Abstract
Transcription of messenger(m) RNA occurs in the nucleus, making the translocation of mRNA across the nuclear envelope (NE) boundary a critical determinant of proper gene expression and cell survival. A major mRNA export route occurs via the NXF1-dependent pathway through the nuclear pore complexes (NPCs) embedded in the NE. However, recent findings have discovered new evidence supporting the existence of multiple mechanisms for crossing the NE, including both NPC-mediated and NE budding-mediated pathways. An analysis of the trans-acting factors and cis components that define these pathways reveals shared elements as well as mechanistic differences. We review here the current understanding of the mechanisms that characterize each pathway and highlight the determinants that influence mRNA transport fate.
Keywords: mRNA export, nuclear envelope, nuclear pore complex, transport, exportin, lamin
Transport across the nuclear envelope
Eukaryotic cells are distinguished from their prokaryotic predecessors by the presence of a nuclear envelope (NE), a double lipid bilayer encasing the heritable genome [1]. In the simplest terms, the NE functions as a physical barrier that separates the contents of the nucleus from those of the cytoplasm. Transcription of messenger (m)RNA occurs in the nucleus, whereas translation of mRNA into functional protein occurs in the cytoplasm [2, 3]. This spatial issue is resolved by mechanisms that efficiently export mRNA across the NE.
An emerging body of work supports the concept that the NE is not simply a static structure that divides subcellular compartments, but rather plays roles in chromatin organization and gene regulation as recently reviewed in [4]. Structurally, the NE outer nuclear membrane (ONM) is continuous with the endoplasmic reticulum and the inner nuclear membrane (INM). Distinct subsets of integral membrane proteins specifically localize to the ONM and the INM [5]. Physical connections in the NE lumen between ONM and INM-localized transmembrane proteins maintain the structural integrity of the NE and establish cytoskeleton-chromatin communications [6, 7]. In higher eukaryotes, the INM is associated with an essential intermediate filament-based structure called the nuclear lamina [8, 9]. Access to the INM requires association with or unraveling of the lamin meshwork. A key process in NE dynamics is the fusion of the ONM and INM to create pores [10]. Nuclear pore complexes (NPCs), large ∼60 megadalton protein assemblies, are embedded in these pores and serve as selective portals for bidirectional transport [11, 12]. Water, sugar, ions, and small molecules freely diffuse through the central NPC channel. In contrast, molecules between ∼5–40nm in diameter, such as mRNPs, ribosomal RNA and proteins, require facilitated transport mechanisms to cross the NPC’s selectivity barrier [11, 13]. Together, all these NE components are differentially leveraged in distinct mRNA export pathways.
A long-standing tenet in the field posits that the NPC is the sole route for nucleocytoplasmic transport through the NE. The identification of a novel vesicular, NE budding-mediated mRNA export mechanism has elicited a restructuring of this view [14]. Combined with additional evidence for multiple mRNA export pathways within the NPC, several interesting mechanistic questions are raised: what are the unique features that distinguish these pathways and what are the fates of the associated mRNAs? We will discuss here how mRNA intrinsic elements such as mRNA structure, sequence, and length, as well as mRNA-binding proteins/adaptors and transport receptors, effectively serve as postage for the mRNA and dictate which pathways are utilized to cross the NE. We aim to summarize our current understanding of mRNA export pathways and emphasize the outstanding questions yet to be addressed.
Common elements for mRNP trafficking across the NE
Regardless of the transport pathway utilized to cross the NE, the mRNA must be packaged in the nucleus into an mRNA ribonucleoprotein (mRNP) complex. It is well established that assembly of the mRNP is tightly integrated with many aspects of mRNA biogenesis including transcription, processing, and quality surveillance (reviewed in [3, 15]). Nascent mRNAs are transcribed in the nucleus by RNA polymerase II (RNAPII). The carboxyl-terminal domain of the largest subunit of RNAPII orchestrates the co-transcriptional loading of accessory factors onto the growing transcript. Dynamic association and disassociation of these accessory protein factors with the mRNA leads to the production of an export-competent mRNP [16]. Proper assembly of an mRNP directly impacts the transport of mRNAs and their associated RNA-binding proteins across the NE [15, 16].
Multiple common events occur if the mRNP is to be targeted to the NPC for export. First, during mRNP biogenesis, a transport receptor is recruited to the complex. At least four NPC-mediated mRNA export mechanisms have been characterized in higher eukaryotic systems (Figure 1). One of these pathways has been extensively analyzed in S. cerevisiae (Figure 1d). There are two major transport receptors implicated in two distinct mRNA export pathways across the NPC: CRM1 (also known as exportin-1; S. cerevisiae (y) Xpo1) (Figure 1a–c) [17, 18] and the heterodimer NXF1/NXT1 (also known as TAP/p15; yMex67/Mtr2) (Figure 1d) [19, 20]. The majority of the constitutively expressed mRNAs are thought to utilize the NXF1/NXT1 pathway. In contrast, specialized subsets of mRNAs as well as UsnRNAs (uridine-rich small nuclear ribonucleoprotein particles), rRNAs (ribosomal ribonucleoproteins), and SRP RNA (signal recognition particle), are exported via a CRM1-dependent pathway (reviewed in [21]). Unlike NXF1/NXT1, which does not rely on the Ran system to mediate mRNA export, CRM1 is a karyopherin that functions by binding cargo in the presence of the GTP bound form of Ran GTPase (reviewed in [11, 13]). Importantly, the transport receptors do not bind to mRNA independently; rather, they require adaptor proteins to facilitate incorporation into the mRNP. These adaptor proteins effectively function as trans-acting factors in the mRNA export mechanism and are considered in further detail below (Table 1).
Figure 1.
NPC-mediated nuclear mRNA export pathways. The major components of the nuclear envelope are the outer nuclear membrane (ONM), inner nuclear membrane (INM), nuclear pore complexes (NPCs), and the nuclear lamina (shaded grey). Of note, lamins are not present in the unicellular organism, S. cerevisiae. The NPC can be divided into three parts: nuclear basket, central channel lined with FG-Nups (brown wavy lines), and cytoplasmic filaments. Export-competent mRNPs assemble in the nucleus. The fate of these mRNAs is dependent on the factors that bind in the nucleus prior to export through the NPC. Major nuclear export receptors are indicated in yellow, whereas adaptor proteins for these receptors are indicated in blue. Higher eukaryotic factors are indicated; however, S. cerevisiae homologues exist for many of these factors. References are cited in the corresponding text. (a) CRM1-mediated mRNA export is RanGTP dependent. LRPPRC binds eIF4E and the RNA element 4E–SE as part of the eIF4E-dependent CRM1-mediated mRNA export pathway. (b) AU-rich element (ARE)-containing mRNAs are bound by the mRNA binding protein HuR that recruits CRM1 via the adaptor proteins, pp32 and APRIL. (c) The tissue-specific factor NXF3 directly interacts with CRM1 and may serve as an adaptor protein for CRM1-mediated mRNA export. (d) The major mRNA export receptor, NXF1 coupled with its heterodimeric partner, NXT1, is required for formation of an export-competent mRNP. REF/Aly serves as an adaptor protein for NXF1. The TREX/THO complex is co-transcriptionally recruited to the mRNP, linking transcription to export. At the cytoplasmic face, NXF1 and NXT1 are remodeled off of the mRNP via the concerted action of DDX19, Gle1, and inositol hexakisphosphate IP6.
Table 1.
Major proposed functions of mRNA nuclear export factors
| NPC-mediated pathways | |||
|---|---|---|---|
| Metazoan | Yeast (S. cerevisiae) | Role | References |
| NXF1-mediated pathway | |||
| NXF1 (TAP) | Mex67 | Major transport receptor for mRNA export |
[19, 20] |
| NXT1 (p15) | Mtr2 | mRNA export factor, binds to NXF1 to form heterodimeric complex |
[82, 83] |
| REF/Aly | Yra1 | Forms part of a complex that binds export-competent mRNPs |
[23–25] |
| CRM1-mediated pathway | |||
| CRM1 | Xpo1 | Major export receptor for proteins and some specific mRNAs |
[17, 18] |
| NXF3 | Tissue specific adaptor for CRM1 |
[51] | |
| pp32 (ANP32a) | Adaptor for CRM1, interacts with HuR |
[50] | |
| APRIL (ANP32b) | Adaptor for CRM1, interacts with HuR |
[50] | |
| HuR | Adaptor for CRM1 binds to AU-rich elements in 3’UTR |
[50] | |
| hCG1 | Nup42 | FG-Nup localized to cytoplasmic face; required for heat shock mRNA export |
[71–73] |
| Gle1 | Gle1 | Export factor that binds to hCG1; involved in mRNP remodeling by activating Dbp5; binds inositol hexakisphosphate (IP6) |
[36, 37, 72, 84] |
| Nup214 | Nup159 | FG-Nup localized to cytoplasmic face; triggers ADP release from Dbp5 |
[39, 41, 85, 86] |
| DDX19 | Dbp5 | RNA-dependent DEAD-box ATPase; involved in mRNP remodeling; ATPase cycle regulated by a by Gle1-IP6 and Nup159 |
[38, 39, 87] |
| Nup358/RanBP2 | Major component of NPC cytoplasmic filaments; potential role in CRM1- mediated export |
[44, 88] | |
| NE budding-mediated pathways | |||
| aPKC | Phosphorylates and remodels nuclear lamins |
[14] | |
| DFrizzled2 | Wnt-1 receptor whose cleavage product, DFz2C, localizes to intranuclear foci |
[89, 90] | |
| LamC | Nuclear lamin protein, required for localization of RNA granules to perinuclear space |
[14] | |
Second, quality control steps that monitor the fidelity of mRNA processing and mRNP assembly occur prior to translocation into the NPC, some at the NPC nuclear face. Myosin-like protein 1, (yMlp1; vertebrate TPR) a protein anchored to the nucleoplasmic face of the NPC, has been shown to facilitate nuclear retention of intron-containing transcripts [22]. Posttranslational modifications have also been implicated in mRNP surveillance and can be influenced by the co-transcriptional recruitment of the TREX complex, a transcription elongation/mRNA export complex consisting of a core THO complex (yHpr1, yTho2, yMft1, yThp2) and associated factors (ySub2, yYra1, and yTho1) (reviewed in [15]). yYra1 (REF/Aly), a component of TREX required for recruitment of yMex67 (NXF1) is ubiquitinated by the E3 ubiquitin ligase, yTom1 [23–26]. The ubiquitination of yYra1 promotes its dissociation from the mRNP prior to export. Failure of yYra1 to dissociate from the mRNP is thought to generate an improperly assembled mRNP that is then targeted to the mRNA surveillance/degradation machinery [23]. In contrast, CRM1 has been implicated in the export of unspliced or partially spliced viral transcripts [27]. Thus, the quality control mechanisms regulating CRM1-dependent RNA export are probably distinct from those of NXF1-dependent export.
Third, NXF1/NXT1 and CRM1 associated mRNPs are targeted to and traverse the NPC by virtue of the transport receptor’s interactions with specific NPC proteins lining the central transport channel [28–31]. NPC proteins (nucleoporins, Nups) with extended unstructured domains harboring multiple phenylalanine-glycine (FG) repeats serve as functional mediators of Mex67-dependent mRNP exit through the NPC (reviewed in [32]). FG repeat domains reside throughout the NPC central transport channel as well as on both the nuclear basket and cytoplasmic filaments [11]. yMex67/Mtr2 directly binds FG Nups during NPC translocation [29, 30]. In addition, the FG repeat-containing Nup98 has been implicated in facilitating CRM1-mediated export of mRNPs [31]. The impact that the nuclear and cytoplasmic NPC faces have on mRNP dynamics was elegantly demonstrated by recent in vivo imaging studies of labeled endogenous mRNAs traversing the NPC [33, 34]. These studies revealed that mRNPs dwell primarily at the nuclear basket while docking and at the cytoplasmic filaments upon release, compared with a relatively short time interval spent in the NPC central transport channel.
Lastly, as the mRNP exits the NPC and enters the cytoplasm, it undergoes significant conformational and compositional changes, referred to as “remodeling,” that contribute to export efficiency and directionality [35]. For the yMex67/Mtr2 pathway, remodeling at the cytoplasmic face of the NPC requires several proteins including the mRNA export factor Gle1 and its cofactor inositol hexakisphosphate (IP6) that associate with the DEAD-box protein yDbp5 (vertebrate DDX19) [36, 37]. yDbp5 is positioned at the cytoplasmic filaments of the NPC by binding specifically to the NPC protein yNup159 (vertebrate Nup214) [38, 39]. The cycle of mRNP remodeling is dependent on both Gle1-IP6 stimulation of the RNA dependent yDbp5 ATPase activity [40–42] and yNup159 triggered ADP release [41]. The prevailing view asserts that this remodeling alters the composition of the mRNP, allowing recycling of the transport receptors and adaptors into the nucleus, and ensuring unidirectional transport of the mRNP cargo into the cytoplasm [35, 41, 43]. While the same vertebrate players reside at the cytoplasmic filaments of the NPC, it is as yet unknown whether the regulatory mechanisms are fully conserved. In contrast, CRM1-mediated cargo release is directed by RanGTP hydrolysis coordinated by RanBP1, Nup358/RanBP2, and RanGTPase activating protein (RanGAP) (reviewed in [21]). In the cytoplasm, generation of RanGDP presumably releases the mRNP from CRM1. Whether mRNPs transported by CRM1 also require DDX19-mediated remodeling remains unknown. However, similar to Nup214, Nup358/RanBP2 is asymmetrically localized at the NPC cytoplasmic filaments [44]. In summary, the NXF1-mediated and CRM1-mediated pathways exhibit both common themes and mechanistic differences.
The role of trans-acting factors in directing mRNA export pathways
There are several criteria utilized to distinguish the transport pathway fate for a given mRNA. In terms of direct RNA specific recognition, NXF1 was indeed first identified as a direct RNA binding protein for the export of constitutive transport element (CTE) viral RNAs [19, 45]. Export of tRNA and miRNA is also mediated via direct RNA recognition by the karyopherins Exportin-t (yLos1) and Exportin-5 (yMsn5), respectively as elegantly illustrated by recent crystal structures [46, 47]. However, direct mRNA recognition by NXF1/NXT1 (yMex67/Mtr2) or CRM1 (yXpo1) is not thought to occur. Instead, the selectivity of the transport receptors is believed to be dictated by trans-acting adaptor proteins that directly bind the mRNA. There is evidence of at least three distinct adaptor mechanisms that regulate mRNP recognition by a specific transport receptor: direct binding to a single adaptor, binding facilitated by multiple adaptor interfaces, and adaptor-mediated conformational changes to allow mRNA binding.
CRM1 recruitment to mRNA is mediated by single adaptor proteins through CRM1 interaction with their nuclear export sequence (NES). Several adaptor proteins have been identified for CRM1 (reviewed in [21]). These include leucine-rich pentatricopeptide repeat protein (LRPPRC), RNA-binding protein human antigen R (HuR) and nuclear export factor 3 (NXF3). LRPPRC, a protein with several putative NESs, has been shown to serve as an adaptor facilitating the interaction between CRM1 and the RNA element 4E–SE [48] (Figure 1a). In fact, compelling evidence has been reported that a subset of nuclear mRNAs relies upon LRPPRC to recruit both CRM1 and eIF4E as part of a unique-eIF4E-dependent export pathway [49]. AU-rich element (ARE)-containing mRNAs interact with CRM1 through its interaction with the nucleocytoplasmic shuttling proteins pp32 and APRIL that are recruited to this class of RNAs by HuR [50] (Figure 1b). Lastly, the CRM1 adaptor NXF3, a tissue-specific factor closely related to NXF1 but lacking its C-terminal Nup interaction domain, contains an NES that recruits CRM1 to mediate export [51] (Figure 1c).
In contrast to CRM1’s recognition of a single NES-containing protein adaptor, NXF1 (yMex67) utilizes one of at least two different mechanisms to associate with the mRNP. Multiple adaptors for NXF1 have been identified that play roles in mRNP maturation and export (Figure 1d). Many of the principle components of mRNA export are conserved from S. cerevisiae to humans; however, the mechanisms coordinating mRNA export may be slightly different. Notably, in higher eukaryotes, the majority of transcripts are spliced and the deposition of the exon-junction complex affects mRNA export. In contrast, in S. cerevisiae, export is primarily coordinated with transcription and polyadenylation [15]. Studies in S. cerevisiae have revealed that yYra1 and yNab2 concomitantly serve as adaptors to enhance binding of yMex67 to the mRNA [23, 52]. ySub2, an RNA helicase co-transcriptionally deposited on the pre-mRNA via its association with the TREX complex, interacts with the same domain of yMex67 as yYra1 [53]. This mutually exclusive interaction dictates that ySub2 must be remodeled off of the mRNP prior to recruitment of yYra1 and yMex67. In addition, the serine-arginine rich (SR) protein, yNpl3, has also been implicated as an mRNA export adaptor and can function independently of ySub2 and yYra1 [54]. SR proteins have also been found to function as adaptors for NXF1 in higher eukaryotes. Many of these adaptor proteins are RNA binding proteins that perform additional functions in mRNA biogenesis suggesting the tight coordination of mRNA processing and export events (reviewed in [55]).
Some mRNA export adaptors function cooperatively to mediate conformational or structural changes that are required for proper mRNP processing. A recent study presented evidence for NXF1 intramolecular interactions that inhibit its own RNA binding activity [56]. Recruitment of two TREX components, REF/Aly and Thoc5, trigger a conformational change in NXF1 exposing NXF1’s RNA binding domain and allowing for binding to the mRNP. Notably, S. cerevisiae does not have an ortholog of Thoc5, suggesting that the mechanisms controlling mRNP export have evolved to include additional levels of regulation in higher eukaryotes. Other structural alterations include those induced by regulatory posttranslational modifications of adaptor proteins. For example, yNpl3 function in export is dependent on its phosphorylation status. Dephosphorylation of yNpl3 by yGlc7 facilitates the association of yMex67 with the mRNP, whereas phosphorylation of yNpl3 in the cytoplasm promotes its disassociation from the mRNP and re-import into the nucleus [57, 58]. In addition, yNab2 is phosphorylated following heat shock stress by the serine/threonine MAP kinase ySlt2, impacting yNab2 association with the transport receptor yMex67 [52]. Taken together, an intricate web of transport receptor interactions with their trans-acting adaptors is required to direct the export pathway.
Evidence for cis-acting mRNA elements in mRNA export
Unique features that distinguish mRNA export pathway fates also include RNA-intrinsic properties such as ribonucleotide structure, sequence, and length. Early knowledge of RNA elements affecting mRNA export originated from experiments that analyzed viral pre-mRNA processing. The human immunodeficiency virus type 1 (HIV-1) mRNA contains a defined RNA structural element, referred to as the HIV-1 Rev Response Element (RRE), which is bound specifically by the nuclear export signal (NES)-containing protein, Rev (reviewed in [27]). HIV-1 mRNA nuclear export is mediated by CRM1 which specifically binds Rev via its NES [17, 59]. A similar pathway has been described for the SM response element (SMRE), an RNA element that is specifically bound by SM, a nuclear phosphoprotein produced by Epstein-Barr virus (EBV) [60]. Additional evidence supporting a role for cis-acting elements in export comes from type D retroviruses where CTE-containing RNAs are exported via direct binding of NXF1 to the CTE [19, 45]. Interestingly, the RNA transport element (RTE) identified in mouse transposons is a CTE-related element that is exported via the NXF1-mediated pathway; however, it is not bound directly by NXF1 but rather is bound by the novel mRNA export factor, RBM15 [61]. More recently, the type D murine LTR retrotransposon (musD) transport element (MTE) was identified and although tertiary interactions have been documented, much remains to be learned regarding the mechanism of export [62].
RNA elements have also been implicated in the export of specialized classes of transcripts. Most vertebrate mRNAs that encode for secretory or mitochondrial-targeted proteins contain RNA elements positioned at the 5’ end of the transcript that promote export. Two specific sequence motifs, signal sequence coding region (SSCR) and mitochondrial targeting sequence coding region (MSCR), have been defined as mRNA identity elements that promote export in a process known as ALREX (alternative mRNA export) [63]. Similarly, mRNAs containing AREs in their 3’UTRs that bind to HuR are substrates for CRM1-mediated export [50]. Furthermore, an elF4E-dependent CRM1-mediated mRNA export pathway has also been shown to be mediated by an RNA element. The mRNA targets of this specific pathway contain a 50-nucleotide structural element in the 3’UTR termed 4E–SE, the eIF4E sensitivity element [49]. Finally, studies examining the heat shock stress response in S. cerevisiae have revealed distinct sequences within the 5’ and 3’UTRs of heat shock transcripts to be sufficient for export during heat shock stress conditions [64]. Hence, despite heterogeneity in sequence and structure, RNA elements play a significant role as part of the postage for the export of diverse classes of transcripts.
One recent study has suggested that the mechanism of export can be designated by RNA length [65]. To evaluate this possibility, nucleocytoplasmic export mechanisms of UsnRNA and mRNA were compared. Both UsnRNAs and mRNAs are transcribed in the nucleus and are subsequently bound by the cap-binding complex (CBC) (reviewed in [66]). However, the adaptor protein PHAX (phosphorylated adaptor for RNA export) is recruited exclusively to UsnRNAs, whereas mRNAs associate with the adaptor REF/Aly prior to recruitment of NXF1 [67]. In an in vitro system, recombinant hnRNP C1/C2 heterotetramers selectively bind RNAs of greater than 200–300 nucleotides in length and inhibit PHAX binding to these longer RNAs [65]. Thus, PHAX binding and hnRNP C1/C2 binding are mutually exclusive. In this way, the intrinsic property of RNA length can impact competition between PHAX and hnRNP C proteins and can allow sorting of RNA for the respective export mechanisms. Together, these studies highlight the function of RNA elements in mRNA export.
Determinants of NE budding-mediated mRNA export
In addition to the translocation of mRNPs through the NPC, a provocative new study has uncovered an mRNA export mechanism that bypasses the NPC [14]. This is accomplished by budding of the INM into the NE lumen and subsequent vesicular fusion with the ONM. From a historical perspective, the molecular mechanism of this pathway might be similar to the vesicle-mediated transport of herpesvirus (HSV) capsids in mammalian cells, often referred to as nuclear egress (reviewed in [68, 69]) (Box 1).
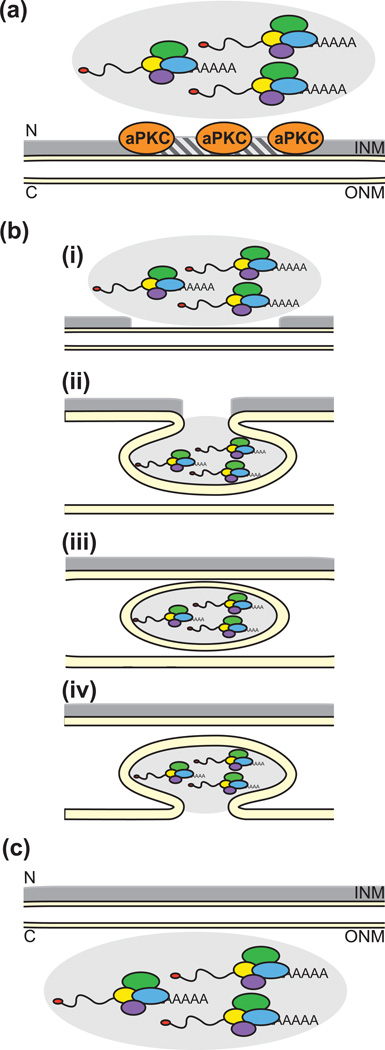
The discovery of a novel NE budding-mediated pathway for endogenous mRNP export was made while studying Wnt-dependent neuromuscular junction (NMJ) synapse development in Drosophila larval body wall muscles [14]. During synapse development, a C-terminal deletion product of the DFrizzled-2 receptor (DFz2C) is incorporated into prominent intranuclear foci containing large mRNP molecules. These large mRNP molecules then translocate from the nucleus to the cytoplasm by a NE budding-mediated pathway rather than via the NPC (Figure 2). Of note, atypical protein kinase C (PKC) is required for formation of the INM invaginations, suggesting that phosphorylation of the nuclear lamins is important in this pathway analogous to the viral nuclear egress pathway (Table 1).
Figure 2.
Proposed steps in the NE budding-mediated mRNA export pathway based on [14]. C, cytoplasm; INM, inner nuclear membrane; N, nucleus; ONM, outer nuclear membrane. (a) Formation of intranuclear granules (grey shading) containing endogenous mRNPs is observed, and local disruption of the nuclear lamins is initiated via activity of atypical PKC (aPKC). (b) Changes in the nuclear lamina allow for positioning of the mRNP granule at the INM (i). The INM invaginates to envelope the mRNP granule (ii). Membrane-bound mRNP granule localizes to the perinuclear space between the ONM and INM (iii). The mRNP granule membrane fuses with ONM (iv). (c) The mRNP granule is released into the cytoplasm.
Many unanswered questions remain regarding this mRNA export pathway. The mRNP components (adaptor proteins and/or intrinsic RNA elements) that direct mRNAs to this pathway will need to be defined. Further, the INM and ONM membrane fusion and fission machineries are unknown. Both RNA binding proteins and NE localized proteins are predicted to play roles, in addition to PKC. As such, this NE budding-mediated pathway likely does not function in isolation of NPCs and may be inherently dependent on NPC-mediated import pathways for proper localization of soluble and INM factors, thereby making it challenging to study the NE budding-mediated export mechanism. It will also be important to pinpoint how the adaptors or transport factors involved in NE budding-mediated mRNP export dictate transport directionality and are recycled after release into the cytoplasm.
Function of mRNA export pathways in environmental stress, disease, and development
The existence of multiple mRNA export pathways opens the possibilities for differential regulation amongst the routes. In particular, there is clear potential for adaptive and selective impacts on specific mRNA transit pathways during times of environmental stress, disease, and development. It is well documented that cells adapt and survive in non-optimal growth conditions through modulation of gene expression (reviewed in [70]). The heat shock stress response in S. cerevisiae serves as an excellent paradigm for how environmental changes specifically alter the mRNA export pathways. One of the major hallmarks of this response is that transcripts encoding for heat shock proteins (Hsps) are efficiently exported, while non-heat shock transcripts are retained in the nucleus [64, 71]. This selectivity in mRNA export during heat shock is dependent on both specific NPC proteins and concurrent impacts on the mRNA-binding protein adaptors for the transport receptor yMex67. The export of transcripts encoding Hsps specifically requires yNup42 (hCG1) on the NPC cytoplasmic face [71–73]; however, yNup42 is not required for the export of non-heat shock mRNAs. yMex67 is required for both heat shock and non-heat shock mRNA export whereas its trans-adaptors, yYra1, ySub2, yNpl3, and yNab2, all of which are essential for non-heat shock mRNA export, are dispensable for heat shock mRNA export [52, 74, 75]. Moreover, yNab2 phosphorylation following heat shock stress is coincident with co-localization of yNab2 and yYra1 to yMlp1-dependent intranuclear foci [52]. Further investigation of the changes to mRNA export pathways that result from environmental stresses, such as heat shock, will be required to fully define how the molecular mechanisms are regulated.
CRM1-mediated mRNA export can also be viewed as an adaptive export mechanism for a specialized set of cellular mRNAs. Importantly, CRM1’s adaptor proteins bind in a sequence-specific manner and can recruit CRM1 to incompletely spliced mRNAs, such as HIV-1 mRNA, that would normally be retained in the nucleus by proofreading mechanisms [27]. The demonstrated ability of CRM1 to bypass nuclear quality control mechanisms might provide the cell with an advantage during times of stress. Interestingly, another CRM1 adaptor, NXF3, exhibits tissue-specific expression [51], suggesting roles in coordinating gene expression in a tissue-specific manner. Further support for a model of adaptive export is provided by studies finding differential regulation of ARE-containing mRNAs by ARE-binding proteins (AUBPs) in response to stress (reviewed in [76]).
Recent reviews have documented the relationship between mRNA export dysregulation and disease [77, 78]. An intriguing series of studies involves the eIF4E/CRM1-dependent mRNA export pathway. The oncogene, eIF4E, is elevated in 30% of cancers and is associated with poor prognosis [79]. Correspondingly, mRNAs involved in cell proliferation and survival have been identified as substrates for the eIF4E-dependent mRNA export pathway [49]. eIF4E/CRM1- dependent export correlates with changes in NPC composition, which is believed to be the means by which eIF4E promotes oncogenic transformation of cells [80]. Several modifications to the cytoplasmic face of the NPC are observed upon eIF4E overexpression. Specifically, these include reductions in the levels of Nup358/RanBP2 and Nup214, as well as a 2–3 fold increase in levels of RanBP1, DDX19, and Gle1. This indicates that the CRM1 pathway might be responsible for the export of mRNAs encoding essential factors for the NXF1 pathways: GLE1 and DDX19 mRNA. As such, overexpression of eIF4E not only impacts CRM1-dependent mRNA export, but also NXF1-dependent export. Just as the NE budding-mediated mRNA export pathway might be dependent on NPC import, this strongly suggests that the different NPC-mediated mRNA export pathways are also functionally interconnected.
The critical role of mRNA export factors and proper gene expression during development is supported by recent work. Functional characterization of vertebrate Gle1 has revealed an essential role in the survival of spinal neural precursor cells [81]. More in depth analysis of the NE budding-mediated mRNP export mechanism promises to strengthen and expand the links between mRNA export and development [14]. In considering why the NE budding-mediated pathway is required, it is possibly utilized for especially large mRNPs that would otherwise require extensive remodeling for export through the NPC. This mechanism might also facilitate co-transport of mRNAs encoding functionally related proteins and regulate subsequent translation of these mRNAs at their proper subcellular destination.
Concluding remarks
There is a growing body of evidence for multiple interconnected mRNA export pathways in the cell. NPC and NE budding-mediated mRNA export share common elements as well as distinguishing features. Work to date fully supports a model in which the selectivity and the postage for export is dictated by mRNA determinants for the NPC pathways, such as mRNA intrinsic elements and trans-acting factors. In contrast, the NE budding-mediated mRNA export pathway has only very recently been identified; hence, many outstanding questions remain unanswered (Box 2).
Going forward, it will be critical to consider how all of these pathways work together to impact cellular gene expression. We further postulate that the NPC-mediated and NE budding-mediated pathways are physiologically interdependent and that defining these relationships will provide critical insight to advance therapeutics for cancer as well as other diseases. As illustrated by the recent identification of the messages targeted by the eIF4E-dependent mRNA export pathway [80], it is clear that gaining a better understanding of the specific mRNPs that are exported by each pathway will be needed for insights into human health and disease. We anticipate that continued innovations in technical approaches across a number of model systems will be key to uncovering precise molecular mechanisms and developing a fully integrated perspective on nuclear mRNA export.
Box 1. Viral nuclear egress.
Nuclear egress is characterized by a sequence of envelopment, deenvelopment, and reenvelopment steps at the NE. To access the INM, local lamina remodeling is triggered by the recruitment of kinases to the nuclear lamina and lamin phosphorylation (reviewed in [69]). The primary enveloped virions reside in the NE perinuclear space until translocation occurs via fusion of the primary envelope with the ONM. Vesicle-mediated nucleocytoplasmic transport, formally believed to be a viral-specific pathway, has now been identified in Drosophila, suggesting that nuclear egress is a more common mechanism for export of mRNPs, hitherto largely uncharted. Importantly, much remains to be learned about the nature of this pathway.
Box 2. Outstanding questions.
What is the level of crosstalk between NPC-mediated and NE budding-mediated pathways? What is the extent to which these pathways are functionally interconnected?
What are the growth and survival benefits of multiple cellular mRNA export pathways? Are all the different mRNA export mechanisms conserved amongst eukaryotic organisms?
What controls transport directionality in the NE budding pathway? Do the mRNPs transcripts exported by the NE budding pathway undergo remodeling? If so, are any of the factors involved in the NXF1 and CRM1 pathways required?
Are distinct types of mRNA exported by each pathway?
What are the necessary and sufficient, intrinsic and extrinsic, factors that dictate mRNA export fate?
Are the different pathways differentially controlled under different environmental, developmental or disease states?
Highlights.
Multiple mRNA export mechanisms exist utilizing both NPC and NE-budding pathways
RNA intrinsic elements and mRNA adaptors specify the mRNA export pathway
Pathways are differentially regulated during stress, disease, and development
Recent evidence suggests that these export pathways are functionally interconnected
Acknowledgements
The authors were supported by grants from the National Institute of Health (R37GM051219 (S.R.W.) and T32CA11925 (B.J.N.)). We apologize to those colleagues whose work we could not cite due to space limitations.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hetzer MW. The nuclear envelope. Cold Spring Harb Perspect Biol. 2010;2 doi: 10.1101/cshperspect.a000539. a000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hocine S, et al. RNA processing and export. Cold Spring Harb Perspect Biol. 2010;2 doi: 10.1101/cshperspect.a000752. a000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore MJ, Proudfoot NJ. Pre-mRNA processing reaches back to transcription and ahead to translation. Cell. 2009;136:688–700. doi: 10.1016/j.cell.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Van de Vosse DW, et al. Role of the nuclear envelope in genome organization and gene expression. Wiley Interdiscip Rev Syst Biol Med. 2011;3:147–166. doi: 10.1002/wsbm.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonin W, et al. Traversing the NPC along the pore membrane: targeting of membrane proteins to the INM. Nucleus. 2011;2:87–91. doi: 10.4161/nucl.2.2.14637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guttinger S, et al. Orchestrating nuclear envelope disassembly and reassembly during mitosis. Nat Rev Mol Cell Biol. 2009;10:178–191. doi: 10.1038/nrm2641. [DOI] [PubMed] [Google Scholar]
- 7.Starr DA, Fridolfsson HN. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu Rev Cell Dev Biol. 2010;26:421–444. doi: 10.1146/annurev-cellbio-100109-104037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dechat T, et al. Nuclear lamins. Cold Spring Harb Perspect Biol. 2010;2 doi: 10.1101/cshperspect.a000547. a000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerace L, Huber MD. Nuclear lamina at the crossroads of the cytoplasm and nucleus. J Struct Biol. 2012;177:24–31. doi: 10.1016/j.jsb.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capelson M, et al. Nuclear pore complexes: guardians of the nuclear genome. Cold Spring Harb Symp Quant Biol. 2010;75:585–597. doi: 10.1101/sqb.2010.75.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aitchison JD, Rout MP. The yeast nuclear pore complex and transport through it. Genetics. 2012;190:855–883. doi: 10.1534/genetics.111.127803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grossman E, et al. Functional architecture of the nuclear pore complex. Annu Rev Biophys. 2012;41:557–584. doi: 10.1146/annurev-biophys-050511-102328. [DOI] [PubMed] [Google Scholar]
- 13.Guttler T, Gorlich D. Ran-dependent nuclear export mediators: a structural perspective. Embo J. 2011;30:3457–3474. doi: 10.1038/emboj.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Speese SD, et al. Nuclear envelope budding enables large ribonucleoprotein particle export during synaptic Wnt signaling. Cell. 2012;149:832–846. doi: 10.1016/j.cell.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Navarro S, Hurt E. Linking gene regulation to mRNA production and export. Curr Opin Cell Biol. 2011;23:302–309. doi: 10.1016/j.ceb.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Kelly SM, Corbett AH. Messenger RNA export from the nucleus: a series of molecular wardrobe changes. Traffic. 2009;10:1199–1208. doi: 10.1111/j.1600-0854.2009.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fornerod M, et al. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 18.Stade K, et al. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell. 1997;90:1041–1050. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- 19.Gruter P, et al. TAP, the human homolog of Mex67p, mediates CTE-dependent RNA export from the nucleus. Mol Cell. 1998;1:649–659. doi: 10.1016/s1097-2765(00)80065-9. [DOI] [PubMed] [Google Scholar]
- 20.Segref A, et al. Mex67p, a novel factor for nuclear mRNA export, binds to both poly(A)+ RNA and nuclear pores. Embo J. 1997;16:3256–3271. doi: 10.1093/emboj/16.11.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutten S, Kehlenbach RH. CRM1-mediated nuclear export: to the pore and beyond. Trends Cell Biol. 2007;17:193–201. doi: 10.1016/j.tcb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Galy V, et al. Nuclear retention of unspliced mRNAs in yeast is mediated by perinuclear Mlp1. Cell. 2004;116:63–73. doi: 10.1016/s0092-8674(03)01026-2. [DOI] [PubMed] [Google Scholar]
- 23.Iglesias N, et al. Ubiquitin-mediated mRNP dynamics and surveillance prior to budding yeast mRNA export. Genes Dev. 2010;24:1927–1938. doi: 10.1101/gad.583310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stutz F, et al. REF, an evolutionary conserved family of hnRNP-like proteins, interacts with TAP/Mex67p and participates in mRNA nuclear export. Rna. 2000;6:638–650. doi: 10.1017/s1355838200000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strasser K, Hurt E. Yra1p, a conserved nuclear RNA-binding protein, interacts directly with Mex67p and is required for mRNA export. Embo J. 2000;19:410–420. doi: 10.1093/emboj/19.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zenklusen D, et al. The yeast hnRNP-Like proteins Yra1p and Yra2p participate in mRNA export through interaction with Mex67p. Mol Cell Biol. 2001;21:4219–4232. doi: 10.1128/MCB.21.13.4219-4232.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cullen BR. Nuclear mRNA export: insights from virology. Trends Biochem Sci. 2003;28:419–424. doi: 10.1016/S0968-0004(03)00142-7. [DOI] [PubMed] [Google Scholar]
- 28.Terry LJ, Wente SR. Nuclear mRNA export requires specific FG nucleoporins for translocation through the nuclear pore complex. J Cell Biol. 2007;178:1121–1132. doi: 10.1083/jcb.200704174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strasser K, et al. Binding of the Mex67p/Mtr2p heterodimer to FXFG, GLFG, and FG repeat nucleoporins is essential for nuclear mRNA export. J Cell Biol. 2000;150:695–706. doi: 10.1083/jcb.150.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strawn LA, et al. The GLFG regions of Nup116p and Nup100p serve as binding sites for both Kap95p and Mex67p at the nuclear pore complex. J Biol Chem. 2001;276:6445–6452. doi: 10.1074/jbc.M008311200. [DOI] [PubMed] [Google Scholar]
- 31.Oka M, et al. The mobile FG nucleoporin Nup98 is a cofactor for Crm1-dependent protein export. Mol Biol Cell. 2010;21:1885–1896. doi: 10.1091/mbc.E09-12-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terry LJ, Wente SR. Flexible gates: dynamic topologies and functions for FG nucleoporins in nucleocytoplasmic transport. Eukaryot Cell. 2009;8:1814–1827. doi: 10.1128/EC.00225-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mor A, et al. Dynamics of single mRNP nucleocytoplasmic transport and export through the nuclear pore in living cells. Nat Cell Biol. 2010;12:543–552. doi: 10.1038/ncb2056. [DOI] [PubMed] [Google Scholar]
- 34.Grunwald D, Singer RH. In vivo imaging of labelled endogenous beta-actin mRNA during nucleocytoplasmic transport. Nature. 2010;467:604–607. doi: 10.1038/nature09438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Folkmann AW, et al. Dbp5, Gle1-IP6 and Nup159: a working model for mRNP export. Nucleus. 2011;2:540–548. doi: 10.4161/nucl.2.6.17881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alcazar-Roman AR, et al. Inositol hexakisphosphate and Gle1 activate the DEAD-box protein Dbp5 for nuclear mRNA export. Nat Cell Biol. 2006;8:711–716. doi: 10.1038/ncb1427. [DOI] [PubMed] [Google Scholar]
- 37.Weirich CS, et al. Activation of the DExD/H-box protein Dbp5 by the nuclear-pore protein Gle1 and its coactivator InsP6 is required for mRNA export. Nat Cell Biol. 2006;8:668–676. doi: 10.1038/ncb1424. [DOI] [PubMed] [Google Scholar]
- 38.Snay-Hodge CA, et al. Dbp5p/Rat8p is a yeast nuclear pore-associated DEAD-box protein essential for RNA export. Embo J. 1998;17:2663–2676. doi: 10.1093/emboj/17.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitt C, et al. Dbp5, a DEAD-box protein required for mRNA export, is recruited to the cytoplasmic fibrils of nuclear pore complex via a conserved interaction with CAN/Nup159p. Embo J. 1999;18:4332–4347. doi: 10.1093/emboj/18.15.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montpetit B, et al. A conserved mechanism of DEAD-box ATPase activation by nucleoporins and InsP6 in mRNA export. Nature. 2011;472:238–242. doi: 10.1038/nature09862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noble KN, et al. The Dbp5 cycle at the nuclear pore complex during mRNA export II: nucleotide cycling and mRNP remodeling by Dbp5 are controlled by Nup159 and Gle1. Genes Dev. 2011;25:1065–1077. doi: 10.1101/gad.2040611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hodge CA, et al. The Dbp5 cycle at the nuclear pore complex during mRNA export I: dbp5 mutants with defects in RNA binding and ATP hydrolysis define key steps for Nup159 and Gle1. Genes Dev. 2011;25:1052–1064. doi: 10.1101/gad.2041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tran EJ, et al. The DEAD-box protein Dbp5 controls mRNA export by triggering specific RNA:protein remodeling events. Mol Cell. 2007;28:850–859. doi: 10.1016/j.molcel.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 44.Bernad R, et al. Nup358/RanBP2 attaches to the nuclear pore complex via association with Nup88 and Nup214/CAN and plays a supporting role in CRM1-mediated nuclear protein export. Mol Cell Biol. 2004;24:2373–2384. doi: 10.1128/MCB.24.6.2373-2384.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bachi A, et al. The C-terminal domain of TAP interacts with the nuclear pore complex and promotes export of specific CTE-bearing RNA substrates. Rna. 2000;6:136–158. doi: 10.1017/s1355838200991994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cook AG, et al. Structures of the tRNA export factor in the nuclear and cytosolic states. Nature. 2009;461:60–65. doi: 10.1038/nature08394. [DOI] [PubMed] [Google Scholar]
- 47.Okada C, et al. A high-resolution structure of the pre-microRNA nuclear export machinery. Science. 2009;326:1275–1279. doi: 10.1126/science.1178705. [DOI] [PubMed] [Google Scholar]
- 48.Topisirovic I, et al. Molecular dissection of the eukaryotic initiation factor 4E (eIF4E) export-competent RNP. Embo J. 2009;28:1087–1098. doi: 10.1038/emboj.2009.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Culjkovic B, et al. eIF4E is a central node of an RNA regulon that governs cellular proliferation. J Cell Biol. 2006;175:415–426. doi: 10.1083/jcb.200607020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brennan CM, et al. Protein ligands to HuR modulate its interaction with target mRNAs in vivo. J Cell Biol. 2000;151:1–14. doi: 10.1083/jcb.151.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang J, et al. Two closely related human nuclear export factors utilize entirely distinct export pathways. Mol Cell. 2001;8:397–406. doi: 10.1016/s1097-2765(01)00303-3. [DOI] [PubMed] [Google Scholar]
- 52.Carmody SR, et al. The mitogen-activated protein kinase Slt2 regulates nuclear retention of non-heat shock mRNAs during heat shock-induced stress. Mol Cell Biol. 2010;30:5168–5179. doi: 10.1128/MCB.00735-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strasser K, Hurt E. Splicing factor Sub2p is required for nuclear mRNA export through its interaction with Yra1p. Nature. 2001;413:648–652. doi: 10.1038/35098113. [DOI] [PubMed] [Google Scholar]
- 54.Lee MS, et al. A protein that shuttles between the nucleus and the cytoplasm is an important mediator of RNA export. Genes Dev. 1996;10:1233–1246. doi: 10.1101/gad.10.10.1233. [DOI] [PubMed] [Google Scholar]
- 55.Tutucci E, Stutz F. Keeping mRNPs in check during assembly and nuclear export. Nat Rev Mol Cell Biol. 2011;12:377–384. doi: 10.1038/nrm3119. [DOI] [PubMed] [Google Scholar]
- 56.Viphakone N, et al. TREX exposes the RNA-binding domain of Nxf1 to enable mRNA export. Nat Commun. 2012;3:1006. doi: 10.1038/ncomms2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gilbert W, Guthrie C. The Glc7p nuclear phosphatase promotes mRNA export by facilitating association of Mex67p with mRNA. Mol Cell. 2004;13:201–212. doi: 10.1016/s1097-2765(04)00030-9. [DOI] [PubMed] [Google Scholar]
- 58.Gilbert W, et al. Phosphorylation by Sky1p promotes Npl3p shuttling and mRNA dissociation. Rna. 2001;7:302–313. doi: 10.1017/s1355838201002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neville M, et al. The importin-beta family member Crm1p bridges the interaction between Rev and the nuclear pore complex during nuclear export. Curr Biol. 1997;7:767–775. doi: 10.1016/s0960-9822(06)00335-6. [DOI] [PubMed] [Google Scholar]
- 60.Verma D, et al. Epstein-Barr Virus SM protein utilizes cellular splicing factor SRp20 to mediate alternative splicing. J Virol. 2010;84:11781–11789. doi: 10.1128/JVI.01359-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lindtner S, et al. RNA-binding motif protein 15 binds to the RNA transport element RTE and provides a direct link to the NXF1 export pathway. J Biol Chem. 2006;281:36915–36928. doi: 10.1074/jbc.M608745200. [DOI] [PubMed] [Google Scholar]
- 62.Legiewicz M, et al. The RNA transport element of the murine musD retrotransposon requires long-range intramolecular interactions for function. J Biol Chem. 2010;285:42097–42104. doi: 10.1074/jbc.M110.182840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Palazzo AF, Akef A. Nuclear export as a key arbiter of “mRNA identity” in eukaryotes. Biochim Biophys Acta. 2012;1819:566–577. doi: 10.1016/j.bbagrm.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 64.Saavedra C, et al. Regulation of mRNA export in response to stress in Saccharomyces cerevisiae. Genes Dev. 1996;10:1608–1620. doi: 10.1101/gad.10.13.1608. [DOI] [PubMed] [Google Scholar]
- 65.McCloskey A, et al. hnRNP C tetramer measures RNA length to classify RNA polymerase II transcripts for export. Science. 2012;335:1643–1646. doi: 10.1126/science.1218469. [DOI] [PubMed] [Google Scholar]
- 66.Kohler A, Hurt E. Exporting RNA from the nucleus to the cytoplasm. Nat Rev Mol Cell Biol. 2007;8:761–773. doi: 10.1038/nrm2255. [DOI] [PubMed] [Google Scholar]
- 67.Ohno M, et al. PHAX, a mediator of U snRNA nuclear export whose activity is regulated by phosphorylation. Cell. 2000;101:187–198. doi: 10.1016/S0092-8674(00)80829-6. [DOI] [PubMed] [Google Scholar]
- 68.Mettenleiter TC, et al. The way out: what we know and do not know about herpesvirus nuclear egress. Cell Microbiol. 2012 doi: 10.1111/cmi.12044. [DOI] [PubMed] [Google Scholar]
- 69.Johnson DC, Baines JD. Herpesviruses remodel host membranes for virus egress. Nat Rev Microbiol. 2011;9:382–394. doi: 10.1038/nrmicro2559. [DOI] [PubMed] [Google Scholar]
- 70.Morano KA, et al. The response to heat shock and oxidative stress in Saccharomyces cerevisiae. Genetics. 2012;190:1157–1195. doi: 10.1534/genetics.111.128033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saavedra CA, et al. Yeast heat shock mRNAs are exported through a distinct pathway defined by Rip1p. Genes Dev. 1997;11:2845–2856. doi: 10.1101/gad.11.21.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kendirgi F, et al. Interaction between the shuttling mRNA export factor Gle1 and the nucleoporin hCG1: a conserved mechanism in the export of Hsp70 mRNA. Mol Biol Cell. 2005;16:4304–4315. doi: 10.1091/mbc.E04-11-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stutz F, et al. The yeast nucleoporin rip1p contributes to multiple export pathways with no essential role for its FG-repeat region. Genes Dev. 1997;11:2857–2868. doi: 10.1101/gad.11.21.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krebber H, et al. Uncoupling of the hnRNP Npl3p from mRNAs during the stress-induced block in mRNA export. Genes Dev. 1999;13:1994–2004. doi: 10.1101/gad.13.15.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rollenhagen C, et al. Following temperature stress, export of heat shock mRNA occurs efficiently in cells with mutations in genes normally important for mRNA export. Eukaryot Cell. 2007;6:505–513. doi: 10.1128/EC.00317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.von Roretz C, et al. Turnover of AU-rich-containing mRNAs during stress: a matter of survival. Wiley Interdiscip Rev RNA. 2011;2:336–347. doi: 10.1002/wrna.55. [DOI] [PubMed] [Google Scholar]
- 77.Siddiqui N, Borden KL. mRNA export and cancer. Wiley Interdiscip Rev RNA. 2012;3:13–25. doi: 10.1002/wrna.101. [DOI] [PubMed] [Google Scholar]
- 78.Kohler A, Hurt E. Gene regulation by nucleoporins and links to cancer. Mol Cell. 2010;38:6–15. doi: 10.1016/j.molcel.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 79.Borden KL, Culjkovic-Kraljacic B. Ribavirin as an anti-cancer therapy: acute myeloid leukemia and beyond? Leuk Lymphoma. 2010;51:1805–1815. doi: 10.3109/10428194.2010.496506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Culjkovic-Kraljacic B, et al. The oncogene eIF4E reprograms the nuclear pore complex to promote mRNA export and oncogenic transformation. Cell Rep. 2012;2:207–215. doi: 10.1016/j.celrep.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jao LE, et al. A zebrafish model of lethal congenital contracture syndrome 1 reveals Gle1 function in spinal neural precursor survival and motor axon arborization. Development. 2012;139:1316–1326. doi: 10.1242/dev.074344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Santos-Rosa H, et al. Nuclear mRNA export requires complex formation between Mex67p and Mtr2p at the nuclear pores. Mol Cell Biol. 1998;18:6826–6838. doi: 10.1128/mcb.18.11.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guzik BW, et al. NXT1 (p15) is a crucial cellular cofactor in TAP-dependent export of intron-containing RNA in mammalian cells. Mol Cell Biol. 2001;21:2545–2554. doi: 10.1128/MCB.21.7.2545-2554.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murphy R, Wente SR. An RNA-export mediator with an essential nuclear export signal. Nature. 1996;383:357–360. doi: 10.1038/383357a0. [DOI] [PubMed] [Google Scholar]
- 85.Hodge CA, et al. Rat8p/Dbp5p is a shuttling transport factor that interacts with Rat7p/Nup159p and Gle1p and suppresses the mRNA export defect of xpo1-1 cells. Embo J. 1999;18:5778–5788. doi: 10.1093/emboj/18.20.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kraemer DM, et al. The essential yeast nucleoporin NUP159 is located on the cytoplasmic side of the nuclear pore complex and serves in karyopherin-mediated binding of transport substrate. J Biol Chem. 1995;270:19017–19021. doi: 10.1074/jbc.270.32.19017. [DOI] [PubMed] [Google Scholar]
- 87.Tseng SS, et al. Dbp5p, a cytosolic RNA helicase, is required for poly(A)+ RNA export. Embo J. 1998;17:2651–2662. doi: 10.1093/emboj/17.9.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hutten S, Kehlenbach RH. Nup214 is required for CRM1-dependent nuclear protein export in vivo. Mol Cell Biol. 2006;26:6772–6785. doi: 10.1128/MCB.00342-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ataman B, et al. Rapid activity-dependent modifications in synaptic structure and function require bidirectional Wnt signaling. Neuron. 2008;57:705–718. doi: 10.1016/j.neuron.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mathew D, et al. Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science. 2005;310:1344–1347. doi: 10.1126/science.1117051. [DOI] [PMC free article] [PubMed] [Google Scholar]