Abstract
Nanoparticles (NPs), due to their size-dependent physical and chemical properties, have shown remarkable potential for a wide range of applications over the past decades. Particularly, the biological compatibilities and functions of NPs have been extensively studied for expanding their potential in areas of biomedical application such as bioimaging, biosensing, and drug delivery. In doing so, surface functionalization of NPs by introducing synthetic ligands and/or natural biomolecules has become a critical component in regards to the overall performance of the NP system for its intended use. Among known examples of surface functionalization, the construction of an artificial cell membrane structure, based on phospholipids, has proven effective in enhancing biocompatibility and has become a viable alternative to more traditional modifications, such as direct polymer conjugation. Furthermore, certain bioactive molecules can be immobilized onto the surface of phospholipid platforms to generate displays more reminiscent of cellular surface components. Thus, NPs with membrane-mimetic displays have found use in a range of bioimaging, biosensing, and drug delivery applications. This review herein describes recent advances in the preparations and characterization of integrated functional NPs covered by artificial cell membrane structures and their use in various biomedical applications.
Keywords: Nanoparticle, Membrane-Mimetic, Drug Delivery, Bioimaging, Biosensing, Lipid
1. Introduction
Nanoparticles, existing in the rapidly evolving field of nanotechnology, have shown tremendous potential for a variety of biomedical applications due to their size-dependent physical and chemical properties. The nanoparticles of major biomedical importance are magnetic nanoparticles, quantum dots, metal nanoparticles, silica nanoparticles, and polymeric nanoparticles, as summarized in Table 1, with their intrinsic properties contributing to their use in specific applications. It is noticeable that nanoparticles alone cannot be used directly because most of them pose certain harmful effects to the surrounding biological environment. To address this problem, surface functionalization of nanoparticles has been extensively explored and has proven to play a critical role in nanoparticle development for practical applications. Particularly, surface functionalization has been extensively explored for biological and biomedical applications, such as biomolecular probing, biological imaging, and drug delivery applications [1-6]. In general, surface functionalization with synthetic ligands and natural biomolecules to impart specific biofunctions and enhance biocompatibility of nanoparticles has become a very essential component in regards to the intended biological application [7]. Careful and selective surface treatment can meld the nanoparticles of choice, depending on whether the nanoparticles are to be used for analysis, sensing, imaging, therapeutics or diagnostics, into a successfully applied system. For these nanoparticle systems, a variety of different ligands may be attached to nanoparticles depending on what effect is to be achieved allowing for either a single function or the development of multimodal capabilities [8, 9]. For example, ligands containing bulky hydrophobic molecules may be attached to nanomaterial surfaces to prevent agglomeration of the nanoparticle core, while surfaces for use in aqueous environments can be coated with water-soluble polymers such as poly (ethylene glycol) (PEG) to enhance solubility and biocompatibility. Ligands may also be attached to act as “tags” for molecular recognition properties that can be exploited in drug targeting and bio-imaging applications [10]. In addition, ligands may also be attached to nanoparticles surface to define the properties of the nanoparticles themselves.
Table 1.
Property of typical nanoparticles for biomedical applications*
| Category | Example | Intrinsic properties | Biomedical applications |
|---|---|---|---|
| Magnetic | Fe3O4 | Magnetic attraction | MRI, drug delivery |
| Semiconductor/Quantum | CdS, CdSe | Fluorescence, Luminescence |
Immunoassays, bioimaging, bio-sensing |
| Metal | Au, Ag | Electron dynamics | Bio-sensing, drug delivery |
| Polymeric | PLGA, Polystyrene |
Encapsulation Covalent conjugation |
Drug and gene delivery |
Modified from Ref. 6.
Common strategies of nanoparticle surface functionalization involve noncovalent physisorption, bioaffinity or covalent conjugation with desired ligands. Cell surface membranes have become a great model for nanoparticle surface functionalization due to its intrinsic anti-adhesion properties and specific biological activity across the membrane surface. Therefore, the construction of an artificial cell membrane structure, based on phospholipids, has proven effective in preventing the non-specific biological reactions at nanoparticle surfaces [11, 12]. Furthermore, certain bioactive molecules can be immobilized on the surface of the phospholipid platform to generate biologically active nanoparticles more representative of the natural display of these molecules on cell membrane surfaces (Figure 1) [13]. This review describes recent advances in membrane mimetic surface functionalization of nanoparticles for biomedical applications (Table 2). The review is organized with the contents of functionalization method, characterization and application of membrane mimetic surface functionalized nanoparticles, in the order of magnetic nanoparticles, quantum dots, gold nanoparticles, silica nanoparticles and polymeric nanoparticles. The final section includes a summary and the concluding remarks.
Figure 1.
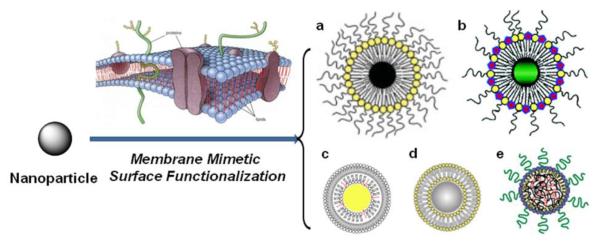
Schematic representation of typical membrane mimetic surface functionalization of nanoparticles. a. magnetic nanoparticle, b. Quantum dot, c. gold nanoparticle, d, silica nanoparticle, and e. polymeric nanoparticle. Modified from Ref. [50, 82, 93, 136].
Table 2.
Membrane-mimetic surface functionalized nanoparticles in biomedical applications
| NPs | Lipid | Application | Reference |
|---|---|---|---|
|
Magnetic
Nanoparticle |
PE-based Lipids: DSPE, DOPE, DMPE, DPPE, DSPE-PEGx |
MRI Contrast Agents Drug/Gene Delivery |
29-31, 33, 42, 47, 48, 53 5, 32, 45, 48 |
|
PC-Based Lipids: DOPC, DPPC, DSPC DLPC |
MRI Contrast Agents Hyperthermia |
53 33, 34 |
|
|
Cationic Lipids: DOTAP |
MRI Contrast Agents | 42 | |
| Quantum Dot |
PE-based Lipids: DSPE, DOPE, DMPE DSPE-PEGx |
Imaging, Diagnostics Drug/Gene Delivery |
3, 70-73, 79, 81, 82 73, 79, 82 |
|
PC-Based Lipids: DOPC, DPPC, DSPC, MHPC |
Imaging, Diagnostics Drug/Gene Delivery |
3, 70-72, 74, 79 79 |
|
|
Cationic Lipids: DOTAP |
Imaging, Diagnostics Drug/Gene Delivery |
73, 74, 79 73, 79 |
|
|
Gold
nanoparticle |
PE-based Lipids: DSPE, DMPE DSPE-PEGx |
Imaging Diagnostics | 92 |
|
PC-Based Lipids: DOPC, DPPC, DSPC, MHPC |
Imaging, Diagnostics Drug/Gene Delivery |
90-94 88 |
|
|
Silica
Nanoparticle |
PE-based Lipids: DSPE, DOPE, DMPE DSPE-PEGx |
Drug/Gene Delivery | 116, 117 |
|
PC-Based Lipids: DOPC, DPPC, DSPC, DMPC, MHPC, POPC |
Imaging, Diagnostics Drug/Gene Delivery |
107, 113, 115 112-121 |
|
|
Cationic Lipids: DOTAP |
Drug/Gene Delivery | 107, 116, 117, 121 | |
|
Polymeric
Nanoparticle |
PE-based Lipids: DSPE, DOPE, DMPE DSPE-PEGx |
Drug/Gene Delivery | 128-131 |
|
PC-Based Lipids: DOPC, DPPC, DSPC, DMPC, DLPC |
Drug/Gene Delivery |
127, 129, 130, 132 134-136 |
|
| Cationic Lipids: DPTAP | Drug/Gene Delivery | 127, 132 |
2. Membrane mimetic surface functionalization of magnetic nanoparticles
Magnetic nanoparticles of iron oxides, that exhibit magnetic moments in the vicinity of an external magnetic field, have attracted increasing interest and have been widely explored in the life sciences [14]. In view of the fact that the magnetic nanoparticles obey the Coulomb’s law, they can be guided to a specific target site by means of an external magnetic field. This unique property of magnetic nanoparticles makes them applicable in the transportation and delivery of molecular markers, various drugs and can also facilitate in biological purifications [14, 15]. Fe3O4 nanoparticles are the most promising type of magnetic nanoparticles and have already been approved by FDA (i.e. Feridex I.V.®) for usage in liver imaging [16]. The chemical and physical properties of the iron oxide nanoparticles play an important role in their usage. By controlling and adjusting the particle core size, particle shape, bio-distribution and magnetic properties, one can meet specific parameters necessary for a variety of applications [17]. Therefore, suitable techniques must be chosen for the synthesis of magnetic iron oxide nanoparticles. There are many synthetic techniques available for the production of magnetic iron oxide nanoparticles. The most commonly utilized techniques are (i) co-precipitation of iron salts [18, 19], (ii) thermal-decomposition of iron precursors [20-24] and (iii) micro-emulsion formation [25, 26].
Although there have been many significant developments in the synthesis of magnetic nanoparticles, maintaining the stability of these particles for a long time without agglomeration or precipitation is an important issue. Surfactants or polymers are often employed to passivate the surface of the nanoparticles during or after the synthesis to avoid agglomeration. In addition, for applications in biomedicine, it is necessary to functionalize nanoparticles with appropriate biocompatible coatings because iron oxide nanoparticles on their own, can pose certain harmful effects to the surrounding biological environment. This is due to the large surface to volume ratios and hydrophobic interactions between the unmodified bare iron oxide nanoparticles that leads to aggregation of magnetic nanoparticles forming larger agglomerates, opsonization, and, if not rapidly cleared by the reticulo-endothelial system (RES), inflammation and potentially cellular damage [27]. Therefore, it is necessary to surface coat the iron oxide nanoparticles with organic or inorganic monolayers in order to reduce their toxicity and to further stabilize the nanoparticles by preventing aggregation. The monolayer coating acts as a barrier between the inner iron oxide core and the surrounding environments. They also govern factors like solubility, reactivity, interactions with targeting biomolecules, and also determine the biological function of the nanoparticles. The introduction of different functional groups/linkers onto the surface of the nanoparticles can enable the conjugation of different biomolecules such as antibodies, carbohydrates, peptides, enzymes, etc., making them applicable for many biomedical applications [28] such as magnetic resonance imaging (MRI) [29, 30, 31], drug delivery [5, 32], hyperthermia [33, 34], in vivo monitoring of tumor cell growth, and cell labeling [35], as depicted in Figure 2.
Figure 2.
Depiction of magnetic nanoparticles and surface functionalization (a,b) prior to application (c,d) [28].
2.1. Methods for membrane mimetic surface functionalization of magnetic nanoparticles
Lipids such as phospholipids, glycolipids, and cholesterol are naturally occurring amphiphillic molecules that constitute the major structural elements of biological membranes. Many phospholipid-based membrane mimetic systems, such as liposomes, have been widely developed for biomedical applications. A liposome encapsulation technique, in which iron oxide nanoparticles, quantum dots, silica and polystyrene nanoparticles are encapsulated into liposomes, have been investigated for a variety of different applications [36]. On the other hand, lipid membrane as a micellular shell has been employed to nanoparticle surface functionalization for various biomedical applications. In general, hydrophobic-ligand stabilized metal cored nanoparticles such as iron oxide, quantum dots, and gold, can be entrapped in a micellular shell of lipids-poly(ethylene glycol) (PEG) derivatives. The bulky hydrophillic PEG molecule acts as a steric barrier and prevents the adsorption of the plasma proteins and uptake by the macrophages, thus facilitating longer circulation times of nanoparticles with enhanced overall biocompability and biodistribution times of nanoparticles [27, 37-41]. In addition, incorporation of lipid-PEG provides the nanoparticle system drug encapsulation capacity, making it applicable for therapeutic/imaging purposes. Depending on the type of application, lipid-PEG derivatives are frequently mixed with different ratios of other lipids such as DOPC, DOPS, DOTAP, DOPE, DPPC or DPPE to form a membrane mimetic system. In some cases, the PEG end chain can be modified with certain targeting moieties such as antibodies, drug molecules, and other biological entities, dependent on the desired application [20, 34, 42, 43].
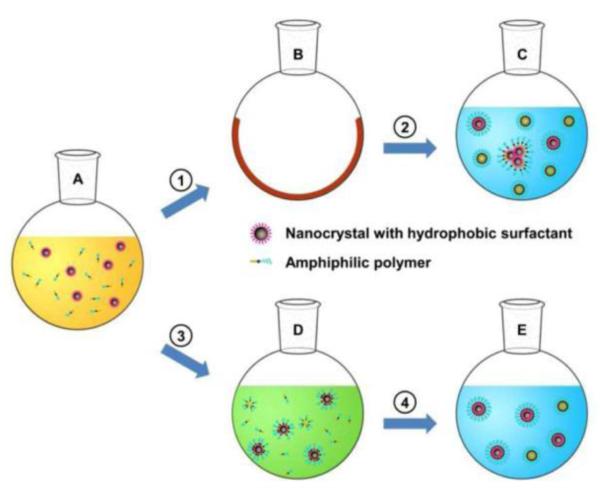
So far, two major methods have been developed for membrane-mimetic surface functionalization of magnetic nanoparticles. First, a thin film hydration and sonication process was reported by Bao and co-workers [29], in which amphiphillic lipids self-assemble on the hydrophobic ligand-coated iron oxide nanoparticles surface in aqueous conditions. In general, a mixture of lipids is added to the hydrophobic iron oxide nanoparticles in chloroform or other organic solvent to allow even mixing. Later, the organic solvent is completely removed by either rotary evaporation or evaporation under an inert gas to afford a thin film of lipids and nanoparticles. This thin film mixture is then hydrated with distilled water and subjected to sonication at 600 C for 20 min to ensure the removal of any residual organic solvent, if present. After sonication the lipid-coated iron oxide nanoparticles are collected by either centrifugation or by means of magnetic field attraction. Finally, the obtained pellet via centrifugation or the attracted lipid coated nanoparticles are re-suspended in distilled water and are further filtered through a 0.22 μm filter and stored at 4 0C in argon gas [29, 42, 44]. The main advantages of this technique is the easiness of lab production under mild conditions, use of biocompatible amphiphilic lipids, and the probability to obtain high encapsulation and loading capacity for lipophilic drugs.
Recently, Bao and co-workers developed a solvent exchange method to obtain a very stable DSPE-PEG coated magnetic nanoparticle [45]. Briefly, iron oxide nanoparticles (in toluene) were mixed with DSPE-PEG in chloroform (1:1 ratio). To the solution, DMSO was gradually added. Later, the toluene and chloroform were completely vaporized under vacuum and therein DMSO was substituted by water using an ultra-filtration centrifugal device with a polyethersulfone membrane. This technique is advantageous when compared with the traditional thin film hydration and sonication technique as it does not require extensive sonication and heating steps and also helps in preventing aggregation of the nanoparticles.
2.2. Characterization techniques for membrane mimetic functionalized magnetic nanoparticles
The surface characteristics of magnetic iron oxide nanoparticles determine the function of the nanoparticles for various biomedical applications. There are a number of analytical techniques used to characterize the iron oxide nanoparticles [46]. These include any combination of X-ray diffraction (XRD) [2, 20], small-angle X-ray scattering (SAXS) [20], transmission electron microscopy (TEM), atomic force microscopy (AFM) [2], giving details regarding basic rheology, size, shape, and surface composition characteristics of the synthesized metal cores. Other methods include light scattering techniques such as quasielastic or dynamic light scattering (QELS or DLS) measurements assuring colloidal stability [29, 42, 45]. Of the aforementioned techniques, DLS, TEM, have become valuable tools translating into analysis of lipid functionalized iron oxide nanoparticles in regards to size distributions, surface composition, and surface thickness of the nanoparticle of surface modifications. DLS technique can provide information about the particle size and the size distribution at a much lower cost and with less time when compared to TEM. In this technique the distribution of the diffusion coefficients are calculated and are converted to measurements of the hydrodynamic or the total diameter of the particles. Though this technique is simple and fast, it has certain disadvantages like inaccurate data from contamination due to dust particles or presence of aggregates in the sample solution. TEM, on the other hand, can be used to determine the total particle size, shape, inner metal core, the surface coating agent/monolayer and also for obtaining detailed information on the size distribution of the magnetic nanoparticles [29, 30, 45-49]. Particularly, high resolution TEM can provide information on the atomic arrangements of the magnetic iron oxide nanoparticles and also allows for enhanced characterization between the inner metal core and the monolayer surrounding it. From the diffraction patterns of the nanoparticles, the lattice and the surface atomic arrangements of the crystalline structures can also be determined. Briefly, a small portion of the sample is mounted on a coated copper grid and allowed to air dry before imaging. Though, TEM is a highly advanced technique, it has several disadvantages such as change in particle characteristics during the drying process, operator bias, and improper contrasting of the sample. When this technique is used to determine the surface characteristics of lipid coated iron oxide nanoparticles, additional staining of the sample is needed needs for better contrast of the lipid monolayer surrounding the inner metal core. The combined use of two stains such as a negative staining agent (phosphotungstic acid) and a positive staining agent (osmium tetroxide) provides for a better and more complete morphological and compositional characterization of the lipid coated iron oxide nanoparticles [29, 50].
2.3. Applications of membrane mimetic functionalized magnetic nanoparticles
Magnetic resonance is one of the leading diagnostic imaging modality as it can give detailed information about soft tissues with high spatial resolution. Recently, lipid-functionalized nanoparticles have been investigated for contrast-enhanced MRI and molecular imaging [50]. For example, enhanced colloidal stability and high r2 relaxivity was observed for water-dispersible ferromagnetic iron oxide nanocubes (WFIONs) when coated with lipid membrane [29]. MR imaging comparison studies performed between the commercially available T2 MRI contrast agents such as Feridex I.V.® and the synthesized PEG-phospholipid coated WFIONs showed that the WFIONs provided a superior T2 contrast effect, thereby, allowing much scope for the magnetic nanoparticles to play crucial roles in the early diagnosis and detection of tumor metastasis [29]. Similarly Tong et al. developed phospholipid coated superparamagnetic nanoparticles with a high T2 relaxivity of 385 s−1 mM−1 conjugated with antibodies against mouse VEGF receptor-1 that was used for the in vivo tumor detection (Figure 4) [45]. Furthermore, Hultman et al. demonstrated a application of immunotargeted superparamagnetic iron oxide nanoparticles (ITSIONs), with in vivo magnetic resonance diagnostic and potential drug delivery capability for kidney disease [47]. In this study, RT1 anti-MHC Class II antibodies were conjugated to lipid-coated ITSIONs, which targets the renal medulla of the rat, a section of the kidney in which MHC Class II is specifically expressed. Enhanced binding of the RT1 ITSIONS to the renal medulla was confirmed and indicated potential for disease detection or drug delivery.
Figure 4.
Red arrow shows the location of the subcutaneous tumor. (b &c) MR images of the tumor before probe injection. (d &e) MR images collected after 1 hr following the injection of 14 nm SPIOs conjugated with antibodies against mouse VEGFR-1. The red dotted line in (b) and (d) outline the tumor (Scale bar represents 5 mm) [45].
The feasibility to incorporate fluorescent labels in the nanoparticle system allows for optical imaging as well allowing for biomodal imaging. Huang et al. have developed a novel cationic lipid-coated superparamagnetic iron oxide nanoparticle with trace amount DOPE-Rhodamine as the probe for in vivo imaging [42]. In this study, low cytotoxicity, long-term imaging signals and efficient cellular uptake into HeLa, PC-3 and Neuro-2a cells were observed. Furthermore, the tumor growth status could be monitored using optical and MR images when the nanoparticle-loaded CT-26 tumor cells were injected into Balb/c mice. The results suggested these newly formulated non-toxic lipid-coated magnetic nanoparticles as a versatile image probe for cell tracking. Zhao et al. conjugated an apoptotic targeting moiety like C2 domain of Synaptotagmin I onto the surface of magnetic nanoparticles for the detection of apoptotic cells through magnetic resonance imaging. [51]. In a similar approach van Tilborg et al. developed Annexin V functionalized micellular iron oxide nanoparticles for the detection of apoptotic cells as well [52]. They found that these types of lipidated magnetic nanoparticles could serve as potential contrast agents for the detection of many apoptotic cells in many neurotic processes such as ischemic reperfusion injury, artherosclerosis, and tumors. These studies clearly demonstrated the significance that the phospholipid coated magnetic nanoparticles could be used for the in vivo imaging of pathological sites. Therefore, designed lipid coated magnetic nanoparticles with tunable surface properties such as size, magnetism, and targeting ligands will be very useful not only for MRI signal enhancement but also for early diagnosis of many diseases.
Another promising application of magnetic iron oxide nanoparticles is the site–specific delivery of both hydrophobic and hydrophilic drugs and genes [53]. Many anti-cancer drugs have been shown to exhibit nonspecific cytotoxicities that limit their therapeutic potential. Therefore, surface modified iron oxide nanoparticles have been investigated as drug carriers for efficient drug delivering to the pathological site and limiting the negative systemic toxicity effects. Most likely, a pharmaceutical drug of interest can be encapsulated by or entrapped in the iron oxide nanoparticles that could be driven to the target organ by means of an external magnetic field where they can be released. Prasad and co-workers reported the magnetophoretically guided drug delivery of light-activated photodynamic therapy using a lipid micelle-magnetic hybrid [5]. Specifically, the nanocarrier consisted of polymeric micelles of PE-PEG co-loaded with the photosensitizing drug 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a (HPPH), and magnetic Fe3O4 nanoparticles. The loading efficiency of HPPH was unaffected upon co-loading of the magnetic nanoparticles, and its phototoxicity was retained. The nanocarrier showed excellent stability and activity and efficient cellular uptake confirmed via confocal laser scanning microscopy. Furthermore, the magnetic response of the nanocarriers was demonstrated by their magnetically directed delivery to tumor cells in vitro. These multifunctional nanocarriers opened many new doors in the development of much more effective drug delivery systems.
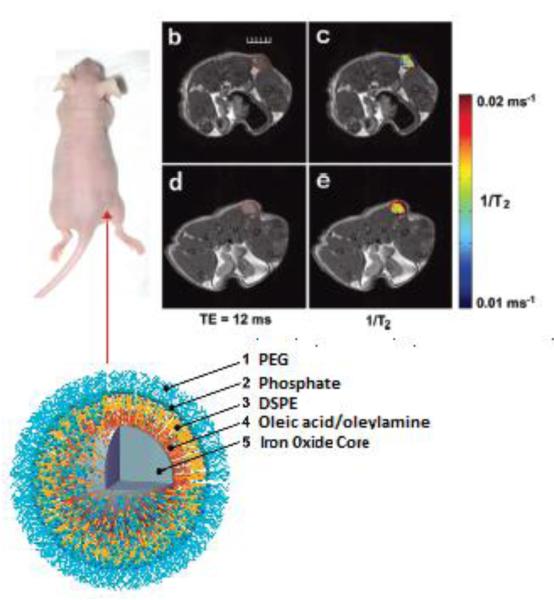
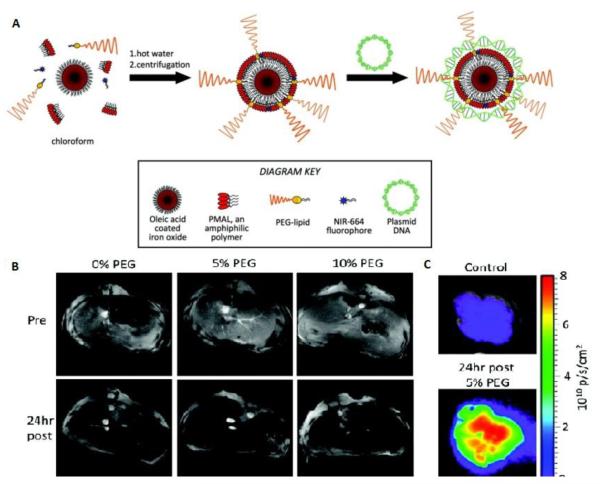
Mulder and co-workers synthesized lipid-coated, fluorescent iron oxide nanoparticles to deliver DNA and to produce a contrast reagent for magnetic resonance imaging (MRI), fluorescence imaging, and transmission electron microscopy (TEM) [48]. In this study, magnetic nanoparticles coated with DSPE-PEG, from 0% to 5%, 10%, or 25% concentrations, were prepared with the aim of reducing opsonization but maintaining DNA binding. They investigated the effect of the nanoparticle coating on DNA binding, cell uptake, cell transfection, and opsonization in vitro. Furthermore, they exploited MRI, fluorescence imaging, and TEM to monitor the distribution of the different formulations in the liver of mice. MRI and fluorescence imaging showed that each formulation was heavily taken up in the liver at 24 h, the 10% PEG formulation was taken up by the therapeutically relevant hepatocytes more extensively than either the 0% PEG or the 5% PEG, indicating its potential for delivery of therapeutics to the liver (Figure 5). This platform may be applied to the delivery of therapeutics such as proteins, small molecules, DNA, siRNAs, and microRNAs.
Figure 5.
A) Depiction of nanoparticle synthesis and subsequent complexation with plasmid DNA. (B) MR images of the livers of mice pre- and 24 h post-injection with plasmid carrying iron oxide nanoparticles. (C) Cy5.5 channel fluorescence images of liver of mice [modified from Ref 48].
3. Membrane mimetic surface functionlization of quantum dots (QDs)
With the advent of modern day solid-state electronics, a variety of materials were investigated for their use in semi-conductors, particularly atoms from groups II–IV or III–V elements in the periodic table [54]. In 1983, AT&T Bell Laboratories began to study the 3-D evolution of these elements, and combinations thereof, from the molecular to the bulk as semiconductor clusters recognizing that single electrons created by HOMO-LUMO promotion move rapidly in response to an electric field [55, 56]. In the absence of defects, the excited states of these semiconductor clusters, such as CdSe, decay radiatively exhibiting near unity fluorescence quantum yield and offer partial protection from quenching [56]. However, the synthetic optimization of such clusters and control of surface formation remains very empirical with little understanding of what influences size, shape and growth limiting the ability for these materials to be produced at a manufacturing level with any consistency [57]. This limitation has kept semiconductor clusters, or quantum dots (QDs), from being regularly implemented in most devices. However, the recognition of their small size, brightness, high photostability, and size-tunable spectral properties of semiconductor quantum dots have spawned interest for their use as potential chromophores in a variety of applications within the fields of biology and medicine, [56, 58].
Of the many semiconductors studied (including InP, GaAs, and Si), Cd-based QDs (CdSe, CdTe, CdS, CdSe-ZnS, CdSe-Si) are the most regularly synthesized, the smallest averaging ~ 20 nm in diameter, and implemented in research studies [55, 60]. However, the traditional high-temperature organometallic synthesis creates QDs with surfaces passivated by trioctylphosphine oxide (TOPO) and trioctylphosphine selenide (TOPSe) groups which, apart from the inner particle itself, can be toxic to most cells [60-64]. As a result, prior to use in any biological system, QDs must be modified with stable hydrophilic surfaces in an attempt to reduce toxicity and enhance biocompatibility. QDs thus have been modified with carboxylic acid groups and even encapsulated into polymer nanoparticles to increase biocompatibility and water solubility [65-67]. Another approach that has been taken to enhance the biocompatibility of QDs is their encapsulation within lipid micelles [3, 68-79]. Further bimolecular modifications of the lipidated QDs facilitate targeted imaging of various tissues [80-83].
3.1. Methods for membrane mimetic surface functionalization of QDs
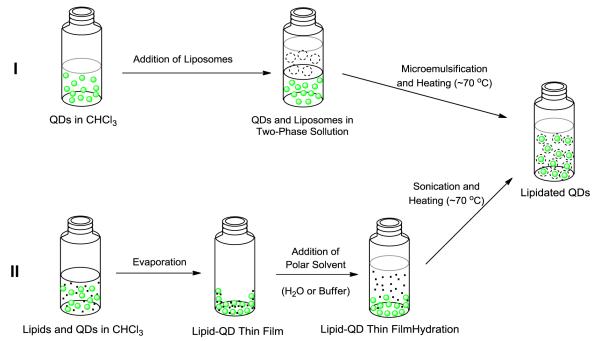
Lipidation of QDs in the formation of micelles is solely governed by supramolecular forces. The van der Waals interactions between alkyl chains of the TOPO and TOPSe assisted by the hydrophobic-hydrophilic interactions induced by polar solvent causes these chains to interdigitate forming a lipid coating, or micelle. Two methods have been established to induce such phenomena (Figure 7) [3, 69]. In the method established by De and associates, QDs in chloroform are added to an aqueous solution containing lipid surfactants and mixed vigorously to form an oil-in-water emulsion with heating, removing the chloroform and forcing transfer to the aqueous phase [69, 70]. On the other hand, Dubertret’s method, and its variations, mixes the QDs and lipids in an organic solvent system that is slowly evaporated to give a thin film followed by rigorous heating and agitation in the presence of aqueous solution [3]. This thin-film hydration method is one of the most regularly used methods for the lipidation of quantum dots [71-74]. Of the lipids used to coat QDs the most frequently used are phospholipids such as DOPE, DOTAP, DSPE, DPPC, and DPPE-PEG and DSPE-PEG, which are often mixed in different ratios dependent upon the intended application. Particularly, lipid PEG derivatives help in stabilizing nanoparticles because the PEG chains form a hydrophilic coating at the QD surface. In addition, it protects the QD from interactions with plasma proteins and rapid clearance by the liver. In general, incorporation of a large quantity of PEG lipids in a lipid mixture leads to the formation of micelles, which are required for obtaining a monolayer lipid coating around the QD. In addition, the PEG end chain can be modified to contain certain biomolecules for targeting applications. Whatever the lipid formulation, three basic requirements must be met to produce a usable lipidated QD reagent: (1) The lipidated QDs have to be monodisperse, (2) they must be stable, and (3) there should be little to no decrease in the quantum yield.
Figure 7.
Representation of De’s (I) and Dubertret’s (II) methods to achie e lipidated QDs [3, 69].
3.2. Characterization techniques for membrane mimetic functionalized QDs
In comparison to the lipid-modified surfaces of these metal-cored nanoparticle systems, the core is readily analyzed using analytical tools commonly used to measure bulk properties of materials [46]. These analytical methods include any combination of XRD, TEM, AFM, UV-Vis spectroscopy and X-ray photoelectron spectroscopy (XPS) and auger electron spectroscopy (AES) giving details regarding basic surface composition characteristics and the synthesized metal cores [77, 78]. Of the aforementioned techniques, TEM, XPS and AES have become valuable tools translating into analysis of lipid functionalized quantum dots in regards size distributions of the nanoparticle, surface modification composition, and surface thickness of the nanoparticle modifications [46, 73, 74]. Other analysis methods include thermogravimetric analysis (TGA), to determine weight compositions, light scattering techniques such as QELS or DLS measurements assuring colloidal stability, and fluorescence microscopy and spectroscopy [71-73]. Due to the superior optical properties of QDs as fluorescence emitters compared to that of conventional organic dyes used in fluorescence-based applications, fluorescence analyses of QDs have become a standard evolving into fluorescence correlation spectroscopy (FCS) [71]. FCS allows for the measurement and identification of QDs of varying size and surface functionalization as well as concentration and kinetic reaction rates by measuring the fluctuations in fluorescence intensity [79]. This analytical method can not only be employed in the characterization of lipidated-QDs but can be used to study the interactions of such particles with biosystems.
3.3. Applications of membrane mimetic functionalized QDs
As previously mentioned, QDs, with the ability to perform multimodal sensing without concern for photobleaching, have become an alternative to traditional chromophores used for bioapplications. However, due to issues of toxicity in incidences of QD core leakage, the real world application in medicine of lipidated QDs has primarily lied within development of fluorescent probes for in vitro biolabeling and biosensing [75]. The earliest investigation of lipidated QDs as fluorescent imaging agents was performed by Fan and associates in 2005 demonstrating the passive targeting, uptake and fluorescent imaging of cultured rat hippocampal neurons in vitro ( Figure 8) [70]. These results, and similar results produced by Gopalakrishnan on a different cell line, soon paved the way for investigators to attempt using functionalized lipids for directed targeting and imaging of various tissue types [80, 81]. Shortly, Mulder and his associates demonstrated the direct targeting of functionalized lipidated QDs fluorescently staining cultured human umbilical vein endothelial cells (HUVEC), exhibiting that lipidated QDs functionalized with αvβ3-specific RGD peptides allowed for significantly more intense fluorescence imaging of the cells versus unfunctionalized lipidated QDs [82]. In a recent report by Lin et al. [83], lipid-coated QDs in combination with harmonic generation microscopy (HGM) make it possible to detect tumors with molecular imaging. LQDs served as surrogate markers to detect and track the tumor cells in vivo with manifest brightness and high resolution under HGM. Without photo-damage and photobleaching to the imaged tissues, HGM with LQDs proved to be excellent for cellular imaging when compared to confocal scanning microscopy. Furthermore, the excitation wavelength enabled HGM with LQDs to observe tumor tissues both deeply and noninvasively. Overall, many studies to date have demonstrated the abilities of various functionalized lipidated QDs as excellent molecular probes not just for fluorescent imaging of particular cell types but for cellular tracking studies of specific molecule.
Figure 8.
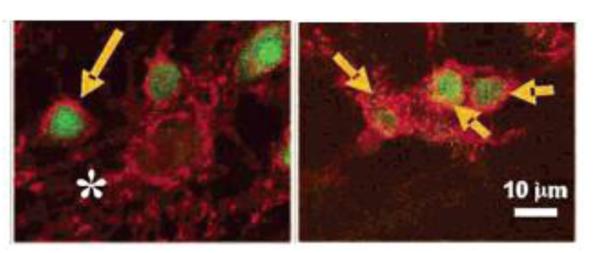
Uptake of lipid-stabilized QD micelles (accumulation marked by arrows) in rat hippocampal neurons visualized via confocal fluorescent microscopy [70].
4. Membrane mimetic surface functionalization of gold nanoparticles (GNPs)
Gold nanoparticles (GNPs) have been extensively studied for many applications due to their unique electrical, chemical, and optical properties. Of specific interest is the manipulation of their optical properties and the ability to fine-tune the localized surface plasmon resonance which arises from the surface plasmon oscillation of free electrons in gold [84]. These oscillations can be measured and quantified and used to identify binding events across the surfaces of planar gold and gold nanoparticles. The surface of gold nanoparticles can therefore be tailored by ligand functionalization to selectively bind specific biomarkers. Another aspect that is advantageous of this nanoparticle system, once functionalized, is the fact that GNPs exhibit low toxicity to most cells upon uptake making them more amenable to in vitro and in vivo use and are excellent contrast agents for electron microscopy [85]. Thus, over the past few decades, gold nanoparticles have emerged as promising platform for many biomedical applications such as biological imaging, drug and gene delivery, and also for photothermal therapy [86-88].
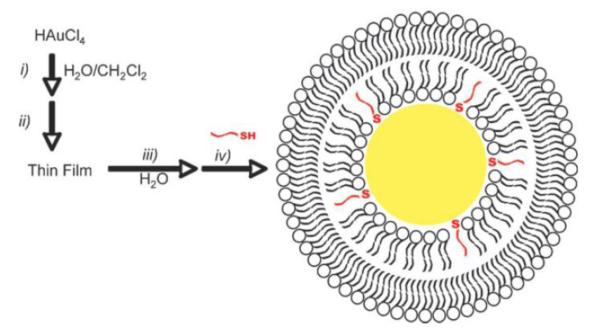
The simplest and the most commonly utilized method for the synthesis of monodispersed gold nanoparticles is the aqueous reduction of HAuCl4 by sodium citrate at boiling point [89, 90]. Another approach for the synthesis of gold nanoparticles is the use of NaBH4 as the reducing agent in the presence of organomercaptans to obtain GNPs that are coated with thiol since in its reduced form, gold has a strong affinity to bind sulfur [91]. This approach has remained quite attractive, allowing for the synthesis and attachment of thiol-based derivatives that allow for subsequent modifications across the GNP’s surface. Thus, a myriad number of molecular entities have been introduced onto the surfaces of GNPs, such as biocompatible block copolymers and biomolecules, in the synthesis of sterically stabilized GNPs in aqueous solutions. The most commonly use biocompatible polymer employed on the surface of GNPs, and many other nanoparticle systems, is PEG. However, in recent years, to more naturally mimic the presentation of targeting moieties at the cellular level researchers are beginning to turn towards lipidation of GNP surfaces as a more biomimetic approach. In making these nanoparticles more membrane-mimetic, the GNPs receive the advantage of being more biocompatible and less likely to induce an inflammatory response and also potentially opsonization followed by rapid clearance [92].
4.1. Methods for membrane mimetic surface functionalization and characterization of GNPs
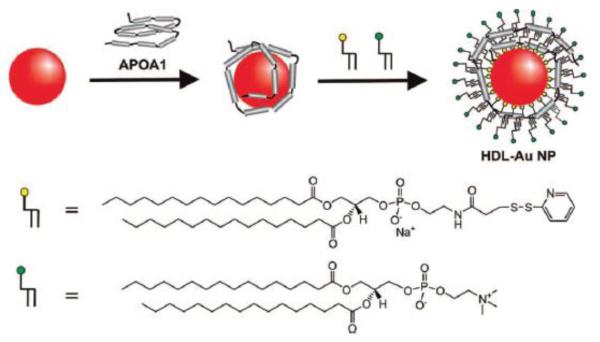
Scott Reed’s lab in 2008 were able to perform the transfer of lipids onto the surface of GNPs via partial ligand exchange through a thin film method as depicted in Figure 9 [93]. Later, they would apply the same method in the formation of lipid-GNP mimics of lipoprotein particles to study the interactions of C-reactive protein with PC membranes via shift in the localized surface plasmon resonance using UV-vis spectroscopy [94]. A slight variant of this method was put into practice by Chad Mirkin’s group. In their communication, using a DPPE-derivate, 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[3-(2-pyridyldithio)propionate] which bears a disulfide bridged head group, and DPPC, the formation of a lipid bilayer across the GNP surface in the presence of the apolipoprotein A-I was achieved via a two-phase transfer [95]. The resulting hybrid system, with a structure similar to HDL, was then tested to see if it could bind NBD-cholesterol to determine if it has potential as a therapeutic agent (Figure 10). As the NBD-cholesterol moved from the more polar aqueous environment, an increase in fluorescence was observed confirming the notion that the HDL-GNPs can absorb free cholesterol onto their surface.
Figure 9.
Depiction of lipid-GNP hybrid preparation involving (i) biphasic borohydride reduction of gold to form PC-coated nanoparticles, (ii) removal of solvent in vacuo, (iii) resuspension in water, and (iv) addition of alkane thiols for stabilization [93].
Figure 10.
Synthesis of HDL-GNPs and NBD-cholesterol binding isotherm [95].
Several others have adopted similar approaches to study the mechanism of this reassembly processes in the development of lipid-bilayer coated GNP hybrids to be potentially used to develop sensors and diagnostic technologies based on surface-enhanced raman scattering spectroscopies (SERs) [96]. For instance, Tam et al. synthesized a colloidal SERS gold nanoparticle that encapsulates Raman dye molecules adsorbed on 60 nm gold with a nonthiol phospholipid coating. They used 1:1 ratio of DMPC and MHPC phospholipids to coat the gold nanoparticles after adsorption of the Raman dye. After testing the sensing capabilities of their lipid-GNP hybrid, they found that the phospholipid bilayer coating provided enhanced biocompatibility and water solubility, thereby retaining the SERS signals for detection and thus making it suitable for in vivo application [97].
Like many other metal-cored nanoparticle systems, the core is readily analyzed using analytical tools commonly used to measure bulk properties of materials [46]. These analytical methods typically include any combination of TEM, AFM, and XPS and AES giving details regarding basic surface composition characteristics and the synthesized metal cores [77, 78]. Of the aforementioned techniques, TEM, XPS and AES have become valuable tools translating into analysis of lipid coating in regards size distributions of the nanoparticle, surface modification composition and surface thickness of the nanoparticle modifications [46, 73, 74]. Other analysis methods include TGA, to determine weight compositions, light scattering techniques such as QELS or DLS measurements assuring colloidal stability, and UV-visible light and surface enhanced raman (SER) spectroscopies [71-73, 84, 87]. Due to the optical properties of GNPs, when incident light irradiates the surface of gold nanoparticles, it excites the conduction electrons in the metal and further induces excitation of surface plasmons leading to enormous electromagnetic enhancement of spectral signature, UV-vis and SER spectroscopies are most commonly employed [93-98]. This is especially the case in instances where GNPs are being developed for ultrasensitive biological detection and imaging.
4.2. Application of membrane mimetic surface functionalized GNPs
Apart from the development of lipid-GNP hybrids for diagnostics, many suggest that the encapsulation of hydrophobic drugs into the lipid bilayer coatings or complexation of genetic material is feasible [86, 92-99]. However, very few have done so until recently when Kong et al. demonstrated that cationic lipid-coated GNPs could be used to deliver small interfering RNAs (siRNA) [98]. Using a modified emulsification/solvent evaporation methods, as previously mentioned, GNPs were coated with a mixture of DOPE and DC-Chol, 3β-[N-(N’,N’-dimethylaminoethane)-carbomyl]-cholesterol, which both displaying cationic head groups at physiological pH (pH 7.4). Using this positive surface charge, siRNAs for silencing of green fluorescence protein (GFP) were assemble on the surface and the siRNA-lipid-GNPs were tested on a cell line overexpressing GFP protein. The results were a success demonstrating that this delivery vehicle suppressed GFP expression down to ~48% as compared to the polyelectrolyte polyethylenimine used to transport siRNA suppressing expression down to ~83%. Thus, the lipid-GNP hybrid system clearly demonstrated a significant advantage. However, with limited studies available, the use of lipid-GNP hybrids is a relatively infantile research area within the area of hybrid nanoparticle systems. As more efforts are being put forth to understand the assembly process to obtain consistent, reproducible lipid-GNPs hybrid systems we should see this research area readily expand.
5. Membrane mimetic surface functionalization of silica nanoparticles (SiNps)
Monodispersed silica nanoparticles (SiNps) have been synthesized for many years over a wide range of sizes ranging from 20–1000 nm in diameter [100, 101]. With the ability to synthesize anything from solid silica nanoparticles to silica nanoparticle mesoporous shells, it is of no surprise that such a nanoparticle system has been implemented so widely in the development of many hybrid nanoparticle systems, such as Si-coated gold, iron oxide, and quantum dot nanoparticles, for applications ranging from microelectronics to diagnostics and therapeutics [54, 102, 103]. However, though many studies have been performed using SiNps, due to their inherently low toxicity, silica by itself has low biocompatibility and is only stabilized in solution by charge repulsion which, in vivo, is disrupted by opsonins causing aggregation and removal by the reticuloendothelial system [104]. To prevent this, a variety of surface modifications to enhance biocompatibility have been employed often involving the chemical attachment via the silanol group of either small molecules, such as thiols, carboxyl, or phosphonate groups, or the attachment of large molecules, such as block copolymers or PEG [105-107]. Both strategies employed though have limitations. Functionalization with small molecules can still lead to opsonization and, dependent on the moiety, may not be stable against aggregation, while attachment of large molecules leads to low-density surface coatings which can adversely affect the application for which the nanoparticle was designed [104]. Alternatively, the use of lipids to coat silica surfaces has been demonstrated for over a decade in an attempt to biomimetically emulate the lipid coating of cellular membranes and improve SiNps biocompatibility [108].
5.1 Methods for membrane mimetic surface functionalization and characterization of SiNps
Several methods for coating lipids onto silica surfaces were investigated and demonstrated the ability that lipids could be deposited onto solid planar silica surfaces forming continuous, defect-free, fluid-like lipid membranes without direct chemical conjugation due to the stabilizing charge interactions of the lipid head group and silica surface [109-112]. This researched phenomenon has been employed to develop one of two common strategies in the lipidation of SiNps to create biocompatible membrane-like surface coatings. This strategy encompasses the fact that if small unilamellar vesicles (SUVs) can spontaneous form membrane bilayers on planar surfaces, the same phenomena should occur across a particle surface [113, 114]. This in fact, was demonstrated by Mornet, when SUVs composed of a 4:1 DOPC: DOPS were mixed with SiNps in a highly ionically charged buffer solution spontaneously forming lipid bilayers across the particle surfaces as seen in Figure 11 [109]. Others would soon employ similar methods to create lipid bilayer encapsulated SiNps as well to study various phenomena and potential bioapplications [115-125].
Figure 11.
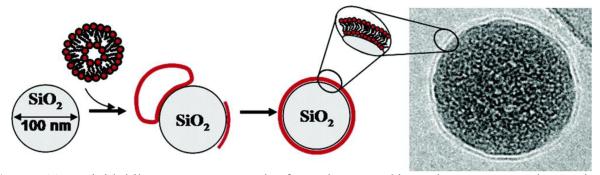
Lipid bilayers spontaneously formed across SiNps by Mornet and associates [adapted from Ref.109].
The second strategy employed for lipidation of SiNps is induction of lipid monolayer formation across the nanoparticle surface via van der Waals and hydrophobic interactions. This was demonstrated by Mulder and others, where the surface of the SiNp was functionalized with octadecanol via a condensation reaction and then lipids were introduced via thin-film hydration methods [104, 126]. Of the lipids used to coat SiNps, or any type of nanoparticle for that matter, the most frequently used are phospholipids, particularly those containing phosphoethanolamine headgroups and their PEG derivatives which help in stabilizing micelles [118-127]. These are often mixed in different ratios and combinations of different lipids dependent upon the intended application. In comparison to the lipid-modified surfaces of other metal-cored nanoparticle systems, the SiNps are readily analyzed using analytical tools commonly used to measure bulk properties of materials [46]. These analytical methods include any combination of XRD, TEM, UV-Vis spectroscopy, Fourier Transfer Infrared spectroscopy (FTIR), TGA, differential scanning calorimetry (DSC) and DLS. XRD, TEM, and DLS often provide the best characterizations in the solid state in regards to the particles size, shape, and atomic surface composition [104, 115-119, 126-128]. Other analysis methods such as TGA, DSC, FTIR and UV-Vis spectroscopy are used to gain even further insight into the structure of the lipid coated surface contributing to the overall understanding of the rheology of such hybrid nanoparticle systems [115-118, 128, 129].
5.2. Application of membrane mimetic surface functionalized SiNps
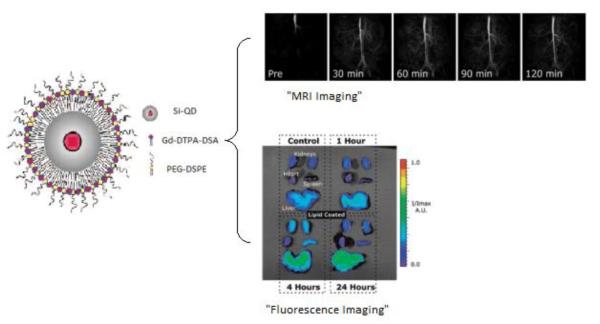
SiNps have been readily investigated in their ability to be modified for a myriad number of potential bioapplications, however the actual number of studies performed in vitro and in vivo to put these applications in practice are limited. Using lipid-coated, monolayer or bilayer, silica nanoparticles, researchers have just recently began to explore the use of such hybrid systems for biomedical applications. In 2008, SiNps, encapsulating quantum dots, modified with alkyl chains were shown to effectively stabilize the formation of a lipid monolayer across the surface [104]. The lipids used were modified with Gadolinium to produce a nanoparticle exhibiting multimodality enabling the MRI in vivo and fluorescent imaging in vitro of mouse organ tissues as depicted in Figure 12 [104]. Others have employed lipid-coated mesoporous SiNps as delivery vehicles. Liu et al. demonstrated this by producing lipid bilayer coated mesoporous SiNps loaded with calcein, a membrane impermeable fluorescent probe, to study liposome fusion with Chinese hamster ovary (CHO) cells. After incubation with the cells, they demonstrated significantly higher fluorescence compared to the controls incubated with free calcein [119]. In their continued work, they demonstrated the uptake and transfection of DsRed fluorescent protein encoding DNA loaded in mesoporous SiNps [120].
Figure 12.
Si-QD hybrids coated with modified lipids for dual in vivo MRI and in vitro fluorescent imaging [adapted from Ref. 104].
Other studies with lipidated SiNps look towards the development of improved drug delivery systems. Prestidge and associates would investigate the encapsulation of hydrophobic drugs, such as Celebrex® and Indomethacin, into such mesoporous silica-lipid hybrid nanoparticles for drug delivery applications [127, 128]. Cauda went as far to use colchicine-loaded lipid bilayer coated mesoporous SiNps to induce microtubule depolymerization [129]. Recently, Ashley and Carnes using an engineered mesoporous silica-lipid hybrid nanoparticle system, depicted in Figure 13, were able to delivery protein-based anticancer reagents and small interfering RNAs in two separate investigations [124, 125]. With continuing research, such systems are no longer limited to release of the therapeutics via passive diffusion but can be designed with internal and external triggering mechanisms. For instance, Stroeve and associates developed a lipid-SiNp containing superparamagnetic iron oxide nanocrystals (SPION) and methylene blue. By applying an alternating magnetic field the SPIONs generate local internal heating which eventually leads to disruption of the coating lipid bilayer. Upon this disruption release of the Nps contents occurs [122]. With very intricate external triggering mechanism, this system could have great future potential. Others have designed internal triggering mechanisms that rely on chemical changes in the external environment to control the release of reagents. Trewyn et al. accomplished this using a system that employs disulfide linkages in the stabilization of a lipid bilayer across the mesoporous SiNp entrapping the reagent. Whether internalized or not, upon exposure to a reducing environment, these disulfide linkages are broken causing destabilization of the lipid bilayer and release of the reagent from the mesoporous SiNp in a controlled fashion [123]. Thus, the release can be tailored towards a specific internal cellular environment as demonstrated using HeLa cells. Lipidated-mesoporous SiNp systems are not just limited to one drug, or probe for that matter, but have great potential to develop a multifunctional theranostic device that can not only recognize and treat but allow for constant monitoring of the disease state as well [130].
Figure 13.
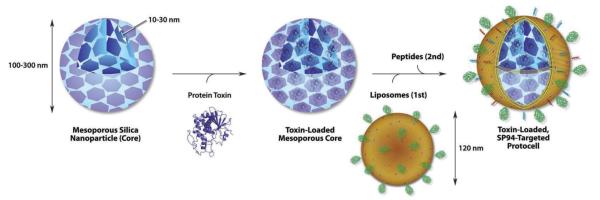
Mesoporous SiNp therapeutic loading and lipidation to form lipidated-SiNp hydrids [124].
6. Membrane mimetic surface functionalization of polymeric nanoparticles
Polymer-based nanoparticle systems were investigated as delivery devices when many recognized that though traditional liposomes display outstanding properties in the encapsulation of reagents, yet lack the ability to store high concentrations of lipophilic reagents and the stability to be prepared and stored for long durations [131]. Polymeric nanoparticles, or nanocapsules, regardless of the synthetic method employed, typically consist of cross-linked polymer chains condensed in a particle/micelle type structure. Control over types of the monomers and crosslinkers used in the polymerization process allows for manipulation of the properties of the particles in terms of biodegradability, swelling kinetics, and release rates [132]. Examples of polymers that have been employed in the formulation of nanocapsules are amphiphilic block copolymers, polysaccharides, poly-(lactic acid) and (glycolic acids), and a multitude of designed anionic and cationic polymers for layer-by-layer assembling [131, 132].
Though polymeric nanocapsules can be readily be synthesized in a consistent manner in regards to chemical composition and size, like any other nanoparticle system they are subject to clearance by the reticuloendothelial system (RES) [133]. Therefore, much thought must go into the design of surface functionalization strategies to enhance biocompatibility and overall biodistribution times of polymeric nanoparticles. Perhaps the most conventional method of polymeric nanocapsule biocompatibility enhancement is PEGylation, the addition of PEG to the nanocapsule surface [134]. The addition of PEG, like any other direct chemical modification, is subject to limitations. As previously mentioned, when synthetically attaching large molecules to particle surfaces disparities in surface densities across different particle surfaces occur, therefore creating an uneven distribution of targeting moieties which may be undesired for the intended application. Another issue, even though low molecular weight PEG is water-soluble and considered non-toxic, is the fact that PEG itself is a synthetic polymer [134]. Thus, investigators began to look towards lipid deposition across polymer nanoparticle surfaces as an alternative strategy for biocompatibility enhancement [135]. Lipids are not only advantageous for the fact that they are biocompatible, but they can help the coated polymeric particles to display biomimetic behaviors, such as adhesion and fusion, and to interact with a wide variety of molecules displayed on cells [135].
6.1 Methods for membrane mimetic surface functionalization and characterization of polymeric nanoparticles
The synthesis of lipid-coated polymeric nanocapsules varies from formulation to formulation depending on the polymer used to create core, the reagents encapsulated, and the lipids employed to coat the surface. However, regardless of the method, only two types of lipid assembling exist for these systems consisting either of a lipid monolayer or lipid bilayer. Dependent on the type of polymer core employed, the formation of lipid monolayer typically involves melding of the lipid alkyl chains with the hydrophobic portions of the carbon backbone of the polymer, followed by removing organic solvent and free molecules by washing the NP solution using an ultracentrifugal filter [4, 136-143]. For example, in a typical protocol developed by Zhang et al. [138], PLGA polymer was dissolved in acetonitrile with concentrations ranging from 1 to 5 mg/mL. Lecithin/ DSPE-PEG (8.5/1.5, molar ratio) with a weight ratio of 15% to the PLGA polymer was dissolved in 4 wt % ethanol aqueous solution. The desired amounts of DSPE-PEG and lecithin solutions were added into deionized water. Then the PLGA solution was carefully pipetted into the resulting aqueous solution. Finally, the amount of deionized water used was adjusted so that the final volume ratio of aqueous to organic solution was 10:1. The resulting mixture solution was sonicated (Figure 14A). Purification was done by washing the solution in PBS buffer (pH=7.4, 1x) 3 times using a Millipore (Amicon Ultra) centrifuge filter with a molecular weight cutoff of 10 kDa. Most recently, Zhang and co-workers have developed large-scale synthesis of lipid–polymer hybrid nanoparticles by using a multi-inlet vortex reactor (MIVR) (Figure 14B) [144]. Hybrid nanoparticles synthesized using the MIVR were comparable to lab-scale particles in both their physicochemical properties and their stability in PBS and serum. Use of the MIVR appeared to be a viable strategy for producing hybrid nanoparticles in clinically significant quantities.
Figure 14.
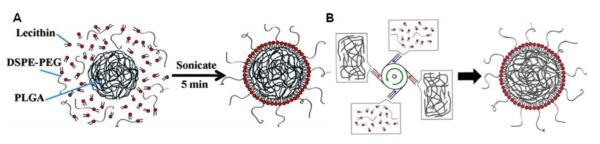
Synthesis of lipid-polymer hybrid nanoparticles. (A) single-step sonication method to synthesize lipid-PLGA hybrid nanoparticles [136], (B) synthesis via multi-inlet vortex reactor [139].
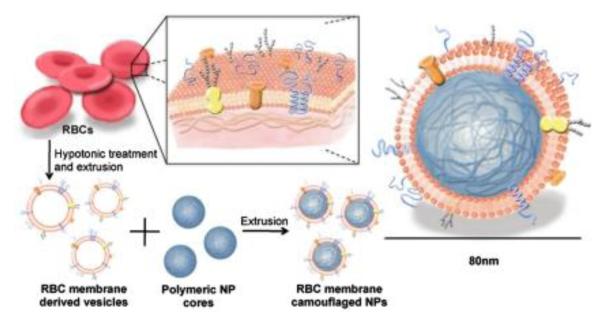
Of the lipids the commonly used for coating polymeric nanoparticles are DPTAP, DSPE, DSPE-PEG, DPPC, DLPC, and cholesterol (Chol) [4, 131, 135-138,144]. Interestingly, Zhang and co-workers reported a top-down biomimetic approach in particle functionalization by coating biodegradable polymeric nanoparticles with natural erythrocyte membranes, including both membrane lipids and associated membrane proteins for long-circulating cargo delivery [145] (Figure 15). In this study, fluorophore-loaded nanoparticles showed superior circulation half-life by the erythrocyte mimicking nanoparticles in mice as compared to control particles coated with the state-of-the-art synthetic stealth materials. Using natural cellular membranes, their associated proteins, and the corresponding functionalities to the surface of synthetic particles represents a unique approach in nanoparticle functionalization.
Figure 15.
Schematics of the preparation process of the RBC-membrane-coated PLGA nanoparticles [145].
Similar to other nanoparticle systems, lipid-coated polymeric nanoparticle systems are readily analyzed using analytical tools commonly used to measure bulk properties of materials [46]. These analytical methods include any combination of transmission/scanning electron microscopies (TEM/SEM) [4, 131, 135-138], UV-Vis spectroscopy [131, 135], TGA [139], DSC [144], AFM [141], light scattering techniques such as QELS or DLS [4, 131, 135-144], and XPS [4, 134, 142] giving details regarding basic rheology and surface composition characteristics of the particles.
6.2 Applications of membrane mimetic surface functionalization and characterization of polymeric nanoparticles
Various types of polymeric nanocapsules have been investigated in regards to their ability to be lipid coated and potentially used in bioapplications, specifically reagent delivery. Kontermann and Messerschmidt employed lipid-coated poly(styrene) nanocapsules as delivery agents using tumor necrosis factor for targeting tumor cells in vitro [137]. However, poly(styrene) nanocapsules are not biodegradable and can potentially cause immune response as the hybrid particle is broken down therefore rendering its use as an in vivo delivery device highly unlikely. Polymeric nanoparticle systems used in vivo are typically composed of FDA-approved biodegradable polymers, such as poly(lactic acid) (PLA), poly(glycolic acid) (PGA), and poly(ε-caprolactone) (PCL) [131]. Of these biodegradable polymers, researchers have combined PLA and PGA creating poly(d,l-lactic-co-glycolic acid) (PGLA) block copolymer nanocapsules. Using lipid-coated PGLA nanocapsules, Langer and Farokhzad demonstrated the successful encapsulation and time controlled release of Docetaxel, a widely used cancer therapeutic [136]. Feng and co-workers took this platform and modify it further using folic acid functionalized Docetaxel loaded lipid-coated PLGA nanocapsules exhibiting directed targeting and uptake by MCF7 cancer cells [4]. Others soon followed suite developing lipid-coated PLGA nanocarriers for the targeted delivery of other drugs as well as fluorescent probes, therapeutic proteins and genetic vectors [142, 143]. It is worth noting that though PLGA nanocarriers are favored in developing delivery platforms because of FDA approval, others have examined and are currently investigating lipid-coated polysaccharide nanoparticles offering as an alternative class of biodegradable lipid-coated polymeric nanocarriers [140, 141].
7. Summary and future perspective
Nanoparticles due to their unique chemo-physical properties have been extensively explored for potential biomedical applications. However, surface functionalization with biomolecules is often times required for most applications of nanoparticles under biological conditions, otherwise the desired function of the nanoparticles may be disrupted by varying biological responses, which may be induced by nonspecific protein adsorption. Thus, control of interactions between nanoparticles and the biological system is essential for effective utilization of nanoparticles for their desired purpose. In general, nanoparticles are mainly composed of metals, metal oxides, silicon, and polymers. Surface functionalization of nanoparticles can impart the enhanced cellular internalization ability, impart biocompatibility, and improve payload binding capacity necessary for effective intracellular delivery. Furthermore, surface functionalities can be tuned to provide the selective or specific recognition required for tissue-specific distributions for specific therapeutic and diagnostic applications.
During the past decades, tremendous efforts to expand nanoparticle performance in vivo have inspired many strategies in particle surface modifications. Many important investigations have demonstrated that membrane mimetic surface functionalization of nanoparticles would extend nanoparticle residence time in vivo, particularly to bypass macrophage uptake and systemic clearance. Mostly, a top-down biomimetic approach in particle functionalization by coating nanoparticles with natural cell membranes, including both membrane lipids and associated membrane proteins for long-circulating cargo delivery would be the future direction for the advanced application of nanoparticles. On the other hand, further modification of membrane mimetic functionalized nanoparticles with bioactive molecules would facilitate targeted delivery of the nanoparticles to the specific site of disease have prove effective for biomedical applications of nanoparticles.
So far, phospholipids such as PC and PE and their PEG derivatives have been mostly explored for membrane mimetic surface functionaliztion of nanoparticles. Functional membrane lipids, such as glycolipids, proteolipids and cholesterol, that have important physiological roles, constitute a vast plethora of lipids that exist in cell membranes. However, these lipids have not been fully investigated and represent a major limitation that membrane-mimetic nanoparticle research faces. The foremost limitation in using mimetic nanoparticles to investigate physiologically relevant functional lipids is accessibility. To date, there are two methods to access these lipids. One is to isolate them from a natural source, which represents a daunting task in regards to separation science, or to undergo the rigor of synthetically producing these ever complex biomacromolecules. Even if these two options are achievable, one then must investigate whether the functional lipids are amenable to incorporation on the nanoparticle surface dependent on their chemical structure and amphiphilic nature. Another limitation that membrane mimetic nanoparticles face, like any other nanotechnology, is reproducibility and the cost for practical large scale applications. Thus, future efforts on investigations of the feasibility of a membrane mimetic system based on a wide variety of functional lipids related to their biological functions of interest, stability and fabrication techniques are much needed. In addition, it has been known that lipid domain structural features of cell membranes strongly affect the functions of membrane embedded biomolecules, such as proteins and carbohydrates. Therefore, studying the membrane effects in regards to the surrounding lipid environment on embedded biomolecules’ functions is necessary. In the same time, nanoparticle technology may contribute the understanding of membrane effects on embedded biomolecules’ functions.
Overall, many progresses and important knowledge for applications of nanoparticles have been obtained so far, continued efforts are still needed for well designed, engineered, and fabricated nanoparticles and their evaluated system, that are the collaborative work from chemists, bioengineers, biologists and physicians. Particularly, detailed knowledge of the complex interaction of nanoparticles with biosystems on the basis of surface functionalities and cellular uptake mechanism of a surface functionalized nanoparticle would establish nanoparticles as an efficient tool for diagnosis and treatment of diseases.
Highlights.
Nanoparticles, due to their size-dependent physical and chemical properties, have shown remarkable potential for a wide range of biomedical applications.
Lipid-based membranes mimetics offer an alternative to nanoparticle functionalization enhancing biocompatibility and specific biological interactions.
Nanoparticles with membrane-mimetic displays have found use in a range of bioimaging, biosensing, and drug delivery applications.
Figure 3.
Membrane mimetic surface functionalization of magnetic nanoparticles via thin-film hydration (1) and (2) and Solvent exchange method (3) and (4) [45].
Figure 6.
Representation of the lipidation of TOPO/TOPSe capped QDs.
Acknowledgement
This work was supported by grants from the NIH (1R01HL102604-04, X.-L. Sun), National Science Foundation MRI Grant (CHE-1126384, X.-L. Sun) and Cleveland State University Faculty Research Development Grant.
Appendix
Abbreviations
- AES
Auger electron spectroscopy
- AFM
Atomic force microscopy
- Chol
Cholesterol
- DLPC
1,2-didecanoyl-sn-glycero-3-phosphocholine
- DLS
Dynamic light scattering
- DMPA
1,2-dimyristoyl-sn-glycero-3-phosphote monosodium salt DMPC 1,2-dimyristoyl-sn-glycero-3-phosphotidylcholine
- DOPC
1,2-dioleolyl-sn-glycero-3-phosphotidylcholine
- DOPE
1,2-dioleoyl-sn-glycero-3-phosphatidylethanolamine DOPS 1,2-dioleoyl-sn-glycero-3-phosphatidylserine
- DOTAP
1,2-dioleoyloxy-3-(trimethylammonium) propane chloride DPPC 1,2-dipalmitoyl-sn-glycero-3-phosphatidycholine DPPE-PEG2000 1,2-dipalmitoyl-sn-glycero-3-phosphatidylethanolamine-N-[methoxy(poly(ethyleneglycol))-2000]
- DPTAP
1,2-dipalmitoyl-3-trimethylammonium propane chloride
- DSPE
1,2-distearoyl-sn-glycero-3-phosphatidylethanolamine DSPE-PEG2000 1,2-distearoyl-sn-glycero-3-phosphatidylethanolamine-N-[methoxy-(poly(ethyleneglycol))-2000]
- FCS
Fluorescence correlation spectroscopy
- GNPs
Gold nanoparticles
- ITSIONs
Immunotargeted superparamagnetic iron oxide nanoparticles MHPC 1-myristoyl-2-hydroxy-sn-glycero-3-phosphocholine
- NPs
Nanoparticles
- PDT
Photodynamic therapy
- PEG
Poly (ethylene) glycol
- QDs
Quantum dots
- QELS
Quasielastic light scattering
- RES
Reticulo-endothelial system
- SPIONs
Superparamagnetic iron oxide nanoparticles
- TEM
Transmission electron microscopy
- TGA
Thermogravimetric analysis
- TOPO
Trioctylphosphine oxide
- TOPSe
Trioctylphosphine selenide
- WFIONs
Water-dispersible ferromagnetic iron oxide nanocubes
- XPS
X-ray photoelectron spectroscopy
- XRD
X-ray diffraction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Senarath-Yapa MD, Phimphivong S, Coym JW, Wirth MJ, Aspinwall CA, Saavedra S. Preparation and characterization of poly(lipid)-coated, fluorophore-doped silica nanoparticles for biolabeling and cellular imaging. Langmuir. 2007;23:12624–12633. doi: 10.1021/la701917w. [DOI] [PubMed] [Google Scholar]
- 2.Senpan A, Caruthers SD, Rhee I, Mauro NA, Pan D, Hu G, et al. Conquering the dark side: colloidal iron oxide nanoparticles. ACS Nano. 2009;3(12):3917–3926. doi: 10.1021/nn900819y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science. 2002;298:1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Li K, Pan J, Liu B, Feng SS. Folic acid conjugated nanoparticles of mixed lipid monolayer shell and biodegradable polymer core for targeted delivery of Docetaxel. Biomaterials. 2010;31:330–338. doi: 10.1016/j.biomaterials.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 5.Cinteza LO, Ohulchanskyy TY, Sahoo Y, Bergey EJ, Pandey RK, Prasad PN. Diacyllipid micelle-based nanocarrier for magnetically guided delivery of drugs in photodynamic therapy. Mol Pharmaceutics. 2006;3:415–423. doi: 10.1021/mp060015p. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Liu LH, Ramström O, Yan M. Engineering nanomaterial surfaces for biomedical applications. Exp Biol Med. 2009;234:1128–1139. doi: 10.3181/0904-MR-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy LH, Arias JL, Nicolas J, Couvreur P. Magnetic nanoparticles: design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem Rev. 2012;112:5818–5878. doi: 10.1021/cr300068p. [DOI] [PubMed] [Google Scholar]
- 8.Mout R, Moyano DF, Rana S, Rotello VM. Surface functionalization of nanoparticles for nanomedicine. Chem Soc Rev. 2012;41:2539–2544. doi: 10.1039/c2cs15294k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thanh NTK, Green LAW. Functionalization of nanoparticles for biomedical applications. Nanotoday. 2010;5:213–230. [Google Scholar]
- 10.Mulder WJM, Strijkers GJ, van Tilborg GAF, Cormode DP, Fayad ZA, Nicolay K. Nanoparticulate assemblies of amphiphiles and diagnostically active materials for multimodality imaging. Acc Chem Res. 2009;42:904–914. doi: 10.1021/ar800223c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuno R, Ishihara K. Integrated functional nanocolloids covered with artificial cell membranes for biomedical applications. Nanotoday. 2011;6:61–74. [Google Scholar]
- 12.Gong YK, Winnik FM. Strategies in biomimetic surface engineering of nanoparticles for biomedical applications. Nanoscale. 2012;4:360–368. doi: 10.1039/c1nr11297j. [DOI] [PubMed] [Google Scholar]
- 13.Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Arruebo M, Fernandez-Pacheco R, Ibarra MR, Santamaria J. Magnetic nanoparticles for drug delivery. Nanotoday. 2007;2:22–32. [Google Scholar]
- 15.Pankhurst QA, Connolly J, Jones SK, Dobson J. Applications of magnetic nanoparticles in biomedicine. J Phys D: Appl Phys. 2003;36:167–181. [Google Scholar]
- 16.Wang YXJ, Hussain SM, Krestin GP. Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur Radiol. 2001;11:2319–2331. doi: 10.1007/s003300100908. [DOI] [PubMed] [Google Scholar]
- 17.Roca AG, Morales MP, Grady KO, Serna CJ. Structural and magnetic properties of uniform magnetite nanoparticles prepared by high temperature decomposition of organic precursors. Nanotechnology. 2006;17:2783–2788. [Google Scholar]
- 18.Qiao R, Yang C, Gao M. Superparamagnetic iron oxide nanoparticles: from preparations to in vivo MRI applications. J Mater Chem. 2009;19:6274–6293. [Google Scholar]
- 19.Laurent S, Forge D, Port M. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev. 2008;108:2064–2110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 20.Shtykova EV, Huang X, Remmes N, Baxter D, Stein B, Dragnea B, et al. Structure and properties of iron oxide nanoparticles encapsulated by phospholipids with poly (ethylene glycol) tails. J Phys Chem C. 2007;111:18078–18086. [Google Scholar]
- 21.Park J, An K, Hwang Y, Park JG, Noh HJ, Kim JY, et al. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat Mater. 2004;3:891–895. doi: 10.1038/nmat1251. [DOI] [PubMed] [Google Scholar]
- 22.Sahoo Y, Goodarzi A, Swihart MT, Ohulchanskyy TY, Kaur N, Furlani EP, Prasad PN. Aqueous ferrofluid of magnetite nanoparticles: fluorescence labeling and magnetophoretic control. J Phys Chem B. 2005;109:3879–3885. doi: 10.1021/jp045402y. [DOI] [PubMed] [Google Scholar]
- 23.Bronstein LM, Huang X, Retrum J, Schmucker A, Pink M, Stein BD, Dragnea B. Influence of iron oleate complex structure on iron oxide nanoparticle formation. Chem Mater. 2007;19:3624–3632. [Google Scholar]
- 24.Yue-Jian C, Juan T, Fei X, Jia-Bi Z, Ning G, Yi-Hua Z, et al. Synthesis, self-assembly, and characterization of PEG-coated iron oxide nanoparticles as potential MRI contrast agent. Drug Dev Ind Pharm. 2010;36:1235–1244. doi: 10.3109/03639041003710151. [DOI] [PubMed] [Google Scholar]
- 25.Husein MM, Nassar NN. Nanoparticle preparation using the single microemulsions scheme. Curr Nanosci. 2008;4:370–380. [Google Scholar]
- 26.Lopez-Perez JA, Lopez-Quintela MA, Mira J, Rivas J, Charles SW. Advances in the preparation of magnetic nanoparticles by the microemulsion method. J Phys Chem B. 1997;101:8045–8047. [Google Scholar]
- 27.Kievit FM, Zhang M. Surface engineering of iron oxide nanoparticles for targeted cancer therapy. Acc Chem Res. 2011;44:853–862. doi: 10.1021/ar2000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jun YW, Lee JH, Cheon J. Chemical design of nanoparticle probes for high-performance magnetic resonance imaging. Angew Chem Int Ed. 2008;47:5122–5135. doi: 10.1002/anie.200701674. [DOI] [PubMed] [Google Scholar]
- 29.Nitin N, LaConte LE, Zurkiya O, Hu X, Bao G. Functionalization of peptide-based delivery of magnetic nanoparticles as an intracellular MRI contrast agent. J Biol Inorg Chem. 2004;6:706–712. doi: 10.1007/s00775-004-0560-1. [DOI] [PubMed] [Google Scholar]
- 30.Lee N, Choi Y, Lee Y, Park M, Moon WK, Choi SH, Hyeon T. Water-dispersible ferrimagnetic iron oxide nanocubes with extremely high r2 relaxivity for highly sensitive in vivo MRI of tumors. Nano Lett. 2012;12:3127–3131. doi: 10.1021/nl3010308. [DOI] [PubMed] [Google Scholar]
- 31.Glaus C, Rossin R, Welch MJ, Bao G. In vivo evaluation of 64Cu-labeled magnetic nanoparticles as a dual-modality PET/MR imaging agent. Bioconjugate Chem. 2010;21:715–722. doi: 10.1021/bc900511j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohan P, Rapoport N. Doxorubicin as a molecular nanotheranostic agent: effect of doxorubicin encapsulation in micelles or nanoemulsions on the ultrasound-mediated intracellular delivery and nuclear trafficking. Mol Pharmaceutics. 2010;7:1959–1973. doi: 10.1021/mp100269f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silva AC, Oliveira TR, Mamani JB, Malheiros SMF, Malavolta L, Pavon LF, et al. Application of hyperthermia induced by superparamagnetic iron oxide nanoparticles in glioma treatment. Int J Nanomed. 2011;6:591–603. doi: 10.2147/IJN.S14737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang G, Liao Y, Baker I. Surface engineering of core/shell iron/iron oxide nanoparticles from microemulsions for hyperthermia. Mater Sci Eng C. 2010;30:92–97. doi: 10.1016/j.msec.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Tilborg GAF, Cormode DP, Jarzyna PA, van der Toorn A, van der Pol SMA, van Bloois L, et al. Nanoclusters of iron oxide: effect of core composition on structure, biocompatibility, and cell labeling efficacy. Bioconjugate Chem. 2012;23:941–950. doi: 10.1021/bc200543k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Jamal WT, Kostarelos K. Liposome–nanoparticle hybrids for multimodal diagnostic and therapeutic applications. Nanomedicine. 2007;2:85–98. doi: 10.2217/17435889.2.1.85. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Kohler N, Zhang M. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials. 2002;23:1553–1561. doi: 10.1016/s0142-9612(01)00267-8. [DOI] [PubMed] [Google Scholar]
- 38.Depalo N, Carrieri P, Comparelli R, Striccoli M, Agostiano A, Bertinetti L, et al. Biofunctionalization of anisotropic nanocrystalline semiconductor–magnetic heterostructures. Langmuir. 2011;27:6962–6970. doi: 10.1021/la200822b. [DOI] [PubMed] [Google Scholar]
- 39.Gupta AK, Wells S. Surface-modified superparamagnetic nanoparticles for drug delivery: preparation, characterization, and cytotoxicity studies. IEEE Trans Nanobiosci. 2004;3:66–73. doi: 10.1109/tnb.2003.820277. [DOI] [PubMed] [Google Scholar]
- 40.Kaaki K, Herve-Aubert K, Chiper M, Shkilnyy A, Souce M, Benoit R, et al. magnetic Nanocarriers of doxorubicin coated with poly(ethylene glycol) and folic acid: relation between coating structure, surface properties, colloidal stability, and cancer cell targeting. Langmuir. 2012;28:1496–1505. doi: 10.1021/la2037845. [DOI] [PubMed] [Google Scholar]
- 41.Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanoparticle PEGylation for imaging and therapy. Nanomedicine. 2011;6:715–728. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang HC, Chang PY, Chang K, Chen CY, Lin CW, Chen JH, et al. Formulation of novel lipid-coated magnetic nanoparticles as the probe for in vivo imaging. J Biomed Sci. 2009;16:86–95. doi: 10.1186/1423-0127-16-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jarzyna PA, Deddens LH, Kann BH, Ramachandran S, Calcagno C, Chen W, et al. Tumor angiogenesis phenotyping by nanoparticle-facilitated magnetic resonance and near-infrared fluorescence molecular imaging. Neoplasia. 2012;14:964–973. doi: 10.1593/neo.121148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LaConte LEW, Nitin N, Zurkiya O, Caruntu D, O’Connor CJ, Hu X, Bao G. Coating thickness of magnetic iron oxide nanoparticles affects R2 relaxivity. J Magn Reson Imaging. 2007;26:1634–1641. doi: 10.1002/jmri.21194. [DOI] [PubMed] [Google Scholar]
- 45.Tong S, Hou S, Zheng Z, Zhou J, Bao G. Coating optimization of superparamagnetic iron oxide nanoparticles for high T2 relaxivity. Nano Lett. 2010;10:4607–4613. doi: 10.1021/nl102623x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baer DR, Gaspar DJ, Nachimuthu P, Techane SD, Castner DG. Application of surface chemical analysis tools for characterization of nanoparticles. Anal Bioanal Chem. 2010;396:983–1002. doi: 10.1007/s00216-009-3360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hultman KL, Raffo AJ, Grzenda AL, Harris PE, Brown TR, O’Brien S. Magnetic resonance imaging of major histocompatibility class ii expression in the renal medulla using immunotargeted superparamagnetic iron oxide nanoparticles. ACS Nano. 2008;2:477–484. doi: 10.1021/nn700400h. [DOI] [PubMed] [Google Scholar]
- 48.Cormode DP, Skajaa GO, Delshad A, Parker N, Jarzyna PA, Calcagno C, et al. A versatile and tunable coating strategy allows control of nanocrystal delivery to cell types in the liver. Bioconjugate Chem. 2011;22(3):353–361. doi: 10.1021/bc1003179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang X, Bronstein LM, Retrum J, Dufort C, Tsvetkova I, Aniagyei S, et al. Self-assembled virus-like particles with magnetic cores. Nano Lett. 2007;7(8):2407–2416. doi: 10.1021/nl071083l. [DOI] [PubMed] [Google Scholar]
- 50.Mulder WJ, Strijkers GJ, van Tilborg GA, Griffioen AW, Nicolay K. Lipid-based nanoparticles for contrast-enhanced MRI and molecular imaging. NMR Biomed. 2006;19:142–164. doi: 10.1002/nbm.1011. [DOI] [PubMed] [Google Scholar]
- 51.Zhao M, Beauregard DA, Loizou L, Davletov B, Brindle KM. Non-invasive detection of apoptosis using magnetic resonance imaging and a targeted contrast agent. Nat Med. 2001;7:1241–1244. doi: 10.1038/nm1101-1241. [DOI] [PubMed] [Google Scholar]
- 52.van Tilborg GA, Mulder WJ, Deckers N, Storm G, Reutelingsperger CPM, Strijkers GJ, Nicolay K. Annexin a5-functionalized bimodal lipid-based contrast agents for the detection of apoptosis. Bioconjugate Chem. 2006;17:741–749. doi: 10.1021/bc0600259. [DOI] [PubMed] [Google Scholar]
- 53.Wahajuddin, Arora S. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int J Nanomedicine. 2012;7:3445–3471. doi: 10.2147/IJN.S30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Edmund AR, Kambalapally S, Wilson TA, Nicolosi RJ. Encapsulation of cadmium selenide quantum dots using a self-assembling nanoemulsion (SANE) reduces their in vitro toxicity. Toxicol In Vitro. 2011;25:185–190. doi: 10.1016/j.tiv.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 55.Steigerwald ML, Brus LE. Semiconductor crystallites: a class of large molecules. Acc Chem Res. 1990;23:183–188. [Google Scholar]
- 56.Nirmal M, Brus L. Luminescence photophysics in semiconductor nanocrystals. Acc Chem Res. 1999;32:407–414. [Google Scholar]
- 57.Sharma CVK. Crystal engineering–where do we go from here? Cryst Growth Des. 2002;2:467. [Google Scholar]
- 58.Kikkeri R, Laurino P, Odedra A, Seeberger PH. Synthesis of carbohydrate-functionalized quantum dots in microreactors. Angew Chem Int Ed. 2010;49:2054. doi: 10.1002/anie.200905053. [DOI] [PubMed] [Google Scholar]
- 59.Willard DM, Carillo LL, Jung J, Van Orden A. CdSe–ZnS Quantum dots as resonance energy transfer donors in a model protein–protein binding assay. Nano Lett. 2001;1:469–474. [Google Scholar]
- 60.Peralta-Videa JR, Zhao L, Lopez-Moreno ML, de la Rosa G, Hong J, Gardea-Torresdey JL. Nanomaterials and the environment: a review for the biennium 2008–2010. J Hazard Mater. 2011;186:1–15. doi: 10.1016/j.jhazmat.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 61.Murray CB, Norris DJ, Bawendi MG. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J Am Chem Soc. 1993;115:8706–8715. [Google Scholar]
- 62.Bowen-Katari JE, Colvin VL, Alivisatos AP. X-ray photoelectron spectroscopy of CdSe nanocrystals with applications to studies of the nanocrystal surface. J Phys Chem. 1994;98:4109–4117. [Google Scholar]
- 63.Ramachandran S, Kumar GL, Blick RH, van der Weide DW. Current bursts in lipid bilayers initiated by colloidal quantum dots. Appl Phys Lett. 2005;86:083901. doi: 10.1016/j.bios.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 64.Yan M, Zhang Y, Xu K, Fu T, Qin H, Zheng X. An in vitro study of vascular endothelial toxicity of CdTe quantum dots. Toxicology. 2011;282:94–103. doi: 10.1016/j.tox.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 65.Rogach AL, Kornowski A, Gao MY, Eychmuller A, Weller H. Synthesis and characterization of a size series of extremely small thiol-stabilized CdSe nanocrystals. J Phys Chem B. 1999;103:3065–3069. [Google Scholar]
- 66.Gerion D, Pinaud F, Williams SC, Parak WJ, Zanchet D, Weiss S, Alivisatos AP. Synthesis and properties of biocompatible water-soluble silica-coated CdSe/ZnS semiconductor quantum dots. J Phys Chem, B. 2001;105:8861–8871. [Google Scholar]
- 67.Yang XT, Zhang Y. Encapsulation of quantum nanodots in polystyrene and silica micro-/nanoparticles. Langmuir. 2004;20:6071–6073. doi: 10.1021/la049610t. [DOI] [PubMed] [Google Scholar]
- 68.Chen YF, Rosenzweig Z. Luminescent CdSe quantum dot doped stabilized micelles. Nano Lett. 2002;2:1299–1302. [Google Scholar]
- 69.De GC, Roy AM, Saba S, Aditya SJ. Semiconductor nanoparticles (CdS, ZnS and ZnxCdS1-x) in reverse micellar media. J Ind Chem Soc. 2003;80:551–557. [Google Scholar]
- 70.Fan H, Leve EW, Scullin C, Gabaldon J, Tallant D, Bunge S, et al. Surfactant-assisted synthesis of water-soluble and biocompatible semiconductor quantum dot micelles. Nano Lett. 2005;5:645–648. doi: 10.1021/nl050017l. [DOI] [PubMed] [Google Scholar]
- 71.Murcia MJ, Minner DE, Mustata GM, Ritchie K, Naumann CA. Design of quantum dot-conjugated lipids for long-term, high-speed tracking experiments on cell surfaces. J Am Chem Soc. 2008;130:15054–15062. doi: 10.1021/ja803325b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Skajaa T, Zhao Y, van den Heuvel DJ, Gerritsen HC, Cormode DP, Koole R, et al. Quantum dot and Cy5.5 labeled nanoparticles to in estigate lipoprotein biointeractions via förster resonance energy transfer. Nano Lett. 2010;10:5131–5138. doi: 10.1021/nl1037903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sigot V, Arndt-Jovin DJ, Jovin TM. Targeted cellular delivery of quantum dots loaded on and in biotinylated liposomes. Bioconjugate Chem. 2010;21:1465–1472. doi: 10.1021/bc100054c. [DOI] [PubMed] [Google Scholar]
- 74.Luccardini C, Tribet C, Vial F, Marchi-Artzner V, Dahan M. Size, charge, and interactions with giant lipid vesicles of quantum dots coated with an amphiphilic macromolecule. Langmuir. 2006;22:2304–2310. doi: 10.1021/la052704y. [DOI] [PubMed] [Google Scholar]
- 75.Liu W, He Z, Liang J, Zhu Y, Xu H, Yang X. Preparation and characterization of novel fluorescent nanocomposite particles: CdSe/ZnS core-shell quantum dots loaded solid lipid nanoparticles. J Biomed Mater Res. 2008;84A:1018–1025. doi: 10.1002/jbm.a.31205. [DOI] [PubMed] [Google Scholar]
- 76.Galloway JF, Winter A, Lee KH, Park JH, Dvoracek CM, Devreotes P, Searson PC. Quantitative characterization of the lipid encapsulation of quantum dots for biomedical applications. Nanomed. 2012;8:1190–1100. doi: 10.1016/j.nano.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnson RL, Johnson GO, Nurmi JT, Tratnyek PG. Natural organic matter enhanced mobility of nano zerovalent iron. Environ Sci Technol. 2009;43:5455–5460. doi: 10.1021/es900474f. [DOI] [PubMed] [Google Scholar]
- 78.Jablonski A, Powell CJ. Information depth and the mean escape depth in Auger electron spectroscopy and x-ray photoelectron spectroscopy. J Vac Sci Techno, A. 2003;21:274–283. [Google Scholar]
- 79.Murcia MJ, Shaw DL, Woodruff H, Naumann CA, Young BY, Long EL. Facile sonochemical synthesis of highly luminescent ZnS-shelled CdSe quantum dots. Chem Mater. 2006;18:2219–2225. [Google Scholar]
- 80.Gopalakrishnan G, Danelon C, Izewska P, Prummer M, Bolinger PY, Geissbuhler I, et al. Multifunctional lipid/quantum dot hybrid nanocontainers for controlled targeting of live cells. Angew Chem Int Ed. 2006;45:5478–5483. doi: 10.1002/anie.200600545. [DOI] [PubMed] [Google Scholar]
- 81.Cuenca AG, Jiang H, Hochwald SN, Delano M, Cance WG, Grobmyer SR. Emerging implications of nanotechnology on cancer diagnostics and therapeutics. Cancer. 2006:107459–466. doi: 10.1002/cncr.22035. [DOI] [PubMed] [Google Scholar]
- 82.Mulder WJM, Koole R, Brandwijk RJ, Storm G, Chin PTK, Strijkers GJ, et al. Quantum dots with paramagnetic coating as a bimodal molecular imaging probe. Nano Lett. 2006;6(1):1–6. doi: 10.1021/nl051935m. [DOI] [PubMed] [Google Scholar]
- 83.Lin CY, Liu TM, Chen CY, Huang YL, Huang WK, Sun CK, et al. Quantitative and qualitative investigation into the impact of focused ultrasound with microbubbles on the triggered release of nanoparticles from vasculature in mouse tumors. J Controlled Release. 2010;146:291–298. doi: 10.1016/j.jconrel.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 84.El-Sayed MA. Some interesting properties of metals confined in time and nanometer space of different shapes. Acc Chem Res. 2001;34:257–264. doi: 10.1021/ar960016n. [DOI] [PubMed] [Google Scholar]
- 85.Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute toxicity. Small. 2005;1:325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 86.Murphy CJ, Gole AM, Stone JW, Sisco PN, Alkilany AM, Goldsmith EC, Baxter SC. Gold nanoparticles in biology: beyond toxicity to cellular imaging. Acc Chem Res. 2008;41:1721–1730. doi: 10.1021/ar800035u. [DOI] [PubMed] [Google Scholar]
- 87.Qian X, Peng XH, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, et al. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat Biotech. 2008;26:83–90. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- 88.Keren S, Zavaleta C, Cheng Z, de la Zerda A, Gheysens O, Gambhir SS. Noninvasive molecular imaging of small living subjects using Raman spectroscopy. Proc Natl Acad Sci USA. 2008;105:5844–5849. doi: 10.1073/pnas.0710575105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Turkevitch J, Stevenson PC, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Faraday Soc. 1951;11:55–75. [Google Scholar]
- 90.Enustun BV, Turkevich J. Coagulation of colloidal gold. J Am Chem Soc. 1963;85:3317–3328. [Google Scholar]
- 91.Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R. Synthesis of thiol-derivatized gold nanoparticles in a two-phase liquid-liquid system. J Chem Soc Chem Commun. 1994:801–802. [Google Scholar]
- 92.Yang JA, Murphy CJ. Evidence for patchy lipid layers on gold nanoparticle surfaces. Langmuir. 2012;28:5404–5416. doi: 10.1021/la300325p. [DOI] [PubMed] [Google Scholar]
- 93.Sitaula S, Mackiewicz MR, Reed SM. Gold nanoparticles become stable to cyanide etch when coated with hybrid lipid bilayers. Chem Commun. 2008:3013–3015. doi: 10.1039/b801525b. [DOI] [PubMed] [Google Scholar]
- 94.Mackiewicz MR, Hodges HL, Reed SM. C-reactive protein induced rearrangement of phosphatidylcholine on nanoparticle mimics of lipoprotein particles. J Phys Chem B. 2010;114:5556–5562. doi: 10.1021/jp911617q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thaxton CS, Daniel WL, Giljohann DA, Thomas AD, Mirkin CA. Templated spherical high density lipoprotein nanoparticles. J Am Chem Soc. 2009;131(4):1384–1385. doi: 10.1021/ja808856z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Genc R, Clergeaud G, Ortiz M, O’Sullivan CK. Green synthesis of gold nanoparticles using glycerol-incorporated nanosized liposomes. Langmuir. 2011;27:10894–10900. doi: 10.1021/la201771s. [DOI] [PubMed] [Google Scholar]
- 97.Tam NCM, Scott BTM, Voicu D, Wilson BC, Zheng G. Facile synthesis of Raman active phospholipid gold nanoparticles. Bioconjugate Chem. 2010;21(12):2178–2182. doi: 10.1021/bc100386a. [DOI] [PubMed] [Google Scholar]
- 98.Kong WH, Bae KH, Jo SD, Kim JS, Park TG. Cationic lipid-coated gold nanoparticles as efficient and non-cytotoxic intracellular siRNA delivery vehicles. Pharm Res. 2012;29:362–374. doi: 10.1007/s11095-011-0554-y. [DOI] [PubMed] [Google Scholar]
- 99.Cormode DP, Skajaa T, van Schooneveld MM, Koole R, Jarzyna P, Lobatto ME, et al. Nanocrystal core high-density lipoproteins: a multimodality contrast agent platform. Nano Lett. 2008;8:3715–3723. doi: 10.1021/nl801958b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stober W, Fink A, Bohn E. Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interface Sci. 1968;26:62–69. [Google Scholar]
- 101.Osseoasare K, Arriagada F. Preparation of SiO2 nanoparticles in a nonionic reverse micellar system. J Colloids Surf. 1990;50:321–339. [Google Scholar]
- 102.Yong KT, Roy I, Swihart MT, Prasad PN. Multifunctional nanoparticles as biocompatible targeted probes for human cancer diagnosis and therapy. J Mater Chem. 2009;19:4655–4672. doi: 10.1039/b817667c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rai P, Mallidi S, Zheng X, Rahmanzadeh R, Mir Y, Elrington S, Khurshid A, Hasan T. Development and applications of photo-triggered theranostic agents. Adv Drug Delivery Rev. 2010;62:1094–1124. doi: 10.1016/j.addr.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Schooneveld MM, Vucic E, Koole R, Zhou Y, Stocks J, Cormode DP, et al. Improved biocompatibility and pharmacokinetics of silica nanoparticles by means of lipid coating: a multimodality investigation. Nano Lett. 2008;8:2517–2525. doi: 10.1021/nl801596a. [DOI] [PubMed] [Google Scholar]
- 105.Kumar R, Roy I, Ohulchanskyy TY, Goswami LN, Bonoiu AC, Bergey EJ, et al. Covalently dye-linked, surface-controlled, and bioconjugated organically modified silica nanoparticles as targeted probes for optical imaging. ACS Nano. 2008;2:449–456. doi: 10.1021/nn700370b. [DOI] [PubMed] [Google Scholar]
- 106.Bagwe RP, Hilliard LR, Tan W. Surface modification of silica nanoparticles to reduce aggregation and nonspecific binding. Langmuir. 2006;22:4357–4362. doi: 10.1021/la052797j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huo Q, Liu J, Wang LQ, Jiang Y, Lambert TN, Fang E. A new class of silica cross-linked micellar core-shell nanoparticles. J Am Chem Soc. 2006;128:6447–6453. doi: 10.1021/ja060367p. [DOI] [PubMed] [Google Scholar]
- 108.Yang CY, Cai SJ, Liu H, Pidgeon C. Immobilized artificial membranes – screens for drug membrane interactions. Adv Drug Delivery Rev. 1997;23:229–256. [Google Scholar]
- 109.Mornet S, Lambert O, Duguet E, Brisson A. The formation of supported lipid bilayers on silica nanoparticles revealed by cryoelectron microscopy. Nano Lett. 2005;5:281–285. doi: 10.1021/nl048153y. [DOI] [PubMed] [Google Scholar]
- 110.Radler J, Strey H, Sackmann E. Phenomenology and kinetics of lipid bilayer spreading on hydrophilic surfaces. Langmuir. 1995;11:4539–4548. [Google Scholar]
- 111.Sackmann E. Supported membranes: scientific and practical applications. Science. 1996;271:43–48. doi: 10.1126/science.271.5245.43. [DOI] [PubMed] [Google Scholar]
- 112.Carmona-Ribeiro AM. Biomimetic nanoparticles: preparation, characterization and biomedical applications. Int J Nanomed. 2010;5:249–259. doi: 10.2147/ijn.s9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang H, Drazenovic J, Luo Z, Zhang J, Zhou H, Wunder SL. Mechanism of supported bilayer formation of zwitterionic lipids on SiO2 nanoparticles and structure of the stable colloids. RSC Adv. 2012;2:11336–11348. [Google Scholar]
- 114.Ahmed S, Savarala S, Chen Y, Bothun G, Wunder SL. Formation of lipid sheaths around nanoparticle-supported lipid bilayers. Small. 2012;8:1740–1751. doi: 10.1002/smll.201101833. [DOI] [PubMed] [Google Scholar]
- 115.Troutier AL, Ladaviere C. An overview of lipid membrane supported by colloidal particles. Adv Colloid Interface Sci. 2007;133(1):1–21. doi: 10.1016/j.cis.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 116.Ahmed S, Wunder SL. Effect of high surface curvature on the main phase transition of supported phospholipid bilayers on SiO2 nanoparticles. Langmuir. 2009;25:3682–3691. doi: 10.1021/la803630m. [DOI] [PubMed] [Google Scholar]
- 117.Savarala S, Ahmed S, Ilies MA, Wunder SL. Formation and colloidal stability of DMPC supported lipid bilayers on SiO2 nanobeads. Langmuir. 2010;26:12081–12088. doi: 10.1021/la101304v. [DOI] [PubMed] [Google Scholar]
- 118.Savarala S, Monson F, Ilies MA, Wunder SL. Supported lipid bilayer nanosystems: stabilization by undulatory-protrusion forces and destabilization by lipid bridging. Langmuir. 2011;27:5850–5861. doi: 10.1021/la200636k. [DOI] [PubMed] [Google Scholar]
- 119.Savarala S, Ahmed S, Ilies MA, Wunder SL. Stabilization of soft lipid colloids: competing effects of nanoparticle decoration and supported lipid bilayer formation. ACS Nano. 2011;5:2619–2628. doi: 10.1021/nn1025884. [DOI] [PubMed] [Google Scholar]
- 120.Liu J, Stace-Naughton A, Jiang X, Brinker CJ. Silica nanoparticle supported lipid bilayers for gene delivery. J Am Chem Soc. 2009;131:1354–1355. doi: 10.1021/ja808018y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu J, Stace-Naughton A, Brinker CJ. Silica nanoparticle supported bilayers for gene delivery. Chem Commun. 2009:5100–5102. doi: 10.1039/b911472f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bringas E, Koysuren O, Quach DV, Mahmoudi M, Aznar E, Roehling JD, et al. Triggered release in lipid bilayer-capped mesoporous silica nanoparticles containing SPION using an alternating magnetic field. Chem Commun. 2012;48:5647–5649. doi: 10.1039/c2cc31563g. [DOI] [PubMed] [Google Scholar]
- 123.Roggers RA, Lin VSY, Trewyn BG. Chemically reducible lipid bilayer coated mesoporous silica nanoparticles demonstrating controlled release and HeLa and normal mouse liver cell biocompatibility and cellular internalization. Mol Pharmaceutics. 2012;9:2770–2777. doi: 10.1021/mp200613y. [DOI] [PubMed] [Google Scholar]
- 124.Epler K, Padilla D, Phillips G, Crowder P, Castillo R, Wilkinson D. Delivery of ricin toxin a-chain by peptide-targeted mesoporous silica nanoparticle-supported lipid bilayers. Adv Healthc Mater. 2012;1:348–353. doi: 10.1002/adhm.201200022. er al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ashley CE, Carnes EC, Epler KE, Padilla DP, Phillips GK, Castillo RE, et al. Delivery of small interfering RNA by peptide-targeted mesoporous silica nanoparticle-supported lipid bilayers. ACS Nano. 2012;6:2174–2188. doi: 10.1021/nn204102q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang Y, Song W, Wang A, Zhu P, Fei J, Li J. Lipid coated mesoporous silica nanoparticles as photosensitive drug carriers. Phys Chem Chem Phys. 2010;12:4418–4422. doi: 10.1039/b924370d. [DOI] [PubMed] [Google Scholar]
- 127.Tan A, Simovic S, Davey AK, Rades T, Prestidge CA. Silica-lipid hybrid (SLH) microcapsules: a novel oral delivery system for poorly soluble drugs. J Controlled Release. 2009;134:62–70. doi: 10.1016/j.jconrel.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 128.Simovic S, Hui H, Song Y, Davey AK, Rades T, Prestidge CA. An oral delivery system for indomethicin engineered from cationic lipid emulsions and silica nanoparticles. J Controlled Release. 2010;143:367–373. doi: 10.1016/j.jconrel.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 129.Cauda V, Engelke H, Sauer A, Arcizet D, Brachle C, Radler J, Bein T. Colchicine-loaded lipid bilayer-coated 50 nm mesoporous nanoparticles efficiently induce microtubule depolymerization upon cell uptake. Nano Lett. 2010;10:2484–2492. doi: 10.1021/nl100991w. [DOI] [PubMed] [Google Scholar]
- 130.Irvine DJ. Drug delivery: one nanoparticle, one kill. Nat Mater. 2011;10:342–343. doi: 10.1038/nmat3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Goutayer M, Dufort S, Josserand V, Royere A, Heinrich E, Vinet F, et al. Tumor targeting of functionalized lipid nanoparticles: Assessment by in vivo fluorescence imaging. Eur J Pharm BioPharm. 2010;75:137–147. doi: 10.1016/j.ejpb.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 132.Sridaeng D, Weingart JJ, Chantarasiri N, Zhe J, Hu JJ. Postsynthetic surface functionalization, encapsulation, and releasing studies of a novel polymer nanocapsule. J Applied Polym Sci. 2010;117:706–713. [Google Scholar]
- 133.Yan GP, Hua L, Cheng SX, Bottle SE, Wang XG, Yew YK, Zhou RX. Preparation, properties, and mathematical modeling of microparticle drug delivery systems based on biodegradable amphiphilic triblock copolymers. J Appl Polym Sci. 2004;92:3869. [Google Scholar]
- 134.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharm Rev. 2001;53:296. [PubMed] [Google Scholar]
- 135.Troutier AL, Delair T, Pichot C, Ladaviere C. Physicochemical and interfacial investigation of lipid/polymer particle assemblies. Langmuir. 2005;21:1305–1313. doi: 10.1021/la047659t. [DOI] [PubMed] [Google Scholar]
- 136.Zhang L, Chan JM, Gu FX, Rhee JW, Wang AZ, Radovic-Moreno AF, et al. Self-assembled lipid-polymer hybrid nanoparticles: a robust drug delivery platform. ACS Nano. 2008;2:1696–1702. doi: 10.1021/nn800275r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Messerschmidt SKE, Musyanovych A, Altvater M, Scheurich P, Pfizenmaier K, Landfester K, Kontermann RE. Targeted lipid-coated nanoparticles: Delivery of tumor necrosis factor-functionalized particles to tumor cells. J Controlled Release. 2009;137:69–77. doi: 10.1016/j.jconrel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 138.Fang RH, Aryal S, Hu CMJ, Zhang L. Quick synthesis of lipid-polymer hybrid nanoparticles with low polydispersity using a single-step sonication method. Langmuir. 2010;26:16958–16962. doi: 10.1021/la103576a. [DOI] [PubMed] [Google Scholar]
- 139.Troutier AL, Veron L, Delair T, Pichot C, Ladaviere C. New insights into self-organization of a model lipid mixture and quantification of its adsorption on spherical polymer particles. Langmuir. 2005;21:9901–9910. doi: 10.1021/la050796l. [DOI] [PubMed] [Google Scholar]
- 140.De Miguel I, Imbertie L, Rieumajou V, Major M, Kravtzoff R, Betbeder D. Proofs of the structure of lipid coated nanoparticles (SMBV™) used as drug carriers. Pharm Res. 2000;17:817–824. doi: 10.1023/a:1007504124603. [DOI] [PubMed] [Google Scholar]
- 141.Gomes JFPS, Rocha S, do Carmo Pereira M, Peres I, Moreno S, Toca-Herrera J, Coelho MAN. Lipid/particle assemblies based on maltodextrin-gum arabic core as biocarriers. Colloids Surf, B. 2010;76:449–455. doi: 10.1016/j.colsurfb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 142.Wang H, Zhao P, Su W, Wang S, Liao Z, Niu R, Chang J. PLGA/polymeric liposome for targeted drug and gene co-delivery. Biomaterials. 2010;31:8741–8748. doi: 10.1016/j.biomaterials.2010.07.082. [DOI] [PubMed] [Google Scholar]
- 143.Zheng Y, Yu B, Weecharangsan W, Piao L, Darby M, Mao Y, et al. Transferrin-conjugated lipid-coated PLGA nanoparticles for targeted delivery of aromatase inhibitor 7alpha-APTADD to breast cancer cells. Int J Pharm. 2010;390:234–341. doi: 10.1016/j.ijpharm.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fang RH, Chen KNH, Aryal S, Hu CMJ, Zhang K, Zhang L. Large-scale synthesis of lipid-polymer hybrid nanoparticles using a multi-inlet vortex reactor. Langmuir. 2012;28:13824–13829. doi: 10.1021/la303012x. [DOI] [PubMed] [Google Scholar]
- 145.Hu CMJ, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci USA. 2011;108:10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]