Abstract
Humans are home to complex microbial communities, whose aggregate genomes and their encoded metabolic activities are referred to as the human microbiome. Recently, researchers have begun to appreciate that different human body habitats and the activities of their resident microorganisms can be better understood in ecological terms, as a range of spatial scales encompassing single cells, guilds of microorganisms responsive to a similar substrate, microbial communities, body habitats, and host populations. However, the bulk of the work to date has focused on studies of culturable microorganisms in isolation or on DNA sequencing-based surveys of microbial diversity in small to moderately sized cohorts of individuals. Here, we discuss recent work that highlights the potential for assessing the human microbiome at a range of spatial scales, and for developing novel techniques that bridge multiple levels: for example, through the combination of single cell methods and metagenomic sequencing. These studies promise to not only provide a much-needed epidemiological and ecological context for mechanistic studies of culturable and genetically tractable microorganisms, but may also lead to the discovery of fundamental rules that govern the assembly and function of host-associated microbial communities.
Keywords: Single cell analysis, metagenomics, human microbiome, microbiota, metabolic activity, microbial ecology
Introduction
Studies of the human microbiome have re-shaped our view of the human body and what it means to be part of a community (Gordon, 2012). Although the current world population is vast (nearly 1010 people), each individual harbors approximately 1,000 times as many microbial cells, representing thousands of species from all three domains of life (the human microbiota). Each individual’s microbiota extends over a wide range of body habitats, including, but not limited to, the skin, the urogenital tract, the nose, the mouth, and the gastrointestinal tract (Costello, et al., 2009, Consortium, 2012). Importantly, the vast majority of these microorganisms are not thought to be dormant or transient members; they extend our human genome by producing essential vitamins and amino acids, metabolizing dietary compounds (Turnbaugh, et al., 2010, Turnbaugh, et al., 2010), and modifying xenobiotics (compounds foreign to a living organism, including host-targeted drugs and antibiotics) (Haiser & Turnbaugh, 2012, Haiser & Turnbaugh, 2013). These host-microbial interactions help develop the immune system (Ivanov, et al., 2009, Round & Mazmanian, 2010, Atarashi, et al., 2011) and protect against invasion by pathogens (Pfeiffer & Sonnenburg, 2011, Kamada, et al., 2012), while also contributing to inflammatory bowel disease (Cadwell, et al., 2010, Devkota, et al., 2012) and obesity (Turnbaugh, et al., 2008, Turnbaugh, et al., 2009, Vijay-Kumar, et al., 2010).
The past decade has seen an unprecedented set of studies aimed at identifying the microbial members of the human body and determining their associations with disease, largely due to the advent of high-throughput DNA sequencing methods (Gill, et al., 2006, Kurokawa, et al., 2007, Costello, et al., 2009, Turnbaugh, et al., 2009, Qin, et al., 2010, Arumugam, et al., 2011, Consortium, 2012, Qin, et al., 2012). These studies typically focus on a single body habitat (e.g. feces, representing the distal gut), and compare patterns of microbial community composition (who’s there), community structure (relative abundance of microbial members), and/or gene content (what genes are there) over time or between individuals. The number of subjects began quite modestly (n=2–3 unrelated individuals) (Eckburg, et al., 2005, Gill, et al., 2006) and rapidly expanded to hundreds of individuals, and in some cases cohorts from multiple populations around the globe (Nasidze, et al., 2009, Yatsunenko, et al., 2012).
Conversely, the bulk of our mechanistic insight into host-associated microbial genomes and their metabolic activities is derived from the genetic and biochemical dissection of a small number of culturable model organisms; e.g. Escherichia coli (Taniguchi, et al., 2010, De Paepe, et al., 2011), Bacteroides thetaiotaomicron (Benjdia, et al., 2011, Marcobal, et al., 2011, Martens, et al., 2011, Cameron, et al., 2012), and Bacteroides fragilis (Round & Mazmanian, 2010, Round, et al., 2011, Shen, et al., 2012). Despite the fact that these microorganisms only represent a small fraction of the microbial diversity in a given individual, they continue to provide an invaluable context for understanding the metabolic activities of the less well-characterized and potentially uncultivable members of the microbiota.
Here, we discuss the emerging evidence that the dynamics and functions of the human microbiome might be better understood by bridging the gap between studies of single microorganisms and surveys of complex microbial communities (Fig. 1). This will require the development and application of tools for assessing microbial diversity and metabolic activity at multiple spatial scales, ranging from single cells to human populations. Although many of these approaches are still in their infancy, we highlight a number of recent studies that demonstrate the potential of using single-cell methods, mock communities of culturable isolates, enrichment procedures, and metagenomics, to tackle these daunting challenges. We will focus on notable examples of these approaches in application to human- or other host-associated microbial communities, building up from single cells to epidemiological studies.
Figure 1. Viewing the human microbiome across multiple spatial scales.

Information gathered at each successive scale may inform the others: ranging from single cell methods to analyses of microbial guilds active on a common substrate, microbial communities (e.g. tongue, ileum, or axilla), body habitats (e.g. the entire gastrointestinal tract), individuals, small to moderately sized cohorts, and finally large-scale epidemiological studies. A variety of techniques exist to assess microbial diversity and function at each scale (Table 1), but more work needs to be done to develop methods for integrating findings across different levels.
Together, these studies promise to address a number of outstanding questions. For example, are all host-associated microbial cells active, or are only a few microbial groups responsible for the bulk of the metabolic activity in a given body habitat? Who are these active, dormant, or damaged cells? Are there preferential ‘hot spots’ of microbial activity along the gastrointestinal tract or in other body habitats? Is it possible to link each metabolic activity to a single member of the human microbiota, or does each function require the concerted efforts of many members of a given community (i.e. a microbial guild)? To what degree have the members of each microbial community adapted to a specific niche; can we identify the specialists and generalists? And perhaps most importantly, will an increased understanding of the fundamental principles that govern host-associated microbial communities lead to new opportunities for therapeutic interventions or diagnostics?
1. Zooming in to the single cell level
Single-cell approaches can be considered as one end of the many spatial scales relevant to the human microbiome, providing information about the physical location, physiological status, and/or metabolic activity of a single cell within a microbial community. Similar to how these emerging techniques have been harnessed to reveal the basic biology of culturable microorganisms (Taniguchi, et al., 2010), researchers studying the human microbiome have begun to apply these methods to obtain a high-resolution view of host-associated microbial communities (Fig. 2). Together, these efforts promise to highlight intra- and inter-cell variations in metabolic activity, cell damage, growth rate, gene content, and transcription.
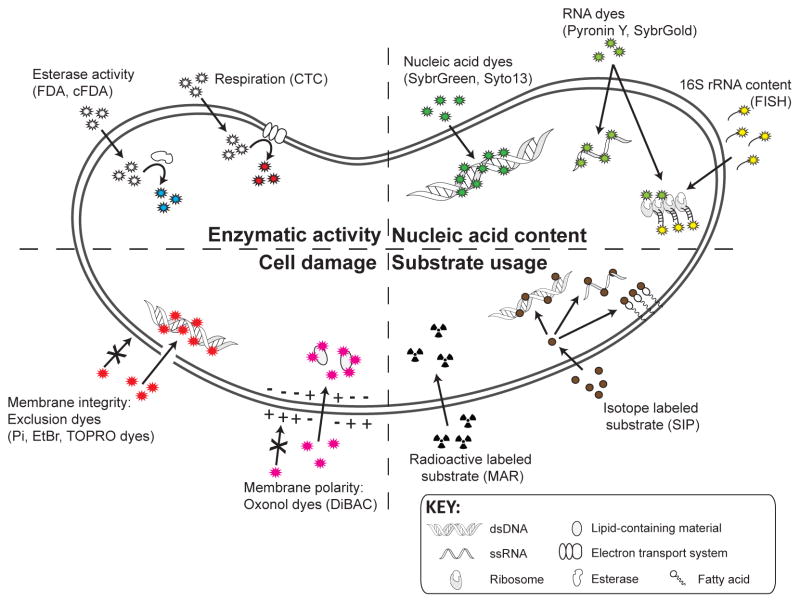
Figure 2. Quantifying metabolic activity with single cell methods.
Single cell tools enable the measurement of (i) enzymatic activity, (ii) nucleic acid content, (iii) cell damage, and (iv) substrate usage. FDA and cFDA are enzymatically activated by esterases to their fluorescent form; CTC becomes fluorescent upon reduction by the electron transport system. Nucleic acid content (DNA, RNA, or 16S ribosomal RNA) can be determined with multiple fluorescent dyes or FISH. Cell damage can be monitored by nucleic acid dyes that are excluded by microbial cells with an intact membrane because of their size, structure, and/or insolubility in the hydrophobic membrane phase. Oxonol dyes are anionic dyes binding to lipid-containing intracellular material, generally excluded from healthy cells by their membrane polarity. Substrates of interest can be either radioactively or stable isotope labeled, prior to their uptake and conversion to nucleic acids and lipids. Abbreviations include the following: double-stranded deoxyribonucleic acid (dsDNA); ethidium bromide (EtBr); fluorescein diacetate (FDA); carboxyfluoresceine diacetate (cFDA); 5-cyano-2,3-ditolyltetrazolium chloride (CTC); fluorescence in situ hybridization (FISH); Propidium iodide (Pi); microautoradiography (MAR); single-stranded ribonucleic acid (ssRNA); and stable isotope probing (SIP).
Flow cytometric measurement of microbial activity and cell damage
Flow cytometry (FCM) is a powerful tool that allows the rapid analysis of millions of individual microbial cells. Microbial cells in suspension are exposed to an intense source of light, generally an argon laser, and light scatter and fluorescence emission signals for each cell can be obtained (Shapiro, 1995). Bacterial abundance and characteristics such as cell size, shape, intracellular content, or granularity can be determined directly, and cells of interest can then be sorted based on such criteria. When combined with specific fluorescent stains, additional relevant information such as relative nucleic acid content, the state of the bacterial membrane, respiration, and enzymatic activity can be assessed. While FCM does not provide any structural or spatial information on microbial communities, it allows for the rapid quantification of cells with distinct physiology and activity levels, which can then be sorted and characterized in more detail.
Flow cytometry has been used extensively in environmental microbial communities, but has only recently been applied to the human microbiome (Del Giorgio & Gasol, 2008, Maurice, et al., 2013). For example, loss of membrane polarity, indicative of cell damage, can be assessed with the use of oxonol dyes [e.g. DiBAC4(3)] (Jepras, et al., 1995, Shapiro, 2000), while severe membrane damage can be assessed with propidium iodide (Pi). Both dyes have been used to determine the physiology of microbial cells from the human gut (Apajalahti, et al., 2003, Ben-Amor, et al., 2005, Maurice, et al., 2013). In our recent study (Maurice, et al., 2013), we showed that damaged cells can make up to approximately one third of the gut microbiota: severely damaged cells (Pi+) were on average just below 17% of the gut microbiota, whereas moderately damaged cells (DiBAC+) represented 27% (Fig. 3a). These results indicate that in healthy individuals, the distal gut microbiota is predominantly composed of cells that have intact membrane polarity, suggesting that these microorganisms are well adapted to this body habitat. In addition, the physiology of these microbial cells is rapidly responsive to environmental and chemical perturbations. Short-term exposure to a panel of xenobiotics, including 6 host-targeted drugs and 8 antibiotics, resulted in detectable increases in cell damage. None of the host-targeted drugs increased cell damage, whereas antibiotic exposure increased the proportions of severely damaged cells (Pi+) and moderately damaged cells (DiBAC+) to on average 23% and 44% of the total cells, respectively (Fig. 3b). These results highlight the dynamic structure of this community, and illustrate the often-unintended consequences of antibiotics on the gut microbiota (Maurice, et al., 2013).
Figure 3. Assessing activity and cell damage in the human gut microbiota.
(A) Baseline proportions of cells with detectable damage, as indicated by loss of membrane polarity (DiBAC) and/or membrane integrity [propidium iodide (Pi)]. Pi+ cells are included here as a subset of the DiBAC+ population. The split between highly active (HNA) and less active (LNA) cells is also shown based on SybrGreen staining of total nucleic acids. (B) Increased cell damage after exposure to antibiotics. (C) The abundance of bacterial phyla in each physiological subset. Data for each panel represents the average values across 3 unrelated healthy individuals (Maurice, et al., 2013).
Studies of environmental microbial communities have suggested that replication, transcriptional activity, and/or metabolic activity can be measured using fluorescent stains that enter all bacterial cells and bind double- and single-stranded nucleic acids (e.g. Syto13, PicoGreen, TOTO, SybrGreen). Interestingly, environmental microbial communities can be grouped into two clusters of cells: high (HNA) and low (LNA) nucleic acid content cells. Numerous studies support the claim that HNA cells generally exhibit an increased rate of cell division and higher metabolic activity than LNA cells (Gasol, et al., 1999, Lebaron, et al., 2002, Zubkov, et al., 2004, Bouvier, et al., 2007, Wang, et al., 2009). Our recent work suggests that these two cell populations also exist in the human gut microbiota (Maurice, et al., 2013). When stained with SybrGreen I, microbial cells from fresh fecal samples clustered into two populations with distinct green fluorescence values, and somewhat different light scatter values. The differences in fluorescence of these two populations could not be explained by increased cell or genome size, suggesting that they reflect differences in metabolic activity. On average, 55% of the microbial cells in fecal samples collected from unrelated healthy individuals are HNA cells (Fig. 3a), suggesting that most of the cells within the human gut are highly active, comparable to highly productive aquatic ecosystems such as coastal tidal marshes (Bouvier, et al., 2007).
A number of methods exist to test the metabolic activity and rate of cell division of these distinct populations. For example, the specific activity of each group might be measured using radiolabeled compounds, or single cell genomics might be harnessed to compare their metabolic potential (discussed in detail below). Recent studies of cultured isolates may also provide a clue as to the nature of the HNA and LNA subsets (Taniguchi, et al., 2010). Quantitative analysis of the E.coli transcriptome at single molecule sensitivity demonstrated that the average coding gene is expressed at <10 copies per cell. If this observation holds true for the dominant members of the gut microbiota, it may stand to reason that most of the differences in nucleic acid content are driven by the differential expression of non-coding RNAs, e.g. the rRNA operon. Follow-up studies applying these high-resolution methods to additional strains isolated from the human gut are necessary.
Active microbial cells can also be quantified with the tetrazolium salt 5-cyano-2,3-ditolyltetrazolium chloride CTC (Rodriguez, et al., 1992, Sherr, et al., 1999, Sieracki, et al., 1999). This salt competes with oxygen as an electron donor and is reduced by the active electron transport system of respiring cells to an insoluble red compound, formazan (Kaprelyants & Kell, 1993, Smith & McFeters, 1997). Initially thought to be restricted to aerobic bacteria, this dye was successfully applied to a variety of facultative and obligate anaerobes (Walsh, et al., 1995, Smith & McFeters, 1997, Bhupathiraju, et al., 1999). Preliminary tests suggest that members of the gut microbiota can reduce CTC, representing an average of 12% of the total cells in fecal samples from 3 unrelated individuals (Maurice and Turnbaugh, unpublished data). These values are comparable to aquatic systems (Del Giorgio & Gasol, 2008). Additional studies utilizing CTC might allow the identification of metabolically active microorganisms, or enable researchers to screen metagenomic libraries or culture collections for novel genes involved in respiration.
Intracellular enzymatic activity can be assessed with fluorescein diacetate (FDA) or carboxyfluoresceine diacetate (cFDA) (Dive, et al., 1988, Petit, et al., 1993, Del Giorgio & Gasol, 2008). These lipophilic non-fluorescent compounds diffuse through the bacterial membrane and are then converted to a membrane impermanant fluorescent form by esterases (Petit, et al., 1993). Thus, fluorescent cells detected by flow cytometry are active cells with intact membranes. These dyes have been used with a variety of bacterial cultures (Ritz, et al., 2001, Ben Amor, et al., 2002, Nguefack, et al., 2004, Tracy, et al., 2010, Arku, et al., 2011), but their application to determine bacterial activity in microbial communities remains controversial, as many bacteria cannot uptake FDA, the fluorescence signals tend to be weak, and damaged cells can still retain some level of esterase activity (Diaper, et al., 1992, Jepras, et al., 1995).
Measuring the intracellular amount of RNA can also serve as a proxy for metabolic activity. For example, pyronin Y was used in combination with flow cytometry and cell sorting to explore the active microbial cells in fecal samples from four healthy unrelated individuals (Peris-Bondia, et al., 2011). The authors identified Clostridiales (Firmicutes) as the most active cells, whereas the Bacteroidetes were less represented in the pyronin Y cell population. The RNA dyes SybrGreen II and Sybr Gold remain to be validated for application to the human microbiome. Sybr Gold has a high affinity for RNA and double- and single-stranded DNA (Tuma, et al., 1999, McKee & Thomson, 2004), whereas SybrGreen II exhibits a stronger quantum yield when bound to RNA than to double stranded DNA, although it is not selective for RNA staining (Haugland, 2005). Both are very stable and sensitive fluorochromes, extensively used for detecting RNA in denaturating gels, single stranded conformation polymorphism, and reverse transcription analyses.
Flow cytometry promises to be a valuable tool in quantifying and identifying the metabolically active cells, and in determining the relevant factors structuring our host-associated microbial communities. However, additional technical challenges remain to be resolved before widely applying this technique to the study of the human microbiome. Sampling procedures from different body habitats should be tested and standardized to minimize their effect on microbial physiology to ensure comparable results. In addition, the unbiased uptake of the different dyes by diverse microorganisms, irrespective of their phyla, or body habitat, remains to be validated.
Fluorescent in situ hybridization
Fluorescent in situ hybridization (FISH) is a technique based on the use of fluorescently labeled oligonucleotide probes targeting the bacterial 16S rRNA, although the use of polynucleotide probes and peptide nucleic acid molecules have also been reported (Cottrell & Kirchman, 2000, Amann, et al., 2001). FISH is a widely used tool for the direct, cultivation-independent enumeration, localization, and identification of individual microbial cells within complex communities, and has proven to be invaluable for the identification of symbiotic microorganisms [e.g. plant or animal-associated microorganisms, (Horn, et al., 2000, Tokuda, et al., 2000, Amann, et al., 2001)]. However, FISH-based techniques rely on successful probe entrance into the microbial cell, and accessibility and number of copies of the target. Several protocols have been tested to optimize probe penetration, resorting to additional enzymatic (generally lysozyme, lysostaphine, or achromopeptidase) or chemical (diluted acids or detergents) pretreatments for certain Gram-positive or even archaeal cells (Moter & Gobel, 2000). In addition, FISH signals are sensitive to cellular growth rates and nutritional status, a critical point for communities with low microbial activity or from nutrient-depleted systems, with low ribosomal probe target concentrations. This has led to the development of fluorescent signal amplification procedures, such as the CAtalyzed Reported Deposition amplification (CARD-FISH) using tyramide signal amplification (Amann, et al., 2001, Pernthaler, et al., 2004).
Despite these limitations, FISH has been extensively applied to quantify the bacteria present in the human gut microbiota (Langendijk, et al., 1995, Franks, et al., 1998, Harmsen, et al., 2000, Schwiertz, et al., 2000, Zoetendal, et al., 2002, Rigottier-Gois, et al., 2003), and the number of FISH probes applicable to the gut microbiota continues to expand (Harmsen, et al., 2002, Heilig, et al., 2002). In addition, several improvements have been made for the accurate FISH detection of specific bacterial groups, such as the combined use of FISH and flow cytometry (flow-FISH), which allows the high-throughput quantification of microbial community structure (Zoetendal, et al., 2002, Rigottier-Gois, et al., 2003, Vaahtovuo, et al., 2005, Dinoto, et al., 2006)
More recently, researchers have developed methods for multi-labeling experimental set-ups with sensitive image analysis, named Combinatorial Labeling and Spectral Imaging (CLASI-FISH), allowing the simultaneous detection of up to 28 different microbial targets (Valm, et al., 2011). When applied to microbial biofilms growing on human dental plaque, the spatial arrangement of 15 different bacterial taxa was revealed. Of note, Actinomyces and Prevotella had the most interspecies interactions, suggestive of a central role in biofilm development and maintenance (Valm, et al., 2011). These novel methods promise to be of particular interest to explore community structure, dynamics, and interactions in a wide range of host-associated microbial communities.
FISH with rRNA-targeted oligonucleotides not only allows the identification of individual cells within a complex assemblage, but also could potentially provide information about cellular activity, due to the relationship between fluorescence intensity and the concentration of the target of interest (Schaechter, et al., 1958, Amann, et al., 1995). This type of analysis has proven useful for cultured isolates and activated sludge from wastewater plants (DeLong, et al., 1989, Wallner, et al., 1993, Wallner, et al., 1995); however, it has, to the best of our knowledge, not yet been tested on the human microbiome.
Microautoradiography FISH
Microautoradiography FISH (MAR-FISH) was one of the earliest techniques developed to link cellular identity and activity. The method relies on the uptake of radiolabeled compounds, such as thymidine, leucine, glucose, acetate, N-acetylglucosamine, or 14CO2 (Ouverney & Fuhrman, 1999, Nielsen, et al., 2002, Hesselsoe, et al., 2005, Nielsen & Nielsen, 2005). Microbial cells are incubated with one, or several, of these radioactive compounds before being exposed to a photographic emulsion. Cells that uptake the compound(s) of interest can be identified using transmission light microscopy by the deposition of silver grains around them. Grain density can also provide further information on the level of substrate uptake by the individual cells (Cottrell & Kirchman, 2000, Sintes & Herndl, 2006). Despite the successful application of this technique to a wide range of environmental microbial communities, MAR-FISH has yet to be applied to the human microbiome. Multiple experimental limitations of this approach exist, including low throughput, general challenges with FISH, the difficulty of downstream molecular analysis, and the radioactive nature of the substrate, precluding its use in vivo. Alternative non-radioactive procedures may be more promising for the study of the human microbiome.
Single cell genomics
A number of approaches have been developed for the genomic analysis of single cells, including whole genome sequencing methods (Kalisky, et al., 2011). These approaches have been successfully applied to difficult to culture members of the human and mouse microbiota. For example, an uncultivated human oral bacterium from the TM7 phylum was isolated using a microfluidic device designed to isolate single cells with a rod-like morphology characteristic of this taxonomic group (Marcy, et al., 2007). Whole genome amplification and sequencing of 2 distinct cells, isolated from different microfluidic devices, resulted in the identification of >1,000 genes, revealing pathways for carbohydrate metabolism, arginine metabolism, and virulence factors. These results provided an important technical proof-of-principle, while also revealing genetic information that might be used to aid the in vitro growth of TM7 strains.
More recently, researchers have used single cell genomics to gain insight into segmented filamentous bacteria (SFB), a taxonomic group within the Firmicutes phylum that grows in filaments that tightly adhere to the intestinal epithelial layer. These fascinating microorganisms are of great interest due to their role in the induction of pro-inflammatory helper T cells (Th17) and the protection against bacterial pathogens. Five SFB filaments were isolated from a fecal sample collected from a single mono-associated mouse using a microfluidic device, subjected to whole genome amplification, and sequenced (Pamp, et al., 2012). The results revealed putative genes involved in the interaction between the SFB filament and the host, as well as those potentially responsible for the lifecycle of the SFB filament.
The ability to sequence individual microbial cells from a given body habitat promises to address many of the key limitations of metagenomic sequencing, namely the challenges of “binning” the observed genes into their strain or species of origin. Many of the initial challenges, such as reagent contamination with residual nucleic acids, are now well documented, and can be circumvented (Blainey, 2013). However, more systematic analyses need to be done to quantify the extent of amplification bias, replication errors, and the formation of chimeras. Multiple new amplification methods, such as degenerate oligonucleotide primed PCR, displacement degenerate oligonucleotide primed PCR, and multiple annealing and looping based amplification cycles, have been developed with the goal of reducing these errors (Blainey, 2013). Ongoing efforts in this area promise to continue to improve the reliability and throughput of single cell genomics.
Single cell mass spectrometry
In addition to inferring metabolic activity through whole genome sequencing, single cell mass spectrometry can be used to directly quantify metabolic activities. Recent applications of single cell mass spectrometry have utilized stable and radioactive isotope labeling, cell identification by FISH, Raman microspectrometry, and/or secondary ion mass spectrometry (SIMS) (Wagner, 2009, Musat, et al., 2012). The choice between the different spectrometry techniques relies mainly on the necessary downstream analyses, the spatial resolution needed, and the available equipment, as both Raman microspectrometry and SIMS require expensive analytical tools.
Raman microspectrometry is a nondestructive vibrational method, which amongst other applications, has been used to characterize the intra-species variation in pathogenic bacteria, the chemical composition of microbial biofilms, and the metabolic heterogeneities within bacterial cultures (Jarvis & Goodacre, 2004, Huang, et al., 2007, Patel, et al., 2008, Ivleva, et al., 2009). Briefly, the uptake of stable isotope markers by microbial cells causes a shift in the observed resonance spectra when compared to the spectra of the same unlabeled marker [for a more detailed description, see (Wagner, 2009)]. Raman spectral shifts correlate with the cellular marker concentration, providing a quantitative measurement of activity, and Raman microspectrometry can be combined with the downstream DNA-based processing of the microbial cells of interest (Huang, et al., 2004, Huang, et al., 2007, Wagner, 2009).
Although this approach has not been widely applied yet to the human microbiome, it has been used to develop novel methods for pathogen identification. For example, this method has enabled the identification of the pathogens involved in bacterial meningitis from cerebrospinal fluid, significantly reducing the time required for microbial identification relative to conventional methods (Harz, et al., 2009). Others have used surface-enhanced Raman spectroscopy (SERS) to increase the spectral intensity and fingerprint bacterial pathogens responsible for urinary tract infections (Jarvis & Goodacre, 2004) and pneumonia (Hennigan, et al., 2010). In the latter case, closely-related strains of M.pneumonia could be accurately differentiated (Hennigan, et al., 2010). Furthermore, Raman spectrometry was recently combined with optical trapping to selectively sort yeast and bacterial cells metabolizing 13C, without significant loss of cell viability, providing a proof-of-principle that this technique can be also be used to enrich for cells performing a metabolic function of interest (Huang, et al., 2009). The preservation of sample integrity and cell viability for further analysis constitutes the major advantage of Raman microspectrometry over other spectrometry-based approaches, and as the number of available isotopes increases, this method promises to significantly increase our understanding of the metabolic activities of uncultured microorganisms (Wagner, 2009).
Secondary Ion Mass Spectrometry (SIMS) relies on the production of secondary particles after exposure of microbial cells to an intense primary ion beam. The charged secondary ions produced are focused into a secondary beam analyzed by mass spectrometry, allowing the very fine identification of the elemental, isotopic, or molecular composition of the microbial cells (Benninghoven, et al., 1987). There are two main SIMS approaches available today, depending on the instrument design: time-of-flight SIMS (TOF-SIMS), and NanoSIMS (Kuypers & Jorgensen, 2007, Musat, et al., 2012). Both provide very fine resolution imaging of elements or isotopes within an eukaryotic or microbial cell, but the exposure to the intense ion beam can lead to sample degradation and electron-stimulated ion emission (Guerquin-Kern, et al., 2005, Lechene, et al., 2006, Vickerman & Gilmore, 2009). When combined with stable or radioactive isotope labeling, SIMS provides activity, diversity, and spatial information about microbial cells, key elements for a better understanding of the mutualistic or antagonist interactions between members of the same community or with the host (Huang, et al., 2007, Huang, et al., 2009, Wagner, 2009).
The first combination of NanoSIMS and stable isotope labeling to host-microbial interactions identified the symbiotic relationship between nitrogen-fixing bacteria and their host, the shipworm Lyrodus pedicellatus (Lechene, et al., 2007). In a recent application to the human microbiota, NanoSIMS and a modified version of FISH with halogen-containing tyramides were applied to bacterial cultures and a consortium of two microbial strains, before being used to study a human gingival biofilm (Behrens, et al., 2008). By identifying Cytophaga-Flavobacterium species as metabolizing 13C-labeled amino acids, the authors provided an initial demonstration of the potential for using NanoSIMS to better understand the human microbiome (Behrens, et al., 2008).
More recently, single cell mass spectrometry and highly sensitive imaging techniques have been applied to animal models. TOF-SIMS was combined in an untargeted way to a nanodesorption electrospray ionization spectrometry method, named MALDI-TOF-IMS. Researchers compared samples collected from adult germ-free mice, animals monoassociated with Bacteroides thetaiotaomicron, and mice colonized with both B.thetaiotaomicron and Bifidobacterium longum (Rath, et al., 2012). Multiple samples were analyzed for each of these three treatment groups from host connective tissue, the large intestinal epithelium, and the lumen of the large and small intestine. The results provide evidence for clear location-specific chemical markers; for example, choline was localized to the luminal contents of the small and large intestine, whereas carnitine was specific to host tissue. Colonization- and location-specific patterns were observed for glycans and secondary bile acids, the latter of which was confirmed by comparing bile acid transformations of the two isolates in pure culture (Rath, et al., 2012). Together, the results from this study clearly illustrate the potential of SIMS to explore the metabolic activity of the human microbiome with unprecedented spatial information, although no further molecular characterization or cell isolation is possible.
2. Designing and identifying microbial guilds
While single cell analyses can enable the dissection of community structure and metabolic activity from intact microbial communities, there are also a variety of approaches that allow for the construction and identification of groups of microorganisms that interact with each other or are involved in a shared metabolic process (referred to as a guild, a group of species that exploit a similar resource). Here, we discuss a number of approaches that have been recently used to explore microbial interactions at the strain level, at the level of 2–15 microorganisms, through enrichment strategies aimed at identifying groups of microorganisms responsive to a given substrate, and through computational analysis of co-occurrence networks.
Assembling microbial communities from a given body habitat
One of the grand challenges in microbial ecology is the potential for doing “community genetics”, i.e. selectively adding or removing individual strains, genes, or metabolic pathways. Recent studies have demonstrated the utility of establishing culture collections from a given body habitat (i.e. feces), which can then be used to construct a range of different mock communities, representing distinct combinations of strains (Faith, et al., 2010). These approaches are largely limited to the ability to isolate and grow microorganisms in pure culture, a relatively minor problem in the human gut, wherein the majority of the abundant bacterial species can be isolated using a single rich medium (Goodman, et al., 2011). In other body habitats, it may be necessary to use culture-independent methods, which can then inform attempts to isolate microorganisms of interest in pure culture.
Insertion sequencing methods (INSeq) have been used to monitor the abundance of transposon mutants constructed in a genetically tractable member of the human gut microbiota, Bacteroides thetaiotaomicron (Goodman, et al., 2009). Comparison of the abundance of each mutant prior to and after colonization of germ-free mice revealed 370 genes with significantly altered representation, ~50% of which could not be explained by a growth advantage in vitro, including a set of genes involved in vitamin B12 acquisition. Furthermore, co-colonization of these mice with representative members of the 3 dominant bacterial phyla (Firmicutes, Bacteroidetes, and Actinobacteria) revealed that community composition influences the selection pressures imposed on B.theta, providing an initial view of how the interactions between community members can alter the in vivo fitness of this common gut bacterium.
These mock communities have also been used to dissect the response of the human gut microbiota to a common perturbation, the consumption of bacterial strains in a commercially available fermented milk product (McNulty, et al., 2011). Germ-free mice were colonized with a 15-member gut microbiota meant to represent the dominant taxonomic and functional groups present in the human gastrointestinal tract. Consumption of the fermented milk product did not markedly perturb the abundance of each member of this simplified gut microbiota, but did result in the increased expression of genes for carbohydrate metabolism, notably the catabolism of xylooligosaccharides. These changes, initially observed in gnotobiotic mice, were supported by similar interventions in human participants and by metabolomics (McNulty, et al., 2011).
One of the major goals of these studies is to build quantitative models that might be used to predict the composition of a given microbial community given a defined perturbation. Substantial progress has recently been made in linking the composition of the diet to the structure of a 10-member gut microbiota in gnotobiotic mice (Faith, et al., 2011). Refined diets were designed that systematically varied the composition of 4 major ingredients: casein (protein), corn oil (fat), corn starch (polysaccharide), and sucrose (simple sugar). The results enabled the development of a statistical model that explained 60% of the variation in species abundance, highlighting the importance of dietary protein (casein) in shaping the gut microbiota. This approach may be generally applicable to a wide range of perturbations, as demonstrated for the consumption of more complex diets spanning a range of baby foods (Faith, et al., 2011).
A major challenge moving forward is the ability to reproducibly construct mock communities of greater complexity, harboring hundreds of strains. A recent study highlighted the ability to construct “personalized culture collections”, representing hundreds of strains from a single donor (Goodman, et al., 2011). Comparisons of germ-free mice colonized with the resulting culture collection to “humanized mice” colonized with an intact human fecal microbiota, demonstrated consistent responses to a high-fat/high-sugar diet. These methods have the benefit over experiments in humanized or conventionally raised mice, as they allow for the reproducible colonization of germ-free animals from frozen culture stocks, enabling the mechanistic dissection of host-microbial interactions. However, is important to note that recent studies have demonstrated the humanization fails to induce the same degree of immune maturation that occurs following colonization with a murine gut microbiota (Chung, et al., 2012), emphasizing the point that not all host-microbial interactions are conserved between mice and men.
Together, these studies highlight the immense power of re-constructing a given microbial community in the lab, allowing for a range of community genetics experiments. On the other hand, attempts at removing or adding strains, genes, or metabolic activities to an existing microbial community have been more challenging, although numerous studies underscore the many potential avenues to pursue. Notable examples include (i) the “fecal transplantation” of an intact donor gut microbiota to Clostridium difficile patients (Khoruts, et al., 2010), (ii) the lateral gene transfer of genetic loci for polysaccharide metabolism from algal bacteria to the gut microbiome (Hehemann, et al., 2010, Hehemann, et al., 2012), and (iii) the inhibition of bacterial β-glucuronidases responsible for the gastrointestinal side effects of chemotherapy (Wallace, et al., 2010).
Using enrichment culturing to identify microorganisms active on a given substrate
Enrichment culturing, whereby specific microorganisms are isolated by their ability to grow on a selective medium, has been extensively used to characterize the metabolism of a number of compounds. For example, many members of the human gut microbiota produce lactic acid, but this fermentation product is rarely detected in human feces or gut contents, while it accumulates in individuals with ulcerative colitis or with gut resections (Hove, et al., 1994, Kaneko, et al., 1997). Enrichment culturing on media with lactate as a sole carbon source identified nine strains of bacteria capable of subsisting on lactate (Duncan, et al., 2004), suggesting that members of the gut microbiota can readily consume this common substrate.
In addition to experiments using standard microbiological methods, it is possible to construct in vitro models that simulate the nutritional milieu, biochemical parameters, and fluid dynamics of the human gastrointestinal tract. Notably, the Simulator of the Human Intestinal Microbial Ecosystem (SHIME) has been used to link members of the gut microbiota to the metabolism of a given substrate of interest (Molly, et al., 1993, Possemiers, et al., 2008). While this system does not provide the direct identification of cells active on a given substrate, it can be used to identify compounds of interest with contrasting effects on the structure and dynamics of the gut microbiota. For example, the addition of organophosphate pesticides, which can be ingested through our diet, to fecal communities in SHIME increased the abundance of Enterococcus and Bacteroides, while at the same time decreasing Lactobacillus and Bifidobacterium (Joly, et al., 2012). Similar models of other body habitats may enable the rapid and controlled analysis of the human microbiome, complementing studies in animal models and humans.
Stable-isotope probing
Stable-isotope probing (SIP) is a powerful tool to identify microbial cells involved in specific metabolic pathways (Radajewski, et al., 2003, Dumont & Murrell, 2005). Complex microbial communities are incubated with substrates labeled with 13C or 15N, which are then incorporated into phospholipid-derived fatty acids (PFLA-SIP), nucleic acids (DNA-SIP), and ribonucleic acids (RNA-SIP) (Fig. 2) (Lechene, et al., 2007, Neufeld, et al., 2007a, Neufeld, et al., 2007b). These labeled products are ‘heavier’ than their non-labeled counterparts, allowing for their isolation via cesium chloride gradient centrifugation. This enables the culture-independent enrichment of members metabolizing one or more compound(s) of interest, which can then be characterized using metagenomics (Reichardt, et al., 2011).
RNA-SIP has been successfully applied to in vitro models of the human gut to study the metabolism of simple sugars (monosaccharides like glucose) and complex plant polysaccharides (i.e. starch). The more rapid incorporation of isotopic RNA over isotopic DNA [nearly 10-fold (Whitby, et al., 2005)] results in shorter incubation times, a major advantage given the possible modifications of activity and diversity of microbial cells when using in vitro model systems. Incubations of human fecal communities with isotope-labeled glucose revealed that Streptococcus bovis and Clostridium perfringens are both active glucose-fermenters (Egert, et al., 2007). Similar studies using isotope-labeled starch highlighted the ability of Ruminococcus bromii to metabolize complex carbohydrates, whereas other microorganisms (Prevotella sp., Bifidobacterium adolescentis and Eubacterium rectale) were shown to benefit from the byproducts of polysaccharide utilization by these primary fermenters. Researchers have also identified bacteria capable of metabolizing galacto-oligosaccharides, including members of the Bifidobacteria (B.longum, B.bifidum, B.catenulatum) and Lactobacillus (L.gasseri, L.salivarus) genera (Maathuis, et al., 2012). These examples illustrate how SIP can be used to identify the microbial players involved in a key metabolic activity in the human gut, and how their downstream interactions with other community members can be de-convoluted.
One major limitation to RNA-SIP for the study of the human gut microbiome is the availability of labeled substrates. For example, polysaccharides are a major energy source for the gut microbiota, but are difficult and/or expensive to label (Reichardt, et al., 2011). To overcome this problem, an alternative RNA-SIP procedure was developed and validated in vitro, using 13C-labeled precursors of de novo RNA synthesis (Reichardt, et al., 2011). These precursors limit the phylogenetic biases induced by the labeled substrate, and have provided ecologically relevant information about the activity of carbohydrate-metabolizing microbial cells of the human gut microbiome. Although stable isotope probing approaches require extensive tests to determine the extend of cross-feeding, validation of the appropriate isotope concentration, and the synthesis of labeled substrates, its use in the context of the human microbiome studies will be critical for linking microbial identity and function. The development of new biologically relevant labeled substrates or alternatives such as the substrates’ precursors will be crucial in applying in vivo SIP to animal models or even human subjects.
Inferring microbial niches from co-occurrence networks
An alternative approach to identifying microbial strains or species with a similar niche is to determine the pattern of co-occurrence within and between individuals. For example, we recently applied an unbiased approach for identifying non-linear associations to identify relationships between species-level bacterial phylotypes found in humanized mice fed a low-fat, plant-polysaccharide-rich diet or a high-fat/high-sugar “Western” diet (Reshef, et al., 2011). As expected, many of these relationships could be explained by phylotypes that responded similarity to the dietary intervention; however, the remaining associations may represent inter-species competition or additional selective factors that shape microbial ecology.
A notable application of this approach analyzed a co-occurrence network based on the relative abundances of 155 bacterial species across the fecal microbiomes of 124 unrelated European adults (Lozupone, et al., 2012). These analyses identified a group of co-occurring Clostridium cluster XIVa members (Anaerostipes caccae, C.symbiosum, and C.boltae). Members of this group were more abundant in samples from patients with inflammatory bowel disease, rapidly colonized the infant and germ-free mouse gut, and were enriched for genes related to virulence and oxidative stress resistance (Lozupone, et al., 2012).
Similar analyses have assessed co-occurrence relationships across multiple body habitats (Faust, et al., 2012), and have led to increasingly sophisticated models of microbial community structure and function (Faust & Raes, 2012). A major challenge moving forward will be to develop predictive models that can be experimentally validated and computational methods to disentangle shared responses to host, microbial, or environmental factors from shared metabolic activities.
3. Linking multiple body habitats
Most studies to date have focused on a single body habitat, leaving many open questions as to the degree of communication and transfer between microbial communities associated with the same host, i.e. along the length and width of the gastrointestinal tract, within the oral cavity, or across the skin surface. Furthermore, it is unclear to what degree microorganisms in a given body habitat may have wide ranging effects on host physiology, immune status, and/or behavior, which may shape microbial ecology throughout the body.
A number of studies of the mammalian gut have highlighted microbial biogeography at multiple spatial scales. Indeed, the first large-scale 16S rRNA gene sequencing-based survey of the human colon (Eckburg, et al., 2005), revealed quantitative differences between the stool and mucosal surfaces, although these were largely representative of inter-individual variations (Ley, et al., 2005). These spatial differences have been appreciated for some time, most notably in the cases of Helicobacter pylori and Lactobacillus reuteri, which are found at much higher relative abundance in the stomach and proximal intestine, respectively (Bik, et al., 2006, Frese, et al., 2011). More recently, laser-capture microdissection was used to compare the microbiota in the lumen (gut contents) and associated with colonic folds in conventionally raised mice, revealing significantly more members of the Firmicutes phylum (families: Lachnospiraceae and Ruminococcaceae) in the interfold regions (Nava, et al., 2011).
Our own studies of humanized mice have revealed differences between the proximal and distal intestinal tract, with significantly increased levels of Bacteroidetes in the cecum and colon (Turnbaugh, et al., 2009). However, attempts to stratify the gut microbiota by luminal contents and mucosal adherent populations did not reveal clear differences. Furthermore, the consumption of a high-fat/high-sugar diet consistently altered the gut microbiota throughout the length of the gastrointestinal tract; a result that was also observed in conventionally raised mice following gastric bypass surgery (Liou, et al., 2013). Together, these results suggest that variations in dietary consumption and/or other environmental exposures may mask microbial biogeography.
Zooming out even further, it may be useful to consider the entire human body and all of its associated microbial communities. No studies have comprehensively surveyed a single individual; however, recent efforts have assessed 27 distinct body habitats (Costello, et al., 2009). The results revealed that differences in microbial community composition are more strongly influenced by gross anatomy (gut, skin, oral cavity), than by inter-individual variations, despite high levels of the latter for a given body habitat even in identical twins (Turnbaugh, et al., 2009, Turnbaugh, et al., 2010). Results from the Human Microbiome Project, a survey of 242 healthy individuals, confirmed the habitat-specificity of microbial colonization and also identified metabolic pathways associated with each body habitat (Consortium, 2012).
Are these differences between body habitats intrinsic to the characteristics of the underlying host tissue, or can they be shaped by each individual’s history of exposure to microorganisms? Results from studies of the skin microbiota suggest that at least some body habitats may be susceptible to invasion: sites on the forehead and forearm were disinfected and inoculated with microorganisms from the tongue (Costello, et al., 2009). The recipient forearms, even after 8 hours, remained more similar to the tongue microbiota; however, the forehead reverted to its original composition within this time frame. These “body habitat transfers” could have critical implications in newborns, as evidenced by a recent comparison of neonates born by Caesarean section and vaginal delivery. Ten neonates were sampled immediately after delivery (oral, skin, and nose) and <24 hours later fecal samples were collected, and then compared to the mother’s skin, oral, and vaginal samples collected 1 hour before delivery. Unlike the adults, there was no differentiation by body habitat within each neonate. Furthermore, neonates born by vaginal delivery resembled their mother’s vaginal microbiota, whereas those born by C-section more closely resembled the skin microbiota (Dominguez-Bello, et al., 2010). Together, these results highlight the potential for microorganisms to be transferred, at least temporarily, from one body habitat to another, even between individuals.
Although the overall picture of microbial colonization is becoming clearer, there are a number of outstanding questions remaining to be addressed. Importantly, most of the studies to date have focused on microbial community composition and gene content. Very little is known about the relevant variations in microbial growth rate or metabolic activity between different body habitats. Does the transfer of microorganisms from one location to another shape the metabolic activities of the resident microbiota? Are microorganisms equally active throughout a given organ or are certain locations, such as the proximal colon, hot spots of activity? What is the distribution of dietary and host-derived substrates along the length of the gastrointestinal tract? Can the chemical properties of a given xenobiotic compound be used to predict its bioavailability, both to the host and to the gut microbiota? Can information about the microbiota in one body habitat be used to predict another?
Finally, it is interesting to consider whether or not the microbial activities in one body habitat might have far-reaching effects on other locations. An illustrative example comes from the co-infection of mice with the small intestinal nematode (Heligmosomoides polygyrus) and the Gram-negative bacterial pathogen Citrobacter rodentium. Infection with H.polygyrus triggers a systematic immune response that decreases host protection and increases intestinal injury in the colon, exacerbating C.rodentium-induced colitis (Weng, et al., 2007). Analogous studies need to be performed on the non-pathogenic members of the gut microbiota to determine to what degree parasites and immunomodulatory bacteria (Ivanov, et al., 2009, Round & Mazmanian, 2010, Atarashi, et al., 2011) can broadly shape the human microbiota across multiple body sites.
4. Zooming out to epidemiological studies of the human microbiome
Although each spatial scale provides its own challenges and insights, a major goal in the foreseeable future is to integrate findings at multiple levels into a “systems biology” framework. There have been numerous studies providing substantial evidence for a link between the human microbiome and the onset of certain diseases, but despite the vast amount of data generated in the past 10 years, it remains a challenge to combine epidemiological studies and metagenomics. One of the issues is that the number of samples per study remains relatively small by epidemiological standards. The first surveys of the human microbiome were on a handful of individuals (Eckburg, et al., 2005, Gill, et al., 2006, Kurokawa, et al., 2007), which quickly increased to hundreds of individuals (Turnbaugh, et al., 2009, Qin, et al., 2010, Consortium, 2012, Qin, et al., 2012, Yatsunenko, et al., 2012). Even by the standards of genome-wide association studies, which may sample thousands of individuals in well-characterized cohorts (Zeggini, et al., 2008), there remains substantial room for growth. The need for broader sampling efforts becomes even clearer when one considers the degree of inter-individual variation within a single body habitat, the human gut microbiota, where only ~50% of the species-level bacterial phylotypes can be shared between identical twins (Turnbaugh, et al., 2009, Turnbaugh, et al., 2010), and substantial genomic variation exists even between members of the same microbial “species” (Hansen, et al., 2011). Despite these challenges, recent efforts linking the human microbiome to obesity, atopic disorders, chronic inflammation, and cancer have begun to show how insights at multiple scales can inform one another, leading to the beginnings of a more comprehensive understanding of human health and disease.
Work over the past 10 years has emphasized that the gut microbiome can alter both sides of the energy balance equation, by liberating additional calories from the diet (Turnbaugh, et al., 2006), increasing intestinal absorption (Semova, et al., 2012), and promoting storage in adipose tissue (Backhed, et al., 2004). In mouse models, obesity is associated with an altered gut microbiota, and accompanied by increased dietary energy harvest, total body fat, and short-chain fatty acids (SCFA), the major products of microbial fermentation (Ley, et al., 2005, Turnbaugh, et al., 2006, Turnbaugh, et al., 2008, Turnbaugh, et al., 2009). In studies of moderately sized human cohorts, significant associations between obesity, microbial community structure, gene content, and metabolic network topology have been observed (Ley, et al., 2006, Turnbaugh, et al., 2009, Arumugam, et al., 2011, Greenblum, et al., 2012).
The disruption of the gut microbiome through exposure to antibiotics may cause increased weight gain and/or adiposity, potentially contributing to obesity and metabolic syndrome. The administration of sub-therapeutic doses of broad-spectrum antibiotics to conventionally raised mice significantly increased body fat, accompanied by increased dietary energy harvest and SCFAs, along with an altered microbial community structure and changes to host lipid metabolism and gut hormone signaling (Cho, et al., 2012). Additionally, a large-scale epidemiological study of 11,532 children suggested that exposure to antibiotics early in life (<6 months of age) is significantly associated with increased body mass (Trasande, et al., 2012).
Other large-scale studies have begun to explore whether a “Western” lifestyle defined by frequent antibiotic use, a high-fat/high-sugar diet, extensive personal hygiene, and limited exposure to livestock may predispose children to develop atopic disorders (i.e. eczema, wheezing, and allergies), potentially by disrupting the human microbiome (Linneberg, et al., 2003, Fukuda, et al., 2004, Penders, et al., 2007). In one of the largest studies to date, researchers used quantitative PCR to measure the abundance of 5 members of the gut microbiota (Bifidobacterium sp., Escherichia coli, Clostridium difficile, Bacteroides fragilis, and Lactobacillus sp.) in fecal samples from 957 1-month old children (Penders, et al., 2007). C.difficile was significantly associated with atopic eczema, recurrent wheeze, allergic sensitization, and dermatitis. In addition, because of the investigation of family history and the young age of the enrolled children, this study suggests that an altered composition of the gut microbiota may precede the manifestation of atopic symptoms.
Similar connections are emerging between the human microbiome and a wide range of diseases, including inflammatory bowel disease (Cadwell, et al., 2010, Devkota, et al., 2012), cancer (Kostic, et al., 2012), and atherosclerosis (Wang, et al., 2011). Together, these studies emphasize the importance of viewing the human microbiome from a broader perspective than that of the individual niche, body habitat, or cohort. They also provide a cautionary note that the comprehensive study of the human microbiome will require the development of a novel framework, including distinct phenotypic information and controls, as opposed to the direct application of standard methods for the study of pathogens or human genetics. In order to successfully accomplish these efforts, individuals from a variety of disciplines, including microbial ecology, genomics, human genetics, epidemiology, and anthropology (Benezra, et al., 2012) will need to work together. In particular, researchers will need to determine the adequate methods and amount of metadata to collect (Yilmaz, et al., 2011), the level of phenotypic analysis of the host cohort, the best spatial and temporal scale at which to sample a given body habitat, and the appropriate statistical tools for calculating power. Importantly, these epidemiological studies stand to benefit greatly from mechanistic insights obtained from much smaller scale studies in humans and animal models.
Concluding remarks: Integrating multiple scales for a more comprehensive view of the human microbiome
With the recent completion of the Human Microbiome Project (Consortium, 2012), it now seems like an opportune time to consider the pros and cons of the available techniques for analyzing microbial activity (Table 1), and how one might intentionally design efforts linking multiple ecological scales for some of these emerging examples. No single method described here can provide all the information needed to comprehensively quantify the diverse metabolic activities found within the human microbiome, but their combined application promises to lead to more robust, and perhaps unexpected, results. Furthermore, it will remain critical to develop approaches to test the in vivo relevance of hypotheses based on in vitro, ex vivo, or animal models.
Table 1.
Advantages and limitations of various techniques for quantifying the metabolic activities of complex microbial communities.
| Method | Advantages | Limitations |
|---|---|---|
| Flow cytometry and Fluorescence-Activated Cell Sorting (FCM/FACS) |
|
|
| Fluorescence in situ hybridization (FISH) |
|
|
| Microautoradiography FISH (MAR-FISH) |
|
|
| Single cell genomics |
|
|
| Single cell mass spectrometry |
|
|
| Stable isotope probing (SIP) |
|
|
| Metatranscriptomics |
|
|
Recent efforts have demonstrated some emerging techniques that might allow researchers to analyze the human microbiome at multiple scales in a single study. For example, we have recently combined single-cell approaches and metatranscriptomics to characterize the highly active and damaged subset of the gut microbiome (Maurice, et al., 2013). The fecal microbiota of unrelated healthy individuals was stained with SybrGreen, DiBAC, and Pi. Cells from each subset were sorted into replicate wells using fluorescence activated cell sorting (FACS), and the composition of each subset was determined using 16S rRNA gene sequencing (Fig. 3c). The community composition and structure of each physiological category was then compared to that of the total microbial community. Bacteria from the order Clostridiales (phylum: Firmicutes) dominated both the highly active and damaged subsets, whereas the less active subset was enriched for Bacteroidetes and Bifidobacteria (phylum: Actinobacteria). These results were consistent with RNA-Seq analysis of community-wide gene expression. The concordance between the highly active and damaged subsets seems to suggest that the Firmicutes may have a higher turnover than other members of the gut microbiota, potentially driven by an increased susceptibility to the many environmental stresses present in the gastrointestinal tract.
The combination of single cell methods, metatranscriptomics, and metagenomics allowed us to determine the different levels of response of the gut microbiota to a short-term exposure to a panel of 14 different xenobiotics, including antibiotics and host-targeted drugs. Antibiotics significantly modified the physiology, gene expression, and community structure of the microbiota, with strong effects on the Clostridiales, consistent with the hypothesis that increased activity may lead to an enhanced susceptibility to these common clinical perturbations. We also detected a number of changes to microbial gene expression in response to host-targeted drugs, representing candidate genes for xenobiotic metabolism (Maurice, et al., 2013). Such studies provide an initial view of the benefits of combining multiple scales of analysis of the human microbiome.
Just as research over the past decade has revealed a wealth of information regarding the structure of the human gut microbiome at a variety of ecological scales, the coming decade promises to expand the field both inwards and outwards, as researchers seek to identify novel metabolic pathways, host-microbial interactions, single cell dynamics, and variations between body habitats, whereas metagenomic surveys of the human microbiome will continue to expand to encompass diverse human populations around the globe. If successful, these efforts will provide novel insights that link together findings across multiple scales, providing predictive models of microbial colonization and metabolism, and new guidelines for the coming generation of microbiome-targeted therapeutics, diagnostics, and nutritional guidelines.
Acknowledgments
This work was supported by the National Institutes of Health (P50 GM068763).
References
- Amann R, Fuchs BM, Behrens S. The identification of microorganisms by fluorescence in situ hybridisation. Curr Opin Biotechnol. 2001;12:231–236. doi: 10.1016/s0958-1669(00)00204-4. [DOI] [PubMed] [Google Scholar]
- Amann RI, Ludwig W, Schleifer KH. Phylogenetic Identification and in-Situ Detection of Individual Microbial-Cells without Cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apajalahti JHA, Kettunen A, Nurminen PH, Jatila H, Holben WE. Selective plating underestimates abundance and shows differential recovery of bifidobacterial species from human feces. Appl Environ Microbiol. 2003;69:5731–5735. doi: 10.1128/AEM.69.9.5731-5735.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arku B, Fanning S, Jordan K. Flow cytometry to assess biochemical pathways in heat-stressed Cronobacter spp. (formerly Enterobacter sakazakii) J Appl Microbiol. 2011;111:616–624. doi: 10.1111/j.1365-2672.2011.05075.x. [DOI] [PubMed] [Google Scholar]
- Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Shima T, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens S, Losekann T, Pett-Ridge J, Weber PK, Ng WO, Stevenson BS, Hutcheon ID, Relman DA, Spormann AM. Linking microbial phylogeny to metabolic activity at the single-cell level by using enhanced element labeling-catalyzed reporter deposition fluorescence in situ hybridization (EL-FISH) and NanoSIMS. Appl Environ Microbiol. 2008;74:3143–3150. doi: 10.1128/AEM.00191-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Amor K, Breeuwer P, Verbaarschot P, Rombouts FM, Akkermans ADL, De Vos WM, Abee T. Multiparametric flow cytometry and cell sorting for the assessment of viable, injured, and dead bifidobacterium cells during bile salt stress. Appl Environ Microbiol. 2002;68:5209–5216. doi: 10.1128/AEM.68.11.5209-5216.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Amor K, Heilig H, Smidt H, Vaughan EE, Abee T, de Vos WM. Genetic diversity of viable, injured, and dead fecal bacteria assessed by fluorescence-activated cell sorting and 16S rRNA gene analysis. Appl Environ Microbiol. 2005;71:4679–4689. doi: 10.1128/AEM.71.8.4679-4689.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benezra A, DeStefano J, Gordon JI. Anthropology of microbes. Proc Natl Acad Sci U S A. 2012;109:6378–6381. doi: 10.1073/pnas.1200515109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjdia A, Martens EC, Gordon JI, Berteau O. Sulfatases and a radical S-adenosyl-L-methionine (AdoMet) enzyme are key for mucosal foraging and fitness of the prominent human gut symbiont, Bacteroides thetaiotaomicron. J Biol Chem. 2011;286:25973–25982. doi: 10.1074/jbc.M111.228841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninghoven A, Rudenauer FG, Werner HW. Secondary ion mass spectrometry: basic concepts, instrumental aspects, applications, and trends. Wiley; New York: 1987. [Google Scholar]
- Bhupathiraju VK, Hernandez M, Landfear D, Alvarez-Cohen L. Application of a tetrazolium dye as an indicator of viability in anaerobic bacteria. J Microbiol Meth. 1999;37:231–243. doi: 10.1016/s0167-7012(99)00069-x. [DOI] [PubMed] [Google Scholar]
- Bik EM, Eckburg PB, Gill SR, Nelson KE, Purdom EA, Francois F, Perez-Perez G, Blaser MJ, Relman DA. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci U S A. 2006;103:732–737. doi: 10.1073/pnas.0506655103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blainey PC. The future is now: single-cell genomics of bacteria and archaea. FEMS Microbiol Rev. 2013 doi: 10.1111/1574-6976.12015. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier T, Del Giorgio PA, Gasol JM. A comparative study of the cytometric characteristics of high and low nucleic-acid bacterioplankton cells from different aquatic ecosystems. Environ Microbiol. 2007;9:2050–2066. doi: 10.1111/j.1462-2920.2007.01321.x. [DOI] [PubMed] [Google Scholar]
- Cadwell K, Patel KK, Maloney NS, Liu TC, Ng AC, Storer CE, Head RD, Xavier R, Stappenbeck TS, Virgin HW. Virus-plus-susceptibility gene interaction determines Crohn’s disease gene Atg16L1 phenotypes in intestine. Cell. 2010;141:1135–1145. doi: 10.1016/j.cell.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron EA, Maynard MA, Smith CJ, Smith TJ, Koropatkin NM, Martens EC. Multidomain Carbohydrate-binding Proteins Involved in Bacteroides thetaiotaomicron Starch Metabolism. J Biol Chem. 2012;287:34614–34625. doi: 10.1074/jbc.M112.397380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H, Pamp SJ, Hill JA, et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149:1578–1593. doi: 10.1016/j.cell.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium THMP. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial Community Variation in Human Body Habitats Across Space and Time. Science. 2009;326:1694–1697. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell MT, Kirchman DL. Community composition of marine bacterioplankton determined by 16S rRNA gene clone libraries and fluorescence in situ hybridization. Appl Environ Microbiol. 2000;66:5116–5122. doi: 10.1128/aem.66.12.5116-5122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paepe M, Gaboriau-Routhiau V, Rainteau D, Rakotobe S, Taddei F, Cerf-Bensussan N. Trade-off between bile resistance and nutritional competence drives Escherichia coli diversification in the mouse gut. PLoS Genet. 2011;7:e1002107. doi: 10.1371/journal.pgen.1002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giorgio PA, Gasol JM. Physiological structure and single-cell activity in marine bacterioplankton. In: Kirchman DL, editor. Microbial ecology of the oceans. Wiley-Blackwell; 2008. pp. 243–285. [Google Scholar]
- DeLong EF, Wickham GS, Pace NR. Phylogenetic stains:Ribosomal RNA-based probes for the identification of single cells. Science. 1989;243:1360–1363. doi: 10.1126/science.2466341. [DOI] [PubMed] [Google Scholar]
- Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature. 2012;487:104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaper JP, Tither K, Edwards C. Rapid assessement of bacterial viability by flow cytometry. Appl Microbiol Biotechnol. 1992;38:268–272. doi: 10.1007/BF00174481. [DOI] [PubMed] [Google Scholar]
- Dinoto A, Marques TM, Sakamoto K, Fukiya S, Watanabe J, Ito S, Yokota A. Population dynamics of Bifidobacterium species in human feces during raffinose administration monitored by fluorescence in situ hybridization-flow cytometry. Appl Environ Microbiol. 2006;72:7739–7747. doi: 10.1128/AEM.01777-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dive C, Cox H, Watson JV, Workman P. Polar Fluorescein Derivatives as Improved Substrate Probes for Flow Cytoenzymological Assay of Cellular Esterases. Mol Cell Probes. 1988;2:131–145. doi: 10.1016/0890-8508(88)90035-7. [DOI] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont MG, Murrell JC. Stable isotope probing - linking microbial identity to function. Nat Rev Microbiol. 2005;3:499–504. doi: 10.1038/nrmicro1162. [DOI] [PubMed] [Google Scholar]
- Duncan SH, Louis P, Flint HJ. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl Environ Microbiol. 2004;70:5810–5817. doi: 10.1128/AEM.70.10.5810-5817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egert M, de Graaf AA, Maathuis A, de Waard P, Plugge CM, Smidt H, Deutz NEP, Dijkema C, de Vos WM, Venema K. Identification of glucose-fermenting bacteria in an in vitro model of the human intestine by RNA-stable isotope probing. FEMS Microbiol Ecol. 2007;60:126–135. doi: 10.1111/j.1574-6941.2007.00281.x. [DOI] [PubMed] [Google Scholar]
- Faith JJ, McNulty NP, Rey FE, Gordon JI. Predicting a human gut microbiota’s response to diet in gnotobiotic mice. Science. 2011;333:101–104. doi: 10.1126/science.1206025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith JJ, Rey FE, O’Donnell D, Karlsson M, McNulty NP, Kallstrom G, Goodman AL, Gordon JI. Creating and characterizing communities of human gut microbes in gnotobiotic mice. ISME J. 2010;4:1094–1098. doi: 10.1038/ismej.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust K, Raes J. Microbial interactions: from networks to models. Nat Rev Microbiol. 2012;10:538–550. doi: 10.1038/nrmicro2832. [DOI] [PubMed] [Google Scholar]
- Faust K, Sathirapongsasuti JF, Izard J, Segata N, Gevers D, Raes J, Huttenhower C. Microbial co-occurrence relationships in the human microbiome. PLoS Comput Biol. 2012;8:e1002606. doi: 10.1371/journal.pcbi.1002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks AH, Harmsen HJ, Raangs GC, Jansen GJ, Schut F, Welling GW. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl Environ Microbiol. 1998;64:3336–3345. doi: 10.1128/aem.64.9.3336-3345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frese SA, Benson AK, Tannock GW, et al. The evolution of host specialization in the vertebrate gut symbiont Lactobacillus reuteri. PLoS Genet. 2011;7:e1001314. doi: 10.1371/journal.pgen.1001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda S, Ishikawa H, Koga Y, Aiba Y, Nakashima K, Cheng L, Shirakawa T. Allergic symptoms and microflora in schoolchildren. J Adolesc Health. 2004;35:156–158. doi: 10.1016/j.jadohealth.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Gasol JM, Zweifel UL, Peters F, Fuhrman JA, Hagstrom A. Significance of size and nucleic acid content heterogeneity as measured by flow cytometry in natural planktonic bacteria. Appl Environ Microbiol. 1999;65:4475–4483. doi: 10.1128/aem.65.10.4475-4483.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AL, Kallstrom G, Faith JJ, Reyes A, Moore A, Dantas G, Gordon JI. Extensive personal human gut microbiota culture collections characterized and manipulated in gnotobiotic mice. Proc Natl Acad Sci U S A. 2011;108:6252–6257. doi: 10.1073/pnas.1102938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman AL, McNulty NP, Zhao Y, Leip D, Mitra RD, Lozupone CA, Knight R, Gordon JI. Identifying genetic determinants needed to establish a human gut symbiont in its habitat. Cell Host Microbe. 2009;6:279–289. doi: 10.1016/j.chom.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JI. Honor thy gut symbionts redux. Science. 2012;336:1251–1253. doi: 10.1126/science.1224686. [DOI] [PubMed] [Google Scholar]
- Greenblum S, Turnbaugh PJ, Borenstein E. Metagenomic systems biology of the human gut microbiome reveals topological shifts associated with obesity and inflammatory bowel disease. Proc Natl Acad Sci U S A. 2012;109:594–599. doi: 10.1073/pnas.1116053109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerquin-Kern JL, Wu TD, Quintana C, Croisy A. Progress in analytical imaging of the cell by dynamic secondary ion mass soectrometry (SIMS microscopy) Biochim Biophys Acta. 2005;1724:228–238. doi: 10.1016/j.bbagen.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Haiser HJ, Turnbaugh PJ. Is it time for a metagenomic basis of therapeutics? Science. 2012;336:1253–1255. doi: 10.1126/science.1224396. [DOI] [PubMed] [Google Scholar]
- Haiser HJ, Turnbaugh PJ. Developing a metagenomic view of xenobiotic metabolism. Pharmacol Res. 2013;69:21–31. doi: 10.1016/j.phrs.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen EE, Lozupone CA, Rey FE, et al. Pan-genome of the dominant human gut-associated archaeon, Methanobrevibacter smithii, studied in twins. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4599–4606. doi: 10.1073/pnas.1000071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen HJ, Raangs GC, He T, Degener JE, Welling GW. Extensive set of 16S rRNA-based probes for detection of bacteria in human feces. Appl Environ Microbiol. 2002;68:2982–2990. doi: 10.1128/AEM.68.6.2982-2990.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen HJM, Wildeboer-Veloo ACM, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, Welling GW. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastr Nutr. 2000;30:61–67. doi: 10.1097/00005176-200001000-00019. [DOI] [PubMed] [Google Scholar]
- Harz M, Kiehntopf M, Stockel S, Rosch P, Straube E, Deufel T, Popp J. Direct analysis of clinical relevant single bacterial cells from cerebrospinal fluid during bacterial meningitis by means of micro-Raman spectroscopy. J Biophoton. 2009;2:70–80. doi: 10.1002/jbio.200810068. [DOI] [PubMed] [Google Scholar]
- Haugland RP. The Handbook : A guide to fluorescent probes and labeling technologies. Invitrogen Corp; 2005. [Google Scholar]
- Hehemann JH, Kelly AG, Pudlo NA, Martens EC, Boraston AB. Bacteria of the human gut microbiome catabolize red seaweed glycans with carbohydrate-active enzyme updates from extrinsic microbes. Proc Natl Acad Sci U S A. 2012;109:19786–19791. doi: 10.1073/pnas.1211002109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehemann JH, Correc G, Barbeyron T, Helbert W, Czjzek M, Michel G. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature. 2010;464:908–912. doi: 10.1038/nature08937. [DOI] [PubMed] [Google Scholar]
- Heilig HG, Zoetendal EG, Vaughan EE, Marteau P, Akkermans AD, de Vos WM. Molecular diversity of Lactobacillus spp. and other lactic acid bacteria in the human intestine as determined by specific amplification of 16S ribosomal DNA. Appl Environ Microbiol. 2002;68:114–123. doi: 10.1128/AEM.68.1.114-123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennigan SL, Driskell JD, Dluhy RA, Zhao Y, Tripp RA, Waites KB, Krause DC. Detection of Mycoplasma pneumoniae in simulated and true clinical throat swab specimens by nanorod array-surface-enhanced Raman spectroscopy. PLoS One. 2010;5:e13633. doi: 10.1371/journal.pone.0013633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselsoe M, Nielsen JL, Roslev P, Nielsen PH. Isotope labeling and microautoradiography of active heterotrophic bacteria on the basis of assimilation of 14CO2. Appl Environ Microbiol. 2005;71:646–655. doi: 10.1128/AEM.71.2.646-655.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn M, Wagner M, Muller KD, Schmid EN, Fritsche TR, Schleifer KH, Michel R. Neochlamydia hartmannellae gen. nov., sp. nov. (Parachlamydiaceae), an endoparasite of the amoeba Hartmannella vermiformis. Microbiology. 2000;146 (Pt 5):1231–1239. doi: 10.1099/00221287-146-5-1231. [DOI] [PubMed] [Google Scholar]
- Hove H, Nordgaard Andersen I, Mortensen PB. Fecal DL-lactate concentration in 100 gastrointestinal patients. Scand J Gastroenterol. 1994;29:255–259. doi: 10.3109/00365529409090473. [DOI] [PubMed] [Google Scholar]
- Huang WE, Ward AD, Whiteley AS. Raman tweezers sorting of single microbial cells. Env Microbiol Rep. 2009;1:44–49. doi: 10.1111/j.1758-2229.2008.00002.x. [DOI] [PubMed] [Google Scholar]
- Huang WE, Griffiths RI, Thompson IP, Bailey MJ, Whiteley AS. Raman microscopic analysis of single microbial cells. Anal Chem. 2004;76:4452–4458. doi: 10.1021/ac049753k. [DOI] [PubMed] [Google Scholar]
- Huang WE, Stoecker K, Griffiths R, Newbold L, Daims H, Whiteley AS, Wagner M. Raman-FISH: combining stable-isotope Raman spectroscopy and fluorescence in situ hybridization for the single cell analysis of identity and function. Environ Microbiol. 2007;9:1878–1889. doi: 10.1111/j.1462-2920.2007.01352.x. [DOI] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivleva NP, Wagner M, Horn H, Niessner R, Haisch C. Towards a nondestructive chemical characterization of biofilm matrix by Raman microscopy. Anal Bioanal Chem. 2009;393:197–206. doi: 10.1007/s00216-008-2470-5. [DOI] [PubMed] [Google Scholar]
- Jarvis RM, Goodacre R. Discrimination of bacteria using surface-enhanced Raman spectroscopy. Anal Chem. 2004;76:40–47. doi: 10.1021/ac034689c. [DOI] [PubMed] [Google Scholar]
- Jepras RI, Carter J, Pearson SC, Paul FE, Wilkinson MJ. Development of a robust flow cytometric assay for determining numbers of viable bacteria. Appl Environ Microbiol. 1995;61:2696–2701. doi: 10.1128/aem.61.7.2696-2701.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly C, Gay-Queheillard J, Leke A, Chardon K, Delanaud S, Bach V, Khorsi-Cauet H. Impact of chronic exposure to low doses of chlorpyrifos on the intestinal microbiota in the Simulator of the Human Intestinal Microbial Ecosystem (SHIME(R)) and in the rat. Environ Sci Pollut Res Int. 2012 doi: 10.1007/s11356-012-1283-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Kalisky T, Blainey P, Quake SR. Genomic analysis at the single-cell level. Annu Rev Genet. 2011;45:431–445. doi: 10.1146/annurev-genet-102209-163607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada N, Kim YG, Sham HP, Vallance BA, Puente JL, Martens EC, Nunez G. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science. 2012;336:1325–1329. doi: 10.1126/science.1222195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Banda Y, Kurihara K, Satomi K, Nonoyama K, Matsuura N. Fecal microflora in a patient with short-bowel syndrome and identification of dominant lactobacilli. J Clin Microbiol. 1997;35:3181–3185. doi: 10.1128/jcm.35.12.3181-3185.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaprelyants AS, Kell DB. Dormancy in stationary-phase cultures of Micrococcus luteus: flow cytometry analysis of starvation and resuscitation. Appl Environ Microbiol. 1993;59:3187–3196. doi: 10.1128/aem.59.10.3187-3196.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol. 2010;44:354–360. doi: 10.1097/MCG.0b013e3181c87e02. [DOI] [PubMed] [Google Scholar]
- Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292–298. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa K, Itoh T, Kuwahara T, et al. Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res. 2007;14:169–181. doi: 10.1093/dnares/dsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers MMM, Jorgensen BB. The future of single-cell environmental microbiology. Environ Microbiol. 2007;9:6–7. doi: 10.1111/j.1462-2920.2006.01222_5.x. [DOI] [PubMed] [Google Scholar]
- Langendijk PS, Schut F, Jansen GJ, Raangs GC, Kamphuis G, Wilkinson MHF, Welling GW. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA targeted probe and its application in fecal samples. Appl Environ Microbiol. 1995;61:3069–3075. doi: 10.1128/aem.61.8.3069-3075.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebaron P, Servais P, Baudoux AC, Bourrain M, Courties C, Parthuisot N. Variations of bacterial-specific activity with cell size and nucleic acid content assessed by flow cytometry. Aquat Microb Ecol. 2002;28:131–140. [Google Scholar]
- Lechene C, Hillion F, McMahon G, et al. High-resolution quantitative imaging of mammalian and bacterial cells using stable isotope mass spectrometry. J Biol. 2006;5:20. doi: 10.1186/jbiol42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechene CP, Luyten Y, McMahon G, Distel DL. Quantitative imaging of nitrogen fixation by individual bacteria within animal cells. Science. 2007;317:1563–1566. doi: 10.1126/science.1145557. [DOI] [PubMed] [Google Scholar]
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linneberg A, Ostergaard C, Tvede M, Andersen LP, Nielsen NH, Madsen F, Frolund L, Dirksen A, Jorgensen T. IgG antibodies against microorganisms and atopic disease in Danish adults: the Copenhagen Allergy Study. J Allergy Clin Immunol. 2003;111:847–853. doi: 10.1067/mai.2003.1335. [DOI] [PubMed] [Google Scholar]
- Liou AP, Paziuk M, Luevano J, Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5:178ra141. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C, Faust K, Raes J, Faith JJ, Frank DN, Zaneveld J, Gordon JI, Knight R. Identifying genomic and metabolic features that can underlie early successional and opportunistic lifestyles of human gut symbionts. Genome Res. 2012;22:1974–1984. doi: 10.1101/gr.138198.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis AJH, ven den Heuvel EG, Schoterman MHC, Venema K. Galacto-oligosaccharides have prebiotic activity in a dynamic in vitro colon model using a 13C-labeling technique. J Nutr. 2012;142:1205–1212. doi: 10.3945/jn.111.157420. [DOI] [PubMed] [Google Scholar]
- Marcobal A, Barboza M, Sonnenburg ED, et al. Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell Host Microbe. 2011;10:507–514. doi: 10.1016/j.chom.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcy Y, Ouverney C, Bik EM, et al. Dissecting biological “dark matter” with single-cell genetic analysis of rare and uncultivated TM7 microbes from the human mouth. Proc Natl Acad Sci U S A. 2007;104:11889–11894. doi: 10.1073/pnas.0704662104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens EC, Lowe EC, Chiang H, et al. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. 2011;9:e1001221. doi: 10.1371/journal.pbio.1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39–50. doi: 10.1016/j.cell.2012.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee DR, Thomson MS. Nucleic acid stain-dependent single strand conformation polymorphisms. BioTechniques. 2004;37:46–50. doi: 10.2144/04371BM04. [DOI] [PubMed] [Google Scholar]
- McNulty NP, Yatsunenko T, Hsiao A, et al. The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Sci Transl Med. 2011;3:106ra106. doi: 10.1126/scitranslmed.3002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molly K, Vande Woestyne M, Verstraete W. Development of a 5-step multi-chamber reactor as a simulator of the human intestinal microbial ecosystem. Appl Microbiol Biotechnol. 1993;39:254–258. doi: 10.1007/BF00228615. [DOI] [PubMed] [Google Scholar]
- Moter A, Gobel UB. Fluorescence in situ hybridization (FISH) for direct visualization of microorganisms. J Microbiol Methods. 2000;41:85–112. doi: 10.1016/s0167-7012(00)00152-4. [DOI] [PubMed] [Google Scholar]
- Musat N, Foster R, Vagner TBA, Kuypers MMM. Detecting metabolic activities in single cells, with emphasis on nanoSIMS. FEMS Microbiol Rev. 2012;36:486–511. doi: 10.1111/j.1574-6976.2011.00303.x. [DOI] [PubMed] [Google Scholar]
- Nasidze I, Li J, Quinque D, Tang K, Stoneking M. Global diversity in the human salivary microbiome. Genome Res. 2009;19:636–643. doi: 10.1101/gr.084616.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava GM, Friedrichsen HJ, Stappenbeck TS. Spatial organization of intestinal microbiota in the mouse ascending colon. ISME J. 2011;5:627–638. doi: 10.1038/ismej.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld JD, Dumont MG, Vohra J, Murrell JC. Methodological considerations for the use of stable isotope probing in microbial ecology. Microb Ecol. 2007;53:435–442. doi: 10.1007/s00248-006-9125-x. [DOI] [PubMed] [Google Scholar]
- Neufeld JD, Vohra J, Dumont MG, Lueders T, Manefield M, Friedrich MW, Murrell JC. DNA stable-isotope probing. Nat Protoc. 2007;2:860–866. doi: 10.1038/nprot.2007.109. [DOI] [PubMed] [Google Scholar]
- Nguefack J, Budde BB, Jakobsen M. Five essential oils from aromatic plants of Cameroon: thier antibacterial activity and ability to permeabilize the cytoplasmic membrane of Listeria innocua examined by flow cytometry. Lett Appl Microbiol. 2004;39:395–400. doi: 10.1111/j.1472-765X.2004.01587.x. [DOI] [PubMed] [Google Scholar]
- Nielsen JL, Nielsen PH. Advances in microscopy: microautoradiography of single cells. Methods Enzymol. 2005;397:237–256. doi: 10.1016/S0076-6879(05)97014-6. [DOI] [PubMed] [Google Scholar]
- Nielsen JL, Juretschko S, Wagner M, Nielsen PH. Abundance and phylogenetic affiliation of iron reducers in activated sludge as assessed by fluorescence in situ hybridization and microautoradiography. Appl Environ Microbiol. 2002;68:4629–4636. doi: 10.1128/AEM.68.9.4629-4636.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouverney CC, Fuhrman JA. Combined microautoradiography-16rRNA probe technique for determination of radioisotope uptake by specific microbial cell types in situ. Appl Environ Microbiol. 1999;65:1746–1752. doi: 10.1128/aem.65.4.1746-1752.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamp SJ, Harrington ED, Quake SR, Relman DA, Blainey PC. Single-cell sequencing provides clues about the host interactions of segmented filamentous bacteria (SFB) Genome Res. 2012;22:1107–1119. doi: 10.1101/gr.131482.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel IS, Premasiri WR, Moir DT, Ziegler LD. Barcoding bacterial cells: A SERS based methodology for pathogen identification. J Raman Spectrosc. 2008;39:1660–1672. doi: 10.1002/jrs.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, Adams H, van Ree R, Stobberingh EE. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA birth cohort study. Gut. 2007;56:661–667. doi: 10.1136/gut.2006.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris-Bondia F, Latorre A, Artacho A, Moya A, D’Auria G. The active human gut microbiota differs from the total microbiota. PLoS One. 2011;6:e22448. doi: 10.1371/journal.pone.0022448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernthaler A, Pernthaler J, Amann R. Molecular microbial ecology manual. Kluwer Academic publishers; 2004. Sensitive multi-color fluorescence in situ hybridization for the identification of environmental microorganisms; pp. 711–726. Vol. 3 ed. [Google Scholar]
- Petit JM, Denis-Gay M, Ratinaud MH. Assessment of fluorochromes for cellular structure and function studies by flow cytometry. Biol Cell. 1993;78:1–13. doi: 10.1016/0248-4900(93)90109-r. [DOI] [PubMed] [Google Scholar]
- Pfeiffer JK, Sonnenburg JL. The intestinal microbiota and viral susceptibility. Front Microbiol. 2011;2:92. doi: 10.3389/fmicb.2011.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possemiers S, Rabot S, Espin JC, Bruneau A, Philippe C, Gonzalez-Sarrias A, Heyerick A, Tomas-Barberan FA, De Keukeleire D, Verstraete W. Eubacterium limosum activates isoxanthohumol from Hops (Humulus lupulus L.) into the potent phytoestrogen 8-prenylnaringenin in vitro and in rat intestine. J Nutr. 2008;138:1310–1316. doi: 10.1093/jn/138.7.1310. [DOI] [PubMed] [Google Scholar]
- Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- Radajewski S, McDonald IR, Murrell JC. Stable-isotope probing of nucleic acids: a window to the function of uncultured microorganisms. Curr Opin Biotechnol. 2003;14:296–302. doi: 10.1016/s0958-1669(03)00064-8. [DOI] [PubMed] [Google Scholar]
- Rath CM, Alexandrov T, Higginbottom SK, Song J, Milla ME, Fischbach MA, Sonnenburg JL, Dorrestein PC. Molecular analysis of model gut microbiotas by imaging mass spectrometry and nanodesorption electrospray ionization reveals dietary metabolite transformations. Anal Chem. 2012;84:9259–9267. doi: 10.1021/ac302039u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt N, Barclay AR, Weaver LT, Morrison DJ. Use of stable isotopes to measure the metabolic activity of the human intestinal microbiota. Appl Environ Microbiol. 2011;77:8009–8014. doi: 10.1128/AEM.05573-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshef DN, Reshef YA, Finucane HK, Grossman SR, McVean G, Turnbaugh PJ, Lander ES, Mitzenmacher M, Sabeti PC. Detecting novel associations in large data sets. Science. 2011;334:1518–1524. doi: 10.1126/science.1205438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigottier-Gois L, Le Bourhis AG, Gramet G, Rochet V, Dore J. Fluorescent hybridisation combined with flow cytometry and hybridisation of total RNA to analyse the composition of microbial communities in human faeces using 16S rRNA probes. FEMS Microbiol Ecol. 2003;43:237–245. doi: 10.1111/j.1574-6941.2003.tb01063.x. [DOI] [PubMed] [Google Scholar]
- Ritz M, Tholozan JL, Federighi M, Pilet MF. Morphological and physiological characterization of Listeria monocytogenes subjected to high hydrostatic pressure. Appl Environ Microbiol. 2001;67:2240–2247. doi: 10.1128/AEM.67.5.2240-2247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez GG, Phipps D, Ishiguro K, Ridgway HF. Use of a fluorescent redox probe for direct visualization of actively respiring bacteria. Appl Environ Microbiol. 1992;58:1801–1808. doi: 10.1128/aem.58.6.1801-1808.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaechter M, Maaloe O, Kjeldgaard NO. Dependency on medium and temperature of cell size and chemical composition during balanced growth of Salmonella typhimurium. J Gen Microbiol. 1958;19:592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- Schwiertz A, Le Blay G, Blaut M. Quantification of different Eubacterium spp. in human fecal samples with species-specific 16S rRNA-targeted oligonucleotide probes. Appl Environ Microbiol. 2000;66:375–382. doi: 10.1128/aem.66.1.375-382.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semova I, Carten JD, Stombaugh J, Mackey LC, Knight R, Farber SA, Rawls JF. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe. 2012;12:277–288. doi: 10.1016/j.chom.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro HM. Pratical Flow Cytometry. John Wiley and Sons; New York: 1995. [Google Scholar]
- Shapiro HM. Membrane potential estimation by flow cytometry. Methods. 2000;21:271–279. doi: 10.1006/meth.2000.1007. [DOI] [PubMed] [Google Scholar]
- Shen Y, Torchia ML, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe. 2012;12:509–520. doi: 10.1016/j.chom.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr BF, del Giorgio P, Sherr EB. Estimating abundance and single-cell characteristics of respiring bacteria via the redox dye CTC. Aquat Microb Ecol. 1999;18:117–131. [Google Scholar]
- Sieracki ME, Cucci TL, Nicinski J. Flow cytometric analysis of 5-cyano-2,3-ditolyl tetrazolium chloride activity of marine bacterioplankton in dilution cultures. Appl Environ Microbiol. 1999;65:2409–2417. doi: 10.1128/aem.65.6.2409-2417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sintes E, Herndl GJ. Quantifying substrate uptake by individual cells of marine bacterioplankton by catalyzed reporter deposition fluorescence in situ hybridization combined with microautoradiography. Appl Environ Microbiol. 2006;72:7022–7028. doi: 10.1128/AEM.00763-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JJ, McFeters GA. Mechanisms of INT (2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyl tetrazolium chloride), and CTC (5-cyano-2,3-ditolyl tetrazolium chloride) reduction in Escherichia coli K-12. J Microbiol Meth. 1997;29:161–175. doi: 10.1111/j.1365-2672.1996.tb03212.x. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Choi PJ, Li GW, Chen H, Babu M, Hearn J, Emili A, Xie XS. Quantifying E. coli proteome and transcriptome with single-molecule sensitivity in single cells. Science. 2010;329:533–538. doi: 10.1126/science.1188308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda G, Yamaoka I, Noda H. Localization of symbiotic clostridia in the mixed segment of the termite Nasutitermes takasagoensis (Shiraki) Appl Environ Microbiol. 2000;66:2199–2207. doi: 10.1128/aem.66.5.2199-2207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy BP, Gaida SM, Papoutsakis ET. Flow cytometry for bacteria: enabling metabolic engineering, synthetic biology and the elucidation of complex phenotypes. Curr Opin Biotechnol. 2010;21:85–99. doi: 10.1016/j.copbio.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Trasande L, Blustein J, Liu M, Corwin E, Cox LM, Blaser MJ. Infant antibiotic exposures and early-life body mass. Int J Obes (Lond) 2012 doi: 10.1038/ijo.2012.132. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuma RS, Beaudet MP, Jin XK, Jones LJ, Cheung CY, Yue S, Singer VL. Characterization of SYBR gold nucleic acid gel stain: A dye optimized for use with 300-nm ultraviolet transilluminators. Anal Biochem. 1999;268:278–288. doi: 10.1006/abio.1998.3067. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Henrissat B, Gordon JI. Viewing the human microbiome through three-dimensional glasses: integrating structural and functional studies to better define the properties of myriad carbohydrate-active enzymes. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010;66:1261–1264. doi: 10.1107/S1744309110029088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Quince C, Faith JJ, et al. Organismal, genetic, and transcriptional variation in the deeply sequenced gut microbiomes of identical twins. Proc Natl Acad Sci U S A. 2010;107:7503–7508. doi: 10.1073/pnas.1002355107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaahtovuo J, Korkeamaki M, Munukka E, Viljanen MK, Toivanen P. Quantification of bacteria in human feces using 16S rRNA-hybridization, DNA-staining and flow cytometry. J Microbiol Meth. 2005;63:276–286. doi: 10.1016/j.mimet.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Valm AM, Mark Welch JL, Rieken CW, Hasegawa Y, Sogin ML, Oldenbourg R, Dewhirst FE, Borisy GG. Systems-level analysis of microbial community organization through combinatorial labeling and spectral imaging. Proc Natl Acad Sci U S A. 2011;108:4152–4157. doi: 10.1073/pnas.1101134108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickerman JC, Gilmore IS. Surface analysis the principle techniques. John Wiley & Sons; Chichester, UK: 2009. [Google Scholar]
- Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M. Single-Cell Ecophysiology of Microbes as Revealed by Raman Microspectroscopy or Secondary Ion Mass Spectrometry Imaging. Annu Rev Microbiol. 2009;63:411–429. doi: 10.1146/annurev.micro.091208.073233. [DOI] [PubMed] [Google Scholar]
- Wallace BD, Wang H, Lane KT, et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science. 2010;330:831–835. doi: 10.1126/science.1191175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner G, Amann R, Beisker W. Optimizing fluorescent in situ hybridization with rRNA-targeted oligonucleotide probes for flow cytometric identification of microorganisms. Cytometry. 1993;14:136–143. doi: 10.1002/cyto.990140205. [DOI] [PubMed] [Google Scholar]
- Wallner G, Erhart R, Amann R. Flow cytometric analysis of activated sludge with rRNA-targeted probes. Appl Environ Microbiol. 1995;61:1859–1866. doi: 10.1128/aem.61.5.1859-1866.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh S, Lappin-Scott HM, Stockdale H, Herbert BN. An assessment of the metabolic activity of starved and vegetative bacteria using two redox dyes. J Microbiol Meth. 1995;24:1–9. [Google Scholar]
- Wang Y, Hammes F, Boon N, Chami M, Egli T. Isolation and characterization of low nucleic acid (LNA)-content bacteria. ISME J. 2009;3:889–902. doi: 10.1038/ismej.2009.46. [DOI] [PubMed] [Google Scholar]
- Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng M, Huntley D, Huang IF, Foye-Jackson O, Wang L, Sarkissian A, Zhou Q, Walker WA, Cherayil BJ, Shi HN. Alternatively activated macrophages in intestinal helminth infection: effects on concurrent bacterial colitis. J Immunol. 2007;179:4721–4731. doi: 10.4049/jimmunol.179.7.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitby C, Bailey M, Whiteley A, Murrell C, Kilham K, Prosser J, Lappin-Scott H. Stable isotope probing links taxonomy with function in microbial communities - Microbial ecologists have a culture-independent means for analyzing sources of metabolic activities among complex mixtures of microbes. ASM News. 2005;71:169–173. [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz P, Kottmann R, Field D, et al. Minimum information about a marker gene sequence (MIMARKS) and minimum information about any (x) sequence (MIxS) specifications. Nat Biotechnol. 2011;29:415–420. doi: 10.1038/nbt.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeggini E, Scott LJ, Saxena R, et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet. 2008;40:638–645. doi: 10.1038/ng.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoetendal EG, Ben-Amor K, Harmsen HJM, Schut F, Akkermans ADL, de Vos WM. Quantification of uncultured Ruminococcus obeum-like bacteria in human fecal samples by fluorescent in situ hybridization and flow cytometry using 16S rRNA-targeted probes. Appl Environ Microbiol. 2002;68:4225–4232. doi: 10.1128/AEM.68.9.4225-4232.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubkov MV, Allen JI, Fuchs BM. Coexistence of dominant groups in marine bacterioplankton community - a combination of experimental and modelling approaches. J Mar Biol Assoc Uk. 2004;84:519–529. [Google Scholar]