Abstract
The estrogen receptor (ERα) is implicated in the progression of breast cancer. Hormonal therapies which block ER functions or local and systemic estrogen production are currently used to treat ERα positive breast cancer. Hormonal therapy shows beneficial effects, however, initial or acquired resistance to endocrine therapies frequently occurs, and tumors recur as metastasis. Emerging evidence suggests in addition to exerting its well-studied nuclear functions, ERα also participates in extranuclear signaling that involve growth factor signaling components, adaptor molecules and stimulation of cytosolic kinases. ERα extranuclear pathways have the potential to activate gene transcription, modulate cytoskeleton, and promote tumor cell proliferation, survival, and metastasis. Cytoplasmic/membrane ERα is detected in a subset of breast tumors and expression of extranuclear components ERα is deregulated in tumors. The extranuclear actions of ER are emerging as important targets for tumorigenic and metastatic control. Inhibition of ERα extranuclear actions has the potential to prevent breast tumor progression and may be useful in preventing ERα positive metastasis. In this review, we summarize the results of recent research into the role of ERα mediated extranuclear actions in breast tumorigenesis and metastasis.
Introduction
Estrogens regulate the expression and activity of key signaling molecules critical in various cellular signaling pathways. The biological effects of estrogen are mediated by its binding to structurally and functionally distinct estrogen receptors, alpha and beta (ERα and ERβ) 1. ER functions as a ligand-activated transcription factor, providing a direct link between intra- and extracellular signaling molecules resulting in the regulation of numerous critical cellular processes including growth, development, differentiation and maintenance within a diverse range of mammalian tissues.
ERs consist of a N-terminal region (A/B domain) containing a constitutively active ligand-independent transactivation (AF1) domain whose activity is regulated by phosphorylation via activation of signaling kinases, DNA-binding domain (C domain) responsible for DNA-binding specificity and ER dimerization, and a C-terminal ligand-dependent transactivation (AF2) binding region 2. Ligand binding to ER results in a conformational change regulating the receptor activity, DNA-binding and interactions with other proteins. The ligand-activated ER functions as a transcription factor, translocates to the nucleus, binds to estrogen responsive element (ERE) within target gene promoters, and stimulates gene transcription (extranuclear/nuclear signaling) 3, 4. Estrogens play an important role in mammary gland development and in the initiation and progression of breast cancer. ERα is the major ER subtype in the mammary epithelium and its importance in mammary gland biology and development has been confirmed in ERα (Esr1) knockout mice, which display grossly impaired ductal epithelial cell proliferation and branching 5, 6.
Emerging evidence suggests that ER signaling is complex, involves cofactors, genomic actions, as well as extranuclear (cytoplasmic and membrane-mediated) actions 7–10. Because of the nature and depth of the information available on estrogen mediated extranuclear actions in different cell types, only representative studies that involve ERα actions in breast cancer cells are included in this review. Here, we focus on summarizing the emerging key evidence for ERα extranuclear signaling in breast cancer progression and discuss the possibility of the targeting ERα extranuclear actions as an additional possible therapeutic target for preventing local and distant progression of estrogen-dependent breast cancer.
Molecular mechanisms of ER extranuclear signaling
Kinase cascades
Emerging evidence suggests that ERα participates in extranuclear signaling via formation of a multiprotein complex collectively called a “signalsome” 11. Even though the complete repertoire of proteins present in the signalosome are not known, evidence suggests that ERα extranuclear signaling utilizes multiple cytosolic kinases. ERα-extranuclear signaling has been linked to rapid responses to E2 through stimulation of the Src kinase, mitogen-activated protein kinase (MAPK), protein kinase B (AKT), phosphatidylinositol- 3-kinase (PI3K), PKA and PKC pathways in the cytosol 12, 13. The proto-oncogene c-Src is a multifunctional intracellular tyrosine kinase implicated in the regulation of a variety of processes including proliferation, differentiation, survival, and motility 14. Src interacts with ERα and is overexpressed in breast tumors 15. ERα exatranuclear actions also involve PKA signaling pathways and functional PKA signaling is needed for optimal activation of MAPK by E2 16. Further, E2 -induced MAPK activation is shown to be mediated by PKC-delta/Ras pathway, that could be crucial for E2-dependent growth-promoting effects in the early stages of tumor progression17. Integrin linked kinase (ILK1) is another ERα interacting kinase; estrogen treatment enhances ILK activity and regulation of ER-ILK1 interaction is dependent on the PI3K pathway 18.
Growth factor signaling
Growth factor receptors EGFR, ErbB2 and IGFR tether ERα to the plasma membrane and are involved in E2 biological actions by interacting with ER signalosome19. Growth factors promote the formation of a multi-protein complex leading to the initiation of MAPK and PI3K signaling pathways in breast cancer cells 20. Activation of the PI3K-AKT pathway has been shown to be an essential step in the estrogenic action of growth factors 21. Signal transducer and activator of transcription (STAT) family of transcription factors play an important role in oncogenesis and signaling crosstalk occurs between ERα, c-Src, EGFR, and STAT5 in ERα positive breast cancer cells and STAT5 plays an integral role in E2-stimulated proliferation22. ERα also interacts with STAT3 and cross-talk between ERα and STAT3 play an important role in leptin-induced STAT3 activation 23. The ILK1 axis is the major signaling node linking integrins and growth factor signaling to a variety of cellular responses regulated by estrogens. ERα interacts with ILK1 enzyme 24 and ILK1 was identified as a novel interacting protein of ERα-coregulator PELP1 25 and ILK functions as a downstream effector of ERα extranuclear signaling, leading to cytoskeleton reorganization.
ERα modifications
ERα undergoes several post-translational modifications including methylation, acetylation, phosphorylation, palmitoylation and S-nitrosylation affecting receptor subcellular localization, stability and ER extranuclear actions. Protein arginine N-methyltransferase 1 (PRMT1) transiently methylates arginine 260 located in the DNA-binding domain of ERα facilitating the interaction of ERα with p85 subunit of PI3K and Src, resulting in ERα extranuclear actions both in normal and malignant epithelial breast cells 26. S-Palmitoylation, a reversible addition of palmitate on non-N-terminal Cys residues is catalyzed by palmitoyl acyl transferase (PAT), facilitates ERα localization to the plasma membrane. Thus enhancing the ERα interaction with adaptor proteins and kinases and activation of the AKT and MAPK pathways 27. ERα and its coregulator’s phosphorylation occurs on tyrosine and serine/threonine residues and such phosphorylation facilitating ERα extranuclear action leading to activation of the AKT pathway28. mTor and MAPK contribute to ERα activation via Serine 167 phosphorylation which has been associated with the development of therapeutic resistance. 29. Serine305 phosphorylation of ER by protein kinase A associates with tamoxifen sensitivity 30. Nitrioxide (NO) can modify ERα via S-nitrosylation at cysteine residue resulting in selective inhibition of DNA-binding of ERα to ERE within target gene promoters. Suggesting, the interaction between NO and ERα favors activation of extranuclear actions and signaling pathways of ERα 31. ERα forms a complex with histonedeacetylase (HDAC) 6 and tubulin at the plasma membrane in ligand dependent manner and promotes rapid deacetylation of tubulin of breast cancer cells. Estrogen-dependent tubulin deacetylation is another mechanism of ER extranuclear actions, and may potentially contributes to the aggressiveness of ERα-positive breast cancer cells 32.
Adaptor molecules
Estrogen is shown to utilize several adaptor molecules to couple ERα with the growth factor signaling axis. Hormonal signaling promotes association of ERα with adaptor protein Shc, which couples additional needed signaling molecules such as Src and growth factor receptors 19. Cytoskeltal associate protein p130Cas, another adaptor protein that associates with ERα signalosome, in a hormonal dependent manner. Over-expression of p130Cas increases estrogen mediated c-Src and MAPK activities 33. Recent studies identified ERα coregulator PELP1, a scaffolding protein coupling ERα with Src kinase leading to activation of the cytosolic kinase pathways including MAPK and AKT. While all the components of the ERα signalosome have yet to be identified, emerging studies suggest that ERα, PELP1 and Src kinase represent key components that facilitating ERα extranuclear signaling 34. Using transgenic mouse model that uniquely express PELP1 in the cytoplasm (MMTV_PELP1cyto mice), it was demonstrated that cytoplasmic localization of ERα coregulator has potential to enhance ERα extranuclear signaling 35. Metastatic tumor antigen 1 (MTA1), an ER coregulator protein and the naturally occurring short form of MTA1 (MTAx) is reported to localize in the cytoplasm, sequesters ERα in the cytoplasm, and thus enhance ER extranuclear responses 36.
Biological functions of ER extranuclear actions
ERα extranuclear actions in gene transcription
Several elegant studies investigated the impact of estrogen mediated extranuclear initiated pathways on global gene expression by using estrogen-dendrimer conjugates (EDCs) 37–40. EDCs are nanoparticles, coated with estradiol (E2) through a 17α-phenylethynyl unit, have a binding affinity similar to estrogen, uniquely localize in the membrane/cytoplasm, and preferably activate ERα extranuclear signaling41, 42. Genome-wide cDNA microarray analysis revealed approximately 25% E2 target genes as EDC responsive. These studies using various assays and pharmacological inhibitors demonstrated that extranuclear signaling cascades have the potential to elicit gene stimulation 39. Aromatase plays a critical role in breast cancer development by converting androgen to estrogen. Estrogen induces aromatase expression without direct binding of ERα to the aromatase promoter and E2 induction could be suppressed by the MAPK inhibitor or growth factor signaling inhibitor. The results from this study suggested that E2 up-regulates aromatase expression by ERα extranuclear actions via crosstalk with growth factor-mediated pathways 43. Estrogen mediated extranuclear actions also promote phosphorylation of several key ERα transcriptional coregulators such as SRC3 and PELP1, thus enhancing their recruitment to target gene promoters and such actions implicate that ER extranuclear signaling may have downstream genomic roles via coactivator signaling 44, 45 Estrogen induced transactivation of a STAT-regulated promoter requires MAPK, Src, and PI3K activity. These results implicate ER mediated extranuclear actions in nuclear transcriptional activation of STAT target genes 46. E2 induces rapid nuclear translocation of MAPK together with cAMP response element binding protein leading to transcriptional activation of gene responsive to cAMP response element binding protein 47. Estrogen mediated extranuclear actions crosstalk with prolactin signaling results in enhanced activity of activating protein 1 and induction of c-fos gene in breast cancer cells 48. Collectively, evolving evidence implicates that inputs from ERα extranuclear pathways in regulating the gene expression of breast cancer cells.
ER extranuclear actions in cytoskeletal remodeling and metastasis
Clinically, estrogen has long been recognized to enhance the development and progression of ERα positive breast cancers. Several studies report a positive effect of ERα signaling on motility 49, 50 as many metastatic tumors retain ERα 51; >80% of lymph node metastases and 65–70% of distant metastases maintain ERα expression 52, 53. A correlation between ERα-positive tumors and development of bone metastasis has been observed clinically 54, 55. Similarly, ERα-mediated signaling enhances lung metastasis by promoting host-compartment response 56. Metastases spawned by malignant tumors that have acquired increased invasiveness are responsible for almost all breast cancer-related morbidity and mortality. Cancer cell metastasis is a multi-stage process involving invasion into surrounding tissue, intravasation, transit in the blood or lymph, extravasation, and growth at a new site; many of these steps require cell motility. This invasive phenotype, characterized by both the loss of cell-cell interactions and increased cellular motility, is driven by cycles of actin polymerization, cell adhesion and acto-myosin contraction.
Tumor cell motility is an essential step in metastasis allowing cancer cells to spread through tissues and migrate to distant organs. Endocrine therapy has also been shown to have a positive effect on the treatment of advanced metastatic disease 57. Recent mechanistic studies have increased our understanding and highlight a role of estrogen-induced rapid ER extra-nuclear signaling in facilitating the metastatic process in breast cancer patients and may provide new targets for therapeutic interventions. ERα activation, by estrogen, induces key features of motile cells including rapid cytoskeletal reorganization and the development of specialized structures. Estrogen triggers rapid and dynamic actin cytoskeleton remodeling leading to increased breast cancer cell horizontal migration and invasion of three-dimensional matrices via the Gα13/RhoA/ROCK/moesin cascade 58. Estrogen-induced effects depend on the rapid recruitment and activation of the actin-binding protein, moesin, and the interaction of ERα with the G protein Gα13, which results in the recruitment of the small GTPase RhoA, subsequent activation of its downstream effector Rho-associated kinase-2 (ROCK-2) and moesin phosphorylation 58.
Recent studies also showed that estrogen-mediated extranuclear signaling promotes formation of signaling complexes containing PELP1, ERα, Src, and ILK1; signaling from this axis plays important roles in promoting cytoskeletal rearrangements, motility and metastasis 25. Extranuclear actions of estrogen facilitate the activation of ILK via the PI3K pathway and inhibition of ILK functions significantly affected the estrogen-mediated migratory potential of breast cancer cells. The proposed signaling pathway, ERα → PELP1 → PI3K → ILK → CDC42, contributes to estrogen-mediated cytoskeleton rearrangements 25. Emerging data regarding the impact of extranuclear signaling of estrogen on cytoskeletal organization suggests, ER-mediated control over cellular movement and invasion related to the catastrophic metastatic events in patients. Collectively, these results may in part explain carcinogenic actions and enhanced metastatic behavior of estrogen-dependent, ER-positive breast cancer seen clinically.
ERα extranuclear actions in cell survival and proliferation
The use of novel ligands with the ability to uniquely activate extranuclear signals demonstrated the distinct biological outcomes of the extranuclear pathway 42. Estrogen-dendrimer conjugate (EDC) which are excluded from the nucleus, verified ERα mediated extranuclear actions stimulates endothelial cell proliferation and migration via ERα direct interaction with Gαi and endothelial NOS (eNOS) activation 59. Estrogen promotes ternary complex formation of ER with Src- and PI3K and the resulting pathways converge on cell cycle progression leading to estrogen induced S-phase entry 25. Estrogen triggers cellular proliferation and survival through the activation of MAPK and AKT pathways respectively. Estrogen stimulation of cyclin D1 gene through ERK or PI3K activation promotes G1/S cell cycle progression in breast cancer cells 60. Estrogen-induced growth of breast and lung cancer cells in vitro correlated closely with acute hormonal activation of MAPK signaling 61. Ligand stimulation causes ERα to dissociate from caveolin-1 allowing the activation of signals to promote cellular proliferation 62–64. A recent study demonstrated that ERα promotes transcription of Bcl-2 via PI3K-AKT crosstalk leading to enhanced cell survival 65.
Significance of ER extranuclear signaling axis in breast cancer progression
Although much is known about ERα genomic actions, the pathobiology of ER extranuclear actions remains unknown. Some evidence suggests that the extranuclear effects of estrogen can regulate different cellular processes, such as proliferation, survival, apoptosis and differentiation functions in diverse cell-types, including breast cancer cells 66. In situ estrogen production by aromatase conversion from androgens plays an important role in breast tumor progression. ERα mediated extranuclear signaling enhances aromatase enzymatic activity via activation of the Src enzyme. These results suggested a possible autocrine loop between E2 and aromatase activity in breast cancer cells and implicate ERα actions in tumor progression 67. Molecular adaptors such as PELP1 which couple ERα to cytosolic signaling axis may play a role in breast tumorigenesis via activation of ERα extranuclear signaling pathways 68. Since breast tumors overexpress Src kinase, deregulation of PELP1 seen in breast tumors can contribute to activation of Src, leading to the progression to metastasis. ERα coregulator PELP1 acts as a scaffolding protein coupling the ERα with Src kinase leading to activation of the ER-Src-MAPK pathway 69. Extranuclear expression of ER/PR occurs frequently in ERα-positive/PR-negative and ER-negative/PR-positive tumors, and in these cases evidence implicates nuclear receptor crosstalk with the PI3K/AKT signaling pathway whose activation by ErbB2 overexpression contributes to the growth of some breast cancers 70. Dysregulation of ErbB2 in breast cancer cells enhances the expression of MTA1s, promotes the cytoplasmic sequestration of ERα and stimulates malignant phenotypes. These study findings implicate that the regulation of the cellular localization of ERα by MTA1s represents a mechanism for enhancing ERα extranuclear actions by nuclear exclusion 36. Methylated ERα is only present in the cytoplasm and arginine methylation is reversed by the demethylase JMJD6, suggesting deregulation of arginine methtylation and demethylation will have consequences in activation of ERα extranuclear actions. In addition, arginine methylation also regulates the balance between coactivator complex assembly and disassembly. Since methylation enzymes such PRMT1 and CARM1 are dysregulated in estrogen-dependent cancers, they are implicated in promoting ER extranuclear signaling 71.
ERα extranuclear actions and hormonal therapy resistance
ERα crosstalk with growth factor signaling play an important role in enhancing ER extranuclear signaling. ErbB2 is an oncogene that has been shown to be over expressed, amplified, or both, in breast tumors. ER expression occurs in ~50% ErbB2 positive breast cancers and crosstalk between the ERα and ErbB2 pathways promotes endocrine therapy resistance 72, 73. ERα-coregulator PELP1 plays an essential role in ERα extranuclear actions by coupling ERα with Src and PI3K pathways 69, 74. PELP1 interacts with growth factor signaling components and participates in ligand independent activation of ERα 75, 76. In our previous studies, we found that in a subset of breast tumors PELP1 is predominantly localized in the cytoplasm, breast cancer model cells mimicking PELP1 cytoplasmic expression showed resistance to tamoxifen via excessive activation of c-Src signaling axis 45. ERα extranuclear pathways have been shown to modify ERα or its coactivators by phosphorylation, resulting in the altered topology of ERα and its coregulator proteins and eventually leading to ligand-independent activation or differential responses to selective estrogen receptor modulators (9, 12). Forced expression of constitutively active AKT in MCF-7 cells promotes estrogen-independent growth as well as tamoxifen response 77. Overexpression of the ERα coactivator SRC3 promoted high tumor incidence, which is associated with the activation of the PI3K-AKT pathway 78. Extranuclear expression of ERα-coregulators such as PELP1 correlates with increases in extranuclear signaling and has the potential to be used as a determinant of hormone sensitivity or vulnerability 35. Recent findings suggest that ILK1 interacts with PELP1 25 and that such interactions enhance ILK1-kinase activity. Since PELP1 expression is commonly deregulated in many hormone-responsive tissues 79, the PELP1-ILK1 interaction is likely to have significant implications in tumor cell survival and therapy resistance. In cells developing resistance to estrogen deprivation by anti-estrogens/aromatase inhibitors, an increased association of ERα with c-Src and EGFR occurs. Further, these conditions promote translocation of ERα out of the nucleus and into the cytoplasm and cell membrane. This study suggested that secondary resistance to hormonal therapy results in usage of both IGFR and EGFR for ERα extranuclear signaling 80.
Therapeutic potential of targeting ER extranuclear actions
ERα extranuclear pathways promote hormone-mediated proliferation and survival of breast tumors making them a promising target for anti-tumor therapy via the combination of anti-estrogens and ER extranuclear signaling blockers 61. ERα extranuclear actions involve kinase cascades and post-translational modifications which can be reversed by pharmacological inhibitors currently in clinical trials. Inhibitors of EGFR, ERBB2, MAPK and AKT pathways could be used to block ER extranuclear signaling in ERα positive tumors that exhibit deregulation of these pathways 72, 81. Pharmacological inhibition of Src using dasatinib inhibits estrogen-mediated extranuclear actions and reduces estrogen-mediated migratory potential suggestive of the therapeutic value of dasatinib in blocking ER-positive metastases 25. ER extranuclear signaling utilizes the ILK axis and ILK inhibitor (QLT-0267) in combination with docetaxel exhibited synergistic effects on reducing the viability of breast cancer cells82. ILK inhibitors also have the potential to down regulate the ILK-mediated EMT phenotype and tumorigenesis. ER extranuclear actions mediate activation of STAT3/5, and ERα-STAT crosstalk is implicated in breast tumorigenesis and therapy resistance 46. STAT inhibitors currently in clinical trials could be used to block ER extranuclear actions. Since arginine methylation is involved in ERα extranuclear signaling, this modification is a possible therapeutic target by using guanidine nitrogen-substituted peptides or the thioglycolic amide, RM65 83, 84. As both ERα genomic and extranuclear signaling are involved in breast tumorigenesis and therapy resistance, a therapeutic approach to inhibit ER extranuclear actions along with current endocrine therapies could have better therapeutic efficacy and delay the on-set of hormonal resistance in advanced breast tumors.
Conclusions/significance
Emerging evidence suggests, in addition to genomic functions, ER participates in extranuclear rapid signaling via the formation of signaling complexes in the cytoplasm with both physiological and pathological consequences. The ability of ERα to participate in extranuclear actions, cytoplasmic localization of ERα and ERα co-activators in breast tumors and ERα-growth factor signaling crosstalk, strongly suggests that ERα extranuclear actions play a key role in breast tumor pathogenesis and development of therapy resistance. Future studies identifying molecular mechanisms of ERα extranuclear signaling and components of the signalosome contributing to ERα extranuclear signaling as well as to examining the prognostic/diagnostic significance of ERα extranuclear signaling using a larger tumor sample size are warranted. Further, elucidation of the normal and pathological roles of ERα extranuclear signaling will have important implications for breast cancer treatment and in the development of next generation estrogen receptor modulators.
Figure 1.
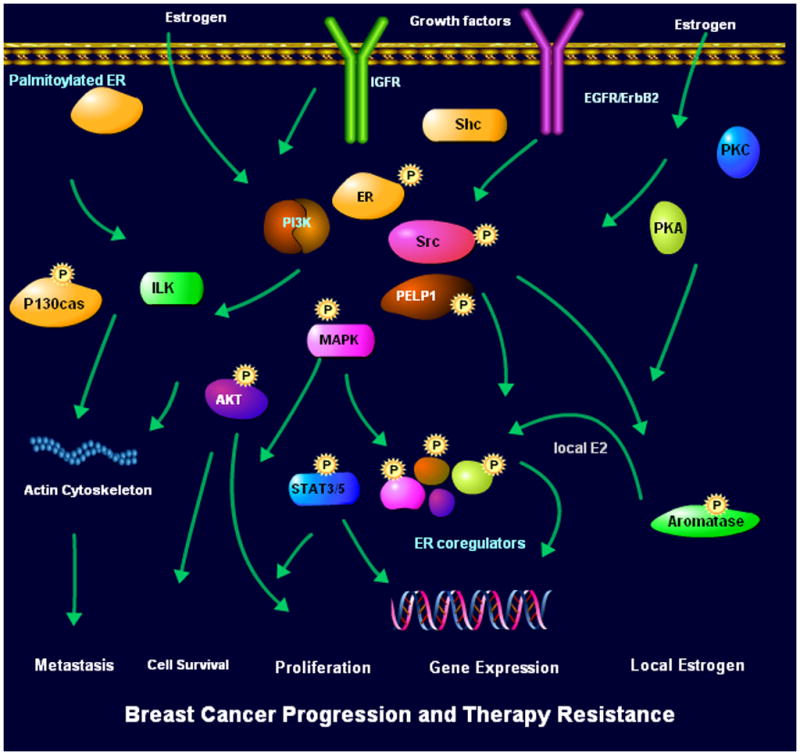
Schematic representation of the current understanding of ER extranuclear signaling. Estrogen and growth factors promote ER complex formation with growth factors signaling components and cytosolic kinases that lead to activation of a number of pathways including MAPK, PI3K and AKT. Extranuclear pathways influence several biological functions including cell survival, proliferation and motility. Deregulation of ER extranuclear signaling will have implications in tumor cell metastasis and tumor progression.
Acknowledgments
Funding: This work was supported by grants from the Susan G. Komen (RKV), DOD BC074432 (RKV), NIH pre-doctoral fellowship CA095681 (VC), NIH 5R01NS050730 (DWB).
Footnotes
Conflicts of interest: None
Reference List
- 1.Warner M, Nilsson S, Gustafsson JA. The estrogen receptor family. Curr Opin Obstet Gynecol. 1999;11:249–254. doi: 10.1097/00001703-199906000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Kumar V, Green S, Stack G, Berry M, Jin JR, Chambon P. Functional domains of the human estrogen receptor. Cell. 1987;51:941–951. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- 3.McKenna NJ, Lanz RB, O’Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 4.McDonnell DP, Norris JD. Connections and regulation of the human estrogen receptor. Science. 2002;296:1642–1644. doi: 10.1126/science.1071884. [DOI] [PubMed] [Google Scholar]
- 5.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bocchinfuso WP, Korach KS. Mammary gland development and tumorigenesis in estrogen receptor knockout mice. J Mammary Gland Biol Neoplasia. 1997;2:323–334. doi: 10.1023/a:1026339111278. [DOI] [PubMed] [Google Scholar]
- 7.Raz L, Khan MM, Mahesh VB, Vadlamudi RK, Brann DW. Rapid estrogen signaling in the brain. Neurosignals. 2008;16:140–53. doi: 10.1159/000111559. [DOI] [PubMed] [Google Scholar]
- 8.Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 9.Carroll JS, Brown M. Estrogen receptor target gene: an evolving concept. Mol Endocrinol. 2006;20:1707–1714. doi: 10.1210/me.2005-0334. [DOI] [PubMed] [Google Scholar]
- 10.Yang LC, Zhang QG, Zhou CF, Yang F, Zhang YD, Wang RM, Brann DW. Extranuclear estrogen receptors mediate the neuroprotective effects of estrogen in the rat hippocampus. PLoS One. 2010;5:e9851. doi: 10.1371/journal.pone.0009851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev. 2007;28:726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- 13.Fox EM, Andrade J, Shupnik MA. Novel actions of estrogen to promote proliferation: integration of cytoplasmic and nuclear pathways. Steroids. 2009;74:622–627. doi: 10.1016/j.steroids.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trevino JG, Summy JM, Gallick GE. SRC inhibitors as potential therapeutic agents for human cancers. Mini Rev Med Chem. 2006;6:681–687. doi: 10.2174/138955706777435724. [DOI] [PubMed] [Google Scholar]
- 15.Russello SV, Shore SK. SRC in human carcinogenesis. Front Biosci. 2004;9:139–144. doi: 10.2741/1138. [DOI] [PubMed] [Google Scholar]
- 16.Belcher SM, Le HH, Spurling L, Wong JK. Rapid estrogenic regulation of extracellular signal- regulated kinase 1/2 signaling in cerebellar granule cells involves a G protein- and protein kinase A-dependent mechanism and intracellular activation of protein phosphatase 2A. Endocrinology. 2005;146:5397–5406. doi: 10.1210/en.2005-0564. [DOI] [PubMed] [Google Scholar]
- 17.Keshamouni VG, Mattingly RR, Reddy KB. Mechanism of 17-beta-estradiol-induced Erk1/2 activation in breast cancer cells. A role for HER2 AND PKC-delta. J Biol Chem. 2002;277:22558–22565. doi: 10.1074/jbc.M202351200. [DOI] [PubMed] [Google Scholar]
- 18.Acconcia F, Manavathi B, Mascarenhas J, Talukder AH, Mills G, Kumar R. An inherent role of integrin-linked kinase-estrogen receptor alpha interaction in cell migration. Cancer Res. 2006;66:11030–11038. doi: 10.1158/0008-5472.CAN-06-2676. [DOI] [PubMed] [Google Scholar]
- 19.Song RX, Barnes CJ, Zhang Z, Bao Y, Kumar R, Santen RJ. The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor alpha to the plasma membrane. Proc Natl Acad Sci U S A. 2004;101:2076–2081. doi: 10.1073/pnas.0308334100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song RX, Fan P, Yue W, Chen Y, Santen RJ. Role of receptor complexes in the extranuclear actions of estrogen receptor alpha in breast cancer. Endocr Relat Cancer. 2006;13(Suppl 1):S3–13. doi: 10.1677/erc.1.01322. [DOI] [PubMed] [Google Scholar]
- 21.Martin MB, Franke TF, Stoica GE, Chambon P, Katzenellenbogen BS, Stoica BA, McLemore MS, Olivo SE, Stoica A. A role for Akt in mediating the estrogenic functions of epidermal growth factor and insulin-like growth factor I. Endocrinology. 2000;141:4503–4511. doi: 10.1210/endo.141.12.7836. [DOI] [PubMed] [Google Scholar]
- 22.Fox EM, Bernaciak TM, Wen J, Weaver AM, Shupnik MA, Silva CM. Signal transducer and activator of transcription 5b, c-Src, and epidermal growth factor receptor signaling play integral roles in estrogen-stimulated proliferation of estrogen receptor-positive breast cancer cells. Mol Endocrinol. 2008;22:1781–1796. doi: 10.1210/me.2007-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Binai NA, Damert A, Carra G, Steckelbroeck S, Lower J, Lower R, Wessler S. Expression of estrogen receptor alpha increases leptin-induced STAT3 activity in breast cancer cells. Int J Cancer. 2010;127:55–66. doi: 10.1002/ijc.25010. [DOI] [PubMed] [Google Scholar]
- 24.Acconcia F, Kumar R. Signaling regulation of genomic and nongenomic functions of estrogen receptors. Cancer Lett. 2005 doi: 10.1016/j.canlet.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Chakravarty D, Nair SS, Santhamma B, Nair BC, Wang L, Bandyopadhyay A, Agyin JK, Brann D, Sun LZ, Yeh IT, Lee FY, Tekmal RR, Kumar R, Vadlamudi RK. Extranuclear Functions of ER Impact Invasive Migration and Metastasis by Breast Cancer Cells. Cancer Res. 2010;70:4092–4101. doi: 10.1158/0008-5472.CAN-09-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le RM, Treilleux I, Leconte N, Robin-Lespinasse Y, Sentis S, Bouchekioua-Bouzaghou K, Goddard S, Gobert-Gosse S, Corbo L. Regulation of estrogen rapid signaling through arginine methylation by PRMT1. Mol Cell. 2008;31:212–221. doi: 10.1016/j.molcel.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 27.Marino M, Ascenzi P, Acconcia F. S-palmitoylation modulates estrogen receptor alpha localization and functions. Steroids. 2006;71:298–303. doi: 10.1016/j.steroids.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Cheskis BJ, Greger J, Cooch N, McNally C, McLarney S, Lam HS, Rutledge S, Mekonnen B, Hauze D, Nagpal S, Freedman LP. MNAR plays an important role in ERa activation of Src/MAPK and PI3K/Akt signaling pathways. Steroids. 2008;73:901–905. doi: 10.1016/j.steroids.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 29.Yamnik RL, Holz MK. mTOR/S6K1 and MAPK/RSK signaling pathways coordinately regulate estrogen receptor alpha serine 167 phosphorylation. FEBS Lett. 2010;584:124–128. doi: 10.1016/j.febslet.2009.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kok M, Zwart W, Holm C, Fles R, Hauptmann M, Van’t Veer LJ, Wessels LF, Neefjes J, Stal O, Linn SC, Landberg G, Michalides R. PKA-induced phosphorylation of ERalpha at serine 305 and high PAK1 levels is associated with sensitivity to tamoxifen in ER-positive breast cancer. Breast Cancer Res Treat. 2010 doi: 10.1007/s10549-010-0798-y. [DOI] [PubMed] [Google Scholar]
- 31.Garban HJ, Marquez-Garban DC, Pietras RJ, Ignarro LJ. Rapid nitric oxide-mediated S-nitrosylation of estrogen receptor: regulation of estrogen-dependent gene transcription. Proc Natl Acad Sci U S A. 2005;102:2632–2636. doi: 10.1073/pnas.0409854102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azuma K, Urano T, Horie-Inoue K, Hayashi S, Sakai R, Ouchi Y, Inoue S. Association of estrogen receptor alpha and histone deacetylase 6 causes rapid deacetylation of tubulin in breast cancer cells. Cancer Res. 2009;69:2935–2940. doi: 10.1158/0008-5472.CAN-08-3458. [DOI] [PubMed] [Google Scholar]
- 33.Cabodi S, Moro L, Baj G, Smeriglio M, Di SP, Gippone S, Surico N, Silengo L, Turco E, Tarone G, Defilippi P. p130Cas interacts with estrogen receptor alpha and modulates non-genomic estrogen signaling in breast cancer cells. J Cell Sci. 2004;117:1603–1611. doi: 10.1242/jcs.01025. [DOI] [PubMed] [Google Scholar]
- 34.Song RX, Santen RJ. Membrane initiated estrogen signaling in breast cancer. Biol Reprod. 2006;75:9–16. doi: 10.1095/biolreprod.105.050070. [DOI] [PubMed] [Google Scholar]
- 35.Kumar R, Zhang H, Holm C, Vadlamudi RK, Landberg G, Rayala SK. Extranuclear coactivator signaling confers insensitivity to tamoxifen. Clin Cancer Res. 2009;15:4123–4130. doi: 10.1158/1078-0432.CCR-08-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar R, Wang RA, Mazumdar A, Talukder AH, Mandal M, Yang Z, Bagheri-Yarmand R, Sahin A, Hortobagyi G, Adam L, Barnes CJ, Vadlamudi RK. A naturally occurring MTA1 variant sequesters oestrogen receptor-alpha in the cytoplasm. Nature. 2002;418:654–657. doi: 10.1038/nature00889. [DOI] [PubMed] [Google Scholar]
- 37.Kim SH, Katzenellenbogen JA. Hormone-PAMAM dendrimer conjugates: polymer dynamics and tether structure affect ligand access to receptors. Angew Chem Int Ed Engl. 2006;45:7243–7248. doi: 10.1002/anie.200601923. [DOI] [PubMed] [Google Scholar]
- 38.Chambliss KL, Wu Q, Oltmann S, Konaniah ES, Umetani M, Korach KS, Thomas GD, Mineo C, Yuhanna IS, Kim SH, Madak-Erdogan Z, Maggi A, Dineen SP, Roland CL, Hui DY, Brekken RA, Katzenellenbogen JA, Katzenellenbogen BS, Shaul PW. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest. 2010;120:2319–2330. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madak-Erdogan Z, Kieser KJ, Kim SH, Komm B, Katzenellenbogen JA, Katzenellenbogen BS. Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol Endocrinol. 2008;22:2116–2127. doi: 10.1210/me.2008-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang QG, Raz L, Wang R, Han D, De SL, Yang F, Vadlamudi RK, Brann DW. Estrogen attenuates ischemic oxidative damage via an estrogen receptor alpha-mediated inhibition of NADPH oxidase activation. J Neurosci. 2009;29:13823–13836. doi: 10.1523/JNEUROSCI.3574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trogden BG, Kim SH, Lee S, Katzenellenbogen JA. Tethered indoles as functionalizable ligands for the estrogen receptor. Bioorg Med Chem Lett. 2009;19:485–488. doi: 10.1016/j.bmcl.2008.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol. 2006;20:491–502. doi: 10.1210/me.2005-0186. [DOI] [PubMed] [Google Scholar]
- 43.Kinoshita Y, Chen S. Induction of aromatase (CYP19) expression in breast cancer cells through a nongenomic action of estrogen receptor alpha. Cancer Res. 2003;63:3546–3555. [PubMed] [Google Scholar]
- 44.Zheng FF, Wu RC, Smith CL, O’Malley BW. Rapid estrogen-induced phosphorylation of the SRC-3 coactivator occurs in an extranuclear complex containing estrogen receptor. Mol Cell Biol. 2005;25:8273–8284. doi: 10.1128/MCB.25.18.8273-8284.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vadlamudi RK, Manavathi B, Balasenthil S, Nair SS, Yang Z, Sahin AA, Kumar R. Functional implications of altered subcellular localization of PELP1 in breast cancer cells. Cancer Res. 2005;65:7724–7732. doi: 10.1158/0008-5472.CAN-05-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bjornstrom L, Sjoberg M. Signal transducers and activators of transcription as downstream targets of nongenomic estrogen receptor actions. Mol Endocrinol. 2002;16:2202–2214. doi: 10.1210/me.2002-0072. [DOI] [PubMed] [Google Scholar]
- 47.Dos Santos EG, Dieudonne MN, Pecquery R, Le MV, Giudicelli Y, Lacasa D. Rapid nongenomic E2 effects on p42/p44 MAPK, activator protein-1, and cAMP response element binding protein in rat white adipocytes. Endocrinology. 2002;143:930–940. doi: 10.1210/endo.143.3.8678. [DOI] [PubMed] [Google Scholar]
- 48.Gutzman JH, Nikolai SE, Rugowski DE, Watters JJ, Schuler LA. Prolactin and estrogen enhance the activity of activating protein 1 in breast cancer cells: role of extracellularly regulated kinase 1/2-mediated signals to c-fos. Mol Endocrinol. 2005;19:1765–1778. doi: 10.1210/me.2004-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sisci D, Aquila S, Middea E, Gentile M, Maggiolini M, Mastroianni F, Montanaro D, Ando S. Fibronectin and type IV collagen activate ERalpha AF-1 by c-Src pathway: effect on breast cancer cell motility. Oncogene. 2004;23:8920–8930. doi: 10.1038/sj.onc.1208098. [DOI] [PubMed] [Google Scholar]
- 50.Thompson EW, Reich R, Shima TB, Albini A, Graf J, Martin GR, Dickson RB, Lippman ME. Differential regulation of growth and invasiveness of MCF-7 breast cancer cells by antiestrogens. Cancer Res. 1988;48:6764–6768. [PubMed] [Google Scholar]
- 51.Koenders PG, Beex LV, Langens R, Kloppenborg PW, Smals AG, Benraad TJ. Steroid hormone receptor activity of primary human breast cancer and pattern of first metastasis. The Breast Cancer Study Group. Breast Cancer Res Treat. 1991;18:27–32. doi: 10.1007/BF01975440. [DOI] [PubMed] [Google Scholar]
- 52.Harrell JC, Dye WW, Allred DC, Jedlicka P, Spoelstra NS, Sartorius CA, Horwitz KB. Estrogen receptor positive breast cancer metastasis: altered hormonal sensitivity and tumor aggressiveness in lymphatic vessels and lymph nodes. Cancer Res. 2006;66:9308–9315. doi: 10.1158/0008-5472.CAN-06-1769. [DOI] [PubMed] [Google Scholar]
- 53.Zheng WQ, Lu J, Zheng JM, Hu FX, Ni CR. Variation of ER status between primary and metastatic breast cancer and relationship to p53 expression*. Steroids. 2001;66:905–910. doi: 10.1016/s0039-128x(01)00121-0. [DOI] [PubMed] [Google Scholar]
- 54.Koenders PG, Beex LV, Langens R, Kloppenborg PW, Smals AG, Benraad TJ. Steroid hormone receptor activity of primary human breast cancer and pattern of first metastasis. The Breast Cancer Study Group. Breast Cancer Res Treat. 1991;18:27–32. doi: 10.1007/BF01975440. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Jarrett J, Huang CC, Satcher RL, Jr, Levenson AS. Identification of estrogen-responsive genes involved in breast cancer metastases to the bone. Clin Exp Metastasis. 2007;24:411–422. doi: 10.1007/s10585-007-9078-6. [DOI] [PubMed] [Google Scholar]
- 56.Banka CL, Lund CV, Nguyen MT, Pakchoian AJ, Mueller BM, Eliceiri BP. Estrogen induces lung metastasis through a host compartment-specific response. Cancer Res. 2006;66:3667–3672. doi: 10.1158/0008-5472.CAN-05-4416. [DOI] [PubMed] [Google Scholar]
- 57.Utsumi T, Kobayashi N, Hanada H. Recent perspectives of endocrine therapy for breast cancer. Breast Cancer. 2007;14:194–199. doi: 10.2325/jbcs.959. [DOI] [PubMed] [Google Scholar]
- 58.Giretti MS, Fu XD, De RG, Sarotto I, Baldacci C, Garibaldi S, Mannella P, Biglia N, Sismondi P, Genazzani AR, Simoncini T. Extra-nuclear signalling of estrogen receptor to breast cancer cytoskeletal remodelling, migration and invasion. PLoS One. 2008;3:e2238. doi: 10.1371/journal.pone.0002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chambliss KL, Wu Q, Oltmann S, Konaniah ES, Umetani M, Korach KS, Thomas GD, Mineo C, Yuhanna IS, Kim SH, Madak-Erdogan Z, Maggi A, Dineen SP, Roland CL, Hui DY, Brekken RA, Katzenellenbogen JA, Katzenellenbogen BS, Shaul PW. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest. 2010;120:2319–2330. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fu XD, Cui YH, Lin GP, Wang TH. Non-genomic effects of 17beta-estradiol in activation of the ERK1/ERK2 pathway induces cell proliferation through upregulation of cyclin D1 expression in bovine artery endothelial cells. Gynecol Endocrinol. 2007;23:131–137. doi: 10.1080/09513590601181457. [DOI] [PubMed] [Google Scholar]
- 61.Pietras RJ, Marquez DC, Chen HW, Tsai E, Weinberg O, Fishbein M. Estrogen and growth factor receptor interactions in human breast and non-small cell lung cancer cells. Steroids. 2005;70:372–381. doi: 10.1016/j.steroids.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 62.Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Mol Biol Cell. 2005;16:231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Galluzzo P, Caiazza F, Moreno S, Marino M. Role of ERbeta palmitoylation in the inhibition of human colon cancer cell proliferation. Endocr Relat Cancer. 2007;14:153–167. doi: 10.1677/ERC-06-0020. [DOI] [PubMed] [Google Scholar]
- 64.Marino M, Ascenzi P. Membrane association of estrogen receptor alpha and beta influences 17beta-estradiol-mediated cancer cell proliferation. Steroids. 2008;73:853–858. doi: 10.1016/j.steroids.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 65.Bratton MR, Duong BN, Elliott S, Weldon CB, Beckman BS, McLachlan JA, Burow ME. Regulation of ERalpha-mediated transcription of Bcl-2 by PI3K-AKT crosstalk: implications for breast cancer cell survival. Int J Oncol. 2010;37:541–550. doi: 10.3892/ijo_00000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Acconcia F, Kumar R. Signaling regulation of genomic and nongenomic functions of estrogen receptors. Cancer Lett. 2005 doi: 10.1016/j.canlet.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 67.Catalano S, Barone I, Giordano C, Rizza P, Qi H, Gu G, Malivindi R, Bonofiglio D, Ando S. Rapid estradiol/ERalpha signaling enhances aromatase enzymatic activity in breast cancer cells. Mol Endocrinol. 2009;23:1634–1645. doi: 10.1210/me.2009-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vadlamudi RK, Kumar R. Functional and biological properties of the nuclear receptor coregulator PELP1/MNAR. Nucl Recept Signal. 2007;5:e004. doi: 10.1621/nrs.05004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barletta F, Wong CW, McNally C, Komm BS, Katzenellenbogen B, Cheskis BJ. Characterization of the interactions of estrogen receptor and MNAR in the activation of cSrc. Mol Endocrinol. 2004;18:1096–1108. doi: 10.1210/me.2003-0335. [DOI] [PubMed] [Google Scholar]
- 70.Kim R, Kaneko M, Arihiro K, Emi M, Tanabe K, Murakami S, Osaki A, Inai K. Extranuclear expression of hormone receptors in primary breast cancer. Ann Oncol. 2006;17:1213–1220. doi: 10.1093/annonc/mdl118. [DOI] [PubMed] [Google Scholar]
- 71.Teyssier C, Le RM, Sentis S, Jalaguier S, Corbo L, Cavailles V. Protein arginine methylation in estrogen signaling and estrogen-related cancers. Trends Endocrinol Metab. 2010;21:181–189. doi: 10.1016/j.tem.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 72.Schiff R, Massarweh SA, Shou J, Bharwani L, Arpino G, Rimawi M, Osborne CK. Advanced concepts in estrogen receptor biology and breast cancer endocrine resistance: implicated role of growth factor signaling and estrogen receptor coregulators. Cancer Chemother Pharmacol. 2005;56(Suppl 1):10–20. doi: 10.1007/s00280-005-0108-2. [DOI] [PubMed] [Google Scholar]
- 73.Marcom PK, Isaacs C, Harris L, Wong ZW, Kommarreddy A, Novielli N, Mann G, Tao Y, Ellis MJ. The combination of letrozole and trastuzumab as first or second-line biological therapy produces durable responses in a subset of HER2 positive and ER positive advanced breast cancers. Breast Cancer Res Treat. 2007;102:43–49. doi: 10.1007/s10549-006-9307-8. [DOI] [PubMed] [Google Scholar]
- 74.Wong CW, McNally C, Nickbarg E, Komm BS, Cheskis BJ. Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with Src/Erk phosphorylation cascade. Proc Natl Acad Sci U S A. 2002;99:14783–14788. doi: 10.1073/pnas.192569699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 75.Manavathi B, Nair SS, Wang RA, Kumar R, Vadlamudi RK. Proline-, glutamic acid-, and leucine-rich protein-1 is essential in growth factor regulation of signal transducers and activators of transcription 3 activation. Cancer Res. 2005;65:5571–5577. doi: 10.1158/0008-5472.CAN-04-4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nagpal J, Nair S, Pothana S, tekmal R, Kumar R, Vadlamudi R. Growth factor regulation of PELP1/MNAR functions:Role of PKA-dependent phosphorylation. 2006:2933. [Google Scholar]
- 77.Faridi J, Wang L, Endemann G, Roth RA. Expression of constitutively active Akt-3 in MCF-7 breast cancer cells reverses the estrogen and tamoxifen responsivity of these cells in vivo. Clin Cancer Res. 2003;9:2933–2939. [PubMed] [Google Scholar]
- 78.Torres-Arzayus MI, Font de MJ, Yuan J, Vazquez F, Bronson R, Rue M, Sellers WR, Brown M. High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell. 2004;6:263–274. doi: 10.1016/j.ccr.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 79.Chakravarty D, Tekmal RR, Vadlamudi RK. PELP1: A novel therapeutic target for hormonal cancers. IUBMB Life. 2010;62:162–169. doi: 10.1002/iub.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song RX, Chen Y, Zhang Z, Bao Y, Yue W, Wang JP, Fan P, Santen RJ. Estrogen utilization of IGF-1-R and EGF-R to signal in breast cancer cells. J Steroid Biochem Mol Biol. 2010;118:219–230. doi: 10.1016/j.jsbmb.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gururaj AE, Rayala SK, Vadlamudi RK, Kumar R. Novel mechanisms of resistance to endocrine therapy: genomic and nongenomic considerations. Clin Cancer Res. 2006;12:1001s–1007s. doi: 10.1158/1078-0432.CCR-05-2110. [DOI] [PubMed] [Google Scholar]
- 82.Kalra J, Warburton C, Fang K, Edwards L, Daynard T, Waterhouse D, Dragowska W, Sutherland BW, Dedhar S, Gelmon K, Bally M. QLT0267, a small molecule inhibitor targeting integrin-linked kinase (ILK), and docetaxel can combine to produce synergistic interactions linked to enhanced cytotoxicity, reductions in P-AKT levels, altered F-actin architecture and improved treatment outcomes in an orthotopic breast cancer model. Breast Cancer Res. 2009;11:R25. doi: 10.1186/bcr2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Spannhoff A, Machmur R, Heinke R, Trojer P, Bauer I, Brosch G, Schule R, Hanefeld W, Sippl W, Jung M. A novel arginine methyltransferase inhibitor with cellular activity. Bioorg Med Chem Lett. 2007;17:4150–4153. doi: 10.1016/j.bmcl.2007.05.088. [DOI] [PubMed] [Google Scholar]
- 84.Lakowski TM, ‘t HP, Ahern CA, Martin NI, Frankel A. N(eta)-Substituted Arginyl Peptide Inhibitors of Protein Arginine N-Methyltransferases. ACS Chem Biol. 2010 doi: 10.1021/cb100161u. [DOI] [PubMed] [Google Scholar]


