Abstract
Natural products are important sources of anti-cancer lead molecules, and high dietary consumption of fruits and vegetables is associated with a reduced risk of certain cancers. Many efforts have been devoted to identifying and developing plant-derived dietary constituents as chemopreventive agents. Among them, apigenin, a naturally occurring flavonoid found in a variety of fruits and leafy vegetables, has been shown to possess remarkable anti-oxidant, anti-inflammatory and anti-carcinogenic properties. This review summarizes the anti-cancer and chemopreventive effects of apigenin at cellular and molecular levels, its chemical structure and properties, with focus on mechanism related to apigenin’s inhibition of the PI3K/Akt/mTOR signaling pathways.
Keywords: Akt, mTOR, apigenin, cancer
1. Introduction
Cancer is a group of diseases characterized by uncontrolled growth and spread of malignant cells. In the United States alone, in addition to more than 2 million skin cancers that are diagnosed annually, a total of 1,638,910 new cancer cases and 577,190 deaths are expected to be reported in the year 2012 [1]. Cancer has become a major public health burden, and currently major treatments for cancer are still surgery, chemotherapy, radiation therapy and immunotherapy [2–4]. However, drug- and radiation-related harmful side effects are common, and not all cancers are surgically curable. In contrast, cancer chemoprevention is a rapidly growing approach that uses naturally occurring or synthetic agents to prevent, inhibit or reverse tumorigenesis or suppress the development of invasive cancer [5–7]. This approach decreases the incidence of cancer and deaths from cancer at an early stage and relies on prevention rather than cure. It is also well known that many human cancers are induced by environmental factors including chemical, radioactive and biological factors, and there are significant differences in the cancer incidence and mortality rates among different racial and ethnic groups that have different lifestyles and have been exposed to different environmental factors [1]. Epidemiologic studies have shown an inverse association between consumption of vegetables/fruits and risk of human cancers at many sites [8–11]. Some dietary components such as dietary fiber, micronutrients and polyphenolic compounds have shown inhibitory effects on human cancers [12–14].
Polyphenolic compounds are produced as the result of the secondary metabolism of plants and are frequently found attached to sugars (glycosides), although occasionally polyphenols occur in plants as aglycones [15]. More than 8000 ployphenolic structures are know so far, and polyphenols are divided into at least 10 different classes based on their structure [16, 17]. Flavonoids are the largest class of polyphenols, comprising about 5000 compounds with a common structure of diphenylpropanes (C6-C3-C6) and one or more hydroxyl substitutes. Flavonoids can be further subdivided into six major subclasses, namely flavones, isoflavones, flavonols, flavanones, flavanols (catechins), and anthocyanidins [17–19]. Flavonoids are widely distributed and ubiquitously present in foods of plant origin, such as vegetables, fruits, tea, cocoa, etc [15]. The subclasses of flavones and flavonols are structurally similar compounds, with flavonols having an extra hydroxyl substitution at the carbon-3 position, and flavone apigenin and flavonol quercetin are the frequently occurring compounds in foods [15].
Flavonoids have been the focus of a great deal of scientific interest because they exert a variety of biological effects, such as free radical scavenging, anti-oxidant activity, anti-inflammation, anti-cancer activity, as well modulating enzymatic activity, inhibiting cellular proliferation, inducing of apoptosis, inhibiting platelet aggregation and reducing plasma levels of low-density lipoproteins [19–22]. These effects may help to explain flavonoids’ potential benefit in cancer chemoprevention. In this article, we explore one common flavonoid – apigenin - and its chemical structure and properties, specifically focusing on mechanism related to apigenin’s anti-cancer and chemopreventive effects at cellular and molecular levels, particularly apigenin’s inhibition of the phosphatidylinositol 3-kinases (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling pathways.
2. Chemical structure and properties of apigenin
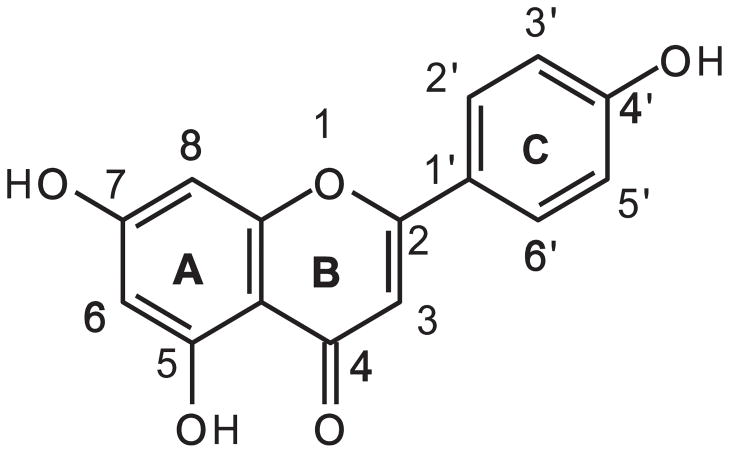
Apigenin (5,7,4′-trihydroxyflavone) is a naturally occurring flavonoid (Fig. (1)) belonging to the flavone subclass and is abundantly present in common fruits (oranges, apples, cherries, grapes), vegetables (onions, parsley, broccoli, sweet green pepper, celery, barley, tomatoes) and beverages (tea, wine) [15, 23, 24]. Apigenin was first structurally identified in 1900, and synthesized in 1939 [25]. In its pure form, apigenin is a yellow crystalline solid, with a melting point of 347.5 °C and molecular weight of 270.24 g/mol [23]. Apigenin is soluble in dimethylsulfoxide (DMSO) (>100 mg/mL), it is slightly soluble in acetone and alcohols such as ethanol, n-octanol, and propylene glycol (1.02–1.63 mg/mL). Apigenin is practically insoluble in highly polar solvents such as water (0.00135 mg/mL), and nonpolar solvents such as silicon fluid (0.0728 mg/mL) and safflower oil (0.0317 mg/mL) [25]. In its natural form, apigenin is present in foods mostly as glycoside conjugates, which are more water soluble than its pure form [15]. These glycosides are efficiently hydrolyzed in vivo by bacterial enzymes in the human intestinal tract to the free flavonoids [26].
Fig. 1.
Chemical structure of apigenin.
Recent reports have shown that bioavailability of specific flavonoids can be quite substantial. Dietary intakes of flavonoids in humans vary from low in Western countries (e.g. 13 mg/day in the USA) to high in Asia (e.g. 64 mg/day in Japan) [27]. Apigenin appears to be absorbable by humans after intake of parsley, an apigenin-rich food. In a randomized crossover study with two one-week intervention periods in succession, volunteers consumed a diet that included 20 g/day parsley. The urinary excretion of apigenin was significantly higher in the parsley-consumption group than in the basic diet control group. The half-life for apigenin was calculated to be on the order of 12 h, although significant individual variation in the bioavailability and excretion of apigenin was observed [28]. Apigenin derived from aqueous alcoholic extracts of chamomile flower heads was found to be concentrated in the stratum corneum within the first 2 h of dermal exposure in human subjects. After 3 h, a steady state was attained, suggesting that apigenin diffuses through deeper skin layers to be absorbed afterwards by cutaneous blood and lymph vessels [29]. Apigenin feeding by gavage to mice at a dose of 20 μg/mouse/day has been reported to achieve 0.63–0.78 μM apigenin in plasma [30, 31], and this dose is comparable to the daily consumption of flavonoid in humans as reported previously [9, 32]. Cai et al. compared tissue and plasma levels of apigenin fed to mice in diet containing apigenin, and reported that feeding 0.2% apigenin in diet for 7 days achieved steady-state tissue concentrations of 1.5 μM and 86 μM in the liver and small intestinal mucosa, respectively [33].
Compared to other flavonoids, such as quercetin, apigenin is relatively nontoxic and nonmutagenic [34, 35]. Birt et al. first reported that apigenin has anti-mutagenesis and anti-promotion properties in mouse skin [36]. Her laboratory further demonstrated that apigenin can inhibit skin cancer induced by either ultraviolet (UV) radiation [37] or chemicals [38] in mice. In these studies they found that topical application of apigenin to mouse skin resulted in inhibition of UV- and 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced ornithine decarboxylase (ODC) activity, a tumor promotion marker, and reduced cancer incidence as well as increased cancer-free survival in mice [37, 38]. These initial studies with apigenin generate further interest in the development of apigenin as a chemopreventive agent.
3. Molecular mechanisms of apigenin’s chemopreventive activity
The chemopreventive effect of apigenin is not limited to the skin carcinogenesis model. Following the reports by Birt et al.[37, 38], studies in numerous laboratories showed that apigenin displayed a wide variety of anti-carcinogenic effects in breast, prostate, colon, and cervical cancer cells, etc (ref. [39] and references therein). Table 1 shows the effect of apigenin on various human cancers. In short, apigenin’s chemopreventive activity in multiple organ sites is likely tied to its anti-mutagenic, anti-oxidant, anti-inflammatory, and anti-carcinogenic properties.
Table 1.
Protective effects of apigenin on different human cancers and cell lines
| Cancer types | Apigenin’s effects | Literature references |
|---|---|---|
| Breast cancer | Inhibition of proliferation, induction of apoptosis, suppression of cell invasion, anti-estrogenic activities | [40–43] |
| Prostate cancer | Induction of apoptosis and G1 phase arrest of cell cycle, inhibition of cell growth, suppression of HIF-1α expression, inhibition of VEGF, inhibition of FAK/src | [44–48] |
| Colon cancer | Inhibition of cell growth, induction of G2/M cell cycle arrest, increase in the stability of p53 protein, induction of apoptosis | [49–51] |
| Cervical cancer | Inhibition of cell growth through G1 cell cycle arrest and apoptosis, suppression of motility and invasion | [52, 53] |
| Skin cancer | Induction of autophagy, enhancement of apoptosis, inhibition of COX-2 expression, suppression of MMP-1 production | [54–57] |
| Lung cancer | Suppression of VEGF transcriptional activation, induction of apoptosis | [58, 59] |
| Ovarian cancer | Inhibition of proliferation and VEGF expression, suppression of migration and invasion | [60–62] |
| Liver cancer | Inhibition of cell growth, induction of apoptosis, enhancement of radiation-induced cell death | [63–65] |
| Pancreatic cancer | Inhibition of Focal Adhesion kinase activation, suppression of HIF-1α, VEGF and Geminin expression, induction of G2/M cell cycle arrest | [66–69] |
| Hematologic cancer | Inhibition of proliferation, induction of apoptosis, suppression of telomerase activity | [70, 71] |
The anti-mutagenic effect of apigenin has been shown to inhibit benzo[a]pyrene and 2-aminoanthracene-induced bacterial mutagenesis [36, 72]. Apigenin was also shown to be anti-mutagenic in the Ames assay and in Chinese hamster V79 cells [36]. Apigenin significantly induces glutathione S-transferase (GST), an enzyme which protects cells against free-radical damage by increasing resistance to oxidative stress caused by hydrogen peroxide [73]. GST also plays a protective role against cancer by detoxifying xenobiotics with mutagenic potential [74]. Therefore, apigenin may both relieve oxidative stress and aid in the detoxification of mutagenic xenobiotics.
The anti-inflammatory effect of apigenin has been demonstrated in numerous studies. Apigenin suppresses TPA-mediated cyclooxygenase-2 (COX-2) expression by blocking Akt signal transduction and arachidonic acid release in human keratinocytes [56]. COX-2 is a key enzyme in the conversion of arachidonic acid to prostaglandins, and COX-2 overexpression plays an important role in carcinogenesis [75]. In mouse keratinocytes, research has shown that one pathway by which apigenin inhibits UV-induced COX-2 expression is through modulation of Upstream Stimulatory Factor (USF) transcriptional activity in the 5′ upstream region of the COX-2 gene [76]. Furthermore, two RNA-binding proteins, HuR and the T-cell-restricted intracellular antigen 1-related protein (TIAR), were found to be associated with endogenous COX-2 mRNA, and apigenin treatment increased their translocation to cell cytoplasm. More importantly, cells expressing reduced TIAR showed marked resistance to apigenin’s ability to inhibit UVB-induced COX-2 expression [77]. Taken together, these results indicate that in addition to transcriptional regulation and inhibition of Akt, another mechanism by which apigenin prevents COX-2 expression is through mediating TIAR suppression of translation. In another study, Nicholas et al. reported that apigenin blocked proinflammatory cytokine expression (such as IL-1beta, IL-8, and TNF) by inactivating NF-kappaB through the suppression of p65 phosphorylation [78].
The anti-carcinogenic properties of apigenin are related to its ability to modulate key targets and pathways involved in cell cycle control, apoptosis, angiogenesis, tumor cell invasion and metastasis, and signal transduction [39]. Studies have provided evidence that apigenin inhibits cell growth by inducing a reversible G2/M arrest and that this arrest was associated, at least in part, with inhibited activity of p34(cdc2) kinase and reduced accumulation of p34(cdc2) and cyclin B1 proteins [49, 79], which was also found independent of p21/WAF1 protein [80]. In addition, apigenin treatment produced a G1 cell cycle arrest by inhibiting cdk2 kinase activity and the phosphorylation of Rb, and inducing the cyclin-dependent kinase (cdk) inhibitor p21/WAF1 [81]. Since p21/WAF1 is a well known downstream effector of the p53 tumor suppressor gene, it is not surprising to find that apigenin increased wild-type p53 protein expression by a mechanism involving p53 protein stabilization [82] and enhancement of p53 mRNA translation through the RNA binding protein HuR [83].
Apigenin has been shown to induce apoptosis in different types of cells [46, 70, 84, 85]. In human keratinocytes and organotypic keratinocyte cultures, apigenin treatment enhanced UVB-induced apoptosis more than 2-fold. In addition, apigenin stimulated changes in Bax localization, and increased the release of cytochrome c from the mitochondria. Overexpression of the antiapoptotic protein Bcl-2 and expression of a dominant-negative form of Fas-associated death domain led to a reduction in apigenin-induced apoptosis, demonstrating that enhancement of UVB-induced apoptosis by apigenin treatment involves both the intrinsic and extrinsic apoptotic pathways [55]. In human prostate cancer cells, apigenin treatment has been shown to alter the Bax/Bcl-2 ratio in favor of apoptosis [46]. In human promyelocytic leukemia HL-60 cells, apigenin induced caspase-3 activity and cleavage of poly-(ADP-ribose) polymerase (PARP), reduced mitochondrial transmembrane potential, released mitochondrial cytochrome c into the cytosol, and subsequently induced procaspase-9 processing [70]. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a promising anticancer agent that kills various tumor cells without damaging normal tissues. However, many cancers remain resistant to TRAIL. Apigenin breaks TRAIL resistance by transcriptional down-regulation of c-FLIP, a key inhibitor of death receptor signaling, and by up-regulation of TRAIL receptor 2 [51].
Tumor angiogenesis is the proliferation of a network of blood vessels that penetrates into cancerous growths, supplying nutrients and oxygen and removing waste products [86]. Given the role of angiogenesis in tumor growth and progression, apigenin has been tested for inhibition of angiogenesis. Apigenin has been reported to be a potent angiogenesis inhibitor through its inhibitory effect on the inflammatory cytokine IL-6/STAT3 pathway. Apigenin also modulated the activation of extracellular signal-regulated kinase-1/2 (ERK) signaling triggered by IL-6, as well markedly reducing proliferation, migration and morphogenic differentiation of endothelial cells. More interestingly, it also modulated the expression of IL-6 signal transducing receptor (IL-6Rα) and the secretion of the extracellular matrix degrading enzyme MMP-2 [87]. Platelet-derived growth factor (PDGF)-dependent recruitment of mural cells such as pericytes and smooth muscle cells plays a central role in the maturation and stabilization of newly formed vasculature during angiogenesis. Lamy et al. demonstrated that apigenin interfered with this event through its inhibitory effect on PDGF-dependent phosphorylation of PDGF receptor beta (PDGFR-beta) [88]. In another study, Fang et al. showed that apigenin inhibits tumor angiogenesis through decreasing hypoxia-inducible factor-1 (HIF-1) and vascular endothelial growth factor (VEGF) expression in different types of cancer cells via the PI3K/Akt/p70S6K1 and HDM/p53 pathways [61].
Most cancer deaths are attributed to metastatic disease rather than primary, organ-confined cancers. Apigenin inhibits migration and invasion in breast cancer cells [89] and melanoma cells [90]. To study the effect of apigenin via oral administration on tumor growth and metastasis, He et al. developed an orthotopic ovarian tumor model in nude mice, and found that apigenin inhibited the micrometastasis of cancer cell by blocking MMP-9 expression, which was mediated by the Akt/p70S6K1 pathway [91]. Apigenin also inhibited expression of focal adhesion kinase (FAK) and migration and invasion of human ovarian cancer A2780 cells [62]. Similarly, Franzen et al. also observed that apigenin inhibited FAK activation and altered cell cytoskeleton in human prostate cancer cells (PC3-M). Overexpression of constitutively active Src blunted the effect of apigenin on cell motility and cytoskeleton remodeling [48].
Apigenin has also been shown to counteract tumor promoter-mediated inhibition of intercellular communication. When exposed to apigenin and either TPA or butylated hydroxytoluene (BHT), rat liver epithelial cells exhibited an increase in gap junctional intercellular communication (GJIC), reversing the GJIC inhibition mediated by the tumor promoters [92].
As mentioned above, anti-carcinogenic properties of apigenin have been attributed to its ability to inhibit UV- and chemical-induced ODC activity [37, 38]. In addition, apigenin has been found to modulate other enzymatic activity related to tumorigenesis. For example, Le Bail et al. demonstrated that apigenin was an effective inhibitor of aromatase (human estrogen synthetase) and 17β-hydroxysteroid dehydrogenase activities in human placental microsomes, suggesting that it may be beneficial in treatment of human breast cancer [93]. In keratinocyte and colon carcinoma cell lines, apigenin induced a dose-dependent phosphorylation of both (ERK) and p38 kinase but had little effect on the phosphorylation of c-jun amino terminal kinase (JNK), and immunoprecipitation-coupled kinase assays showed that apigenin increased the kinase activity of ERK and p38 but not JNK [94]. Apigenin has further been shown to inhibit casein kinase (CK)-2 in both breast and prostate cancer cells [95, 96]. In addition, apigenin significantly inhibited protein kinase C (PKC) activity [97], and in the human anaplastic thyroid carcinoma cell line (ARO), apigenin treatment decreased the expression of EGF receptor tyrosine kinase as well as MAP kinase [98]. Taken together, these studies could provide mechanistic insight into developing novel strategies for cancer chemoprevetion by apigenin.
4. Inhibition of PI3K/Akt and mTOR signaling pathways by apigenin
The PI3K/Akt and the mTOR signaling pathways are two interdependent pathways that play important roles in the regulation of cell growth, proliferation, and survival. Aberrant activation of these pathways has been linked to cancer development and is frequently detected in malignancies. PI3K are lipid kinases divided into three classes and characterized by their substrate specificity and lipid products [99]. Class IA PI3K is composed of a catalytic p110 subunit and a regulating p50/p55/p85 subunit, and converts phosphatidylinositol-4,5-bisphosphate (PIP2) to phosphatidyl-inositol-3,4,5-trisphosphate (PIP3) [100]. Mammalian cells also express three Akt protein kinases (Akt1-3), which share >80% amino acid sequence identity and are encoded by different genes [101]. Akt binds to PIP3 via its pleckstrin homology domain and translocates to the plasma membrane, where 3-phosphoinositide-dependent kinase-1 (PDK1) phosphorylates Akt on T308 and activates Akt [102]. Once activated, Akt phosphorylates a broad range of proteins involved in apoptosis, cell cycle regulation, growth and survival. The PI3K/Akt pathway is negatively controlled by several phosphatases. Among them, the 5′-phosphatase and tensin homologue (PTEN) lipid phosphatase antagonizes the PI3K action on PIP3, and genetic inactivation of PTEN leads to constitutive activation of the PI3K/Akt pathway in many cancer cells [103].
In mammalian cells, mTOR functions as two distinct complexes, mTORC1 and mTORC2. Besides mTOR, mTORC1 contains the regulatory-associated protein of mTOR (Raptor), mLST8, and proline-rich Akt substrate 40 kDa (Pras40). The mTORC1 is sensitive to rapamycin, and regulates cell growth by controlling ribosome biogenesis, protein and lipid synthesis, and autophagy. The two best-known substrates of mTORC1 are the ribosomal protein S6 kinase (P70S6K) and the eukaryotic initiation factor 4E binding protein (4E-BP1). The mTORC1 pathway can be activated by the tuberous sclerosis complex (TSC1/TSC2/Rheb) axis [104]. mTORC2 is composed of mTOR, mLST8, mSIN1, and the rapamycin-insensitive companion of TOR protein (Rictor) [104]. mTORC2 has a PDK2 kinase activity and phosphorylates Akt on S473 that is required for its full activation [105]. mTORC2 also stabilizes Akt by constitutive phosphorylation of its turn motif on T450. mTORC2 is generally thought to be rapamycin-insensitive although a long exposure time to high concentrations of this drug may prevent its assembly and inhibit Akt phosphorylation on S473 [106].
The PI3K/Akt and mTOR signaling pathways are closely interdependent. Akt positively regulates mTORC1 by acting at different levels: First, Akt inactivates TSC1/TSC2 by phosphorylating TSC2, a negative regulator of mTORC1 [107]. Second, Akt inhibits Pras40, another negative regulator of mTORC1 [108]. Third, Akt regulates intracellular ATP levels and suppresses AMP-activated protein kinase (AMPK), which inhibits mTORC1 [109]. On the other hand, mTORC1 activation induces a negative feedback loop toward the PI3K/Akt pathway: Phosphorylated P70S6K, a downstream substrate of mTORC1, phosphorylates insulin receptor substrate 1 (IRS-1), leading to their proteasomal degradation and limits PI3K activation [104]. In addition, as mentioned above, PDK1 phosphorylates Akt on T308, but full activation of Akt kinase activity requires its phosphorylation on S473 by mTORC2 [110].
Apigenin has been shown to inhibit Akt function in different cell types by directly suppressing PI3K activity through blocking the ATP-binding site of PI3K, and subsequently inhibiting Akt kinase activity [111]. Recently, Zhao et al. demonstrated that apigenin inhibited CK2 activity, reduced phosphorylation of Cdc37, disassociated the Hsp90/Cdc37/kinase client complex, and thus induced degradation of multiple kinase clients including Akt [112]. Many of the chemoprevetive effects of apigenin are related to its inhibition of Akt activity. It is well known that PI3K/Akt signaling plays an important role in inhibiting apoptosis for cancer cell survival and growth, and apigenin has been reported in numerous studies in vitro as well as in vivo to enhance apoptosis by inactivation of Akt [113, 114]. Apigenin has also been demonstrated to inhibit ovarian tumor metastasis through down-regulation of MMP-9, which is mediated by Akt signaling [91]. Apigenin also inhibits metastasis in breast cancer cells by blocking PI3K/Akt pathway [89]. In addition, apigenin has been shown to inhibit cancer angiogenesis by suppressing HIF-1α and VEGF expression, which is also related to the inhibition of Akt by apigenin [47, 58]. Deregulation of insulin-like growth factor (IGF)-I signaling has been implicated in the development and progression of prostate cancer. Shukla et al reported that apigenin substantially reduced the levels of IGF-I in the serum and in the dorso-lateral prostate, and this modulation of IGF-I was associated with the inhibition of Akt [115].
Compared to numerous reports in the literature that apigenin inhibits Akt activity, there are only a few reports demonstrating apigenin’s effect on mTOR activity. Tong et al. recently demonstrated that apigenin induced AMPK activation in human keratinocytes (both cultured HaCaT cell line and primary normal human epidermal keratinocytes) [54]. They also found that the activation of AMPK by apigenin was independent of upstream kinase LKB1. Instead, calcium/calmodulin-dependent protein kinase kinase-β (CaMKKβ), another upstream kinase of AMPK, was required for apigenin-induced AMPK activation. Apigenin-induced AMPK activation further inhibited mTOR activity (AMPK phosphorylates TSC2 and enhances its ability to turn off mTOR activity [116]. AMPK can also directly phosphorylate the mTOR binding partner Raptor on two well-conserved serine residues, and this phosphorylation further inhibited mTOR activity [117]) and induced autophagy in human keratinocytes [54]. At early stages of tumorigenesis, autophagy acts as a tumor suppressor. It prevents tumorigenesis by removing damaged organelles and proteins, reducing chromosome instability, and inducing type II programmed cell death [118–120]. Therefore, pharmacological induction of autophagy may provide a new strategy for cancer chemoprevention. Another study by Turktekin and coworkers also demonstrated that apigenin reduced mTOR expression in colon cancer cells [121]. However, in serum-starved malignant neuroblastoma cells, apigenin alone or combined with synthetic retinoid N-(4-hydroxyphenyl) retinamide activated Akt/mTOR signaling pathwaty and the molecular mechanism for the activation is not clear [122].
Since apigenin has been demonstrated to inhibit Akt activity, and Akt has been reported to inhibit AMPK activation, one would wonder whether the activation of AMPK by apigenin is dependent on its inhibition of Akt? The answer is no, at least in human keratinocytes, because neither inhibition of Akt activity nor overexpression of constitutively active Akt had any effect on AMPK activation induced by apigenin [54]. However, the same study also suggested that apigenin treatment could inhibit the mTOR activity by two different mechanisms, either by activation of AMPK and subsequent activation of TSC2, or by direct inhibition of Akt, which is a negative regulator of both TSC2 and Pras40 (Fig. (2)) [54].
Fig. 2.
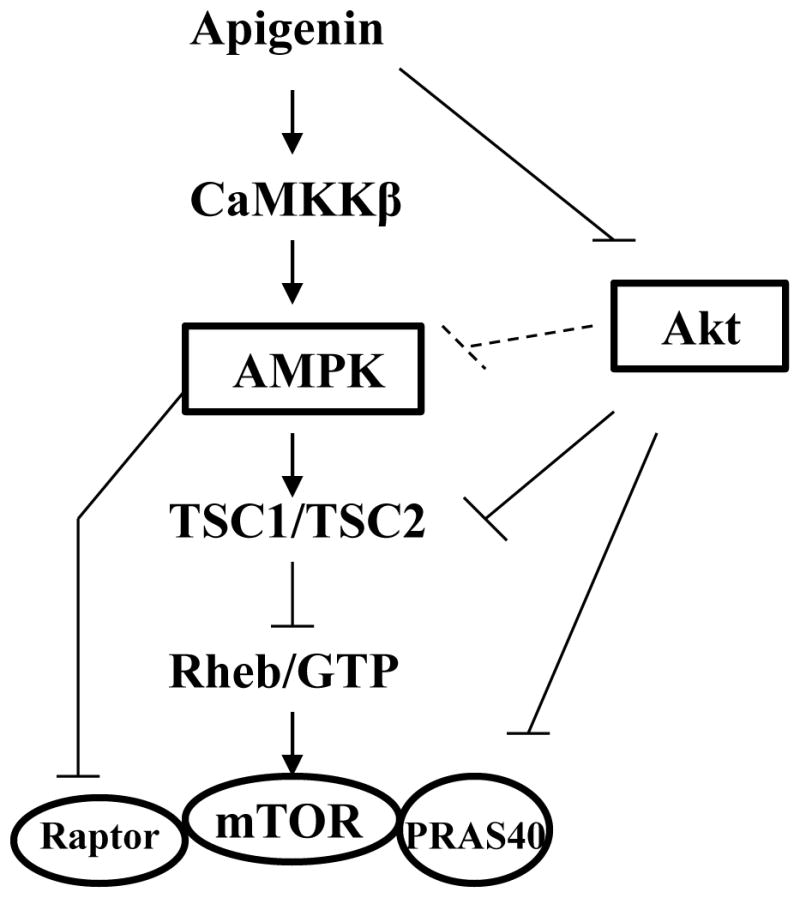
Apigenin inhibits both Akt and mTOR signaling pathways. Apigenin activates AMPK through CaMKKβ, further blocking mTOR activity. Apigenin also inhibits Akt, which activates mTOR by negatively regulating TSC2 and Pras40.
mTOR has emerged as a promising target for cancer treatment, and since the discovery of rapamycin as the first mTOR inhibitor, many rapamycin analogs have been developed to treat cancer. However, the clinical results are modest in many cancers, and it has been suggested that these mTOR inhibitors could activate Akt due to the loss of feedback inhibition of the PI3K pathway by preventing mTORC1-mediated P70S6K activation [123]. Recently, development of the second generation of mTOR inhibitors is underway, and the new mTOR/PI3K dual inhibitors are supposed to be more effective but also more toxic than rapamycin [110]. The ability of apigenin to inhibit both PI3K/Akt and mTOR signaling pathways makes it a unique and distinctive chemopreventive agent, and an added benefit is that apigenin is associated with very little toxicity, making it more attractive for cancer chemoprevention.
Acknowledgments
This work was support in part by NIH (CA161181 to X.T., CA104768 to J.P.), the American Cancer Society, Illinois Division, Inc. (#167567 to X.T.), the Zell Foundation, the Rosenberg Fund, the H Foundation, Northwestern University Skin Disease Research Center, Cell Imaging Facility and the Robert H. Lurie Comprehensive Cancer Center.
References
- 1.American Cancer Society. Cancer Facts and Figures 2012. Atlanta, Ga: American Cancer Society; 2012. [Google Scholar]
- 2.Jhanwar YS, Divgi C. Current status of therapy of solid tumors. J Nucl Med. 2005;46 (Suppl 1):141S–150S. [PubMed] [Google Scholar]
- 3.Guillemard V, Saragovi HU. Novel approaches for targeted cancer therapy. Curr Cancer Drug Targets. 2004;4:313–326. doi: 10.2174/1568009043332989. [DOI] [PubMed] [Google Scholar]
- 4.Antonia S, Mule JJ, Weber JS. Current developments of immunotherapy in the clinic. Curr Opin Immunol. 2004;16:130–136. doi: 10.1016/j.coi.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Smith JJ, Tully P, Padberg RM. Chemoprevention: a primary cancer prevention strategy. Semin Oncol Nurs. 2005;21:243–251. doi: 10.1016/j.soncn.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Sporn MB, Liby KT. Cancer chemoprevention: scientific promise, clinical uncertainty. Nat Clin Pract Oncol. 2005;2:518–525. doi: 10.1038/ncponc0319. [DOI] [PubMed] [Google Scholar]
- 7.Brenner DE, Gescher AJ. Cancer chemoprevention: lessons learned and future directions. Br J Cancer. 2005;93:735–739. doi: 10.1038/sj.bjc.6602765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham S. Results of case-control studies of diet and cancer in Buffalo, New York. Cancer Res. 1983;43(5 Suppl):2409s–2413s. [PubMed] [Google Scholar]
- 9.Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 10.Cohen JH, Kristal AR, Stanford JL. Fruit and vegetable intakes and prostate cancer risk. J Natl Cancer Inst. 2000;92:61–68. doi: 10.1093/jnci/92.1.61. [DOI] [PubMed] [Google Scholar]
- 11.Terry P, Giovannucci E, Michels KB, Bergkvist L, Hansen H, Holmberg L, Wolk A. Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J Natl Cancer Inst. 2001;93:525–533. doi: 10.1093/jnci/93.7.525. [DOI] [PubMed] [Google Scholar]
- 12.Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Ther. 2001;90:157–177. doi: 10.1016/s0163-7258(01)00137-1. [DOI] [PubMed] [Google Scholar]
- 13.Shukla S, Gupta S. Dietary agents in the chemoprevention of prostate cancer. Nutr Cancer. 2005;53:18–32. doi: 10.1207/s15327914nc5301_3. [DOI] [PubMed] [Google Scholar]
- 14.Gupta SC, Kim JH, Prasad S, Aggarwal BB. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010;29:405–434. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 16.Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 17.Croft KD. The chemistry and biological effects of flavonoids and phenolic acids. Ann N Y Acad Sci. 1998;854:435–442. doi: 10.1111/j.1749-6632.1998.tb09922.x. [DOI] [PubMed] [Google Scholar]
- 18.Harbourne JB. The flavonoids: advances in research since 1986. London: Chapman & Hall; 1993. [Google Scholar]
- 19.Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21:381–406. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- 20.O’Prey J, Brown J, Fleming J, Harrison PR. Effects of dietary flavonoids on major signal transduction pathways in human epithelial cells. Biochem Pharmacol. 2003;66:2075–2088. doi: 10.1016/j.bcp.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Thiery-Vuillemin A, Nguyen T, Pivot X, Spano JP, Dufresnne A, Soria JC. Molecularly targeted agents: their promise as cancer chemopreventive interventions. Eur J Cancer. 2005;41:2003–2015. doi: 10.1016/j.ejca.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 23.Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise (review) Int J Oncol. 2007;30:233–245. [PubMed] [Google Scholar]
- 24.Janssen K, Mensink RP, Cox FJ, Harryvan JL, Hovenier R, Hollman PC, Katan MB. Effects of the flavonoids quercetin and apigenin on hemostasis in healthy volunteers: results from an in vitro and a dietary supplement study. Am J Clin Nutr. 1998;67:255–262. doi: 10.1093/ajcn/67.2.255. [DOI] [PubMed] [Google Scholar]
- 25.Li B, Robinson DH, Birt DF. Evaluation of properties of apigenin and [G-3H]apigenin and analytic method development. J Pharm Sci. 1997;86:721–725. doi: 10.1021/js960383s. [DOI] [PubMed] [Google Scholar]
- 26.Eaton EA, Walle UK, Lewis AJ, Hudson T, Wilson AA, Walle T. Flavonoids, potent inhibitors of the human P-form phenolsulfotransferase. Potential role in drug metabolism and chemoprevention. Drug Metab Dispos. 1996;24:232–237. [PubMed] [Google Scholar]
- 27.Hertog MG, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, Giampaoli S, Jansen A, Menotti A, Nedeljkovic S, et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med. 1995;155:381–386. [PubMed] [Google Scholar]
- 28.Nielsen SE, Young JF, Daneshvar B, Lauridsen ST, Knuthsen P, Sandstrom B, Dragsted LO. Effect of parsley (Petroselinum crispum) intake on urinary apigenin excretion, blood antioxidant enzymes and biomarkers for oxidative stress in human subjects. Br J Nutr. 1999;81:447–455. doi: 10.1017/s000711459900080x. [DOI] [PubMed] [Google Scholar]
- 29.Merfort I, Heilmann J, Hagedorn-Leweke U, Lippold BC. In vivo skin penetration studies of camomile flavones. Pharmazie. 1994;49:509–511. [PubMed] [Google Scholar]
- 30.Shukla S, Gupta S. Molecular targets for apigenin-induced cell cycle arrest and apoptosis in prostate cancer cell xenograft. Mol Cancer Ther. 2006;5:843–852. doi: 10.1158/1535-7163.MCT-05-0370. [DOI] [PubMed] [Google Scholar]
- 31.Shukla S, MacLennan GT, Flask CA, Fu P, Mishra A, Resnick MI, Gupta S. Blockade of beta-catenin signaling by plant flavonoid apigenin suppresses prostate carcinogenesis in TRAMP mice. Cancer Res. 2007;67:6925–6935. doi: 10.1158/0008-5472.CAN-07-0717. [DOI] [PubMed] [Google Scholar]
- 32.Hollman PC, Katan MB. Dietary flavonoids: intake, health effects and bioavailability. Food Chem Toxicol. 1999;37:937–942. doi: 10.1016/s0278-6915(99)00079-4. [DOI] [PubMed] [Google Scholar]
- 33.Cai H, Boocock DJ, Steward WP, Gescher AJ. Tissue distribution in mice and metabolism in murine and human liver of apigenin and tricin flavones with putative cancer chemopreventive properties. Cancer Chemother Pharmacol. 2007;60:257–266. doi: 10.1007/s00280-006-0368-5. [DOI] [PubMed] [Google Scholar]
- 34.Brown JP, Dietrich PS. Mutagenicity of plant flavonols in the Salmonella/mammalian microsome test: activation of flavonol glycosides by mixed glycosidases from rat cecal bacteria and other sources. Mutat Res. 1979;66:223–240. doi: 10.1016/0165-1218(79)90083-1. [DOI] [PubMed] [Google Scholar]
- 35.Czeczot H, Tudek B, Kusztelak J, Szymczyk T, Dobrowolska B, Glinkowska G, Malinowski J, Strzelecka H. Isolation and studies of the mutagenic activity in the Ames test of flavonoids naturally occurring in medical herbs. Mutat Res. 1990;240:209–216. doi: 10.1016/0165-1218(90)90060-f. [DOI] [PubMed] [Google Scholar]
- 36.Birt DF, Walker B, Tibbels MG, Bresnick E. Anti-mutagenesis and anti-promotion by apigenin, robinetin and indole-3-carbinol. Carcinogenesis. 1986;7:959–963. doi: 10.1093/carcin/7.6.959. [DOI] [PubMed] [Google Scholar]
- 37.Birt DF, Mitchell D, Gold B, Pour P, Pinch HC. Inhibition of ultraviolet light induced skin carcinogenesis in SKH-1 mice by apigenin, a plant flavonoid. Anticancer Res. 1997;17:85–91. [PubMed] [Google Scholar]
- 38.Wei H, Tye L, Bresnick E, Birt DF. Inhibitory effect of apigenin, a plant flavonoid, on epidermal ornithine decarboxylase and skin tumor promotion in mice. Cancer Res. 1990;50:499–502. [PubMed] [Google Scholar]
- 39.Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res. 2010;27:962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Way TD, Kao MC, Lin JK. Apigenin induces apoptosis through proteasomal degradation of HER2/neu in HER2/neu-overexpressing breast cancer cells via the phosphatidylinositol 3-kinase/Akt-dependent pathway. J Biol Chem. 2004;279:4479–4489. doi: 10.1074/jbc.M305529200. [DOI] [PubMed] [Google Scholar]
- 41.Way TD, Kao MC, Lin JK. Degradation of HER2/neu by apigenin induces apoptosis through cytochrome c release and caspase-3 activation in HER2/neu-overexpressing breast cancer cells. FEBS Lett. 2005;579:145–152. doi: 10.1016/j.febslet.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 42.Lindenmeyer F, Li H, Menashi S, Soria C, Lu H. Apigenin acts on the tumor cell invasion process and regulates protease production. Nutr Cancer. 2001;39:139–147. doi: 10.1207/S15327914nc391_19. [DOI] [PubMed] [Google Scholar]
- 43.Collins-Burow BM, Burow ME, Duong BN, McLachlan JA. Estrogenic and antiestrogenic activities of flavonoid phytochemicals through estrogen receptor binding-dependent and -independent mechanisms. Nutr Cancer. 2000;38:229–244. doi: 10.1207/S15327914NC382_13. [DOI] [PubMed] [Google Scholar]
- 44.Morrissey C, O’Neill A, Spengler B, Christoffel V, Fitzpatrick JM, Watson RW. Apigenin drives the production of reactive oxygen species and initiates a mitochondrial mediated cell death pathway in prostate epithelial cells. Prostate. 2005;63:131–142. doi: 10.1002/pros.20167. [DOI] [PubMed] [Google Scholar]
- 45.Gupta S, Afaq F, Mukhtar H. Selective growth-inhibitory, cell-cycle deregulatory and apoptotic response of apigenin in normal versus human prostate carcinoma cells. Biochem Biophys Res Commun. 2001;287:914–920. doi: 10.1006/bbrc.2001.5672. [DOI] [PubMed] [Google Scholar]
- 46.Shukla S, Gupta S. Molecular mechanisms for apigenin-induced cell-cycle arrest and apoptosis of hormone refractory human prostate carcinoma DU145 cells. Mol Carcinog. 2004;39:114–126. doi: 10.1002/mc.10168. [DOI] [PubMed] [Google Scholar]
- 47.Mirzoeva S, Kim ND, Chiu K, Franzen CA, Bergan RC, Pelling JC. Inhibition of HIF-1 alpha and VEGF expression by the chemopreventive bioflavonoid apigenin is accompanied by Akt inhibition in human prostate carcinoma PC3-M cells. Mol Carcinog. 2008;47:686–700. doi: 10.1002/mc.20421. [DOI] [PubMed] [Google Scholar]
- 48.Franzen CA, Amargo E, Todorovic V, Desai BV, Huda S, Mirzoeva S, Chiu K, Grzybowski BA, Chew TL, Green KJ, Pelling JC. The chemopreventive bioflavonoid apigenin inhibits prostate cancer cell motility through the focal adhesion kinase/Src signaling mechanism. Cancer Prev Res (Phila) 2009;2:830–841. doi: 10.1158/1940-6207.CAPR-09-0066. [DOI] [PubMed] [Google Scholar]
- 49.Wang W, Heideman L, Chung CS, Pelling JC, Koehler KJ, Birt DF. Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol Carcinog. 2000;28:102–110. [PubMed] [Google Scholar]
- 50.Plaumann B, Fritsche M, Rimpler H, Brandner G, Hess RD. Flavonoids activate wild-type p53. Oncogene. 1996;13:1605–1614. [PubMed] [Google Scholar]
- 51.Ding J, Polier G, Kohler R, Giaisi M, Krammer PH, Li-Weber M. Wogonin and related natural flavones overcome tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) protein resistance of tumors by down-regulation of c-FLIP protein and up-regulation of TRAIL receptor 2 expression. J Biol Chem. 2012;287:641–649. doi: 10.1074/jbc.M111.286526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng PW, Chiang LC, Lin CC. Apigenin induced apoptosis through p53-dependent pathway in human cervical carcinoma cells. Life Sci. 2005;76:1367–1379. doi: 10.1016/j.lfs.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 53.Czyz J, Madeja Z, Irmer U, Korohoda W, Hulser DF. Flavonoid apigenin inhibits motility and invasiveness of carcinoma cells in vitro. Int J Cancer. 2005;114:12–18. doi: 10.1002/ijc.20620. [DOI] [PubMed] [Google Scholar]
- 54.Tong X, Smith KA, Pelling JC. Apigenin, a chemopreventive bioflavonoid, induces AMP-activated protein kinase activation in human keratinocytes. Mol Carcinog. 2012;51:268–279. doi: 10.1002/mc.20793. [DOI] [PubMed] [Google Scholar]
- 55.Abu-Yousif AO, Smith KA, Getsios S, Green KJ, Van Dross RT, Pelling JC. Enhancement of UVB-induced apoptosis by apigenin in human keratinocytes and organotypic keratinocyte cultures. Cancer Res. 2008;68:3057–3065. doi: 10.1158/0008-5472.CAN-07-2763. [DOI] [PubMed] [Google Scholar]
- 56.Van Dross RT, Hong X, Pelling JC. Inhibition of TPA-induced cyclooxygenase-2 (COX-2) expression by apigenin through downregulation of Akt signal transduction in human keratinocytes. Mol Carcinog. 2005;44:83–91. doi: 10.1002/mc.20123. [DOI] [PubMed] [Google Scholar]
- 57.Hwang YP, Oh KN, Yun HJ, Jeong HG. The flavonoids apigenin and luteolin suppress ultraviolet A-induced matrix metalloproteinase-1 expression via MAPKs and AP-1-dependent signaling in HaCaT cells. J Dermatol Sci. 2011;61:23–31. doi: 10.1016/j.jdermsci.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 58.Liu LZ, Fang J, Zhou Q, Hu X, Shi X, Jiang BH. Apigenin inhibits expression of vascular endothelial growth factor and angiogenesis in human lung cancer cells: implication of chemoprevention of lung cancer. Mol Pharmacol. 2005;68:635–643. doi: 10.1124/mol.105.011254. [DOI] [PubMed] [Google Scholar]
- 59.Bruno A, Siena L, Gerbino S, Ferraro M, Chanez P, Giammanco M, Gjomarkaj M, Pace E. Apigenin affects leptin/leptin receptor pathway and induces cell apoptosis in lung adenocarcinoma cell line. Eur J Cancer. 2011;47:2042–2051. doi: 10.1016/j.ejca.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 60.Li ZD, Hu XW, Wang YT, Fang J. Apigenin inhibits proliferation of ovarian cancer A2780 cells through Id1. FEBS Lett. 2009;583:1999–2003. doi: 10.1016/j.febslet.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 61.Fang J, Xia C, Cao Z, Zheng JZ, Reed E, Jiang BH. Apigenin inhibits VEGF and HIF-1 expression via PI3K/AKT/p70S6K1 and HDM2/p53 pathways. Faseb J. 2005;19:342–353. doi: 10.1096/fj.04-2175com. [DOI] [PubMed] [Google Scholar]
- 62.Hu XW, Meng D, Fang J. Apigenin inhibited migration and invasion of human ovarian cancer A2780 cells through focal adhesion kinase. Carcinogenesis. 2008;29:2369–2376. doi: 10.1093/carcin/bgn244. [DOI] [PubMed] [Google Scholar]
- 63.Cai J, Zhao XL, Liu AW, Nian H, Zhang SH. Apigenin inhibits hepatoma cell growth through alteration of gene expression patterns. Phytomedicine. 2011;18:366–373. doi: 10.1016/j.phymed.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 64.Choi SI, Jeong CS, Cho SY, Lee YS. Mechanism of apoptosis induced by apigenin in HepG2 human hepatoma cells: involvement of reactive oxygen species generated by NADPH oxidase. Arch Pharm Res. 2007;30:1328–1335. doi: 10.1007/BF02980274. [DOI] [PubMed] [Google Scholar]
- 65.van Rijn J, van den Berg J. Flavonoids as enhancers of x-ray-induced cell damage in hepatoma cells. Clin Cancer Res. 1997;3:1775–1779. [PubMed] [Google Scholar]
- 66.Pham H, Chen M, Takahashi H, King J, Reber HA, Hines OJ, Pandol S, Eibl G. Apigenin Inhibits NNK-Induced Focal Adhesion Kinase Activation in Pancreatic Cancer Cells. Pancreas. 2012;41:1306–1315. doi: 10.1097/MPA.0b013e31824d64d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Melstrom LG, Salabat MR, Ding XZ, Strouch MJ, Grippo PJ, Mirzoeva S, Pelling JC, Bentrem DJ. Apigenin down-regulates the hypoxia response genes: HIF-1alpha, GLUT-1, and VEGF in human pancreatic cancer cells. J Surg Res. 2011;167:173–181. doi: 10.1016/j.jss.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 68.Salabat MR, Melstrom LG, Strouch MJ, Ding XZ, Milam BM, Ujiki MB, Chen C, Pelling JC, Rao S, Grippo PJ, McGarry TJ, Bentrem DJ. Geminin is overexpressed in human pancreatic cancer and downregulated by the bioflavanoid apigenin in pancreatic cancer cell lines. Mol Carcinog. 2008;47:835–844. doi: 10.1002/mc.20441. [DOI] [PubMed] [Google Scholar]
- 69.Ujiki MB, Ding XZ, Salabat MR, Bentrem DJ, Golkar L, Milam B, Talamonti MS, Bell RH, Iwamura T, Jr, Adrian TE. Apigenin inhibits pancreatic cancer cell proliferation through G2/M cell cycle arrest. Mol Cancer. 2006;5:76. doi: 10.1186/1476-4598-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang IK, Lin-Shiau SY, Lin JK. Induction of apoptosis by apigenin and related flavonoids through cytochrome c release and activation of caspase-9 and caspase-3 in leukaemia HL-60 cells. Eur J Cancer. 1999;35:1517–1525. [PubMed] [Google Scholar]
- 71.Jayasooriya RG, Kang SH, Kang CH, Choi YH, Moon DO, Hyun JW, Chang WY, Kim GY. Apigenin decreases cell viability and telomerase activity in human leukemia cell lines. Food Chem Toxicol. 2012;50:2605–2611. doi: 10.1016/j.fct.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 72.Nakasugi T, Nakashima M, Komai K. Antimutagens in gaiyou (Artemisia argyi levl. et vant) J Agric Food Chem. 2000;48:3256–3266. doi: 10.1021/jf9906679. [DOI] [PubMed] [Google Scholar]
- 73.Fiander H, Schneider H. Dietary ortho phenols that induce glutathione S-transferase and increase the resistance of cells to hydrogen peroxide are potential cancer chemopreventives that act by two mechanisms: the alleviation of oxidative stress and the detoxification of mutagenic xenobiotics. Cancer Lett. 2000;156:117–124. doi: 10.1016/s0304-3835(00)00368-2. [DOI] [PubMed] [Google Scholar]
- 74.Dirven HA, Dictus EL, Broeders NL, van Ommen B, van Bladeren PJ. The role of human glutathione S-transferase isoenzymes in the formation of glutathione conjugates of the alkylating cytostatic drug thiotepa. Cancer Res. 1995;55:1701–1706. [PubMed] [Google Scholar]
- 75.Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, Edelstein D, Soslow RA, Koki AT, Woerner BM, Masferrer JL, Dannenberg AJ. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59:991–994. [PubMed] [Google Scholar]
- 76.Van Dross RT, Hong X, Essengue S, Fischer SM, Pelling JC. Modulation of UVB-induced and basal cyclooxygenase-2 (COX-2) expression by apigenin in mouse keratinocytes: role of USF transcription factors. Mol Carcinog. 2007;46:303–314. doi: 10.1002/mc.20281. [DOI] [PubMed] [Google Scholar]
- 77.Tong X, Van Dross RT, Abu-Yousif A, Morrison AR, Pelling JC. Apigenin prevents UVB-induced cyclooxygenase 2 expression: coupled mRNA stabilization and translational inhibition. Mol Cell Biol. 2007;27:283–296. doi: 10.1128/MCB.01282-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nicholas C, Batra S, Vargo MA, Voss OH, Gavrilin MA, Wewers MD, Guttridge DC, Grotewold E, Doseff AI. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-kappaB through the suppression of p65 phosphorylation. J Immunol. 2007;179:7121–7127. doi: 10.4049/jimmunol.179.10.7121. [DOI] [PubMed] [Google Scholar]
- 79.Lepley DM, Li B, Birt DF, Pelling JC. The chemopreventive flavonoid apigenin induces G2/M arrest in keratinocytes. Carcinogenesis. 1996;17:2367–2375. doi: 10.1093/carcin/17.11.2367. [DOI] [PubMed] [Google Scholar]
- 80.McVean M, Weinberg WC, Pelling JC. A p21(waf1)-independent pathway for inhibitory phosphorylation of cyclin-dependent kinase p34(cdc2) and concomitant G(2)/M arrest by the chemopreventive flavonoid apigenin. Mol Carcinog. 2002;33:36–43. doi: 10.1002/mc.10016. [DOI] [PubMed] [Google Scholar]
- 81.Lepley DM, Pelling JC. Induction of p21/WAF1 and G1 cell-cycle arrest by the chemopreventive agent apigenin. Mol Carcinog. 1997;19:74–82. doi: 10.1002/(sici)1098-2744(199707)19:2<74::aid-mc2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 82.McVean M, Xiao H, Isobe K, Pelling JC. Increase in wild-type p53 stability and transactivational activity by the chemopreventive agent apigenin in keratinocytes. Carcinogenesis. 2000;21:633–639. doi: 10.1093/carcin/21.4.633. [DOI] [PubMed] [Google Scholar]
- 83.Tong X, Pelling JC. Enhancement of p53 expression in keratinocytes by the bioflavonoid apigenin is associated with RNA-binding protein HuR. Mol Carcinog. 2009;48:118–129. doi: 10.1002/mc.20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iwashita K, Kobori M, Yamaki K, Tsushida T. Flavonoids inhibit cell growth and induce apoptosis in B16 melanoma 4A5 cells. Biosci Biotechnol Biochem. 2000;64:1813–1820. doi: 10.1271/bbb.64.1813. [DOI] [PubMed] [Google Scholar]
- 85.Hirano T, Oka K, Akiba M. Antiproliferative effects of synthetic and naturally occurring flavonoids on tumor cells of the human breast carcinoma cell line, ZR-75–1. Res Commun Chem Pathol Pharmacol. 1989;64:69–78. [PubMed] [Google Scholar]
- 86.Cao Y, Arbiser J, D’Amato RJ, D’Amore PA, Ingber DE, Kerbel R, Klagsbrun M, Lim S, Moses MA, Zetter B, Dvorak R. Langer, Forty-year journey of angiogenesis translational research. Sci Transl Med. 2011;3:114rv113. doi: 10.1126/scitranslmed.3003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lamy S, Akla N, Ouanouki A, Lord-Dufour S, Beliveau R. Diet-derived polyphenols inhibit angiogenesis by modulating the interleukin-6/STAT3 pathway. Exp Cell Res. 2012;318:1586–1596. doi: 10.1016/j.yexcr.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 88.Lamy S, Bedard V, Labbe D, Sartelet H, Barthomeuf C, Gingras D, Beliveau R. The dietary flavones apigenin and luteolin impair smooth muscle cell migration and VEGF expression through inhibition of PDGFR-beta phosphorylation. Cancer Prev Res (Phila Pa) 2008;1:452–459. doi: 10.1158/1940-6207.CAPR-08-0072. [DOI] [PubMed] [Google Scholar]
- 89.Lee WJ, Chen WK, Wang CJ, Lin WL, Tseng TH. Apigenin inhibits HGF-promoted invasive growth and metastasis involving blocking PI3K/Akt pathway and beta 4 integrin function in MDA-MB-231 breast cancer cells. Toxicol Appl Pharmacol. 2008;226:178–191. doi: 10.1016/j.taap.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 90.Piantelli M, Rossi C, Iezzi M, La Sorda R, Iacobelli S, Alberti S, Natali PG. Flavonoids inhibit melanoma lung metastasis by impairing tumor cells endothelium interactions. J Cell Physiol. 2006;207:23–29. doi: 10.1002/jcp.20510. [DOI] [PubMed] [Google Scholar]
- 91.He J, Xu Q, Wang M, Li C, Qian X, Shi Z, Liu LZ, Jiang BH. Oral Administration of Apigenin Inhibits Metastasis through AKT/P70S6K1/MMP-9 Pathway in Orthotopic Ovarian Tumor Model. Int J Mol Sci. 2012;13:7271–7282. doi: 10.3390/ijms13067271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chaumontet C, Droumaguet C, Bex V, Heberden C, Gaillard-Sanchez I, Martel P. Flavonoids (apigenin, tangeretin) counteract tumor promoter-induced inhibition of intercellular communication of rat liver epithelial cells. Cancer Lett. 1997;114:207–210. doi: 10.1016/s0304-3835(97)04664-8. [DOI] [PubMed] [Google Scholar]
- 93.Le Bail JC, Laroche T, Marre-Fournier F, Habrioux G. Aromatase and 17beta-hydroxysteroid dehydrogenase inhibition by flavonoids. Cancer Lett. 1998;133:101–106. doi: 10.1016/s0304-3835(98)00211-0. [DOI] [PubMed] [Google Scholar]
- 94.Van Dross R, Xue Y, Knudson A, Pelling JC. The chemopreventive bioflavonoid apigenin modulates signal transduction pathways in keratinocyte and colon carcinoma cell lines. J Nutr. 2003;133:3800S–3804S. doi: 10.1093/jn/133.11.3800S. [DOI] [PubMed] [Google Scholar]
- 95.Hessenauer A, Montenarh M, Gotz C. Inhibition of CK2 activity provokes different responses in hormone-sensitive and hormone-refractory prostate cancer cells. Int J Oncol. 2003;22:1263–1270. [PubMed] [Google Scholar]
- 96.Landesman-Bollag E, Song DH, Romieu-Mourez R, Sussman DJ, Cardiff RD, Sonenshein GE, Seldin DC. Protein kinase CK2: signaling and tumorigenesis in the mammary gland. Mol Cell Biochem. 2001;227:153–165. [PubMed] [Google Scholar]
- 97.Lee SF, Lin JK. Inhibitory effects of phytopolyphenols on TPA-induced transformation, PKC activation, and c-jun expression in mouse fibroblast cells. Nutr Cancer. 1997;28:177–183. doi: 10.1080/01635589709514572. [DOI] [PubMed] [Google Scholar]
- 98.Yin F, Giuliano AE, Van Herle AJ. Signal pathways involved in apigenin inhibition of growth and induction of apoptosis of human anaplastic thyroid cancer cells (ARO) Anticancer Res. 1999;19:4297–4303. [PubMed] [Google Scholar]
- 99.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 100.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 102.Alessi DR, Deak M, Casamayor A, Caudwell FB, Morrice N, Norman DG, Gaffney P, Reese CB, MacDougall CN, Harbison D, Ashworth A, Bownes M. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997;7:776–789. doi: 10.1016/s0960-9822(06)00336-8. [DOI] [PubMed] [Google Scholar]
- 103.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 104.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 105.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 106.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 107.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 108.Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 109.Hahn-Windgassen A, Nogueira V, Chen CC, Skeen JE, Sonenberg N, Hay N. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem. 2005;280:32081–32089. doi: 10.1074/jbc.M502876200. [DOI] [PubMed] [Google Scholar]
- 110.Willems L, Tamburini J, Chapuis N, Lacombe C, Mayeux P, Bouscary D. PI3K and mTOR signaling pathways in cancer: new data on targeted therapies. Curr Oncol Rep. 2012;14:129–138. doi: 10.1007/s11912-012-0227-y. [DOI] [PubMed] [Google Scholar]
- 111.Tang Q, Gonzales M, Inoue H, Bowden GT. Roles of Akt and glycogen synthase kinase 3beta in the ultraviolet B induction of cyclooxygenase-2 transcription in human keratinocytes. Cancer Res. 2001;61:4329–4332. [PubMed] [Google Scholar]
- 112.Zhao M, Ma J, Zhu HY, Zhang XH, Du ZY, Xu YJ, Yu XD. Apigenin inhibits proliferation and induces apoptosis in human multiple myeloma cells through targeting the trinity of CK2, Cdc37 and Hsp90. Mol Cancer. 2011;10:104. doi: 10.1186/1476-4598-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Budhraja A, Gao N, Zhang Z, Son YO, Cheng S, Wang X, Ding S, Hitron A, Chen G, Luo J, Shi X. Apigenin induces apoptosis in human leukemia cells and exhibits anti-leukemic activity in vivo. Mol Cancer Ther. 2012;11:132–142. doi: 10.1158/1535-7163.MCT-11-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cheong JW, Min YH, Eom JI, Kim SJ, Jeung HK, Kim JS. Inhibition of CK2{alpha} and PI3K/Akt synergistically induces apoptosis of CD34+CD38− leukaemia cells while sparing haematopoietic stem cells. Anticancer Res. 2010;30:4625–4634. [PubMed] [Google Scholar]
- 115.Shukla S, MacLennan GT, Fu P, Gupta S. Apigenin attenuates insulin-like growth factor-I signaling in an autochthonous mouse prostate cancer model. Pharm Res. 2012;29:1506–1517. doi: 10.1007/s11095-011-0625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 117.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 119.Maiuri MC, Tasdemir E, Criollo A, Morselli E, Vicencio JM, Carnuccio R, Kroemer G. Control of autophagy by oncogenes and tumor suppressor genes. Cell Death Differ. 2009;16:87–93. doi: 10.1038/cdd.2008.131. [DOI] [PubMed] [Google Scholar]
- 120.Steeves MA, Dorsey FC, Cleveland JL. Targeting the autophagy pathway for cancer chemoprevention. Curr Opin Cell Biol. 2010;22:218–225. doi: 10.1016/j.ceb.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Turktekin M, Konac E, Onen HI, Alp E, Yilmaz A, Menevse S. Evaluation of the effects of the flavonoid apigenin on apoptotic pathway gene expression on the colon cancer cell line (HT29) J Med Food. 2011;14:1107–1117. doi: 10.1089/jmf.2010.0208. [DOI] [PubMed] [Google Scholar]
- 122.Mohan N, Banik NL, Ray SK. Combination of N-(4-hydroxyphenyl) retinamide and apigenin suppressed starvation-induced autophagy and promoted apoptosis in malignant neuroblastoma cells. Neurosci Lett. 2011;502:24–29. doi: 10.1016/j.neulet.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huang S. A new clue to explain resistance to mTOR inhibitors. Cell Cycle. 2012:11. doi: 10.4161/cc.11.5.19598. [DOI] [PubMed] [Google Scholar]