Abstract
Bacterial conjugation results in the transfer of DNA of either plasmid or chromosomal origin between microorganisms. Transfer begins at a defined point in the DNA sequence, usually called the origin of transfer (oriT). The capacity of conjugative DNA transfer is a property of self-transmissible plasmids and conjugative transposons, which will mobilize other plasmids and DNA sequences that include a compatible oriT locus. This review will concentrate on the genes required for bacterial conjugation that are encoded within the transfer region (or regions) of conjugative plasmids. One of the best-defined conjugation systems is that of the F plasmid, which has been the paradigm for conjugation systems since it was discovered nearly 50 years ago. The F transfer region (over 33 kb) contains about 40 genes, arranged contiguously. These are involved in the synthesis of pili, extracellular filaments which establish contact between donor and recipient cells; mating-pair stabilization; prevention of mating between similar donor cells in a process termed surface exclusions; DNA nicking and transfer during conjugation; and the regulation of expression of these functions. This review is a compendium of the products and other features found in the F transfer region as well as a discussion of their role in conjugation. While the genetics of F transfer have been described extensively, the mechanism of conjugation has proved elusive, in large part because of the low levels of expression of the pilus and the numerous envelope components essential for F plasmid transfer. The advent of molecular genetic techniques has, however, resulted in considerable recent progress. This summary of the known properties of the F transfer region is provided in the hope that it will form a useful basis for future comparison with other conjugation systems.
Full text
PDF

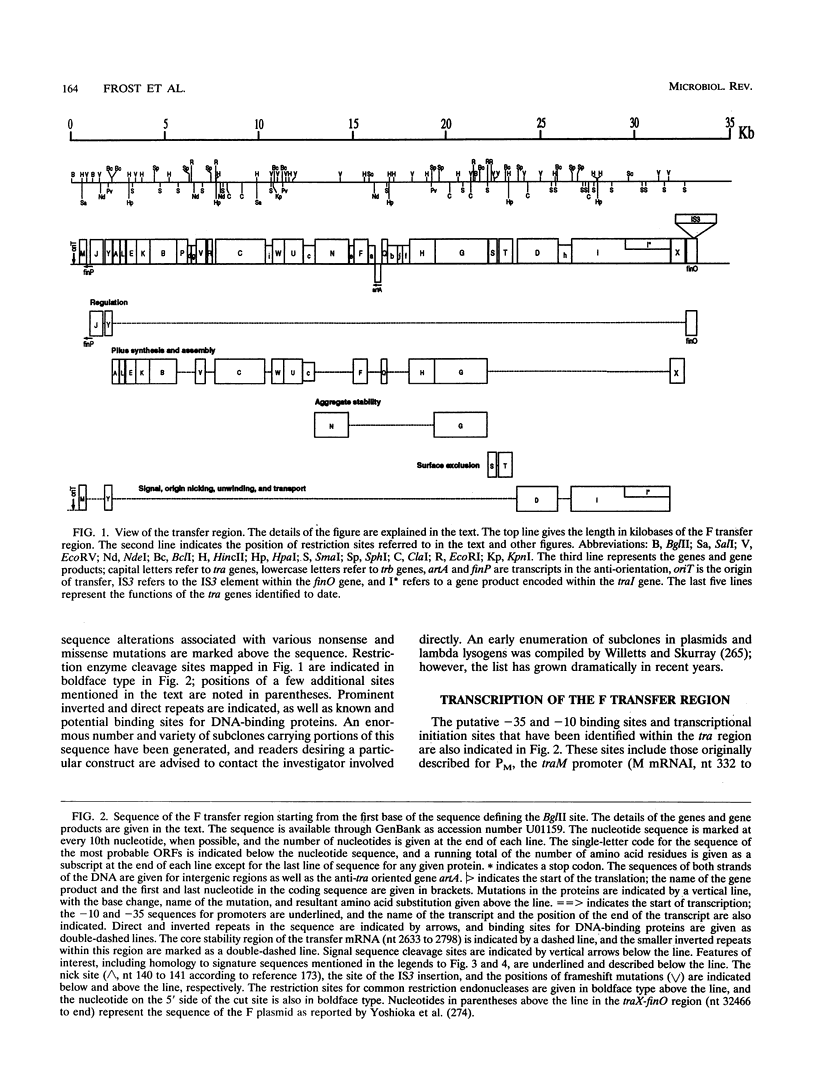


















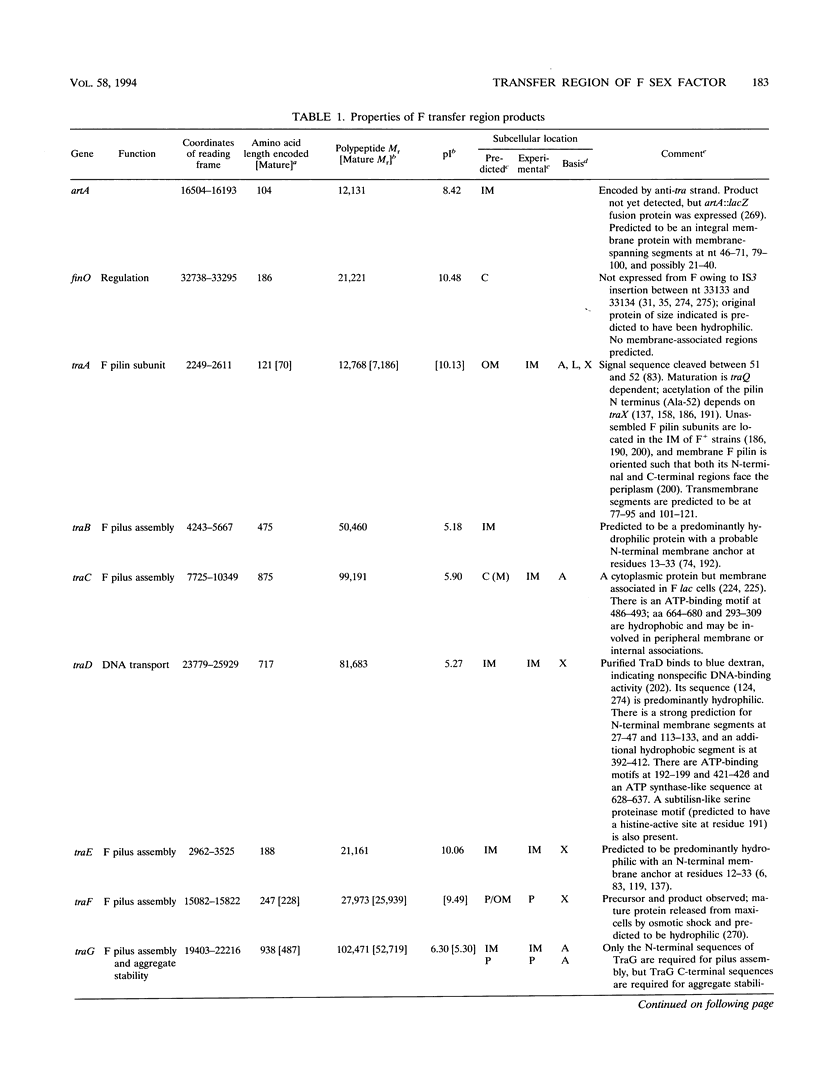
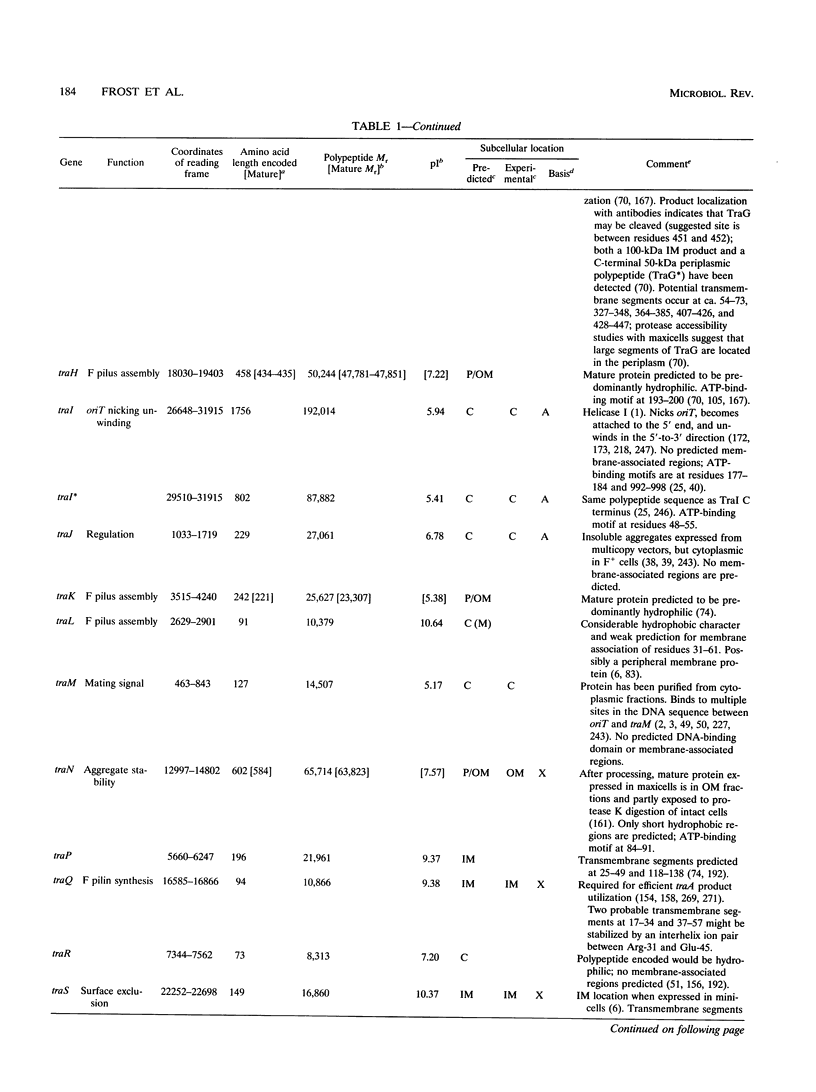
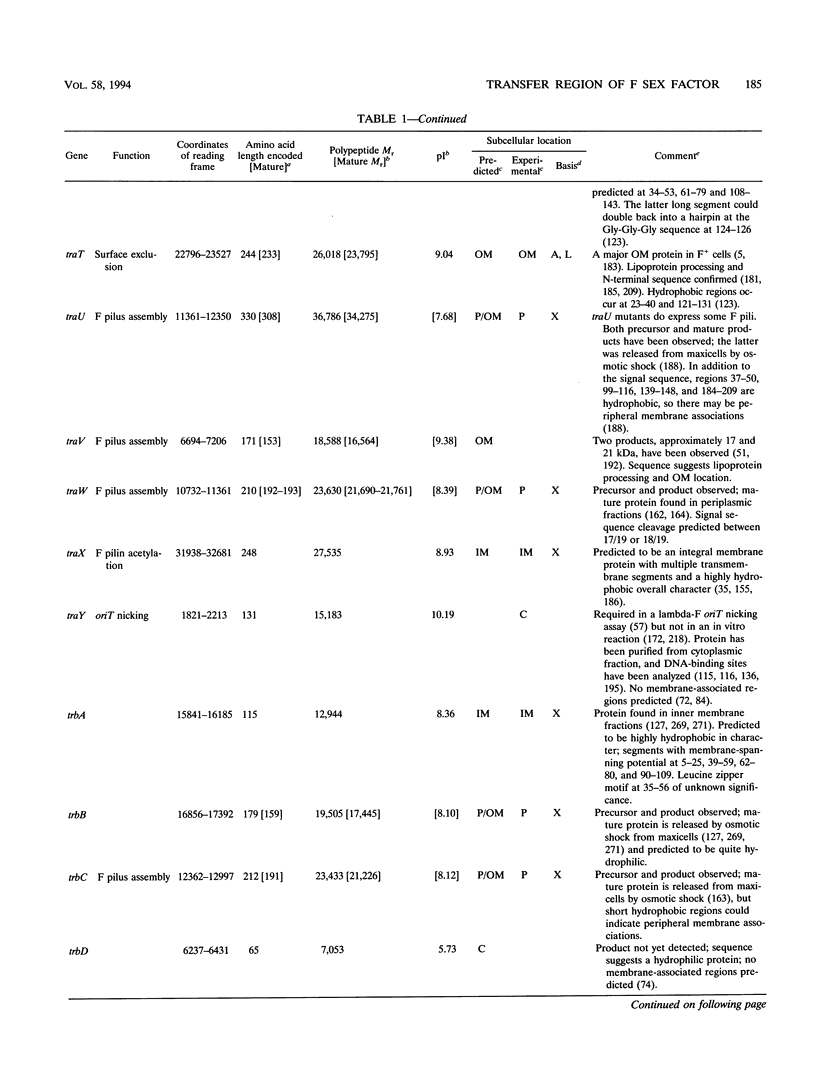





















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Monem M., Taucher-Scholz G., Klinkert M. Q. Identification of Escherichia coli DNA helicase I as the traI gene product of the F sex factor. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4659–4663. doi: 10.1073/pnas.80.15.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abo T., Inamoto S., Ohtsubo E. Specific DNA binding of the TraM protein to the oriT region of plasmid R100. J Bacteriol. 1991 Oct;173(20):6347–6354. doi: 10.1128/jb.173.20.6347-6354.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abo T., Ohtsubo E. Repression of the traM gene of plasmid R100 by its own product and integration host factor at one of the two promoters. J Bacteriol. 1993 Jul;175(14):4466–4474. doi: 10.1128/jb.175.14.4466-4474.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Kennedy N., Skurray R. Cell--cell interactions in conjugating Escherichia coli: role of traT protein in surface exclusion. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5104–5108. doi: 10.1073/pnas.74.11.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Manning P. A., Edelbluth C., Herrlich P. Export without proteolytic processing of inner and outer membrane proteins encoded by F sex factor tra cistrons in Escherichia coli minicells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4837–4841. doi: 10.1073/pnas.76.10.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Manning P. A., Kusecek B., Schwuchow S., Willetts N. A genetic analysis of F sex factor cistrons needed for surface exclusion in Escherichia coli. J Mol Biol. 1980 Apr 25;138(4):779–795. doi: 10.1016/0022-2836(80)90065-0. [DOI] [PubMed] [Google Scholar]
- Achtman M. Mating aggregates in Escherichia coli conjugation. J Bacteriol. 1975 Aug;123(2):505–515. doi: 10.1128/jb.123.2.505-515.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Morelli G., Schwuchow S. Cell-cell interactions in conjugating Escherichia coli: role of F pili and fate of mating aggregates. J Bacteriol. 1978 Sep;135(3):1053–1061. doi: 10.1128/jb.135.3.1053-1061.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Willetts N., Clark A. J. Beginning a genetic analysis of conjugational transfer determined by the F factor in Escherichia coli by isolation and characterization of transfer-deficient mutants. J Bacteriol. 1971 May;106(2):529–538. doi: 10.1128/jb.106.2.529-538.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Willetts N., Clark A. J. Conjugational complementation analysis of transfer-deficient mutants of Flac in Escherichia coli. J Bacteriol. 1972 Jun;110(3):831–842. doi: 10.1128/jb.110.3.831-842.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alifano P., Rivellini F., Limauro D., Bruni C. B., Carlomagno M. S. A consensus motif common to all Rho-dependent prokaryotic transcription terminators. Cell. 1991 Feb 8;64(3):553–563. doi: 10.1016/0092-8674(91)90239-u. [DOI] [PubMed] [Google Scholar]
- Armstrong G. D., Frost L. S., Sastry P. A., Paranchych W. Comparative biochemical studies on F and EDP208 conjugative pili. J Bacteriol. 1980 Jan;141(1):333–341. doi: 10.1128/jb.141.1.333-341.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong G. D., Frost L. S., Vogel H. J., Paranchych W. Nature of the carbohydrate and phosphate associated with ColB2 and EDP208 pilin. J Bacteriol. 1981 Mar;145(3):1167–1176. doi: 10.1128/jb.145.3.1167-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdasarian M., Bailone A., Angulo J. F., Scholz P., Bagdasarian M., Devoret R. PsiB, and anti-SOS protein, is transiently expressed by the F sex factor during its transmission to an Escherichia coli K-12 recipient. Mol Microbiol. 1992 Apr;6(7):885–893. doi: 10.1111/j.1365-2958.1992.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Bagdasarian M., Bailone A., Bagdasarian M. M., Manning P. A., Lurz R., Timmis K. N., Devoret R. An inhibitor of SOS induction, specified by a plasmid locus in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5723–5726. doi: 10.1073/pnas.83.15.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailone A., Bäckman A., Sommer S., Célérier J., Bagdasarian M. M., Bagdasarian M., Devoret R. PsiB polypeptide prevents activation of RecA protein in Escherichia coli. Mol Gen Genet. 1988 Nov;214(3):389–395. doi: 10.1007/BF00330471. [DOI] [PubMed] [Google Scholar]
- Bairoch A. PROSITE: a dictionary of sites and patterns in proteins. Nucleic Acids Res. 1992 May 11;20 (Suppl):2013–2018. doi: 10.1093/nar/20.suppl.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz I., Müller H. Escherichia coli DNA helicase I. Characterization of the protein and of its DNA-binding properties. Eur J Biochem. 1990 Apr 30;189(2):267–276. doi: 10.1111/j.1432-1033.1990.tb15486.x. [DOI] [PubMed] [Google Scholar]
- Beutin L., Manning P. A., Achtman M., Willetts N. sfrA and sfrB products of Escherichia coli K-12 are transcriptional control factors. J Bacteriol. 1981 Feb;145(2):840–844. doi: 10.1128/jb.145.2.840-844.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie J. U., Sauer R. T. TraY proteins of F and related episomes are members of the Arc and Mnt repressor family. J Mol Biol. 1990 Jan 5;211(1):5–6. doi: 10.1016/0022-2836(90)90004-6. [DOI] [PubMed] [Google Scholar]
- Boyd A. C., Archer J. A., Sherratt D. J. Characterization of the ColE1 mobilization region and its protein products. Mol Gen Genet. 1989 Jun;217(2-3):488–498. doi: 10.1007/BF02464922. [DOI] [PubMed] [Google Scholar]
- Bradshaw H. D., Jr, Traxler B. A., Minkley E. G., Jr, Nester E. W., Gordon M. P. Nucleotide sequence of the traI (helicase I) gene from the sex factor F. J Bacteriol. 1990 Jul;172(7):4127–4131. doi: 10.1128/jb.172.7.4127-4131.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton C. C., Jr The properties of sex pili, the viral nature of "conjugal" genetic transfer systems, and some possible approaches to the control of bacterial drug resistance. CRC Crit Rev Microbiol. 1971 May;1(1):105–160. doi: 10.3109/10408417109104479. [DOI] [PubMed] [Google Scholar]
- Buxton R. S., Drury L. S. Cloning and insertional inactivation of the dye (sfrA) gene, mutation of which affects sex factor F expression and dye sensitivity of Escherichia coli K-12. J Bacteriol. 1983 Jun;154(3):1309–1314. doi: 10.1128/jb.154.3.1309-1314.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J. R., Porter R. D. traY and traI are required for oriT-dependent enhanced recombination between lac-containing plasmids and lambda plac5. J Bacteriol. 1991 Feb;173(3):1027–1034. doi: 10.1128/jb.173.3.1027-1034.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. W., Merrill B. M., Williams K. R. F sex factor encodes a single-stranded DNA binding protein (SSB) with extensive sequence homology to Escherichia coli SSB. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5480–5484. doi: 10.1073/pnas.80.18.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield L. K., Orr E., Boulnois G. J., Wilkins B. M. DNA primase of plasmid ColIb is involved in conjugal DnA synthesis in donor and recipient bacteria. J Bacteriol. 1982 Dec;152(3):1188–1195. doi: 10.1128/jb.152.3.1188-1195.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah K. C., Skurray R. The F plasmid carries an IS3 insertion within finO. J Gen Microbiol. 1986 Dec;132(12):3269–3275. doi: 10.1099/00221287-132-12-3269. [DOI] [PubMed] [Google Scholar]
- Close S. M., Kado C. I. A gene near the plasmid pSa origin of replication encodes a nuclease. Mol Microbiol. 1992 Feb;6(4):521–527. doi: 10.1111/j.1365-2958.1992.tb01497.x. [DOI] [PubMed] [Google Scholar]
- Cook D. M., Farrand S. K. The oriT region of the Agrobacterium tumefaciens Ti plasmid pTiC58 shares DNA sequence identity with the transfer origins of RSF1010 and RK2/RP4 and with T-region borders. J Bacteriol. 1992 Oct;174(19):6238–6246. doi: 10.1128/jb.174.19.6238-6246.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton F. A., Hazen E. E., Jr, Legg M. J. Staphylococcal nuclease: proposed mechanism of action based on structure of enzyme-thymidine 3',5'-bisphosphate-calcium ion complex at 1.5-A resolution. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2551–2555. doi: 10.1073/pnas.76.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cram D. S., Loh S. M., Cheah K. C., Skurray R. A. Sequence and conservation of genes at the distal end of the transfer region on plasmids F and R6-5. Gene. 1991 Jul 31;104(1):85–90. doi: 10.1016/0378-1119(91)90469-r. [DOI] [PubMed] [Google Scholar]
- Cram D., Ray A., O'Gorman L., Skurray R. Transcriptional analysis of the leading region in F plasmid DNA transfer. Plasmid. 1984 May;11(3):221–233. doi: 10.1016/0147-619x(84)90028-3. [DOI] [PubMed] [Google Scholar]
- Crothers D. M., Haran T. E., Nadeau J. G. Intrinsically bent DNA. J Biol Chem. 1990 May 5;265(13):7093–7096. [PubMed] [Google Scholar]
- Cuozzo M., Silverman P. M. Characterization of the F plasmid TraJ protein synthesized in F' and Hfr strains of Escherichia coli K-12. J Biol Chem. 1986 Apr 15;261(11):5175–5179. [PubMed] [Google Scholar]
- Cuozzo M., Silverman P. M., Minkley E. G., Jr Overproduction in Escherichia coli K-12 and purification of the TraJ protein encoded by the conjugative plasmid F. J Biol Chem. 1984 May 25;259(10):6659–6666. [PubMed] [Google Scholar]
- Dash P. K., Traxler B. A., Panicker M. M., Hackney D. D., Minkley E. G., Jr Biochemical characterization of Escherichia coli DNA helicase I. Mol Microbiol. 1992 May;6(9):1163–1172. doi: 10.1111/j.1365-2958.1992.tb01555.x. [DOI] [PubMed] [Google Scholar]
- Dempsey W. B., Fee B. E. Integration host factor affects expression of two genes at the conjugal transfer origin of plasmid R100. Mol Microbiol. 1990 Jun;4(6):1019–1028. doi: 10.1111/j.1365-2958.1990.tb00674.x. [DOI] [PubMed] [Google Scholar]
- Dempsey W. B. Integration host factor and conjugative transfer of the antibiotic resistance plasmid R100. J Bacteriol. 1987 Sep;169(9):4391–4392. doi: 10.1128/jb.169.9.4391-4392.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey W. B. Sense and antisense transcripts of traM, a conjugal transfer gene of the antibiotic resistance plasmid R100. Mol Microbiol. 1989 Apr;3(4):561–570. doi: 10.1111/j.1365-2958.1989.tb00202.x. [DOI] [PubMed] [Google Scholar]
- Dempsey W. B. Transcript analysis of the plasmid R100 traJ and finP genes. Mol Gen Genet. 1987 Oct;209(3):533–544. doi: 10.1007/BF00331160. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Laurenzio L., Frost L. S., Finlay B. B., Paranchych W. Characterization of the oriT region of the IncFV plasmid pED208. Mol Microbiol. 1991 Jul;5(7):1779–1790. doi: 10.1111/j.1365-2958.1991.tb01927.x. [DOI] [PubMed] [Google Scholar]
- Di Laurenzio L., Frost L. S., Paranchych W. The TraM protein of the conjugative plasmid F binds to the origin of transfer of the F and ColE1 plasmids. Mol Microbiol. 1992 Oct;6(20):2951–2959. doi: 10.1111/j.1365-2958.1992.tb01754.x. [DOI] [PubMed] [Google Scholar]
- Drolet M., Zanga P., Lau P. C. The mobilization and origin of transfer regions of a Thiobacillus ferrooxidans plasmid: relatedness to plasmids RSF1010 and pSC101. Mol Microbiol. 1990 Aug;4(8):1381–1391. doi: 10.1111/j.1365-2958.1990.tb00717.x. [DOI] [PubMed] [Google Scholar]
- Dutreix M., Bäckman A., Célérier J., Bagdasarian M. M., Sommer S., Bailone A., Devoret R., Bagdasarian M. Identification of psiB genes of plasmids F and R6-5. Molecular basis for psiB enhanced expression in plasmid R6-5. Nucleic Acids Res. 1988 Nov 25;16(22):10669–10679. doi: 10.1093/nar/16.22.10669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürrenberger M. B., Villiger W., Bächi T. Conjugational junctions: morphology of specific contacts in conjugating Escherichia coli bacteria. J Struct Biol. 1991 Oct;107(2):146–156. doi: 10.1016/1047-8477(91)90018-r. [DOI] [PubMed] [Google Scholar]
- Ebright R. H., Cossart P., Gicquel-Sanzey B., Beckwith J. Mutations that alter the DNA sequence specificity of the catabolite gene activator protein of E. coli. Nature. 1984 Sep 20;311(5983):232–235. doi: 10.1038/311232a0. [DOI] [PubMed] [Google Scholar]
- Eguchi Y., Itoh T., Tomizawa J. Antisense RNA. Annu Rev Biochem. 1991;60:631–652. doi: 10.1146/annurev.bi.60.070191.003215. [DOI] [PubMed] [Google Scholar]
- Everett R., Willetts N. Characterisation of an in vivo system for nicking at the origin of conjugal DNA transfer of the sex factor F. J Mol Biol. 1980 Jan 15;136(2):129–150. doi: 10.1016/0022-2836(80)90309-5. [DOI] [PubMed] [Google Scholar]
- Fee B. E., Dempsey W. B. Cloning, mapping, and sequencing of plasmid R100 traM and finP genes. J Bacteriol. 1986 Jul;167(1):336–345. doi: 10.1128/jb.167.1.336-345.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee B. E., Dempsey W. B. Nucleotide sequence of gene X of antibiotic resistance plasmid R100. Nucleic Acids Res. 1988 May 25;16(10):4726–4726. doi: 10.1093/nar/16.10.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Frost L. S., Paranchych W. Localization, cloning, and sequence determination of the conjugative plasmid ColB2 pilin gene. J Bacteriol. 1984 Oct;160(1):402–407. doi: 10.1128/jb.160.1.402-407.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Frost L. S., Paranchych W. Nucleotide sequence of the tra YALE region from IncFV plasmid pED208. J Bacteriol. 1986 Nov;168(2):990–998. doi: 10.1128/jb.168.2.990-998.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Frost L. S., Paranchych W. Origin of transfer of IncF plasmids and nucleotide sequences of the type II oriT, traM, and traY alleles from ColB4-K98 and the type IV traY allele from R100-1. J Bacteriol. 1986 Oct;168(1):132–139. doi: 10.1128/jb.168.1.132-139.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Frost L. S., Paranchych W., Parker J. M., Hodges R. S. Major antigenic determinants of F and ColB2 pili. J Bacteriol. 1985 Jul;163(1):331–335. doi: 10.1128/jb.163.1.331-335.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Frost L. S., Paranchych W., Willetts N. S. Nucleotide sequences of five IncF plasmid finP alleles. J Bacteriol. 1986 Aug;167(2):754–757. doi: 10.1128/jb.167.2.754-757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Paranchych W. Nucleotide sequence of the surface exclusion genes traS and traT from the IncF0 lac plasmid pED208. J Bacteriol. 1986 Jun;166(3):713–721. doi: 10.1128/jb.166.3.713-721.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan D. J., Willetts N. S. Two classes of Flac mutants insensitive to transfer inhibition by an F-like R factor. Mol Gen Genet. 1971;111(3):256–264. doi: 10.1007/BF00433110. [DOI] [PubMed] [Google Scholar]
- Finnegan D., Willetts N. The site of action of the F transfer inhibitor. Mol Gen Genet. 1973 Dec 31;127(4):307–316. doi: 10.1007/BF00267101. [DOI] [PubMed] [Google Scholar]
- Firth N., Skurray R. Characterization of the F plasmid bifunctional conjugation gene, traG. Mol Gen Genet. 1992 Mar;232(1):145–153. doi: 10.1007/BF00299147. [DOI] [PubMed] [Google Scholar]
- Folkhard W., Leonard K. R., Malsey S., Marvin D. A., Dubochet J., Engel A., Achtman M., Helmuth R. X-ray diffraction and electron microscope studies on the structure of bacterial F pili. J Mol Biol. 1979 May 15;130(2):145–160. doi: 10.1016/0022-2836(79)90423-6. [DOI] [PubMed] [Google Scholar]
- Fowler T., Taylor L., Thompson R. The control region of the F plasmid transfer operon: DNA sequence of the traJ and traY genes and characterisation of the traY leads to Z promoter. Gene. 1983 Dec;26(1):79–89. doi: 10.1016/0378-1119(83)90038-0. [DOI] [PubMed] [Google Scholar]
- Fowler T., Thompson R. Shadow promoters in the F plasmid transfer operon. Mol Gen Genet. 1986 Mar;202(3):509–511. doi: 10.1007/BF00333285. [DOI] [PubMed] [Google Scholar]
- Frost L. S., Armstrong G. D., Finlay B. B., Edwards B. F., Paranchych W. N-terminal amino acid sequencing of EDP208 conjugative pili. J Bacteriol. 1983 Feb;153(2):950–954. doi: 10.1128/jb.153.2.950-954.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost L. S., Bazett-Jones D. P. Examination of the phosphate in conjugative F-like pili by use of electron spectroscopic imaging. J Bacteriol. 1991 Dec;173(23):7728–7731. doi: 10.1128/jb.173.23.7728-7731.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost L. S., Finlay B. B., Opgenorth A., Paranchych W., Lee J. S. Characterization and sequence analysis of pilin from F-like plasmids. J Bacteriol. 1985 Dec;164(3):1238–1247. doi: 10.1128/jb.164.3.1238-1247.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost L. S., Lee J. S., Scraba D. G., Paranchych W. Two monoclonal antibodies specific for different epitopes within the amino-terminal region of F pilin. J Bacteriol. 1986 Oct;168(1):192–198. doi: 10.1128/jb.168.1.192-198.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost L. S., Paranchych W. DNA sequence analysis of point mutations in traA, the F pilin gene, reveal two domains involved in F-specific bacteriophage attachment. Mol Gen Genet. 1988 Jul;213(1):134–139. doi: 10.1007/BF00333409. [DOI] [PubMed] [Google Scholar]
- Frost L. S., Paranchych W., Willetts N. S. DNA sequence of the F traALE region that includes the gene for F pilin. J Bacteriol. 1984 Oct;160(1):395–401. doi: 10.1128/jb.160.1.395-401.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost L., Lee S., Yanchar N., Paranchych W. finP and fisO mutations in FinP anti-sense RNA suggest a model for FinOP action in the repression of bacterial conjugation by the Flac plasmid JCFL0. Mol Gen Genet. 1989 Jul;218(1):152–160. doi: 10.1007/BF00330578. [DOI] [PubMed] [Google Scholar]
- Fu Y. H., Tsai M. M., Luo Y. N., Deonier R. C. Deletion analysis of the F plasmid oriT locus. J Bacteriol. 1991 Feb;173(3):1012–1020. doi: 10.1128/jb.173.3.1012-1020.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya N., Komano T. Determination of the nick site at oriT of IncI1 plasmid R64: global similarity of oriT structures of IncI1 and IncP plasmids. J Bacteriol. 1991 Oct;173(20):6612–6617. doi: 10.1128/jb.173.20.6612-6617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney D., Skurray R., Willetts N. Regulation of the F conjugation genes studied by hybridization and tra-lacZ fusion. J Mol Biol. 1983 Jul 25;168(1):103–122. doi: 10.1016/s0022-2836(83)80325-8. [DOI] [PubMed] [Google Scholar]
- Gamas P., Caro L., Galas D., Chandler M. Expression of F transfer functions depends on the Escherichia coli integration host factor. Mol Gen Genet. 1987 May;207(2-3):302–305. doi: 10.1007/BF00331593. [DOI] [PubMed] [Google Scholar]
- Gao Q., Luo Y., Deonier R. C. Initiation and termination of DNA transfer at F plasmid oriT. Mol Microbiol. 1994 Feb;11(3):449–458. doi: 10.1111/j.1365-2958.1994.tb00326.x. [DOI] [PubMed] [Google Scholar]
- Gaudin H. M., Silverman P. M. Contributions of promoter context and structure to regulated expression of the F plasmid traY promoter in Escherichia coli K-12. Mol Microbiol. 1993 Apr;8(2):335–342. doi: 10.1111/j.1365-2958.1993.tb01577.x. [DOI] [PubMed] [Google Scholar]
- Gerdes K., Nielsen A., Thorsted P., Wagner E. G. Mechanism of killer gene activation. Antisense RNA-dependent RNase III cleavage ensures rapid turn-over of the stable hok, srnB and pndA effector messenger RNAs. J Mol Biol. 1992 Aug 5;226(3):637–649. doi: 10.1016/0022-2836(92)90621-p. [DOI] [PubMed] [Google Scholar]
- Gerdes K., Thisted T., Martinussen J. Mechanism of post-segregational killing by the hok/sok system of plasmid R1: sok antisense RNA regulates formation of a hok mRNA species correlated with killing of plasmid-free cells. Mol Microbiol. 1990 Nov;4(11):1807–1818. doi: 10.1111/j.1365-2958.1990.tb02029.x. [DOI] [PubMed] [Google Scholar]
- Gerlitz M., Hrabak O., Schwab H. Partitioning of broad-host-range plasmid RP4 is a complex system involving site-specific recombination. J Bacteriol. 1990 Nov;172(11):6194–6203. doi: 10.1128/jb.172.11.6194-6203.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub E. I., Low K. B. Unrelated conjugative plasmids have sequences which are homologous to the leading region of the F factor. J Bacteriol. 1986 May;166(2):670–672. doi: 10.1128/jb.166.2.670-672.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub E. I., Panzer H. A. The F factor of Escherichia coli carries a locus of stable plasmid inheritance stm, similar to the parB locus of plasmid RI. Mol Gen Genet. 1988 Oct;214(2):353–357. doi: 10.1007/BF00337735. [DOI] [PubMed] [Google Scholar]
- Golub E., Bailone A., Devoret R. A gene encoding an SOS inhibitor is present in different conjugative plasmids. J Bacteriol. 1988 Sep;170(9):4392–4394. doi: 10.1128/jb.170.9.4392-4394.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus-Göldner A., Graus H., Schlacher T., Högenauer G. The sequences of genes bordering oriT in the enterotoxin plasmid P307: comparison with the sequences of plasmids F and R1. Plasmid. 1990 Sep;24(2):119–131. doi: 10.1016/0147-619x(90)90014-4. [DOI] [PubMed] [Google Scholar]
- Graus H., Hödl A., Wallner P., Högenauer G. The sequence of the leading region of the resistance plasmid R1. Nucleic Acids Res. 1990 Feb 25;18(4):1046–1046. doi: 10.1093/nar/18.4.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman T. H., Frost L. S., Silverman P. M. Structure and function of conjugative pili: monoclonal antibodies as probes for structural variants of F pili. J Bacteriol. 1990 Mar;172(3):1174–1179. doi: 10.1128/jb.172.3.1174-1179.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman T. H., Silverman P. M. Structure and function of conjugative pili: inducible synthesis of functional F pili by Escherichia coli K-12 containing a lac-tra operon fusion. J Bacteriol. 1989 Feb;171(2):650–656. doi: 10.1128/jb.171.2.650-656.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiney D. G. Host range of conjugation and replication functions of the Escherichia coli sex plasmid Flac. Comparison with the broad host-range plasmid RK2. J Mol Biol. 1982 Dec 15;162(3):699–703. doi: 10.1016/0022-2836(82)90397-7. [DOI] [PubMed] [Google Scholar]
- Guyer M. S., Reed R. R., Steitz J. A., Low K. B. Identification of a sex-factor-affinity site in E. coli as gamma delta. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):135–140. doi: 10.1101/sqb.1981.045.01.022. [DOI] [PubMed] [Google Scholar]
- Ham L. M., Cram D., Skurray R. Transcriptional analysis of the F plasmid surface exclusion region: mapping of traS, traT, and traD transcripts. Plasmid. 1989 Jan;21(1):1–8. doi: 10.1016/0147-619x(89)90081-4. [DOI] [PubMed] [Google Scholar]
- Ham L. M., Firth N., Skurray R. Nucleotide sequence of the F plasmid transfer gene, traH: identification of a new gene and a promoter within the transfer operon. Gene. 1989 Jan 30;75(1):157–165. doi: 10.1016/0378-1119(89)90392-2. [DOI] [PubMed] [Google Scholar]
- Harrington L. C., Rogerson A. C. The F pilus of Escherichia coli appears to support stable DNA transfer in the absence of wall-to-wall contact between cells. J Bacteriol. 1990 Dec;172(12):7263–7264. doi: 10.1128/jb.172.12.7263-7264.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J. L., Taylor I. M., Platt K., O'Connor C. D. Surface exclusion specificity of the TraT lipoprotein is determined by single alterations in a five-amino-acid region of the protein. Mol Microbiol. 1992 Oct;6(19):2825–2832. doi: 10.1111/j.1365-2958.1992.tb01462.x. [DOI] [PubMed] [Google Scholar]
- Harwood C. R., Meynell E. Cyclic AMP and the production of sex pili by E. coli K-12 carrying derepressed sex factors. Nature. 1975 Apr 17;254(5501):628–660. doi: 10.1038/254628a0. [DOI] [PubMed] [Google Scholar]
- Havekes L. M., Lugtenberg B. J., Hoekstra W. P. Conjugation deficient E. coli K12 F- mutants with heptose-less lipopolysaccharide. Mol Gen Genet. 1976 Jul 5;146(1):43–50. doi: 10.1007/BF00267981. [DOI] [PubMed] [Google Scholar]
- Heinemann J. A., Sprague G. F., Jr Bacterial conjugative plasmids mobilize DNA transfer between bacteria and yeast. Nature. 1989 Jul 20;340(6230):205–209. doi: 10.1038/340205a0. [DOI] [PubMed] [Google Scholar]
- Helmuth R., Achtman M. Operon structure of DNA transfer cistrons on the F sex factor. Nature. 1975 Oct 23;257(5528):652–656. doi: 10.1038/257652a0. [DOI] [PubMed] [Google Scholar]
- Howland C. J., Rees C. E., Barth P. T., Wilkins B. M. The ssb gene of plasmid ColIb-P9. J Bacteriol. 1989 May;171(5):2466–2473. doi: 10.1128/jb.171.5.2466-2473.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihler G., Rupp W. D. Strand-specific transfer of donor DNA during conjugation in E. coli. Proc Natl Acad Sci U S A. 1969 May;63(1):138–143. doi: 10.1073/pnas.63.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamoto S., Ohtsubo E. Specific binding of the TraY protein to oriT and the promoter region for the traY gene of plasmid R100. J Biol Chem. 1990 Apr 15;265(11):6461–6466. [PubMed] [Google Scholar]
- Inamoto S., Yoshioka Y., Ohtsubo E. Identification and characterization of the products from the traJ and traY genes of plasmid R100. J Bacteriol. 1988 Jun;170(6):2749–2757. doi: 10.1128/jb.170.6.2749-2757.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamoto S., Yoshioka Y., Ohtsubo E. Site- and strand-specific nicking in vitro at oriT by the traY-traI endonuclease of plasmid R100. J Biol Chem. 1991 Jun 5;266(16):10086–10092. [PubMed] [Google Scholar]
- Ippen-Ihler K., Moore D., Laine S., Johnson D. A., Willetts N. S. Synthesis of F-pilin polypeptide in the absence of F traJ product. Plasmid. 1984 Mar;11(2):116–129. doi: 10.1016/0147-619x(84)90017-9. [DOI] [PubMed] [Google Scholar]
- Iuchi S., Lin E. C. arcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1888–1892. doi: 10.1073/pnas.85.6.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R., Cram D., Ray A., DiBerardino D., Skurray R. Cloning and analysis of pif, replication and leading regions of the F plasmid. Mol Gen Genet. 1984;197(1):129–136. doi: 10.1007/BF00327933. [DOI] [PubMed] [Google Scholar]
- Jalajakumari M. B., Guidolin A., Buhk H. J., Manning P. A., Ham L. M., Hodgson A. L., Cheah K. C., Skurray R. A. Surface exclusion genes traS and traT of the F sex factor of Escherichia coli K-12. Determination of the nucleotide sequence and promoter and terminator activities. J Mol Biol. 1987 Nov 5;198(1):1–11. doi: 10.1016/0022-2836(87)90452-9. [DOI] [PubMed] [Google Scholar]
- Jalajakumari M. B., Manning P. A. Nucleotide sequence of the traD region in the Escherichia coli F sex factor. Gene. 1989 Sep 30;81(2):195–202. doi: 10.1016/0378-1119(89)90179-0. [DOI] [PubMed] [Google Scholar]
- Jones A. L., Barth P. T., Wilkins B. M. Zygotic induction of plasmid ssb and psiB genes following conjugative transfer of Incl1 plasmid Collb-P9. Mol Microbiol. 1992 Mar;6(5):605–613. doi: 10.1111/j.1365-2958.1992.tb01507.x. [DOI] [PubMed] [Google Scholar]
- Kathir P., Ippen-Ihler K. Construction and characterization of derivatives carrying insertion mutations in F plasmid transfer region genes, trbA, artA, traQ, and trbB. Plasmid. 1991 Jul;26(1):40–54. doi: 10.1016/0147-619x(91)90035-u. [DOI] [PubMed] [Google Scholar]
- Kingsman A., Willetts N. The requirements for conjugal DNA synthesis in the donor strain during flac transfer. J Mol Biol. 1978 Jul 5;122(3):287–300. doi: 10.1016/0022-2836(78)90191-2. [DOI] [PubMed] [Google Scholar]
- Kolodkin A. L., Capage M. A., Golub E. I., Low K. B. F sex factor of Escherichia coli K-12 codes for a single-stranded DNA binding protein. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4422–4426. doi: 10.1073/pnas.80.14.4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koraimann G., Högenauer G. A stable core region of the tra operon mRNA of plasmid R1-19. Nucleic Acids Res. 1989 Feb 25;17(4):1283–1298. doi: 10.1093/nar/17.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koraimann G., Koraimann C., Koronakis V., Schlager S., Högenauer G. Repression and derepression of conjugation of plasmid R1 by wild-type and mutated finP antisense RNA. Mol Microbiol. 1991 Jan;5(1):77–87. doi: 10.1111/j.1365-2958.1991.tb01828.x. [DOI] [PubMed] [Google Scholar]
- Koraimann G., Schroller C., Graus H., Angerer D., Teferle K., Högenauer G. Expression of gene 19 of the conjugative plasmid R1 is controlled by RNase III. Mol Microbiol. 1993 Aug;9(4):717–727. doi: 10.1111/j.1365-2958.1993.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Koronakis V. E., Bauer E., Högenauer G. The traM gene of the resistance plasmid R1: comparison with the corresponding sequence of the Escherichia coli F factor. Gene. 1985;36(1-2):79–86. doi: 10.1016/0378-1119(85)90071-x. [DOI] [PubMed] [Google Scholar]
- Koronakis V., Högenauer G. The sequences of the traJ gene and the 5' end of the traY gene of the resistance plasmid R1. Mol Gen Genet. 1986 Apr;203(1):137–142. doi: 10.1007/BF00330394. [DOI] [PubMed] [Google Scholar]
- Lahue E. E., Matson S. W. Purified Escherichia coli F-factor TraY protein binds oriT. J Bacteriol. 1990 Mar;172(3):1385–1391. doi: 10.1128/jb.172.3.1385-1391.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine S., Moore D., Kathir P., Ippen-Ihler K. Genes and gene products involved in the synthesis of F-pili. Basic Life Sci. 1985;30:535–553. doi: 10.1007/978-1-4613-2447-8_38. [DOI] [PubMed] [Google Scholar]
- Lanka E., Barth P. T. Plasmid RP4 specifies a deoxyribonucleic acid primase involved in its conjugal transfer and maintenance. J Bacteriol. 1981 Dec;148(3):769–781. doi: 10.1128/jb.148.3.769-781.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn A. M., Meynell E., Cooke M. Mixed infections with bacterial sex factors: sex pili of pure and mixed phenotype. Ann Inst Pasteur (Paris) 1971 Jan;120(1):3–8. [PubMed] [Google Scholar]
- Lawn A. M., Meynell E. Serotypes of sex pili. J Hyg (Lond) 1970 Dec;68(4):683–694. doi: 10.1017/s0022172400042625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H., Frost L. S., Paranchych W. FinOP repression of the F plasmid involves extension of the half-life of FinP antisense RNA by FinO. Mol Gen Genet. 1992 Oct;235(1):131–139. doi: 10.1007/BF00286190. [DOI] [PubMed] [Google Scholar]
- Lerner T. J., Zinder N. D. Chromosomal regulation of sexual expression in Escherichia coli. J Bacteriol. 1979 Feb;137(2):1063–1065. doi: 10.1128/jb.137.2.1063-1065.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessl M., Balzer D., Pansegrau W., Lanka E. Sequence similarities between the RP4 Tra2 and the Ti VirB region strongly support the conjugation model for T-DNA transfer. J Biol Chem. 1992 Oct 5;267(28):20471–20480. [PubMed] [Google Scholar]
- Lessl M., Pansegrau W., Lanka E. Relationship of DNA-transfer-systems: essential transfer factors of plasmids RP4, Ti and F share common sequences. Nucleic Acids Res. 1992 Nov 25;20(22):6099–6100. doi: 10.1093/nar/20.22.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B. Very stable prokaryotic messenger RNA in chromosomeless Escherichia coli minicells. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2900–2904. doi: 10.1073/pnas.72.8.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llosa M., Bolland S., de la Cruz F. Structural and functional analysis of the origin of conjugal transfer of the broad-host-range IncW plasmid R388 and comparison with the related IncN plasmid R46. Mol Gen Genet. 1991 May;226(3):473–483. doi: 10.1007/BF00260661. [DOI] [PubMed] [Google Scholar]
- Loh S., Cram D., Skurray R. Nucleotide sequence of the leading region adjacent to the origin of transfer on plasmid F and its conservation among conjugative plasmids. Mol Gen Genet. 1989 Oct;219(1-2):177–186. doi: 10.1007/BF00261174. [DOI] [PubMed] [Google Scholar]
- Loh S., Skurray R., Célérier J., Bagdasarian M., Bailone A., Devoret R. Nucleotide sequence of the psiA (plasmid SOS inhibition) gene located on the leading region of plasmids F and R6-5. Nucleic Acids Res. 1990 Aug 11;18(15):4597–4597. doi: 10.1093/nar/18.15.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Gao Q., Deonier R. C. Mutational and physical analysis of F plasmid traY protein binding to oriT. Mol Microbiol. 1994 Feb;11(3):459–469. doi: 10.1111/j.1365-2958.1994.tb00327.x. [DOI] [PubMed] [Google Scholar]
- López J., Salazar L., Andrés I., Ortiz J. M., Rodríguez J. C. Nucleotide sequence of the oriT-traM-finP region of the haemolytic plasmid pSU316: comparison to F. Nucleic Acids Res. 1991 Jun 25;19(12):3451–3451. doi: 10.1093/nar/19.12.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneewannakul K., Ippen-Ihler K. Construction and analysis of F plasmid traR, trbJ, and trbH mutants. J Bacteriol. 1993 Mar;175(5):1528–1531. doi: 10.1128/jb.175.5.1528-1531.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneewannakul K., Maneewannakul S., Ippen-Ihler K. Synthesis of F pilin. J Bacteriol. 1993 Mar;175(5):1384–1391. doi: 10.1128/jb.175.5.1384-1391.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneewannakul S., Kathir P., Ippen-Ihler K. Characterization of the F plasmid mating aggregation gene traN and of a new F transfer region locus trbE. J Mol Biol. 1992 May 20;225(2):299–311. doi: 10.1016/0022-2836(92)90923-8. [DOI] [PubMed] [Google Scholar]
- Maneewannakul S., Kathir P., Moore D., Le L. A., Wu J. H., Ippen-Ihler K. Location of F plasmid transfer operon genes traC and traW and identification of the traW product. J Bacteriol. 1987 Nov;169(11):5119–5124. doi: 10.1128/jb.169.11.5119-5124.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneewannakul S., Maneewannakul K., Ippen-Ihler K. Characterization of trbC, a new F plasmid tra operon gene that is essential to conjugative transfer. J Bacteriol. 1991 Jun;173(12):3872–3878. doi: 10.1128/jb.173.12.3872-3878.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneewannakul S., Maneewannakul K., Ippen-Ihler K. Characterization, localization, and sequence of F transfer region products: the pilus assembly gene product TraW and a new product, TrbI. J Bacteriol. 1992 Sep;174(17):5567–5574. doi: 10.1128/jb.174.17.5567-5574.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning P. A., Beutin L., Achtman M. Outer membrane of Escherichia coli: properties of the F sex factor traT protein which is involved in surface exclusion. J Bacteriol. 1980 Apr;142(1):285–294. doi: 10.1128/jb.142.1.285-294.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning P. A., Kusecek B., Morelli G., Fisseau C., Achtman M. Analysis of the promoter-distal region of the tra operon of the F sex factor of Escherichia coli K-12 encoded by EcoRI restriction fragments f17, f19, and f2. J Bacteriol. 1982 Apr;150(1):76–88. doi: 10.1128/jb.150.1.76-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning P. A., Morelli G., Achtman M. traG protein of the F sex factor of Escherichia coli K-12 and its role in conjugation. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7487–7491. doi: 10.1073/pnas.78.12.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C. A genetic approach to defining the sites of interaction of a membrane protein with different external agents. J Mol Biol. 1983 Sep 15;169(2):507–519. doi: 10.1016/s0022-2836(83)80063-1. [DOI] [PubMed] [Google Scholar]
- Manoil C., Rosenbusch J. P. Conjugation-deficient mutants of Escherichia coli distinguish classes of functions of the outer membrane OmpA protein. Mol Gen Genet. 1982;187(1):148–156. doi: 10.1007/BF00384398. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Folkhard W. Structure of F-pili: reassessment of the symmetry. J Mol Biol. 1986 Sep 20;191(2):299–300. doi: 10.1016/0022-2836(86)90267-6. [DOI] [PubMed] [Google Scholar]
- Masai H., Nomura N., Kubota Y., Arai K. Roles of phi X174 type primosome- and G4 type primase-dependent primings in initiation of lagging and leading strand syntheses of DNA replication. J Biol Chem. 1990 Sep 5;265(25):15124–15133. [PubMed] [Google Scholar]
- Matson S. W., Morton B. S. Escherichia coli DNA helicase I catalyzes a site- and strand-specific nicking reaction at the F plasmid oriT. J Biol Chem. 1991 Aug 25;266(24):16232–16237. [PubMed] [Google Scholar]
- Matson S. W., Nelson W. C., Morton B. S. Characterization of the reaction product of the oriT nicking reaction catalyzed by Escherichia coli DNA helicase I. J Bacteriol. 1993 May;175(9):2599–2606. doi: 10.1128/jb.175.9.2599-2606.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazodier P., Davies J. Gene transfer between distantly related bacteria. Annu Rev Genet. 1991;25:147–171. doi: 10.1146/annurev.ge.25.120191.001051. [DOI] [PubMed] [Google Scholar]
- McIntire S. A., Dempsey W. B. Fertility inhibition gene of plasmid R100. Nucleic Acids Res. 1987 Mar 11;15(5):2029–2042. doi: 10.1093/nar/15.5.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Ewins A. Effect on exclusion of alterations to the sex pilus. J Bacteriol. 1973 Jan;113(1):71–75. doi: 10.1128/jb.113.1.71-75.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Matthews R. A., Lawn A. M. Antigenic differences between the ends and shafts of sex pili. J Gen Microbiol. 1974 May;82(1):203–205. doi: 10.1099/00221287-82-1-203. [DOI] [PubMed] [Google Scholar]
- Meynell E., Meynell G. G., Datta N. Phylogenetic relationships of drug-resistance factors and other transmissible bacterial plasmids. Bacteriol Rev. 1968 Mar;32(1):55–83. doi: 10.1128/br.32.1.55-83.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T., Horiuchi T., Willetts N. S. Identification and characterization of four new tra cistrons on the E. coli K12 sex factor F. Plasmid. 1978 Jun;1(3):316–323. doi: 10.1016/0147-619x(78)90048-3. [DOI] [PubMed] [Google Scholar]
- Minkley E. G., Jr, Ippen-Ihler K. Identification of a membrane protein associated with expression of the surface exclusion region of the F transfer operon. J Bacteriol. 1977 Mar;129(3):1613–1622. doi: 10.1128/jb.129.3.1613-1622.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkley E. G., Jr, Polen S., Brinton C. C., Jr, Ippen-Ihler K. Identification of the structural gene for F-pilin. J Mol Biol. 1976 Nov;108(1):111–121. doi: 10.1016/s0022-2836(76)80098-8. [DOI] [PubMed] [Google Scholar]
- Minkley E. G., Jr Purification and characterization of pro-TraTp, the signal sequence-containing precursor of a secreted protein encoded by the F sex factor. J Bacteriol. 1984 May;158(2):464–473. doi: 10.1128/jb.158.2.464-473.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkley E. G., Jr, Willetts N. S. Overproduction, purification and characterization of the F traT protein. Mol Gen Genet. 1984;196(2):225–235. doi: 10.1007/BF00328054. [DOI] [PubMed] [Google Scholar]
- Moore D., Hamilton C. M., Maneewannakul K., Mintz Y., Frost L. S., Ippen-Ihler K. The Escherichia coli K-12 F plasmid gene traX is required for acetylation of F pilin. J Bacteriol. 1993 Mar;175(5):1375–1383. doi: 10.1128/jb.175.5.1375-1383.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D., Maneewannakul K., Maneewannakul S., Wu J. H., Ippen-Ihler K., Bradley D. E. Characterization of the F-plasmid conjugative transfer gene traU. J Bacteriol. 1990 Aug;172(8):4263–4270. doi: 10.1128/jb.172.8.4263-4270.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D., Sowa B. A., Ippen-Ihler K. A new activity in the Ftra operon which is required for F-pilin synthesis. Mol Gen Genet. 1982;188(3):459–464. doi: 10.1007/BF00330049. [DOI] [PubMed] [Google Scholar]
- Moore D., Sowa B. A., Ippen-Ihler K. Location of an F-pilin pool in the inner membrane. J Bacteriol. 1981 Apr;146(1):251–259. doi: 10.1128/jb.146.1.251-259.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D., Sowa B. A., Ippen-Ihler K. The effect of tra mutations on the synthesis of the F-pilin membrane polypeptide. Mol Gen Genet. 1981;184(2):260–264. doi: 10.1007/BF00272914. [DOI] [PubMed] [Google Scholar]
- Moore D., Wu J. H., Kathir P., Hamilton C. M., Ippen-Ihler K. Analysis of transfer genes and gene products within the traB-traC region of the Escherichia coli fertility factor, F. J Bacteriol. 1987 Sep;169(9):3994–4002. doi: 10.1128/jb.169.9.3994-4002.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morona R., Klose M., Henning U. Escherichia coli K-12 outer membrane protein (OmpA) as a bacteriophage receptor: analysis of mutant genes expressing altered proteins. J Bacteriol. 1984 Aug;159(2):570–578. doi: 10.1128/jb.159.2.570-578.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullineaux P., Willetts N. Promoters in the transfer region of plasmid F. Basic Life Sci. 1985;30:605–614. doi: 10.1007/978-1-4613-2447-8_42. [DOI] [PubMed] [Google Scholar]
- Nelson W. C., Morton B. S., Lahue E. E., Matson S. W. Characterization of the Escherichia coli F factor traY gene product and its binding sites. J Bacteriol. 1993 Apr;175(8):2221–2228. doi: 10.1128/jb.175.8.2221-2228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura N., Masai H., Inuzuka M., Miyazaki C., Ohtsubo E., Itoh T., Sasamoto S., Matsui M., Ishizaki R., Arai K. Identification of eleven single-strand initiation sequences (ssi) for priming of DNA replication in the F, R6K, R100 and ColE2 plasmids. Gene. 1991 Dec 1;108(1):15–22. doi: 10.1016/0378-1119(91)90482-q. [DOI] [PubMed] [Google Scholar]
- Ogata R. T., Winters C., Levine R. P. Nucleotide sequence analysis of the complement resistance gene from plasmid R100. J Bacteriol. 1982 Aug;151(2):819–827. doi: 10.1128/jb.151.2.819-827.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi Y., Iguma H., Ono T., Nagaishi H., Clark A. J. Genetic mapping of the F plasmid gene that promotes degradation of stable ribonucleic acid in Escherichia coli. J Bacteriol. 1977 Dec;132(3):784–789. doi: 10.1128/jb.132.3.784-789.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. T., Anderson T. F. Role of pili in bacterial conjugation. J Bacteriol. 1970 Jun;102(3):648–654. doi: 10.1128/jb.102.3.648-654.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva W. D., Grossman T., Silverman P. M. Characterization of F-pilin as an inner membrane component of Escherichia coli K12. J Biol Chem. 1992 Dec 25;267(36):26191–26197. [PubMed] [Google Scholar]
- Panicker M. M., Minkley E. G., Jr DNA transfer occurs during a cell surface contact stage of F sex factor-mediated bacterial conjugation. J Bacteriol. 1985 May;162(2):584–590. doi: 10.1128/jb.162.2.584-590.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pansegrau W., Lanka E. Common sequence motifs in DNA relaxases and nick regions from a variety of DNA transfer systems. Nucleic Acids Res. 1991 Jun 25;19(12):3455–3455. doi: 10.1093/nar/19.12.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranchych W., Frost L. S. The physiology and biochemistry of pili. Adv Microb Physiol. 1988;29:53–114. doi: 10.1016/s0065-2911(08)60346-x. [DOI] [PubMed] [Google Scholar]
- Penfold S. S., Usher K., Frost L. S. The nature of the traK4 mutation in the F sex factor of Escherichia coli. J Bacteriol. 1994 Apr;176(7):1924–1931. doi: 10.1128/jb.176.7.1924-1931.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumal N. B., Minkley E. G., Jr The product of the F sex factor traT surface exclusion gene is a lipoprotein. J Biol Chem. 1984 May 10;259(9):5357–5360. [PubMed] [Google Scholar]
- Pradel E., Schnaitman C. A. Effect of rfaH (sfrB) and temperature on expression of rfa genes of Escherichia coli K-12. J Bacteriol. 1991 Oct;173(20):6428–6431. doi: 10.1128/jb.173.20.6428-6431.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi S. Y., Sukupolvi S., O'Connor C. D. Outer membrane permeability of Escherichia coli K12: isolation, cloning and mapping of suppressors of a defined antibiotic-hypersensitive mutant. Mol Gen Genet. 1991 Oct;229(3):421–427. doi: 10.1007/BF00267465. [DOI] [PubMed] [Google Scholar]
- Rashtchian A., Crooks J. H., Levy S. B. traJ independence in expression of traT on F. J Bacteriol. 1983 May;154(2):1009–1012. doi: 10.1128/jb.154.2.1009-1012.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A., Cheah K. C., Skurray R. An F-derived conjugative cosmid: analysis of tra polypeptides in cosmid-infected cells. Plasmid. 1986 Sep;16(2):90–100. doi: 10.1016/0147-619x(86)90067-3. [DOI] [PubMed] [Google Scholar]
- Ray A., Skurray R. Cloning and polypeptide analysis of the leading region in F plasmid DNA transfer. Plasmid. 1983 May;9(3):262–272. doi: 10.1016/0147-619x(83)90004-5. [DOI] [PubMed] [Google Scholar]
- Ray A., Skurray R. Stabilization of the cloning vector pACYC184 by insertion of F plasmid leading region sequences. Plasmid. 1984 May;11(3):272–275. doi: 10.1016/0147-619x(84)90036-2. [DOI] [PubMed] [Google Scholar]
- Rees C. E., Wilkins B. M. Protein transfer into the recipient cell during bacterial conjugation: studies with F and RP4. Mol Microbiol. 1990 Jul;4(7):1199–1205. doi: 10.1111/j.1365-2958.1990.tb00695.x. [DOI] [PubMed] [Google Scholar]
- Rees C. E., Wilkins B. M. Transfer of tra proteins into the recipient cell during bacterial conjugation mediated by plasmid ColIb-P9. J Bacteriol. 1989 Jun;171(6):3152–3157. doi: 10.1128/jb.171.6.3152-3157.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reygers U., Wessel R., Müller H., Hoffmann-Berling H. Endonuclease activity of Escherichia coli DNA helicase I directed against the transfer origin of the F factor. EMBO J. 1991 Sep;10(9):2689–2694. doi: 10.1002/j.1460-2075.1991.tb07812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. P. Preventing the synthesis of unused transcripts by Rho factor. Cell. 1991 Mar 22;64(6):1047–1049. doi: 10.1016/0092-8674(91)90257-y. [DOI] [PubMed] [Google Scholar]
- Riede I., Eschbach M. L. Evidence that TraT interacts with OmpA of Escherichia coli. FEBS Lett. 1986 Sep 15;205(2):241–245. doi: 10.1016/0014-5793(86)80905-x. [DOI] [PubMed] [Google Scholar]
- Rohrer J., Rawlings D. E. Sequence analysis and characterization of the mobilization region of a broad-host-range plasmid, pTF-FC2, isolated from Thiobacillus ferrooxidans. J Bacteriol. 1992 Oct;174(19):6230–6237. doi: 10.1128/jb.174.19.6230-6237.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar L., Lopéz J., Andrés I., Ortiz J. M., Rodríguez J. C. Characterization and nucleotide sequence of the oriT-traM-finP region of the IncFVII plasmid pSU233. Mol Gen Genet. 1992 Sep;234(3):442–448. doi: 10.1007/BF00538704. [DOI] [PubMed] [Google Scholar]
- Schandel K. A., Maneewannakul S., Ippen-Ihler K., Webster R. E. A traC mutant that retains sensitivity to f1 bacteriophage but lacks F pili. J Bacteriol. 1987 Jul;169(7):3151–3159. doi: 10.1128/jb.169.7.3151-3159.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schandel K. A., Maneewannakul S., Vonder Haar R. A., Ippen-Ihler K., Webster R. E. Nucleotide sequence of the F plasmid gene, traC, and identification of its product. Gene. 1990 Nov 30;96(1):137–140. doi: 10.1016/0378-1119(90)90354-t. [DOI] [PubMed] [Google Scholar]
- Schandel K. A., Muller M. M., Webster R. E. Localization of TraC, a protein involved in assembly of the F conjugative pilus. J Bacteriol. 1992 Jun;174(11):3800–3806. doi: 10.1128/jb.174.11.3800-3806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzinger E., Lurz R., Otto S., Dobrinski B. In vitro cleavage of double- and single-stranded DNA by plasmid RSF1010-encoded mobilization proteins. Nucleic Acids Res. 1992 Jan 11;20(1):41–48. doi: 10.1093/nar/20.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab M., Gruber H., Högenauer G. The TraM protein of plasmid R1 is a DNA-binding protein. Mol Microbiol. 1991 Feb;5(2):439–446. doi: 10.1111/j.1365-2958.1991.tb02127.x. [DOI] [PubMed] [Google Scholar]
- Schwab M., Reisenzein H., Högenauer G. TraM of plasmid R1 regulates its own expression. Mol Microbiol. 1993 Mar;7(5):795–803. doi: 10.1111/j.1365-2958.1993.tb01170.x. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Cohen S. N., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. II. Structure of drug resistance (R) factors and F factors. J Mol Biol. 1973 Apr 5;75(2):235–255. doi: 10.1016/0022-2836(73)90018-1. [DOI] [PubMed] [Google Scholar]
- Shirasu K., Kado C. I. Membrane location of the Ti plasmid VirB proteins involved in the biosynthesis of a pilin-like conjugative structure on Agrobacterium tumefaciens. FEMS Microbiol Lett. 1993 Aug 1;111(2-3):287–294. doi: 10.1111/j.1574-6968.1993.tb06400.x. [DOI] [PubMed] [Google Scholar]
- Shirasu K., Kado C. I. The virB operon of the Agrobacterium tumefaciens virulence regulon has sequence similarities to B, C and D open reading frames downstream of the pertussis toxin-operon and to the DNA transfer-operons of broad-host-range conjugative plasmids. Nucleic Acids Res. 1993 Jan 25;21(2):353–354. doi: 10.1093/nar/21.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman P. M., Wickersham E., Harris R. Regulation of the F plasmid traY promoter in Escherichia coli by host and plasmid factors. J Mol Biol. 1991 Mar 5;218(1):119–128. doi: 10.1016/0022-2836(91)90878-a. [DOI] [PubMed] [Google Scholar]
- Silverman P. M., Wickersham E., Rainwater S., Harris R. Regulation of the F plasmid traY promoter in Escherichia coli K12 as a function of sequence context. J Mol Biol. 1991 Jul 20;220(2):271–279. doi: 10.1016/0022-2836(91)90012-u. [DOI] [PubMed] [Google Scholar]
- Silverman P., Nat K., McEwen J., Birchman R. Selection of Escherichia coli K-12 chromosomal mutants that prevent expression of F-plasmid functions. J Bacteriol. 1980 Sep;143(3):1519–1523. doi: 10.1128/jb.143.3.1519-1523.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen L. Dynamics of plasmid transfer on surfaces. J Gen Microbiol. 1990 Jun;136(6):1001–1007. doi: 10.1099/00221287-136-6-1001. [DOI] [PubMed] [Google Scholar]
- Skurray R. A., Hancock R. E., Reeves P. Con--mutants: class of mutants in Escherichia coli K-12 lacking a major cell wall protein and defective in conjugation and adsorption of a bacteriophage. J Bacteriol. 1974 Sep;119(3):726–735. doi: 10.1128/jb.119.3.726-735.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers W. S., Phillips S. E. Crystal structure of the met repressor-operator complex at 2.8 A resolution reveals DNA recognition by beta-strands. Nature. 1992 Oct 1;359(6394):387–393. doi: 10.1038/359387a0. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukupolvi S., O'Connor C. D. TraT lipoprotein, a plasmid-specified mediator of interactions between gram-negative bacteria and their environment. Microbiol Rev. 1990 Dec;54(4):331–341. doi: 10.1128/mr.54.4.331-341.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Ohlendorf D. H., Anderson W. F., Matthews B. W. DNA-binding proteins. Science. 1983 Sep 9;221(4615):1020–1026. doi: 10.1126/science.6308768. [DOI] [PubMed] [Google Scholar]
- Thompson R., Taylor L., Kelly K., Everett R., Willetts N. The F plasmid origin of transfer: DNA sequence of wild-type and mutant origins and location of origin-specific nicks. EMBO J. 1984 May;3(5):1175–1180. doi: 10.1002/j.1460-2075.1984.tb01947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R., Taylor L. Promoter mapping and DNA sequencing of the F plasmid transfer genes traM and traJ. Mol Gen Genet. 1982;188(3):513–518. doi: 10.1007/BF00330058. [DOI] [PubMed] [Google Scholar]
- Thompson T. L., Centola M. B., Deonier R. C. Location of the nick at oriT of the F plasmid. J Mol Biol. 1989 Jun 5;207(3):505–512. doi: 10.1016/0022-2836(89)90460-9. [DOI] [PubMed] [Google Scholar]
- Traxler B. A., Minkley E. G., Jr Evidence that DNA helicase I and oriT site-specific nicking are both functions of the F TraI protein. J Mol Biol. 1988 Nov 5;204(1):205–209. doi: 10.1016/0022-2836(88)90609-2. [DOI] [PubMed] [Google Scholar]
- Traxler B. A., Minkley E. G., Jr Revised genetic map of the distal end of the F transfer operon: implications for DNA helicase I, nicking at oriT, and conjugal DNA transport. J Bacteriol. 1987 Jul;169(7):3251–3259. doi: 10.1128/jb.169.7.3251-3259.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai M. M., Fu Y. H., Deonier R. C. Intrinsic bends and integration host factor binding at F plasmid oriT. J Bacteriol. 1990 Aug;172(8):4603–4609. doi: 10.1128/jb.172.8.4603-4609.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber R. F., Silverman P. M. The cpx proteins of Escherichia coli K12. Structure of the cpxA polypeptide as an inner membrane component. J Mol Biol. 1988 Sep 20;203(2):467–478. doi: 10.1016/0022-2836(88)90013-7. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Johnson F. D., Burns D. L. Molecular characterization of an operon required for pertussis toxin secretion. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2970–2974. doi: 10.1073/pnas.90.7.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S. Characterization of the F transfer cistron, traL. Genet Res. 1973 Apr;21(2):205–213. doi: 10.1017/s0016672300013379. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Moore P. M., Paranchych W. Variant pili produced by mutants of the Flac plasmid. J Gen Microbiol. 1980 Apr;117(2):455–464. doi: 10.1099/00221287-117-2-455. [DOI] [PubMed] [Google Scholar]
- Willetts N. S. The kinetics of inhibition of Flac transfer by R100 in E. coli. Mol Gen Genet. 1974 Mar 14;129(2):123–130. doi: 10.1007/BF00268626. [DOI] [PubMed] [Google Scholar]
- Willetts N. Interactions between the F conjugal transfer system and CloDF13::Tna plasmids. Mol Gen Genet. 1980;180(1):213–217. doi: 10.1007/BF00267372. [DOI] [PubMed] [Google Scholar]
- Willetts N., Maule J., McIntire S. The genetic locations of traO, finP and tra-4 on the E. coli K12 sex factor F. Genet Res. 1975 Dec;26(3):255–263. doi: 10.1017/s0016672300016050. [DOI] [PubMed] [Google Scholar]
- Willetts N., Maule J. Specificities of IncF plasmid conjugation genes. Genet Res. 1986 Feb;47(1):1–11. doi: 10.1017/s0016672300024447. [DOI] [PubMed] [Google Scholar]
- Willetts N., Skurray R. The conjugation system of F-like plasmids. Annu Rev Genet. 1980;14:41–76. doi: 10.1146/annurev.ge.14.120180.000353. [DOI] [PubMed] [Google Scholar]
- Willetts N. The transcriptional control of fertility in F-like plasmids. J Mol Biol. 1977 May 5;112(1):141–148. doi: 10.1016/s0022-2836(77)80161-7. [DOI] [PubMed] [Google Scholar]
- Willetts N., Wilkins B. Processing of plasmid DNA during bacterial conjugation. Microbiol Rev. 1984 Mar;48(1):24–41. doi: 10.1128/mr.48.1.24-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worobec E. A., Frost L. S., Pieroni P., Armstrong G. D., Hodges R. S., Parker J. M., Finlay B. B., Paranchych W. Location of the antigenic determinants of conjugative F-like pili. J Bacteriol. 1986 Aug;167(2):660–665. doi: 10.1128/jb.167.2.660-665.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. H., Ippen-Ihler K. Nucleotide sequence of traQ and adjacent loci in the Escherichia coli K-12 F-plasmid transfer operon. J Bacteriol. 1989 Jan;171(1):213–221. doi: 10.1128/jb.171.1.213-221.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. H., Kathir P., Ippen-Ihler K. The product of the F plasmid transfer operon gene, traF, is a periplasmic protein. J Bacteriol. 1988 Aug;170(8):3633–3639. doi: 10.1128/jb.170.8.3633-3639.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. H., Moore D., Lee T., Ippen-Ihler K. Analysis of Escherichia coli K12 F factor transfer genes: traQ, trbA, and trbB. Plasmid. 1987 Jul;18(1):54–69. doi: 10.1016/0147-619x(87)90078-3. [DOI] [PubMed] [Google Scholar]
- Xiong X. F., Reznikoff W. S. Transcriptional slippage during the transcription initiation process at a mutant lac promoter in vivo. J Mol Biol. 1993 Jun 5;231(3):569–580. doi: 10.1006/jmbi.1993.1310. [DOI] [PubMed] [Google Scholar]
- Yang C. C., Nash H. A. The interaction of E. coli IHF protein with its specific binding sites. Cell. 1989 Jun 2;57(5):869–880. doi: 10.1016/0092-8674(89)90801-5. [DOI] [PubMed] [Google Scholar]
- Yoshioka Y., Fujita Y., Ohtsubo E. Nucleotide sequence of the promoter-distal region of the tra operon of plasmid R100, including traI (DNA helicase I) and traD genes. J Mol Biol. 1990 Jul 5;214(1):39–53. doi: 10.1016/0022-2836(90)90145-C. [DOI] [PubMed] [Google Scholar]
- Yoshioka Y., Ohtsubo H., Ohtsubo E. Repressor gene finO in plasmids R100 and F: constitutive transfer of plasmid F is caused by insertion of IS3 into F finO. J Bacteriol. 1987 Feb;169(2):619–623. doi: 10.1128/jb.169.2.619-623.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegelin G., Pansegrau W., Lurz R., Lanka E. TraK protein of conjugative plasmid RP4 forms a specialized nucleoprotein complex with the transfer origin. J Biol Chem. 1992 Aug 25;267(24):17279–17286. [PubMed] [Google Scholar]
- van Biesen T., Frost L. S. Differential levels of fertility inhibition among F-like plasmids are related to the cellular concentration of finO mRNA. Mol Microbiol. 1992 Mar;6(6):771–780. doi: 10.1111/j.1365-2958.1992.tb01527.x. [DOI] [PubMed] [Google Scholar]
- van Mansfeld A. D., van Teeffelen H. A., Baas P. D., Jansz H. S. Two juxtaposed tyrosyl-OH groups participate in phi X174 gene A protein catalysed cleavage and ligation of DNA. Nucleic Acids Res. 1986 May 27;14(10):4229–4238. doi: 10.1093/nar/14.10.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]


