Enhancing the effects of antimicrobial elements
Silver in its ionized form (Ag+) is well-established as a broad-spectrum antimicrobial agent.93 The mechanism of action is believed to involve disruption of protein structure through interaction with thiols, but the mechanism of action is not fully agreed upon. In 2001, Hagnauer and McManus et al. demonstrated that PAMAM dendrimers with tris(2-hydroxymethyl)-amidomethane (TRIS) or carboxylate termini could be used to complex silver ions, which upon exposure to light resulted in dendrimer conjugates containing silver nanoparticles (AgNPs).94 The materials showed activity comparable to silver nitrate solutions against S. aureus, P. aeruginosa, and E. coli, with improved solubility and stability. Murugan and Vimala reported the use of a three component system containing multiwalled carbon nanotubes (MWCNTs), an amphiphillic poly(propyleneimine) dendrimer (APPI), and AgNPs.95 Covalent conjugation of APPI to the MWCNTs increased the bacteriocidal activity against B. subtilis, S. aureus, and E. coli as compared to unfunctionalized MWCNTs. The addition of AgNPs also increased the activity. Saha et al. prepared and investigated the antibacterial properties of Zn/Te/dendrimer nanocomposites (ZnTe DNCs).96 They found that the ZnTe DNCs were active against clinical isolates of the Gram (-) bacteria V. cholerae and E. coli, but were inactive against Gram (+) bacteria. In addition, they demonstrated that the ZnTe DNC were non-toxic to human erythrocytes at 8-times the MBC, whereas bulk ZnTe is cytotoxic.
Other organic polymer and supramolecular antimicrobial systems
The dendrimers described in this review are just one class of organic nanostructures capable of functioning as antimicrobial agents. Other classes of nanostructures include polymers and liposomes. Because these structures have been actively investigated for decades, they are further developed, with examples in clinical use. For instance, the number of different linear polymer systems that display antimicrobial activity is large and includes polynorbornene97-99 and polyacrylate100-102 derivatives, poly(arylamide),103 poly(β-lactam)104 and pyridinium copolymers.105 These structures, many of them cationic, mimic antimicrobial peptides but can be synthesized more easily, scaled up readily, and produced at low cost. Moreover, the polymers that possess an amphiphilic structure exhibit greater cytotoxicity towards bacteria over erythrocytes.105 Micro- and nanoparticles can also be formulated from polymers to function as antibiotic carriers; several such synthetic polymer systems have shown promising results as antimicrobial agents in vivo, and this area has been extensively reviewed.106 Finally, a recently designed biodegradable polymer that can function as an antimicrobial amphiphilic self-assembling system has sparked further interest in this class of structures for in vivo applications.107, 108
Similarly, liposomes as vehicles to encapsulate antimicrobial drugs have exhibited remarkable antimicrobial activity by improving both drug half-life and drug efficacy.109- 111 For instance, liposome-aminoglycoside antimicrobial systems have been evaluated in preclinical and clinical trials against various infections including intracellular B. canis,112 B. abortus,112, 113 B. melitensis,114 S. tryphimurium,115 S. dublin,116 S. enteritidis,117 M. avium-intracellulare,118 and M. tuberculosis.119 Furthermore, antimicrobial liposomes can be prepared with free fatty acids (FFAs), which are found naturally in the human body, making these nanostructures particularly biocompatible. A variety of these structures have shown antimicrobial activity, most notably against Gram-positive bacterial cell lines.120-122 In a recent study, liposomes containing the FFA oleic acid showed antimicrobial activity against MRSA cells in vitro and could be used to effectively target MRSA infections in vivo without causing skin toxicity.123 The documented successful use of liposomal formulations in the clinic110 provides an example for going forward with other systems to combat infections. The current clinical success of polylysine-based dendrimers as an antiviral agent indicates that dendrimers have the potential to be very successful antimicrobial agents in the near future with continued research/clincial efforts.124
Conclusion
Based on the reports summarized in this review, it is apparent that dendrimers can be used both as antibacterial drug delivery systems, bacteriophobic coatings, and antibacterial agents capable of targeting bacterial cells or bacterial toxins. Due to their globular shape and the positioning of reactive groups at the surface, dendrimers are well suited to engage in multivalent interactions, allowing these compounds to interfere with the function of critical bacterial virulence determinants. Whereas glycodendrimers offer an opportunity for high specificity, cationic dendrimers are promising as broad-spectrum antibiotics, either acting alone or in synergy with known drugs. Antimicrobial peptide dendrimers represent a middle ground between the two strategies. Being composed of a combination of cationic and hydrophobic residues, they act through membrane disruption, but are more selective than classic cationic dendrimer because of their polypeptide nature.
The key findings from this review that highlight the importance of dendrimers as antimicrobial agents are summarized in Figure 10. However, it is important to point out that some unexplored areas remain in this field of research, presenting opportunities for additional studies regarding the use of dendrimers as antimicrobial agents. The number and variety of chemical structures investigated is limited, with PAMAM, PPI, and dendritic polylysine derivatives being the most widely explored. In most cases, insufficient data is available to obtain structure-activity relationships necessary to guide future developments. Generally, only a few papers are reported on each particular dendrimer and the variety in strains of bacteria tested is usually limited. Despite the significant in vitro antimicrobial activity data collected for a variety of dendrimer analogues, the effects of these structures on non-model bacteria systems, including MRSA strains or TB, have been evaluated in only a handful of studies, and only a few studies can be found with regard to the activity of dendritic polymers against fungi.39 Furthermore, the potential for these compounds to be used as drug delivery devices rather than agents via the formation of hydrogels has only been evaluated in a few studies. The ability to use dendrimers to form complex supramolecular structures gives researchers the opportunity to exploit the synergistic effects of dendrimer-drug systems. Finally, in a clinical setting, the potential of biofilm formation poses a serious risk of infection for patients due to the limited activity of many current antimicrobial drugs. The use of dendrimers as a method to target and disrupt these bacterial aggregates on surfaces may allow for reduced prevalence of resistant strains and should be further probed in future studies. In summary, unlike the area of drug and gene delivery where there has been a significant research effort by many groups, the use of dendritic polymers to combat emerging and re-emerging infectious diseases is less established, and, as such, we welcome and encourage others to invest their knowledge, time, and resources to solving this unmet clinical challenge.
Figure 10.
Key findings in the review of dendrimers for use as antimicrobial agents, coatings, or drug delivery devices.
References
- 1.MacDougall C, Polk RE. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev. 2005;18(4):638–656. doi: 10.1128/CMR.18.4.638-656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Projan SJ. Whither antibacterial drug discovery. Drug Discov Today. 2008;13:279–280. doi: 10.1016/j.drudis.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Dunne WMJ. Bacterial adhesion: Seen any good biofilms lately? Clin Microbiol Rev. 2002;15:155–166. doi: 10.1128/CMR.15.2.155-166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davey ME, O’Toole GA. Microbial biofilms: From ecology to molecular genetics. Microbiol Mol Biol Rev. 2000;64:847–867. doi: 10.1128/mmbr.64.4.847-867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial Biofilms: From the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 6.Costerton JW, Lewandowski Z, Caldwell DE, Krober DR, Lappin-Scott HM. Microbial biofilms. Ann Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 7.Newkome GR, Shreiner C. Dendrimers derived from 1→ 3 branching motifs. Chem Rev. 2010;110(10):6338–6442. doi: 10.1021/cr900341m. [DOI] [PubMed] [Google Scholar]
- 8.Newkome GR, Shreiner CD. Poly(amidoamine), polypropylenimine, and related dendrimers and dendrons possessing different 1 → 2 branching motifs: an overview of the divergent procedures. Polymer. 2008;49(1):1–173. [Google Scholar]
- 9.Newkome GR, Yao Z, Baker GR, Gupta VK. Micelles. Part 1. Cascade molecules: a new approach to micelles. a [27]-arborol. J Org Chem. 1985;50(11):2003–2004. [Google Scholar]
- 10.Newkome GR, Yao Z, Baker GR, Gupta VK, Russo PS, Saunders MJ. Chemistry of micelle series. Part 2. Cascade molecules. Synthesis and characterization of a benzene[9]3-arborol. J Am Chem Soc. 1986;108(4):849–850. [Google Scholar]
- 11.Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P. A new class of polymers: starburst-dendritic molecules. Polym J. 1985;17(1):117–132. [Google Scholar]
- 12.Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P. Dendritic macromolecules: synthesis of starburst dendrimers. Macromolecules. 1986;19(9):2466–2468. [Google Scholar]
- 13.Hawker CJ, Frechet JMJ. Preparation of polymers with controlled architecture. A new convergent approach to dendritic macromolecules. J Am Chem Soc. 1990;112(21):7638–7647. [Google Scholar]
- 14.Miller TM, Neenan TX. Convergent synthesis of monodisperse dendrimers based upon 1,3,5-trisubstituted benzenes. Chem Mater. 1990;2(4):346–349. [Google Scholar]
- 15.Carlmark A, Hawker C, Anders H, Malkoch M. New methodologies in the construction of dendritic materials. Chem Soc Rev. 2009;38(2):352–362. doi: 10.1039/b711745k. [DOI] [PubMed] [Google Scholar]
- 16.Buhleier E, Wehner W, Vögtle F. Cascade- and nonskid-chain-like synthesis of molecular cavity topologies. Synthesis. 1978;55:155–158. [Google Scholar]
- 17.de Brabander-van den Berg EMM, Meijer EW. Poly(propylenimine) dendrimers: large-scale synthesis via heterogeneously catalyzed hydrogenation. Angew Chem. 1993;105(9):1370–1372. [Google Scholar]
- 18.Denkewalter RG, Kolc J, Lukasavage WJ. Macromolecular highly branched homogeneous compound based on lysine units. 4289872. U S Patent. 1981
- 19.Green KA, Cifuentes MP, Samoc M, Humphrey MG. Synthesis and NLO properties of metal alkynyl dendrimers. Coordination Chem Rev. 2011;255(17-18):2025–2038. [Google Scholar]
- 20.Astruc D, Boisselier E, Ornelas C. Dendrimers designed for functions: from physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chem Rev. 2010;110(4):1857–1959. doi: 10.1021/cr900327d. [DOI] [PubMed] [Google Scholar]
- 21.Helms B, Frechet JMJ. The dendrimer effect in homogeneous catalysis. Adv Syn Catal. 2006;348(10-11):1125–1148. [Google Scholar]
- 22.Kofoed J, Reymond J-L. Dendrimers as artificial enzymes. Curr Opin Chem Biol. 2005;9(6):656–664. doi: 10.1016/j.cbpa.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Rolland O, Turrin C-O, Caminade A-M, Majoral J-P. Dendrimers and nanomedicine: multivalency in action. New J Chem. 2009;33(9):1809–1824. [Google Scholar]
- 24.Longmire MR, Ogawa M, Choyke PL, Kobayashi H. Biologically optimized nanosized molecules and particles: More than just size. Bioconjugate Chem. 2011;22:993–1000. doi: 10.1021/bc200111p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tolia GT, Choi HH. The role of dendrimers in topical drug delivery. Pharm Technol. 2008;32(22):88–98. [Google Scholar]
- 26.Roeglin L, Lempens EHM, Meijer EW. A synthetic “tour de force”: well-defined multivalent and multimodal dendritic structures for biomedical applications. Angew Chem Int Ed. 2011;50(1):102–112. doi: 10.1002/anie.201003968. [DOI] [PubMed] [Google Scholar]
- 27.Mintzer MA, Grinstaff MW. Biomedical applications of dendrimers: a tutorial. Chem Soc Rev. 2011;40(1):173–190. doi: 10.1039/b901839p. [DOI] [PubMed] [Google Scholar]
- 28.Svenson S, Tomalia DA. Dendrimers in biomedical applications-reflections on the field. Adv Drug Deliver Rev. 2005;57(15):2106–2129. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Lee CC, MacKay JA, Frechet JMJ, Szoka FC. Designing dendrimers for biological applications. Nat Biotech. 2005;23(12):1517–1526. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 30.Castonguay A, Ladd E, van de Ven TGM, Kakkar A. New J Chem. ASAP; Dendrimers as bactericides. [Google Scholar]
- 31.Armstrong GL, Conn LA, Pinner RW. Trends in infectious disease mortality in the United States during the 20th century. J Am Med Assoc. 1999;281(1):61–66. doi: 10.1001/jama.281.1.61. [DOI] [PubMed] [Google Scholar]
- 32.Barrett R, Kuzawa CW, McDade T, Armelagos GJ. Emerging and re-emerging infectious diseases: The third epidemiologic transition. Annu Rev Anthropol. 1998;27:247–271. [Google Scholar]
- 33.Walsh C. Molecular mechanisms that confer antibacterial drug resistance. Nature. 2000;406:775–781. doi: 10.1038/35021219. [DOI] [PubMed] [Google Scholar]
- 34.Davies J. Bacteria on the rampage. Nature. 1996;383:219–220. doi: 10.1038/383219a0. [DOI] [PubMed] [Google Scholar]
- 35.Davies J. Inactivation of antibiotics and the dissemination of resistance genes. Science. 1994;264(5157):375–382. doi: 10.1126/science.8153624. [DOI] [PubMed] [Google Scholar]
- 36.Remaut H, Waksman G. Structural biology of bacterial pathogenesis. Curr Opin Struct Biol. 2004;14:161–170. doi: 10.1016/j.sbi.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Donohue-Rolfe A, Keusch G, Edson C, Thorley-Lawson D, Jacewicz M. Pathogenesis of Shigella diarrhea. IX. Simplified high yield purification of Shigella toxin and characterization of subunit composition and function by the use of subunit-specific monoclonal and polyclonal antibodies. J Exp Med. 1984;160(6):1767–1781. doi: 10.1084/jem.160.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cloninger MJ. Glycodendrimers and other macromolecules bearing multiple carbohydrates. In: Rotello VM, Thayumanavan S, editors. Molecular Recognition and Polymers: Control of Polymer Structure and Self-Assembly. 1. John Wiley & Sons; Hoboken, NJ: 2008. pp. 335–358. [Google Scholar]
- 39.Rojo J, Delgado R. Dendrimers and dendritic polymers as anti-infective agents: New antimicrobial strategies for therapeutic drugs. Anti-infective Agents in Medicinal Chemistry. 2007;6(3):151–174. [Google Scholar]
- 40.Imberty A, Chabre YM, Roy R. Glycomimetics and glycodendrimers as high affinity microbial anti-adhesions. Chem Eur J. 2008;14:7490–7499. doi: 10.1002/chem.200800700. [DOI] [PubMed] [Google Scholar]
- 41.Thompson JP, Schengrund C-L. Oligosaccharide-derivatized dendrimers: defined multivalent inhibitors of the adherence of the cholera toxin B subunit and the heat labile enterotoxin of E. coli to GM1. Glycoconjugate J. 1997;14:837–845. doi: 10.1023/a:1018590021762. [DOI] [PubMed] [Google Scholar]
- 42.Fishman PH, Pacuszka T, Orlandi PA. Gangliosides as receptors for bacterial enterotoxins. Advances in Lipid Res. 1993;25:165–187. [PubMed] [Google Scholar]
- 43.Spangler BD. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiological Rev. 1992;56(4):622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson JP, Schengrund C-L. Inhibition of the adherence of cholera toxin and the heat-labile enterotoxin of Escherichia coli to cell-surface GM1 by oligosaccharide-derivatized dendrimers. Biochem Pharmacol. 1998;56:591–597. doi: 10.1016/s0006-2952(98)00198-1. [DOI] [PubMed] [Google Scholar]
- 45.Vrasidas I, de Mol NJ, Liskamp RMJ, Pieters RJ. Synthesis of lactose dendrimers and multivalency effects in binding to the cholera toxin B subunit. Eur J Org Chem. 2001;2001(24):4685–4692. [Google Scholar]
- 46.Pukin AV, Branderhorst HM, Sisu C, Weijers CAGM, Gilbert M, Liskamp RMJ, Visser GM, Zuilhof H, Pieters RJ. Strong inhibition of cholera toxin by multivalent GM1 derivatives. ChemBioChem. 2007;8:1500–1503. doi: 10.1002/cbic.200700266. [DOI] [PubMed] [Google Scholar]
- 47.Sisu C, Baron AJ, Branderhorst HM, Connell SD, Weijers CAGM, de Vries R, Hayes ED, Pukin AV, Gilbert M, Pieters RJ, Zuilhof H, Visser GM, Turnbull WB. The influence of ligand valency on aggregation mechanisms for inhibiting bacterial toxins. ChemBioChem. 2009;10:329–337. doi: 10.1002/cbic.200800550. [DOI] [PubMed] [Google Scholar]
- 48.Branderhorst HM, Liskamp RMJ, Visser GM, Pieters RJ. Strong inhibition of cholera toxin binding by galactose dendrimers. Chem Commun. 2007;2007:5043–5045. doi: 10.1039/b711070g. [DOI] [PubMed] [Google Scholar]
- 49.Joosten JAF, Loimaranta V, Appeldoorn CCM, Haataja S, El Maate FA, Liskamp RMJ, Finne J, Pieters RJ. Inhibition of Streptococcus suis adhesion by dendritic galabiose compounds at low nanomolar concentration. J Med Chem. 2004;47(26):6499–6508. doi: 10.1021/jm049476+. [DOI] [PubMed] [Google Scholar]
- 50.Staats JJ, Feder I, Okwumabua O, Chengappa MM. Streptococcus suis: Past and present. Vet Res Commun. 1997;21:381–407. doi: 10.1023/a:1005870317757. [DOI] [PubMed] [Google Scholar]
- 51.Salminen A, Loimaranta V, Joosten JAF, Khan AS, Hacker J, Pieters RJ, Finne J. Inhibition of P-fimbriated Escherichia coli adhesion by multivalent galabiose derivatives studied by a live-bacteria application of surface plasmon resonance. J Antimicrob Chemother. 2007;60:495–501. doi: 10.1093/jac/dkm251. [DOI] [PubMed] [Google Scholar]
- 52.Appeldoorn CCM, Joosten JAF, el Maate FA, Dobrindt U, Hacker J, Liskamp RMJ, Khan AS, Pieters RJ. Novel multivalent mannose compounds and their inhibition of the adhesion of type 1 fibriated uropathogenic E. coli. Tetrahedron Assym. 2005;16:361–372. [Google Scholar]
- 53.Lindhorst TK, Kieburg C, Krallmann-Wenzel U. Inhibition of the type I fimbriae-mediated adhesion of Escherichia coli to erythrocytes by multiantennary α-mannosyl clusters: the effect of multivalency. Glycoconjugate J. 1998;15:605–613. doi: 10.1023/a:1006920027641. [DOI] [PubMed] [Google Scholar]
- 54.Kolomiets E, Johansson EMV, Renaudet O, Darbre T, Reymond J-L. Neoglycopeptide dendrimer libraries as a source of lectin binding ligands. Org Lett. 2007;9(8):1465–1468. doi: 10.1021/ol070119d. [DOI] [PubMed] [Google Scholar]
- 55.Lis H, Sharon N. Lectins: Carbohydrate-specific proteins that mediate cellular recognition. Chem Rev. 1998;98(2):637–674. doi: 10.1021/cr940413g. [DOI] [PubMed] [Google Scholar]
- 56.Wagner VE, Iglewski BH. Pseudomonas aeruginosa biofilms in CF infection. Clin Rev Allergy Immunol. 2008;35:124–134. doi: 10.1007/s12016-008-8079-9. [DOI] [PubMed] [Google Scholar]
- 57.Tielker D, Hacker S, Loris R, Strathmann M, Wingeder J, Wilhelm S, Rosenau F, Jaeger K-E. Pseudomonas aeruginosa lectin LecB is located in the outer membrane and is involved in biofilm formation. Microbiology. 2005;151:1313–1323. doi: 10.1099/mic.0.27701-0. [DOI] [PubMed] [Google Scholar]
- 58.Johansson EMV, Crusz SA, Kolomiets E, Buts L, Kadam RU, Cacciarini M, Bartels K-M, Diggle SP, Cámara M, Williams P, Loris R, Nativi D, Rosenau F, Jaeger K-E, Darbre T, Reymond J-L. Inhibition and dispersion of Pseudomonas aeruginosa biofilms by glycopeptide dendrimers targeting the fucose-specific lectin LecB. Chem Biol. 2008;15:1249–1257. doi: 10.1016/j.chembiol.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 59.Johansson EMV, Kadam RU, Rispoli G, Crusz SA, Bartels K-M, Diggle SP, Cámara M, Williams P, Jaeger K-E, Darbre T, Reymond J-L. Inhibition of Pseudomonas aeruginosa biofilms with a glycopeptide dendrimer containing D-amino acids. Med Chem Commun. 2011;2:418–420. [Google Scholar]
- 60.Block S. Disinfection, Sterilization and Preservation. Lea and Febiger; Philadelphia: 1983. [Google Scholar]
- 61.Franklin TJ, Snow GA. Biochemistry of Antimicrobial Actions. 4. Chapman and Hall; London: 1987. [Google Scholar]
- 62.Chen CZ, Tan NCB, Cooper SL. Incorporation of dimethyldodecylammonium chloride functionalities onto poly(propylene imine) dendrimers significantly enhances their antimicrobial properties. Chem Commun. 1999:1585–1586. [Google Scholar]
- 63.Chen CZ, Beck-Tan NC, Dhurjati P, van Dyk TK, LaRossa RA, Cooper SL. Quaternary ammonium functionalized poly(propylene imine) dendrimers as effective antimicrobials: structure-activity studies. Biomacromolecules. 2000;1(3):473–480. doi: 10.1021/bm0055495. [DOI] [PubMed] [Google Scholar]
- 64.Ortega P, Copa-Patiño JL, Muñoz-Fernandez MA, Soliveri J, Gomez R, de la Mata FJ. Amine and ammonium functionalization of chloromethylsilane-ended dendrimers. Antimicrobial activity studies. Org Biomol Chem. 2008;6:3264–3269. doi: 10.1039/b809569h. [DOI] [PubMed] [Google Scholar]
- 65.Rasines B, Hernández-Ros JM, de las Cuevas N, Copa-Patiño JL, Soliver J, Muñoz-Fernandez MA, Gómez R, de la Mata FJ. Water-stable ammonium-terminated carbosilane dendrimers as efficient actibacterial agents. Dalton Trans. 2009;2009(40):8704–8713. doi: 10.1039/b909955g. [DOI] [PubMed] [Google Scholar]
- 66.Ortega P, Cobaleda BM, Hernández-Ros JM, Fuentes-Paniagua E, Sánchez-Nieves J, Tarazona MP, Copa-Patiño J, Soliveri J, de la Mata FJ, Gómez R. Hyperbranched polymers versus dendrimers containing a carbosilane framework and terminal ammonium groups as antimicrobial agents. Org Biomol Chem. 2011;9:5238–5248. doi: 10.1039/c1ob05321c. [DOI] [PubMed] [Google Scholar]
- 67.Yang H, Lopina ST. Penicillin V-Conjugated PEG-PAMAM Star Polymers. J Biomater Sci Polymer Edn. 2003;14(10):1043–1056. doi: 10.1163/156856203769231556. [DOI] [PubMed] [Google Scholar]
- 68.Cheng Y, Qu H, Ma M, Xu Z, Xu P, Fang Y, Xu T. Polyamidoamine (PAMAM) dendrimers as biocompatible carriers of quinolone antimicrobials: An in vitro study. Eur J Med Chem. 2007;42(7):1032–1038. doi: 10.1016/j.ejmech.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 69.Calabretta MK, Kumar A, McDermott AM, Cai C. Antibacterial activities of poly(amidoamine) dendrimers terminated wiht amino and poly(ethylene glycol) groups. Biomacromolecules. 2007;8(6):1807–1811. doi: 10.1021/bm0701088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lopez AI, Reins RY, McDermott AM, Trautner BW, Cai C. Antibacterial activity and cytotoxicity of PEGylated poly(amidoamine) dendrimers. Mol BioSyst. 2009;5:1148–1156. doi: 10.1039/b904746h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang L, Erasquin UJ, Zhao M, Ren L, Zhang MY, Cheng GJ, Wang Y, Cai C. Stability, antimicrobial activity, and cytotoxicity of poly(amidoamine) dendrimers on titanium substrates. ACS Appl Meter Interfaces. 2011;3:2885–2894. doi: 10.1021/am2004398. [DOI] [PubMed] [Google Scholar]
- 72.Patrick LA, Gaudet LM, Farley AE, Rossiter JP, Tomalty LL, Smith GN. Development of a guinea pig model of chorioamnionitis and fetal brain injury. Am J Obstet Gynecol. 2004;191:1205–1211. doi: 10.1016/j.ajog.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 73.Wang B, Navath RS, Menjoge AR, Balakrishnan B, Ballair R, Dai H, Romero R, Kannan S, Kannan RM. Inhibition of bacterial growth and intramniotic infection in a guinea pig model of chorioamnionitis using PAMAM dendrimers. Int J Pharma. 2010;395:298–308. doi: 10.1016/j.ijpharm.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Navath RS, Menjoge AR, Dai H, Romero R, Kannan S, Kannan RM. Injectable PAMAM Dendrimer–PEG Hydrogels for the Treatment of Genital Infections: Formulation and in Vitro and in Vivo Evaluation. Mol Pharmaceutics. 2011;8(4):1209–1223. doi: 10.1021/mp200027z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nguyen PM, Zacharia NS, Verploegen E, Hammond PT. Extended release antibacterial layer-by-layer films incorporating linear-dendritic block copolymer micelles. Chem Mater. 2007;19(23):5524–5530. [Google Scholar]
- 76.Meyers SR, Juhn FS, Griset AP, Luman NR, Grinstaff MW. Anionic amphiphilic dendrimers as antibacterial agents. J Am Chem Soc. 2008;130(44):14444–5. doi: 10.1021/ja806912a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mintzer MA, Grinstaff MW. Anionic amphiphilic dendrimers as broad spectrum antimicrobial agents. 240th ACS National Meeting; Boston, MA. 2010. [Google Scholar]
- 78.Tulu M, Aghatabay NM, Senel M, Dizman C, Parali T, Dulger B. Synthesis, characterization and antimicrobial activity of water soluble dendritic macromolecules. Eur J Med Chem. 2009;44(3):1093–1099. doi: 10.1016/j.ejmech.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 79.Melo MN, Ferre R, Castanho MA. Antimicrobial peptides: linking partition, activity and high membrane-bound concentration. Nat Rev Microbiol. 2009;7:245–250. doi: 10.1038/nrmicro2095. [DOI] [PubMed] [Google Scholar]
- 80.Tam JP. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci USA. 1988;85:5409–5403. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tam JP, Lu Y-A, Yang J-L. Antimicrobial dendrimeric peptides. Eur J Biochem. 2002;269:923–932. doi: 10.1046/j.0014-2956.2001.02728.x. [DOI] [PubMed] [Google Scholar]
- 82.Pini A, Guiliani A, Falciani C, Runci Y, Ricci C, Lelli B, Malossi M, Neri P, Rossolini GM, Bracci L. Antimicrobial activity of novel dendrimeric peptides obtained by phage display selection and rational modification. Antimicrob Agents Chemother. 2005;49(7):2665–2672. doi: 10.1128/AAC.49.7.2665-2672.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bruschi M, Pirri G, Giuliani A, Nicoletto SF, Baster I, Scorciapino MA, Casu M, Rinaldi AC. Synthesis, characterization, antimicrobial activity and LPS-interaction properties of SB041, a novel dendrimeric peptide with antimicrobial properties. Peptides. 2010;31(8):1459–1467. doi: 10.1016/j.peptides.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 84.Liu Z, Young AW, Hu P, Rice AJ, Zhou C, Zhang Y, Kallenbach NR. Tuning the membrane selectivity of antimicrobial peptides by using multivalent design. ChemBioChem. 2007;8:2063–2065. doi: 10.1002/cbic.200700502. [DOI] [PubMed] [Google Scholar]
- 85.Young AW, Liu Z, Zhou C, Totsingan F, Jiwrajka N, Shi Z, Kallenbach NR. Structure and antimicrobial properties of multivalent short peptides. Med Chem Comm. 2011;2:308–314. [Google Scholar]
- 86.Hou S, Zhou C, Liu Z, Young AW, Shi Z, Ren D, Kallenbach NR. Antimicrobial dendrimer active against Eschericia coli biofilms. Bioorg Med Chem Lett. 2009;19:5478–5481. doi: 10.1016/j.bmcl.2009.07.077. [DOI] [PubMed] [Google Scholar]
- 87.Chen X, Zhang M, Zhou C, Kallenbach NR, Ren D. Control of bacterial persister cells by Trp/Arg-containing antimicrobial peptides. Appl Environ Microbiol. 2011;77(14):4878–4885. doi: 10.1128/AEM.02440-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khoo X, O’Toole GA, Nair SA, Snyder BD, Kenan DJ, Grinstaff MW. Staphylococcus aureus resistance on titanium coated with multivalent PEGylated-peptides. Biomaterials. 2010;31:9285–9292. doi: 10.1016/j.biomaterials.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khoo X, Hamilton P, O’Toole GA, Snyder BD, Kenan DJ, Grinstaff MW. Direct assembly of PEGylated-peptide coatings for infection-resistant titanium metal. J Am Chem Soc. 2009;131(31):10992–10997. doi: 10.1021/ja9020827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gristina AG. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237(4822):1588–1595. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- 91.Arciola CR, Campoccia D, Montanaro L. Detection of biofilm-forming strains of Staphylococcus epidermidis and S. aureus. Expert Rev Mol Diagn. 2002;2(5):478–484. doi: 10.1586/14737159.2.5.478. [DOI] [PubMed] [Google Scholar]
- 92.Harris LG, Richards RG. Staphylococcus aureus adhesion to different treated titanium surfaces. J Mater Sci Mater Med. 2004;15(4):311–314. doi: 10.1023/b:jmsm.0000021093.84680.bb. [DOI] [PubMed] [Google Scholar]
- 93.Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27(1):76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 94.Balogh L, Swanson DR, Tomalia DA, Hagnauer GL, McManus AT. Dendrimer–Silver Complexes and Nanocomposites as Antimicrobial Agents. Nano Lett. 2000;1(1):18–21. [Google Scholar]
- 95.Murugan E, Vimala G. Effective functionalization of multiwalled carbon nanotube with amphiphilic poly(propyleneimine) dendrimer carrying silver nanoparticles for better dispersability and antimicrobial activity. J Colloid Interface Sci. 2011;357(2):354–365. doi: 10.1016/j.jcis.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 96.Ghosh S, Ghosh D, Bag PK, Bhattacharya SC, Saha A. Aqueous synthesis of ZnTe/dendrimer nanocomposites and their antimicrobial activity: implications in therapeutics. Nanoscale. 2011;3(3):1139–1148. doi: 10.1039/c0nr00610f. [DOI] [PubMed] [Google Scholar]
- 97.Lienkamp K, Madkour AE, Musante A, Nelson CF, Nüsslein K, Tew GN. Antimicrobial polymers prepared by ROMP with unprecedented selectivity: a molecular construction kit approach. J Am Chem Soc. 2008;130(30):9836–9843. doi: 10.1021/ja801662y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Al-Badri ZM, Sorn A, Lyon S, Nelson CF, Nüsslein K, Tew GN. Investigating the effect of increasing charge density on the hemolytic activity of synthetic antimicrobial polymers. Biomacromolecules. 2008;9(10):2805–2810. doi: 10.1021/bm800569x. [DOI] [PubMed] [Google Scholar]
- 99.Ilker MF, Nüsslein K, Tew GN, Coughlin EB. Tuning the hemolytic and antibacterial activities of amphiphilic polynorbornene derivatives. J Am Chem Soc. 2004;126(48):15870–15875. doi: 10.1021/ja045664d. [DOI] [PubMed] [Google Scholar]
- 100.Kenawy E-R, Worley SD, Broughton R. The chemistry and applications of antimicrobial polymers: a state-of-the-art review. Biomacromolecules. 2007;8(5):1359–1384. doi: 10.1021/bm061150q. [DOI] [PubMed] [Google Scholar]
- 101.Kuroda K, DeGrado WF. Amphiphilic polymethacrylate derivatives as antimicrobial agents. J Am Chem Soc. 2005;127(12):4128–4129. doi: 10.1021/ja044205+. [DOI] [PubMed] [Google Scholar]
- 102.Ivanov I, Vemparala S, Pophristic V, Kuroda K, DeGrado WF, McCammon JA, Klein ML. Characterization of nonbiological antimicrobial polymers in aqueous solution and at water-lipid interfaces from all-atom molecular dynamics. J Am Chem Soc. 2006;128(6):1778–1779. doi: 10.1021/ja0564665. [DOI] [PubMed] [Google Scholar]
- 103.Tew GN, Liu D, Chen B, Doerksen RJ, Kaplan J, Carroll PJ, Klein ML, DeGrado WF. De novo design of biomimetic antimicrobial polymers. Proc Natl Acad Sci U S A. 2002;99(8):5110–5114. doi: 10.1073/pnas.082046199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mowery BP, Lee SE, Kissounko DA, Epand RF, Epand RM, Weisblum B, Stahl SS, Gellman SH. Mimicry of antimicrobial host-defense peptides by random copolymers. J Am Chem Soc. 2007;129(50):15474–15476. doi: 10.1021/ja077288d. [DOI] [PubMed] [Google Scholar]
- 105.Sambhy V, Peterson BR, Sen A. Antibacterial and hemolytic activities of pyridinium polymers as a function of the spatial relationship between the positive charge and the pendant alkyl tail. Angew Chem Int Ed. 2008;47(7):1250–1254. doi: 10.1002/anie.200702287. [DOI] [PubMed] [Google Scholar]
- 106.Pinto-Alphandary H, Andremont A, Couvreur P. Targeted delivery of antibiotics using liposomes and nanoparticles: reserach and applications. Int J Antimicrob Agents. 2000;13:155–168. doi: 10.1016/s0924-8579(99)00121-1. [DOI] [PubMed] [Google Scholar]
- 107.Liu L, Xu K, Wang H, Tan PK, Fan W, Venkatraman SS, Li L, Yang YY. Self-assembled cationic peptide nanoparticles as an efficient antimicrobial agent. Nat Nanotechnol. 2009;4(7):457–463. doi: 10.1038/nnano.2009.153. [DOI] [PubMed] [Google Scholar]
- 108.Nederberg F, Zhang Y, Tan JP, Xu K, Wang H, Yang C, Gao S, Guo XD, Fukushima K, Li L, Hedrick JL, Yang YY. Biodegradable nanostructures with selective lysis of microbial membranes. Nat Chem. 2011;3(5):409–414. doi: 10.1038/nchem.1012. [DOI] [PubMed] [Google Scholar]
- 109.Bakker-Woudenberg IAJM, Schiffelers RM, Storm G, Becker MJ, Guo L. Long-circulating sterically stablilized liposomes in the treatment of infections. Methods Enzymol. 2005;391:228–260. doi: 10.1016/S0076-6879(05)91014-8. [DOI] [PubMed] [Google Scholar]
- 110.Drulis-Kawa Z, Dorotkiewicz-Jach A. Liposomes as delivery systems for antibiotics. Int J Pharma. 2010;387(1-2):187–198. doi: 10.1016/j.ijpharm.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 111.Juliano RL. Liposomes as drug carriers in the therapy of infectious diseases. Horizons Biochem Biophys. 1989;9:249–279. [PubMed] [Google Scholar]
- 112.Fountain MW, Weiss SJ, Fountain AG, Shen A, Lenk RP. Treatment of Brucella canis and Brucella abortus in vitro and in vivo by stable plurilamellar vesicle-encapsulated aminoglycosides. J Infect Dis. 1985;152:529–535. doi: 10.1093/infdis/152.3.529. [DOI] [PubMed] [Google Scholar]
- 113.Vitas AI, Diaz R, Gamazo C. Protective effect of liposomal gentamicin against systemic acute murine brucellosis. Chemother. 1997;43:204–210. doi: 10.1159/000239563. [DOI] [PubMed] [Google Scholar]
- 114.Hernandez-Caselles T, Vera A, Crespo F, Villalain J, Gomez-Fernandez JC. Treatment of Brucella melitensis infection in mice by use of liposome-encapsulated gentamicin. Am J Vet Res. 1989;50:1486–1488. [PubMed] [Google Scholar]
- 115.Swenson CE, Stewart KA, Hammett JL, Fitzsimmons WE, Ginsberg RS. Pharmacokinetics and in vivo activity of liposome-encapsulated gentamicin. Antimicrob Agents Chemother. 1990;34:235–240. doi: 10.1128/aac.34.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fierer J, Hatlen L, Lin JP, Estrella D, Mihalko P, Yau-Young A. Successful treatment using gentamicin liposomes of Salmonella dublin infections in mice. Antimicrob Agents Chemother. 1990;34:343–348. doi: 10.1128/aac.34.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Khalil RM, Murad FE, Yehia SA, El-Ridy MS, Salama HA. Free versus liposome-entrapped streptomycin sulfate in treatment of infections caused by Salmonella enteritidis. Pharmazie. 1996;51:182–184. [PubMed] [Google Scholar]
- 118.Wiley EL, Perry A, Nightingale SD, Lawrence J. Detection of Mycobacterium avium-intracellulare complex in bone marrow specimens of patients with aquired immunodeficiency syndrome. Am J Clin Pathol. 1994;101:446–451. doi: 10.1093/ajcp/101.4.446. [DOI] [PubMed] [Google Scholar]
- 119.Vladimirsky MA, Ladigina GA. Antibacterial activity of liposome-entrapped streptomycin in mice infected with Mycobacterium tuberculosis. Biomed Pharmacother. 1982;36:375–377. [PubMed] [Google Scholar]
- 120.Drake DR, Brogden KA, Dawson DV, Wertz PW. Thematic review series: skin lipids. Antimicrobial lipids at the skin surface. J Lipid Res. 2008;49(1):4–11. doi: 10.1194/jlr.R700016-JLR200. [DOI] [PubMed] [Google Scholar]
- 121.Georgel P, Crozat K, Lauth X, Makrantonaki E, Seltmann H, Sovath S, Hoebe K, Du X, Rutschmann S, Jiang Z, Bigby T, Nizet V, Zouboulis CC, Beutier B. A toll-like receptor 2-responsive lipid effector pathway protects mammals against skin infections with gram-positive bacteria. Infect Immunol. 2005;78(3):4512–4521. doi: 10.1128/IAI.73.8.4512-4521.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Skřivanová E, Marounek M, Dlouhá G, Kaňka J. Susceptibility of Clostridium perfringens to C2-C18 fatty acids. Lett Appl Microbiol. 2005;41(1):77–81. doi: 10.1111/j.1472-765X.2005.01709.x. [DOI] [PubMed] [Google Scholar]
- 123.Huang CM, Chen CH, Pornpattananangkul D, Zhang L, Chan M, Hsieh MF, Zhang L. Eradication of drug resistant Staphylococcus aureus by liposomal oleic acids. Biomaterials. 2011;32(1):214–221. doi: 10.1016/j.biomaterials.2010.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Price CF, Tyssen D, Sonza S, Davie A, Evans S, Lewis GR, Xia S, Spelman T, Hodsman P, Moench TR, Humberstone A, Paull JR, Tachedjian G. SPL7013 gel (VivaGel) retains potent HIV-1 and HSV-2 inhibitory activity following vaginal administration in humans. PLoS One. 2011;6(9):e24095. doi: 10.1371/journal.pone.0024095. [DOI] [PMC free article] [PubMed] [Google Scholar]


