Abstract
Severe burn injury induces a pathophysiological response that affects almost every physiological system within the body. Inflammation, hypermetabolism, muscle wasting, and insulin resistance are all hallmarks of the pathophysiological response to burn injury, with perturbations in metabolism known to persist for several years post injury. Skeletal muscle is the main depot of lean tissue within the body and as the primary site of peripheral glucose disposal, plays an important role in metabolic regulation. Following a large burn, skeletal muscle functions as and endogenous amino acid store, providing substrates for more pressing functions post burn, such as the synthesis of acute phase proteins and the deposition of new skin. Subsequently, burn patients become cachexic, which is associated with poor outcomes in terms of metabolic health and functional capacity. While a loss of skeletal muscle contractile proteins per se will no doubt negatively impact functional capacity, detriments in skeletal muscle quality, i.e. a loss in mitochondrial number and/or function may be quantitatively just as important. The goal of this review article is to summarize the current understanding of the impact of burn injury on skeletal muscle mitochondrial content and function, to offer direction for future research concerning skeletal muscle mitochondrial function in patients with severe burns, and to renew interest in the role of these organelles in metabolic dysfunction following burn injury.
Keywords: burn injury, skeletal muscle, mitochondrial function
Introduction
The pathophysiological response to burn injury is multi-factorial with resulting perturbations in metabolism affecting nearly every physiological system [1–16]. Like most forms of critical illness, burn injury results in an inflammatory and hypermetabolic stress response, but the extent and duration of these responses and their debilitating nature appear unique to burn injury [1, 6, 7, 17–20]. With recent advances in clinical practice such as early wound excision and closure, and robust infection management [21], severe burn injuries are more survivable than ever before. Consequently, there is a real need for effective rehabilitative strategies that mitigate the pathophysiological response to burn injury and restore normal physiological function in order to reduce morbidity and improve quality of life in patients recovering from severe burns. Central to the process of developing novel strategies that impact outcomes in burn patients is a comprehensive understanding of the pathophysiological response to severe burn trauma. While the stress response to burn injury including but not limited to inflammation, the catecholamine surge, hypermetabolism and muscle wasting have been studied in human patients in great detail [1, 5, 6, 9, 10, 17, 19, 20, 22–41], the impact of severe burns on skeletal muscle bioenergetics in human patients has been paid comparatively little attention [42–46]. This is perhaps surprising given the fact that supraphysiological rates of energy expenditure [1, 5, 18, 19, 23, 33, 47–49], insulin resistance [1, 4, 5, 29, 45, 50, 51] and muscle wasting [6, 14, 17, 19, 23, 25, 26, 28, 30–32, 34–40] are all considered as hallmarks of the pathophysiological response to burn injury. Moreover, mitochondria are sensitive to environmental and pharmacological stimuli [52–55], meaning that these intriguing organelles make ideal candidates for interventions aimed at altering the pathophysiology of burn injury. With this in mind, the purpose of this article is to review the current literature pertaining to skeletal muscle bioenergetics in patients with severe burns, with an aim to renewing interest in this field and wherever possible, offer direction to researchers interested in improving metabolic health and functional capacity in patients recovering from severe burns.
Metabolic complications associated with severe burn injury
Hypermetabolism
Severe burns result in profound alterations in energy expenditure. Indeed, resting energy expenditure has been reported to be between 120–180% above normal values in the first one to two months post injury [1, 18, 19, 23]. Moreover, it has previously been reported that energy expenditure was significantly elevated for up to 24 months post injury in burned children and remained elevated, albeit not significantly, at 36 months post injury [1]. It is thought that heat loss through open wounds coupled with a comprised skin barrier and thus an inability to effectively thermoregulate play a role in increased metabolic rate post burn [47–49]. In addition, chronic adrenergic stimulation post burn is also likely to alter the kinetics of numerous metabolic substrates post burn [33, 56, 57], while cellular respiration not linked to ATP production may also increase in response to severe thermal injury [57].
Substrate cycling in patients with severe burn injury
In the aftermath of severe burn trauma numerous ATP consuming reactions linked to the cycling of metabolic substrates appear to be up-regulated (reviewed in detail in [57]). Elevations in circulating lipids moieties following a severe burn injury are most likely the results of the catecholamine surge and subsequent β-adrenergic stimulation elevating lipolysis. However, despite elevated rates of lipolysis in burned patients, the ratio of fatty acid appearance to glycerol appearance is lower relative to healthy individuals. Indeed, while a normal ratio of fatty acid appearance to glycerol appearance would be approximately 3, this value is far lower in patients with severe burns (1.6), suggesting immediate re-esterfication of fatty acids within adipose tissue [58, 59]. Many of the metabolic perturbations associated with burn injury favor increased intracellular fatty acid re-esterfication, such as elevated plasma glucose and insulin concentrations as well as depleted serum albumin concentrations, which are needed to transport hydrophobic fatty acids in the periphery. Regardless of the exact mechanism(s) responsible for this fatty acid cycling, the result is that triglyceride is broken down for fatty acids to be re-esterfied in a seemingly futile cycle. Furthermore, much like lipid metabolism, Wolfe and colleagues also showed that pyruvate production from glycolytic flux was 300% greater in burned patients when compared to healthy controls, suggesting that glycolysis is profoundly elevated in burned patients to the extent that despite elevated pyruvate dehydrogenase activity, plasma lactate concentrations are 2–4 times greater in burned patients when compared to healthy controls [60]. This suggests that the elevated rates of glycolysis within the skeletal muscle of burned patients accounts for peripheral lactic acidosis and not deficits in oxidative pyruvate disposal as suggested elsewhere [44]. Moreover, the observation of increased Cori cycle flux following burn injury in humans [61] suggests that elevated glycolysis and lactate formation in skeletal muscle provides substrate for hepatic gluconeogensis in what appear to be more futile cycling of energy substrates.
While increased substrate cycling seems to be futile, it may be an adaptation to burn injury which provides the liver with gluconeogenic substrates such as glycerol and lactate, which in turn may reduce the amount of alanine released from muscle for the purposes of hepatic gluconeogenesis. However, since severely burned patients are fed enteral formulas which provide the majority of their caloric load in the form of carbohydrate, it is unlikely that glycerol and pyruvate cycling play an important role in hepatic gluconeogenesis, meaning that while patients are receiving adequate burn care, this substrate cycling is indeed futile and is likely to contribute to the hypermetabolic response to burns.
In addition to lipid and carbohydrate substrate cycles, it is thought that there are concurrent increases in muscle protein breakdown and synthesis post burn, where increased intracellular availability of amino acids from protein breakdown simulates increased muscle protein synthesis [17]. Subsequently, it would seem that some amino acids liberated from skeletal muscle are resynthesized back into constituent proteins without ever leaving the muscle cell. Taken together, it would appear that there is seemingly futile cycling of metabolic substrates such as fatty acids, glucose and amino acids. These processes are not without their own metabolic costs. For example, it has been shown that the rates of protein synthesis, urea production, gluconeogenesis and the cycling of glucose and fatty acids are at least 2-fold higher in burned patients relative to unburned individuals, where the rate of intracellular cycling of fatty acids is thought to be over 15 times greater in severely burn patients in comparison to healthy individuals [57]. Collectively, the increase in these ATP consuming processes in patients with severe burn injury has been calculated to increase energy requirements by approximately 10 kcal·kg−1 day−1 [57]. Therefore, cycling of metabolic substrate likely plays a quantitatively important role in the hypermetabolic response to burn injury.
Skeletal muscle cachexia following burn injury
A hallmark of the adaptive response to burn injury is the catabolism of skeletal muscle, which is known to persist for at least 9 months [6]. While excessive erosion of lean tissue impairs functional capacity and metabolic health, thus impeding rehabilitation post burn, it would seem the skeletal muscle is sacrificed to aid wound healing [62]. Indeed, acutely post burn, increasing protein intake does not further increase skeletal muscle protein synthesis. However, increasing dietary provision of protein increased skin FSR in the same group of patients [63]. Furthermore, it appears that the magnitude of skeletal muscle catabolism post burn is comparable to the magnitude of protein synthesis in the burn wound [26]. Moreover, there is marked loss of protein form skeletal muscle whereas there is a net accretion of protein within the burn wound [26], further suggesting that skeletal muscle protein is essentially redistributed post burn, and principally disposed of in healing skin wounds. Interestingly, while protein kinetic studies across the leg suggest that muscle breakdown dominates [26], where skeletal muscle catabolism persist for months post injury [6], studies of whole body protein turnover demonstrate that while protein turnover is greater in burned children compared to unburned children, whole body protein synthesis appears to be quantitatively more responsible for the increase in whole body protein turnover than whole body protein breakdown [24]. The discordance in the findings of studies determining skeletal muscle or whole body protein turnover in burned patients is most probably explained by the fact that skeletal muscle catabolism is not futile in these patients, but merely reflects the necessity for protein to be redistributed from skeletal muscle to healing burn and donor wounds.
While skeletal muscle wasting post burn is not disputed, the relative contribution of different protein pools within skeletal muscle (i.e. myofibrilar, sarcoplasmic, mitochondrial), towards skeletal muscle catabolism is not known with regards to burn injury. While the magnitude and persistence of skeletal muscle catabolism in severely burn patients results in profound alterations in body composition [14], it would perhaps be reasonable to assume that the various protein compartments of skeletal muscle are catabolized to a similar degree. An alternative hypothesis with particular pertinence to skeletal muscle quality is that given their high turnover rates relative to contractile proteins, that mitochondrial proteins are lost in greater abundance. However, at present the lack of experimental evidence prevents any conclusion from being made. Subsequently, future research aimed at addressing the aforementioned questions would be of great interest.
Skeletal muscle mitochondrial function in patients with severe burns
Skeletal muscle mitochondrial function in health
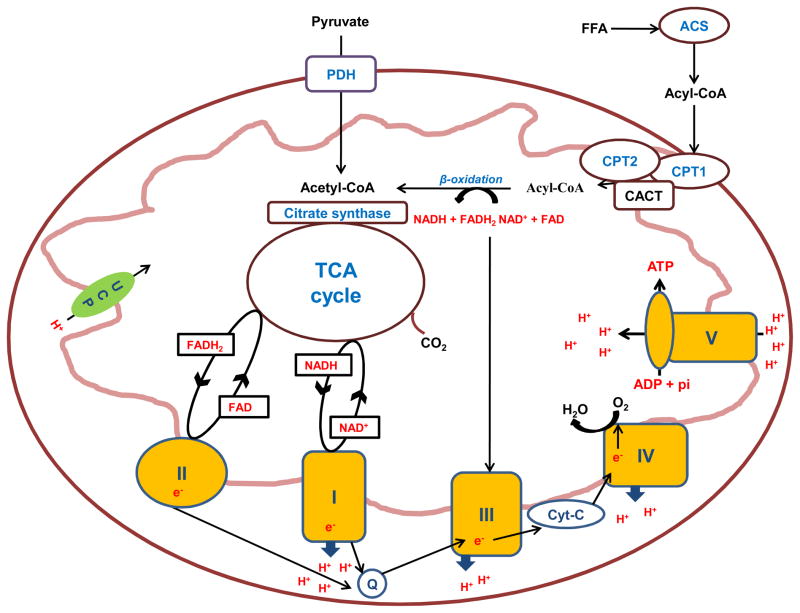
Mitochondria fulfill the role of the combustion engines of respiring cells. In essence these cellular organelles are able to catabolize nutrient derived substrates into a form (acetyl-CoA) which can participate in the tricarboxylic acid (TCA) cycle. In turn, the TCA cycle can generate NADH and succinate, which themselves feed protons (H+) and electrons (e−) to the electron transport chain (ETC) via NADH reductase (complex I) and succinate dehydrogenase (complex II) respectively. The subsequent flow of electrons along the ETC via ubiquinon (Q) and cytochrome C (cyt-C) pumps H+ from the matrix through NADH reductase (complex 1), cytochrome C reductase (complex III) and cytochrome c oxidase (complex IV). A subsequent change in matrix charge drives a proton motive force that transfers H+ back into the mitochondrial matrix through ATP synthase (complex V) which catalyzes the phosphorylation of ADP (Mitchel et al, 1961). As molecular oxygen acts as the final electron acceptor in the ETC the process of mitochondrial ATP synthesis is aerobic. While oxidative phosphorylation is generally considered to be well coupled in healthy mitochondria, H+ can re-enter the mitochondrial matrix via so called uncoupling proteins, which results in a seemingly futile production of heat. Figure 1 shows a schematic depiction of a mitochondrion and the constituents of the ETC.
Figure 1.
Schematic overview of the mitochondrion and its constituents including the tricarboxylic acid (TCA) cycle and the electron transport chain. Abbreviations: FFA, free fatty acids; PDH, pyruvate dehydrogenase; CPT 1, carnitine palmitoyl transferase 1; CPT 2, carnitine palmitoyl transferase 2; CACT, carnitine acylcarnitine translocase; TCA cycle, tricarboxylic acid cycle; NADH, reduced nicotinamide adenine dinucleotide; NAD oxidized nicotinamide adenine dinucleotide; FADH2 reduced flavin adenine dinucleotide; FAD oxidized flavin adenine dinucleotide; UCP, uncoupling protein; I, complex 1 (NADH reductase; II), complex II (succinate dehydrogenase); III, complex III (cytochrome C reductase); IV, complex IV (cytochrome C oxidase); V, complex V (ATP synthase); Q, ubiquinone; Cyt C, cytochrome C, ADP, adenosine diphosphate; ATP adenosine triphosphate; e−, electrons; H+, protons.
Given its important role in locomotion, mitochondria are found in relatively high number in skeletal muscles. As ATP is not stored in tissues like skeletal muscle in great abundance, means that there is a constant requirement for ATP production, a role primarily performed by the mitochondrion. While much research has focused on the impact of reduced skeletal muscle mass on morbidity and mortality in various patient populations, the impact of muscle wasting on skeletal muscle mitochondrial content and function has been paid less attention. This is perhaps surprising given the apparent plasticity of these organelles and therefore the potential for interventions to alter skeletal muscle mitochondrial number and/or function. For example, it has long been known that exercise training can increase mitochondrial enzyme activities in skeletal muscle [53]. Moreover, exercise appears to be a simple means of restoring skeletal muscle mitochondrial content and function in elderly subjects [64]. In addition, pharmacological interventions can also alter skeletal muscle mitochondrial function, where growth hormone [55] [55] and erythropoeitin [54] alter mitochondrial content and function in human skeletal muscle, further suggesting the mitochondria are am ideal target for interventions aimed at altering skeletal muscle bioenergetics in severely burned patients
The impact of burn injury on skeletal muscle mitochondrial function
Severe burn injury is associated with rapid alterations in skeletal muscle mitochondrial function. For example, Yasuhara and colleagues showed in rodents that even as early as 15 minutes after a 40% total body surface area (TBSA) burn there was a concurrent reduction in mitochondrial cytochrome C levels and increase in the concentration of cytochrome C in the cytosol consistent with damage to, and subsequent leaking from, the outer mitochondrial membrane. Furthermore, these same researchers showed that at 1 hour post injury mitochondrial membrane potential was altered in burned animals [65], further suggesting a rapid onset of mitochondrial dysfunction post burn.
In scalded mice, Padfield and co-workers demonstrated a comprehensive shift in skeletal muscle gene expression at six days post injury. Transcripts involved in carbohydrate and lipid metabolism, the TCA cycle, and oxidative phosphorylation were down regulated post injury when compared to control muscle [66]. For example, the expression of CPT1 and PDH mRNA, both thought to be rate limiting enzymes in fatty acid and carbohydrate oxidation, respectively, were significantly down regulated in muscle following burn injury. Moreover, the expression of citrate synthase and malate dehydrogenase, which catalyze the first and last steps of the TCA were both significantly down regulated within a few days of burn injury. Lastly, expression of complexes I, II, IV and V of the respiratory chain were significantly down regulated within 3 days of burn injury [66]. Taken together, it would appear that burn injury results in a genetic signature, and presuming that these changes in transcription translate to differences in protein expression, it is likely that this altered geneotype will also result in a profoundly altered phenotype. Indeed, Padifield and colleagues were able to show that phosphocreatine (PCr) concentrations within burned skeletal muscle were lower than control [66], where reduced phosphagen (PCr) levels are indicative of impaired oxidative phosphorylation. Indeed, these researchers were able to show that ATP production rates were significantly lower in burned muscle compared to control [66]. Taken together, these findings suggest that within a matter of days post injury, there are profound alterations in the expression of numerous transcripts involved in skeletal muscle bioenergetics. Moreover, this is associated with alterations in mitochondrial function, where the capacity of mitochondrial to phosphorylate ADP in vivo is significantly impaired.
The aforementioned data points towards rapid alterations in the expression of numerous proteins, which are central to normal mitochondrial function resulting in alterations in skeletal muscle bioenergetics. These transcriptional and functional changes in skeletal muscle bioenergetics are thought to be regulated in part by alterations in the expression of co-activators involved in mitochondrial biogenesis. Two such co-activators are the peroxisome proliforator-activated receptor gamma co-activators (PGC1) alpha and beta. PGC1α was identified first as a co-activator of brown adipose tissue [67], whereas PGC1β was subsequently identified as a homologue of PGC1α sharing much of the same sequence [68]. In their roles as co-activators PGC1α and PGC1β play numerous roles in energy metabolism and mitochondrial biogenesis. Interestingly, Tzika and colleagues [46] showed a profound reduction in skeletal muscle PGC1β mRNA expression in children with a 64% body surface area burn when compared to healthy controls. This is perhaps most interesting given that PGC1β regulates a number of other genes including nuclear respiratory factors 1 and 2 which themselves are involved in mitochondrial biogenesis by regulating the expression of several nuclear encoded proteins of the electron transport chains as well as well as indirectly inducing the expression on mitochondrial encoded electron transport chain proteins.
While there is an abundance of data concerning the impact of aging and insulin resistance on mitochondrial function in humans, far less data is available relating to human skeletal muscle mitochondrial function following severe burn injury. Colleagues in our laboratory were the first to determine mitochondrial function in permeabilized myofibres obtained from healthy and burned children. They found that following the addition of malate (2mM), ADP (0.5mM) and pyruvate (10mM) to induce state 3 respiration in the presence of pyruvate, mitochondrial oxygen consumption (normalized to mitochondrial content) was 50% lower in permeabilized myofibres of burned children when compared to controls. Similarly, following the addition of malate (2mM), ADP (0.5mM) and palmitoyl-carnitine (1mM) to induce state 3 respiration with parallel FADH2/NADH input from the β-oxidation pathway, mitochondrial oxygen consumption (normalized to mitochondrial content) was 66% lower in permeabilized muscle samples obtained from burned children when compared to healthy children [42]. While the authors concluded that mitochondrial oxidative capacity of both pyruvate and palmitate were reduced significantly in burned patients, it is difficult to definitively say whether this is true given the fact that high resolution respirometry primarily determines various respiratory states as well as the contribution of each respiratory complex to these states. Indeed, when state 3 respiration is achieved with the addition of glutamate, malate and ADP, the subsequent addition of octanoyl-carnitine for example, does not further augment respiratory flux [69]. Moreover, the absence of succinate and subsequently suboptimal electron transfer through complex II of the respiratory chain to ubiquinone and cytochrome C means the true oxidative capacity was not determined in the study of Cree and co-workers [42]. However, with that said, given that respiration was profoundly lower under identical experimental settings in myofibers from burned patients when compared to healthy individuals, it seems clear that burn injury results in profound qualitative changes in mitochondrial function within human skeletal muscle [42].
Does sepsis worsen skeletal muscle mitochondrial function in severely burned patients?
Derangements in skeletal muscle mitochondrial function in critically ill patients are not exclusive to burn injury. Indeed, sepsis induced multiple organ failure is also synonymous with skeletal muscle mitochondrial dysfunction. Maximal rates of citrate synthase and NADH reductase activity (markers of mitochondrial content), and cytochrome C oxidase activity (a marker of mitochondrial function) were significantly lower in skeletal muscle biopsies obtained from intensive care unit patients with sepsis and respiratory failure when compared to biopsy samples obtained from otherwise healthy individuals [70]. In addition, citrate synthase and NADH reductase activity were lower in the intercostal muscles of patients with sepsis induced multiple organ failure compared to age matched healthy controls [71]. Furthermore, in quadriceps muscle biopsies from the same patients, cytochrome C oxidase activity along with ATP and PCr contents were lower, while lactate content was higher when compared to healthy individuals [71]. Collectively, these finding suggest that sepsis results in reduced muscle mitochondrial content as well as derangements in mitochondrial function, leading to phosphagen depletion and lactate accumulation.
Whether patients with severe burn injury who become septic also have further deteriorations in skeletal muscle mitochondrial function remains to be seen. However, in a recent study documenting the cause of death in severely burned children it was found that 47% of mortality in this patient group could be attributed to sepsis [72]. Furthermore, the presence of sepsis is associated with 3-fold higher rates of skeletal muscle catabolism in patients with severe burn injury compared to patients with burn injury alone [19]. As such, it may be quite reasonable to assume that the development of sepsis in severely burned patients will lead to further derangements in skeletal muscle bioenergetics, which itself may be linked to multiple organ failure and death. However, further investigation into the relationship between sepsis and burn injury and the subsequent impact this may have on skeletal muscle mitochondrial function are needed.
Altering skeletal muscle mitochondrial function in patients with severe burns
An interesting aspect of mitochondria is their apparent plasticity in response to environmental and pharmacological stimuli [52–55, 64, 73–75], making them extremely interesting organelles when considering interventions in populations with derangements in skeletal muscle function. With regards to burn injury, Cree and colleagues demonstrated that acute PPARα agonist (fenofibrate) treatment could improve mitochondrial function in burned children. In particular, state 3 respiration (normalized to citrate synthase activity) in the presence of either palmitate or pyruvate was significantly improved in burned children following two weeks of fenofibrate administration (5 mg·kg−1) when compared to a placebo group [43, 44]. Importantly, these changes in mitochondrial function were accompanied by an apparent improvement in skeletal muscle metabolic flexibility and insulin sensitivity as evidenced by an increase in whole body palmitate oxidation [43] and an increase in insulin stimulated glucose disposal during a euglycemic hyperinsulinemic clamp [44]. While there was an apparent qualitative improvement in mitochondrial function following acute fenofibrate administration in burned children, Cree and co-workers also observed marked increases in both maximal rates of the mitochondrial enzymes citrate synthase and cytochrome c oxidase [43, 44], with the former being considered as a robust surrogate of mitochondrial content. Subsequently, it would appear that burn injury results in a reduction in skeletal muscle mitochondrial content and intrinsic function, while acute fenofibrate treatment results in both qualitative and quantitative improvements in skeletal muscle mitochondrial function in severely burned children. Although a mechanistic explanation for the favorable effects of fenofibrate on skeletal muscle mitochondrial content and function in burned patients remains elusive, these positive outcomes warrant further investigation, particularly with regards to the long term impact of fenofibrate treatment in severely burned individuals.
Under fasting conditions, insulin has been shown to play a central role in attenuating muscle protein catabolism in critically ill patients [76] Moreover, in patients with severe burns, hyperinsulinemia stimulates muscle protein synthesis and improves muscle protein balance [28]. Further, in severely burned rodents, insulin treatment attenuates the morphological and functional derangements in hepatic mitochondria [77]. With regards to skeletal muscle bioenergetics, intensive insulin therapy has been associated with an improvement in skeletal muscle mitochondrial capacity in severely burned children which was associated with improved glucose control [45]. Whether changes in mitochondrial physiology can be attributed to better glucose control or whether intensive insulin therapy mitigated post burn skeletal muscle catabolism, thereby maintaining skeletal muscle quality (i.e. mitochondrial mass) remains to be seen. However, this particular study [45] further highlights the importance of skeletal muscle mitochondria in metabolic function of burned patients while demonstrating that altering skeletal muscle mitochondrial function presents a viable means to improve outcomes in severely burned individuals.
While previous work has made significant strides in characterizing skeletal muscle mitochondrial function following severe burn trauma [42, 66], and demonstrating that pharmacological interventions such as PPARα agonists and intensive insulin therapy are a viable means of augmenting skeletal muscle mitochondrial function [43–45], which leads to improved skeletal muscle metabolic flexibility and insulin sensitivity, little is known regarding the potential for other interventions to mitigate the deleterious impact of burn injury on skeletal muscle bioenergetics. More specifically, whether other pharmacological interventions such as propranolol and/or oxandrolone treatment, which both mitigate skeletal muscle catabolism post burn [23, 32, 33, 40], also alter mitochondrial content or function within skeletal muscle remains to be seen. In addition, it has long been know that exercise training increases skeletal muscle oxidative capacity [53]. Exercise also has numerous benefits to the convalescing burn patient, which include improvements in lean mass, muscle strength and endurance, and pulmonary function [78–82]. However, to the best of our knowledge, the impact of exercise training on skeletal muscle mitochondrial content or function in severely burned individuals has yet to be studied.
Summary and conclusions
Skeletal muscle mitochondrial function plays an obligatory role in the functional capacity and metabolic health of an individual. In burn injury, there appears to be rapid and profound reductions in skeletal muscle mitochondrial content and function post injury, which are associated with poorer clinical outcomes. As such, strategies aimed at improving skeletal muscle oxidative capacity in severely burned individuals are likely to be efficacious with regards to aiding patient rehabilitation. Indeed, acute fenofibrate and insulin therapy are both associated with improvements in skeletal muscle oxidative capacity, which is associated with better glycemic control. These interesting observations act as a good starting point, however, it is clear that further robust human research trials are needed to i) further elucidate the role of skeletal muscle mitochondrial dysfunction in the pathophysiology of burn injury and ii) determine the efficacy of nutritional, exercise and pharmacological interventions alone, and in combination, for altering skeletal muscle bioenergetics post burn. Given the apparent plasticity of these organelles, it would be reasonable to assume that this avenue of research will be fruitful with regards to delivering interventions which improve the functional capacity and metabolic health of patients recovering from major burns.
Footnotes
Conflict of Interest statement:
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One. 2011;6:e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams FN, Herndon DN, Suman OE, Lee JO, Norbury WB, Branski LK, et al. Changes in cardiac physiology after severe burn injury. J Burn Care Res. 2011;32:269–74. doi: 10.1097/BCR.0b013e31820aafcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keck M, Herndon DH, Kamolz LP, Frey M, Jeschke MG. Pathophysiology of burns. Wien Med Wochenschr. 2009;159:327–36. doi: 10.1007/s10354-009-0651-2. [DOI] [PubMed] [Google Scholar]
- 4.Gauglitz GG, Herndon DN, Kulp GA, Meyer W, Jr, Jeschke MG. Abnormal insulin sensitivity persists up to three years in pediatric patients post-burn. J Clin Endocrinol Metab. 2009;94:1656–64. doi: 10.1210/jc.2008-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeschke MG, Chinkes DL, Finnerty CC, Kulp G, Suman OE, Norbury WB, et al. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hart DW, Wolf SE, Mlcak R, Chinkes DL, Ramzy PI, Obeng MK, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128:312–9. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 7.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2005;363:1895–902. doi: 10.1016/S0140-6736(04)16360-5. [DOI] [PubMed] [Google Scholar]
- 8.Jeschke MG, Micak RP, Finnerty CC, Herndon DN. Changes in liver function and size after a severe thermal injury. Shock. 2007;28:172–7. doi: 10.1097/shk.0b013e318047b9e2. [DOI] [PubMed] [Google Scholar]
- 9.Finnerty CC, Herndon DN, Przkora R, Pereira CT, Oliveira HM, Queiroz DM, et al. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006;26:13–9. doi: 10.1097/01.shk.0000223120.26394.7d. [DOI] [PubMed] [Google Scholar]
- 10.Jeschke MG, Barrow RE, Herndon DN. Extended hypermetabolic response of the liver in severely burned pediatric patients. Arch Surg. 2004;139:641–7. doi: 10.1001/archsurg.139.6.641. [DOI] [PubMed] [Google Scholar]
- 11.Chrysopoulo M, Jeschke M, Dziewulski P, Barrow R, Herndon D. Acute renal dysfunction in severely burned adults. J Trauma. 1999;46:141–4. doi: 10.1097/00005373-199901000-00024. [DOI] [PubMed] [Google Scholar]
- 12.Markell K, Renz E, White C, Albrecht M, Blackbourne L, Park M, et al. Abdominal complications after severe burns. J Am Coll Surg. 2009;208:940–7. doi: 10.1016/j.jamcollsurg.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 13.Mlcak R, Suman O, Herndon D. Respiratory management of inhalation injury. Burns. 2007;33:2–13. doi: 10.1016/j.burns.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Przkora R, Barrow RE, Jeschke MG, Suman OE, Celis M, Sanford AP, et al. Body composition changes with time in pediatric burn patients. J Trauma. 2006;60:968–71. doi: 10.1097/01.ta.0000214580.27501.19. [DOI] [PubMed] [Google Scholar]
- 15.Klein GL, Chen TC, Holick MF, Langman CB, Price H, Celis MM, et al. Synthesis of vitamin D in skin after burns. Lancet. 2004;363:291–2. doi: 10.1016/S0140-6736(03)15388-3. [DOI] [PubMed] [Google Scholar]
- 16.Klein GL, Langman CB, Herndon DN. Vitamin D depletion following burn injury in children: a possible factor in post-burn osteopenia. J Trauma. 2002;52:346–50. doi: 10.1097/00005373-200202000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Biolo G, Fleming RY, Maggi SP, Nguyen TT, Herndon DN, Wolfe RR. Inverse regulation of protein turnover and amino acid transport in skeletal muscle of hypercatabolic patients. J Clin Endocrinol Metab. 2002;87:3378–84. doi: 10.1210/jcem.87.7.8699. [DOI] [PubMed] [Google Scholar]
- 18.Goran MI, Peters EJ, Herndon DN, Wolfe RR. Total energy expenditure in burned children using the doubly labeled water technique. Am J Physiol. 1990;259:576–85. doi: 10.1152/ajpendo.1990.259.4.E576. [DOI] [PubMed] [Google Scholar]
- 19.Hart DW, Wolf SE, Chinkes DL, Gore DC, Mlcak RP, Beauford RB, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000;232:455–65. doi: 10.1097/00000658-200010000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norbury WB, Herndon DN, Branski LK, Chinkes DL, Jeschke MG. Urinary cortisol and catecholamine excretion after burn injury in children. J Clin Endocrinol Metab. 2008;93:1270–5. doi: 10.1210/jc.2006-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart DW, Wolf SE, Chinkes DL, Beauford RB, Mlcak RP, Heggers JP, et al. Effects of early excision and aggressive enteral feeding on hypermetabolism, catabolism, and sepsis after severe burn. J Trauma. 2003;54:755–61. doi: 10.1097/01.TA.0000060260.61478.A7. [DOI] [PubMed] [Google Scholar]
- 22.Kulp GA, Herndon DN, Lee JO, Suman OE, Jeschke MG. Extent and magnitude of catecholamine surge in pediatric burned patients. Shock. 2010;33:369–74. doi: 10.1097/SHK.0b013e3181b92340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223–9. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 24.Børsheim E, Chinkes DL, McEntire SJ, Rodriguez NR, Herndon DN, Suman OE. Whole body protein kinetics measured with a non-invasive method in severely burned children. Burns. 2010;36:1006–12. doi: 10.1016/j.burns.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrando AA, Sheffield-Moore M, Wolf SE, Herndon DN, Wolfe RR. Testosterone administration in severe burns ameliorates muscle catabolism. Crit Care Med. 2001;29:1936–42. doi: 10.1097/00003246-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Gore DC, Chinkes DL, Wolf SE, Sanford AP, Herndon DN, Wolfe RR. Quantification of protein metabolism in vivo for skin, wound, and muscle in severe burn patients. JPEN J Parenter Enteral Nutr. 2006;30:331–8. doi: 10.1177/0148607106030004331. [DOI] [PubMed] [Google Scholar]
- 27.Gore DC, Wolf SE, Herndon DN, Wolfe RR. Relative influence of glucose and insulin on peripheral amino acid metabolism in severely burned patients. JPEN J Parenter Enteral Nutr. 2002;26:271–7. doi: 10.1177/0148607102026005271. [DOI] [PubMed] [Google Scholar]
- 28.Gore DC, Wolf SE, Sanford AP, Herndon DN, Wolfe RR. Extremity hyperinsulinemia stimulates muscle protein synthesis in severely injured patients. Am J Physiol Endocrinol Metab. 2004;286:529–34. doi: 10.1152/ajpendo.00258.2003. [DOI] [PubMed] [Google Scholar]
- 29.Gore DC, Wolf SE, Sanford A, Herndon DN, Wolfe RR. Influence of metformin on glucose intolerance and muscle catabolism following severe burn injury. Ann Surg. 2005;241:334–42. doi: 10.1097/01.sla.0000152013.23032.d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hart DW, Herndon DN, Klein G, BLS, Celis M, Mohan S, et al. Attenuation of posttraumatic muscle catabolism and osteopenia by long-term growth hormone therapy. Ann Surg. 2001;233:827–34. doi: 10.1097/00000658-200106000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hart DW, Wolf SE, Chinkes DL, Lal SO, Ramzy PI, Herndon DN. Beta-blockade and growth hormone after burn. Ann Surg. 2002;236:450–7. doi: 10.1097/00000658-200210000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hart DW, Wolf SE, Ramzy PI, Chinkes DL, Beauford RB, Ferrando AA, et al. Anabolic effects of oxandrolone after severe burn. Ann Surg. 2001;233:556–64. doi: 10.1097/00000658-200104000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herndon DN, Barrow RE, Rutan TC, Minifee P, Jahoor F, Wolfe RR. Effect of propranolol administration on hemodynamic and metabolic responses of burned pediatric patients. Ann Surg. 1988;208:484–92. doi: 10.1097/00000658-198810000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herndon DN, Ramzy PI, DebRoy MA, Zheng M, Ferrando AA, Chinkes DL, et al. Muscle protein catabolism after severe burn: effects of IGF-1/IGFBP-3 treatment. Ann Surg. 1999;229:713–20. doi: 10.1097/00000658-199905000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jahoor F, Desai M, Herndon DN, Wolfe RR. Dynamics of the protein metabolic response to burn injury. Metabolism. 1988;37:330–7. doi: 10.1016/0026-0495(88)90132-1. [DOI] [PubMed] [Google Scholar]
- 36.Prelack K, Yu YM, Dylewski M, Lydon M, Sheridan RL, Tompkins RG. The contribution of muscle to whole-body protein turnover throughout the course of burn injury in children. J Burn Care Res. 2010;31:942–8. doi: 10.1097/BCR.0b013e3181f938e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakurai Y, Aarsland A, Herndon DN, Chinkes DL, Pierre E, Nguyen TT, et al. Stimulation of muscle protein synthesis by long-term insulin infusion in severely burned patients. Ann Surg. 1995;222:283–94. doi: 10.1097/00000658-199509000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tuvdendorj D, Chinkes DL, Zhang XJ, Ferrando AA, Elijah IE, Mlcak RP, et al. Adult patients are more catabolic than children during acute phase after burn injury: a retrospective analysis on muscle protein kinetics. Intensive Care Med. 2011;37:1317–22. doi: 10.1007/s00134-011-2223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tuvdendorj D, Chinkes DL, Zhang XJ, Sheffield-Moore M, Herndon DN. Skeletal muscle is anabolically unresponsive to an amino acid infusion in pediatric burn patients 6 months postinjury. Ann Surg. 2011;253:592–7. doi: 10.1097/SLA.0b013e31820d9a63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tuvdendorj D, Chinkes DL, Zhang XJ, Suman OE, Aarsland A, Ferrando A, et al. Long-term oxandrolone treatment increases muscle protein net deposition via improving amino acid utilization in pediatric patients 6 months after burn injury. Surgery. 2011;149:645–53. doi: 10.1016/j.surg.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf SE, Thomas S, Dasu MR, Ferrando AA, Chinkes DL, Wolfe RR, et al. Improved net protein balance, lean mass, and gene expression changes with oxandrolone treatment in the severely burned. Ann Surg. 2003;237:801–10. doi: 10.1097/01.SLA.0000071562.12637.3E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cree MG, Fram RY, Herndon DN, Qian T, Angel C, Green JM, et al. Human mitochondrial oxidative capacity is acutely impaired after burn trauma. Am J Surg. 2007;196:234–9. doi: 10.1016/j.amjsurg.2007.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cree MG, Newcomer BR, Herndon DN, Qian T, Sun D, Morio B, et al. PPAR-alpha agonism improves whole body and muscle mitochondrial fat oxidation, but does not alter intracellular fat concentrations in burn trauma children in a randomized controlled trial. Nutr Metab. 2007;23:4, 9. doi: 10.1186/1743-7075-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cree MG, Zwetsloot JJ, Herndon DN, Qian T, Morio B, Fram R, et al. Insulin sensitivity and mitochondrial function are improved in children with burn injury during a randomized controlled trial of fenofibrate. Ann Surg. 2007;245:214–21. doi: 10.1097/01.sla.0000250409.51289.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fram RY, Cree MG, Wolfe RR, Mlcak RP, Qian T, Chinkes DL, et al. Intensive insulin therapy improves insulin sensitivity and mitochondrial function in severely burned children. Crit Care Med. 2010;38:1475–83. doi: 10.1097/CCM.0b013e3181de8b9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tzika A, Mintzopoulos D, Padfield K, Wilhelmy J, Mindrinos M, Yu H, et al. Reduced rate of adenosine triphosphate synthesis by in vivo 31P nuclear magnetic resonance spectroscopy and downregulation of PGC-1beta in distal skeletal muscle following burn. Int J Mol Med. 2008;21:201–8. [PubMed] [Google Scholar]
- 47.Caldwell F, Hammel H, Dolan F. A calorimeter for simultaneous determination of heat production and heat loss in the rat. J Appl Physiol. 1966;21:1665–71. doi: 10.1152/jappl.1966.21.5.1665. [DOI] [PubMed] [Google Scholar]
- 48.Caldwell FJ, Bowser B, Crabtree J. The effect of occlusive dressings on the energy metabolism of severely burned children. Ann Surg. 1981;193:579–91. doi: 10.1097/00000658-198105000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caldwell FJ, Wallace B, Cone J, Manuel L. Control of the hypermetabolic response to burn injury using environmental factors. Ann Surg. 1992;215:485–90. doi: 10.1097/00000658-199205000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fram RY, Cree MG, Wolfe RR, Barr D, Herndon DN. Impaired glucose tolerance in pediatric burn patients at discharge from the acute hospital stay. J Burn Care Res. 2010;31:728–33. doi: 10.1097/BCR.0b013e3181eebe63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cree MG, Fram RY, Barr D, Chinkes D, Wolfe RR, Herndon DN. Insulin resistance, secretion and breakdown are increased 9 months following severe burn injury. Burns. 2009;35:63–9. doi: 10.1016/j.burns.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gibala M. Molecular responses to high-intensity interval exercise. Appl Physiol Nutr Metab. 2009;34:428–32. doi: 10.1139/H09-046. [DOI] [PubMed] [Google Scholar]
- 53.Holloszy J, Oscai L, Don I, Molé P. Mitochondrial citric acid cycle and related enzymes: adaptive response to exercise. Biochem Biophys Res Commun. 1970;40:1368–73. doi: 10.1016/0006-291x(70)90017-3. [DOI] [PubMed] [Google Scholar]
- 54.Plenge U, Belhage B, Guadalupe-Grau A, Andersen PR, Lundby C, Dela F, et al. Erythropoietin treatment enhances muscle mitochondrial capacity in humans. Front Physiol. 2012;3:50. doi: 10.3389/fphys.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Short KR, Moller N, Bigelow ML, Coenen-Schimke J, Nair KS. Enhancement of muscle mitochondrial function by growth hormone. J Clin Endocrinol Metab. 2008;93:597–604. doi: 10.1210/jc.2007-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolfe RR, Klein S, Herndon DN, Jahoor F. Substrate cycling in thermogenesis and amplification of net substrate flux in human volunteers and burned patients. J Trauma. 1990;30:6–9. doi: 10.1097/00005373-199012001-00004. [DOI] [PubMed] [Google Scholar]
- 57.Yu YM, Tompkins RG, Ryan CM, Young VR. The metabolic basis of the increase of the increase in energy expenditure in severely burned patients. JPEN J Parenter Enteral Nutr. 1999;23:160–8. doi: 10.1177/0148607199023003160. [DOI] [PubMed] [Google Scholar]
- 58.Wolfe RR, Herndon DN, Peters EJ, Jahoor F, Desai MH, Holland OB. Regulation of lipolysis in severely burned children. Ann Surg. 1987;206:214–21. doi: 10.1097/00000658-198708000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolfe RR, Herndon DN, Jahoor F, Miyoshi H, Wolfe M. Effect of severe burn injury on substrate cycling by glucose and fatty acids. N Engl J Med. 1987;317:403–8. doi: 10.1056/NEJM198708133170702. [DOI] [PubMed] [Google Scholar]
- 60.Wolfe RR, Jahoor F, Herndon DN, Miyoshi H. Isotopic evaluation of the metabolism of pyruvate and related substrates in normal adult volunteers and severely burned children: effect of dichloroacetate and glucose infusion. Surgery. 1991;110:54–67. [PubMed] [Google Scholar]
- 61.Wolfe RR, Durkot MJ, Allsop JR, Burke JF. Glucose metabolism in severely burned patients. Metabolism. 1979;28:1031–9. doi: 10.1016/0026-0495(79)90007-6. [DOI] [PubMed] [Google Scholar]
- 62.Porter C, Hurren NM, Herndon DN, Børsheim E. Whole body and skeletal muscle protein turnover in recovery from burns. Int J Burns Trauma. 2013;3:9–17. [PMC free article] [PubMed] [Google Scholar]
- 63.Patterson BW, Nguyen T, Pierre E, Herndon DN, Wolfe RR. Urea and protein metabolism in burned children: effect of dietary protein intake. Metabolism. 1997;46:573–8. doi: 10.1016/s0026-0495(97)90196-7. [DOI] [PubMed] [Google Scholar]
- 64.Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci. 2006;61:534–40. doi: 10.1093/gerona/61.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yasuhara S, Perez ME, Kanakubo E, Yasuhara Y, Shin YS, Kaneki M, et al. Skeletal muscle apoptosis after burns is associated with activation of proapoptotic signals. Am J Physiol Endocrinol Metab. 2000;279:1114–21. doi: 10.1152/ajpendo.2000.279.5.E1114. [DOI] [PubMed] [Google Scholar]
- 66.Padfield K, Astrakas L, Zhang Q, Gopalan S, Dai G, Mindrinos M, et al. Burn injury causes mitochondrial dysfunction in skeletal muscle. Proc Natl Acad Sci U S A. 2005;102:5368–73. doi: 10.1073/pnas.0501211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–39. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 68.Lin J, Wu H, Tarr P, Zhang C, Wu Z, Boss O, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 69.Larsen S, Nielsen J, Hansen CN, Nielsen LB, Wibrand F, Stride N, et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol. 2012;590:3349–60. doi: 10.1113/jphysiol.2012.230185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fredriksson K, Tjäder I, Keller P, Petrovic N, Ahlman B, Schéele C, et al. Dysregulation of mitochondrial dynamics and the muscle transcriptome in ICU patients suffering from sepsis induced multiple organ failure. PLoS One. 2008;3:e3686. doi: 10.1371/journal.pone.0003686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fredriksson K, Hammarqvist F, Strigård K, Hultenby K, Ljungqvist O, Wernerman J, et al. Derangements in mitochondrial metabolism in intercostal and leg muscle of critically ill patients with sepsis-induced multiple organ failure. Am J Physiol Endocrinol Metab. 2006;291:1044–50. doi: 10.1152/ajpendo.00218.2006. [DOI] [PubMed] [Google Scholar]
- 72.Williams FN, Herndon DN, Hawkins HK, Lee JO, Cox RA, Kulp GA, et al. The leading causes of death after burn injury in a single pediatric burn center. Crit Care Med. 2009;13:R183. doi: 10.1186/cc8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86:5755–61. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- 74.Menshikova EV, Ritov VB, Toledo FG, Ferrell RE, Goodpaster BH, Kelley DE. Effects of weight loss and physical activity on skeletal muscle mitochondrial function in obesity. Am J Physiol Endocrinol Metab. 2005;288:818–25. doi: 10.1152/ajpendo.00322.2004. [DOI] [PubMed] [Google Scholar]
- 75.Little J, Safdar A, Wilkin G, Tarnopolsky M, Gibala M. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol. 2010;588:1011–22. doi: 10.1113/jphysiol.2009.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang X, Kunkel K, Jahoor F, Wolfe R. Role of basal insulin in the regulation of protein kinetics and energy metabolism in septic patients. JPEN J Parenter Enteral Nutr. 1991;15:394–9. doi: 10.1177/0148607191015004394. [DOI] [PubMed] [Google Scholar]
- 77.Jeschke M, Kraft R, Song J, Gauglitz G, Cox R, Brooks N, et al. Insulin protects against hepatic damage postburn. Mol Med. 2011;17:516–22. doi: 10.2119/molmed.2010.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suman OE, Spies RJ, Celis MM, Mlcak RP, Herndon DN. Effects of a 12-wk resistance exercise program on skeletal muscle strength in children with burn injuries. J Appl Physiol. 2001;91:1168–75. doi: 10.1152/jappl.2001.91.3.1168. [DOI] [PubMed] [Google Scholar]
- 79.Suman OE, Mlcak RP, Herndon DN. Effect of exercise training on pulmonary function in children with thermal injury. J Burn Care Rehabil. 2002;23:288–93. doi: 10.1097/00004630-200207000-00013. [DOI] [PubMed] [Google Scholar]
- 80.Przkora R, Herndon DN, Suman OE. The effects of oxandrolone and exercise on muscle mass and function in children with severe burns. Pediatrics. 2006;119:109–16. doi: 10.1542/peds.2006-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suman OE, Herndon DN. Effects of cessation of a structured and supervised exercise conditioning program on lean mass and muscle strength in severely burned children. Arch Phys Med Rehabil. 2007;88:24–9. doi: 10.1016/j.apmr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 82.Al-Mousawi AM, Williams FN, Mlcak RP, Jeschke MG, Herndon DN, Suman OE. Effects of exercise training on resting energy expenditure and lean mass during pediatric burn rehabilitation. J Burn Care Res. 2010;31:400–8. doi: 10.1097/BCR.0b013e3181db5317. [DOI] [PMC free article] [PubMed] [Google Scholar]