Summary
While infections with methicillin-resistant Staphylococcus aureus (MRSA) were traditionally restricted to the hospital setting, novel MRSA strains emerged over the last two decades that have the capacity to infect otherwise healthy people outside of the hospital setting. These communityassociated (CA-) MRSA strains combine methicillin resistance with enhanced virulence and fitness. Interestingly, CA-MRSA strains emerged globally and from different backgrounds, indicating that the “trade-off” between maintaining sufficient levels of methicillin resistance and obtaining enhanced virulence at a low fitness cost was achieved on several occasions in convergent evolution. However, frequently this process comprised similar changes. First and foremost, all CA-MRSA strains typically carry a novel type of methicillin resistance locus that appears to cause less of a fitness burden. Additionally, acquisition of specific toxin genes, most notably that encoding Panton-Valentine leukocidin (PVL), and adaptation of gene expression of genome-encoded toxins, such as alpha-toxin and phenol-soluble modulins (PSMs), further contributed to the evolution of CA-MRSA. Finally, the exceptional epidemiological success of the USA300 CA-MRSA clone in particular may have been due to yet another gene acquisition, namely that of the speG gene, which is located on the arginine catabolic mobile element (ACME) and involved in detoxifying harmful host-derived polyamines.
Keywords: Staphylococcus aureus, MRSA, community-associated MRSA, alpha-toxin, Panton-Valentine leukocidin, phenol-soluble modulin
Introduction
Antibiotic resistance is a big problem during the treatment of bacterial infections. While antibiotics are an effective means to control such infections, antibiotic overuse triggers the spread of resistant strains in the population. As a result, many strains of dangerous bacterial pathogens nowadays are resistant to antibiotics, with some strains combining even multiple resistances to different antibiotics.
Possibly the most infamous example of an antibiotic-resistant pathogen is Staphylococcus aureus (Lowy, 2003). This bacterium asymptomatically colonizes about a third of the population and may cause moderately severe to severe and occasionally life-threatening infections(Lowy, 1998). Notably, it is the most common cause of nosocomial infections and a leading cause of death in hospitalized patients. This extreme morbidity and mortality is due largely to the fact that many S. aureus strains carry genes that provide resistance to a variety of antibiotics, including the most efficient and widely used anti-staphylococcal drugs.
Penicillin and its derivatives are very effective against staphylococci. However, soon after the introduction of penicillin intro clinical use, penicillin-resistant, penicillinase-containing S. aureus strains spread all over the world(Barber and Rozwadowska-Dowzenko, 1948; Kirby, 1944). As a response to the fact that penicillin became ineffective against many infectious S. aureus strains, the penicillinase-resistant penicillin derivative methicillin was introduced in 1959. However, methicillin-resistant S. aureus (MRSA) was found within a year (Barber, 1961). Beginning in the 1980s, MRSA spread globally to such an extent that many countries now report MRSA rates of 50% or higher among infective S. aureus isolates in hospitals(Diekema et al., 2001). Only some countries, such as the Netherlands and the Scandinavian countries, which have effective search-and-destroy policies and/or control antibiotic overuse, have so far succeeded in keeping MRSA rates at a low level.
Until the mid 1990s, MRSA infections were limited to hospitals, infecting primarily the elderly, very young, and patients with weakened immune systems or undergoing surgery. However, within the last ~ 15 years, MRSA outbreaks were reported in healthy individuals without connection to health care institutions, such as sports teams, army recruits, or prisoners (Chambers, 2001). It soon became clear that these infections were due to the rise of new, distinct strains of MRSA, now called CA-MRSA strains. In the present review I will address the question what makes these strains different from hospital-associated (HA-) MRSA, enabling them to spread sustainably in the population and cause disease in otherwise healthy people.
Epidemiology and CA-MRSA disease manifestations
The first well documented CA-MRSA cases appeared in the upper midwestern United States between 1997 and 1999in children (CDC, 1999). These infections, which were fatal cases of sepsis and severe pneumonia, were caused by strain MW2 (pulsed-field type USA400). In the meantime, closely related strains belonging to pulsed-field type strain USA300 have replaced USA400 strains in the U.S. (Moran et al., 2006), but USA400 CA-MRSA infections are still observed in Alaska (David et al., 2008). While the U.S. has experienced the most pronounced CA-MRSA epidemic, CA-MRSA is also a global problem. Global strains of CA-MRSA belong to a series of different lineages; and specific strains are predominant in different countries (Mediavilla et al., 2012). For example, infections with CA-MRSA strains belonging to sequence type (ST) 80 are common in Europe and ST30 CA-MRSA infections occur predominantly in Australasia.
By far the most frequent disease manifestation associated with CA-MRSA is infection of the skin and soft tissues(Fridkin et al., 2005). Skin and soft tissue infections (SSTI) account for at least 90% of CA-MRSA infections. CA-MRSA SSTI are usually moderately severe to severe, often very painful, and are treated by simple incision and drainage. However, rare cases of very dramatic skin infections, such as necrotizing fasciitis, have been reported with CA- in contrast to HA-MRSA(Miller et al., 2005).CA-MRSA strains also cause infections of the bones and joints, such as osteomyelitis, and respiratory infections, such as pneumonia, sepsis, and urinary tract infections. Depending on the survey, these types of infections each account for <1 and 4% of all CA-MRSA infections.
Antibiotic resistance
With respect to antibiotic resistance, CA-MRSA strains are distinguished from HA-MRSA strains by three characteristics: First, CA-MRSA harbor different types of SCCmec elements, the mobile genetic elements (MGEs) encoding methicillin resistance genes. Traditional SCCmec elements found in HA-MRSA are most frequently of types I, II, and III; in contrast, CA-MRSA have SCCmec elements of types IV and V, which are shorter and thus believed to cause less of a fitness burden(Daum et al., 2002; Hiramatsu et al., 2001; Lee et al., 2007).
Second, CA-MRSA isolates are usually sensitive to most antibiotics other than methicillin and beta-lactams, while multi-resistance is common in HA-MRSA isolates. There have been reports on multi-resistant CA-MRSA; for example, a specific USA300 clone caused an outbreak of multi-resistant CA-MRSA in San Francisco (Diep et al., 2008a). This outbreak clone had accumulated resistance to mupirocin and clindamycin, and most isolates were also resistant to tetracycline. Non-multi-resistant USA300 isolates commonly only are resistant to erythromycin and ciprofloxacin, in addition to methicillin and beta-lactams. Fortunately, multi-resistant CA-MRSA isolates are still an exception.
Third, MIC (minimal inhibitory concentration) values of HA-MRSA clones are usually higher than those of CA-MRSA clones. For example, strain MRSA252 (USA200, EMRSA-16), a typical HA-MRSA clone from the United Kingdom, has an MIC that is > 50 times higher than that of strain LAC, a USA300 CA-MRSA clone isolated from prisoners in Los Angeles that is often used in laboratory research(Cheung et al., 2011).
Virulence characteristics
The observation that CA-MRSA strains have the capacity to infect otherwise healthy people indicates enhanced virulence. Accordingly, Voyich et al. showed in 2005 that the CA-MRSA strains MW2 and LAC are more virulent in a mouse infection model than common HA-MRSA strains such as COL and MRSA252(Voyich et al., 2005). Furthermore, these authors found that the enhanced virulence of CA-MRSA strains was accompanied by enhanced survival in human neutrophils, suggesting that the interaction of the pathogen with neutrophils, a critical step in the establishment of S. aureus infection, determines the increased virulence of CA-MRSA compared to HA-MRSA (Voyich et al., 2005).
It is important to stress that CA-MRSA strains are not more virulent than many MSSA strains. For example, MSSA strains of the ST8 lineage, such as those of the NCTC8325 family that are frequently used for laboratory experiments, are at least as virulent in animal infection models as CA-MRSA strains (Cheung et al., 2011). What distinguishes CA-MRSA strains is the combination of methicillin resistance with virulence characteristics not previously seen with HA-MRSA strains.
Toxins
The species S. aureus is infamous for producing a plethora of toxins, some of which are found virtually in all S. aureus, while others are linked to MGEs and restricted to a subset of strains. Therefore, a specific toxin repertoire or an enhanced production of toxins appeared as a likely basis for the enhanced virulence characteristics of CA-MRSA. As evasion of neutrophil killing was at least one of the predominant factors assumed to be associated with that enhanced virulence, staphylococcal leukocidins were in the center of investigations that were undertaken to delineate the molecular basis of CA-MRSA virulence.
Panton-Valentine leukocidin
Panton-Valentine leukocidin (PVL) is a two-component leukocidin that belongs to a beta-barrel forming family of cytolytic toxins, also comprising several other leukocidins, gamma-toxin, and alpha-toxin (Szmigielski et al., 1999). PVL is encoded by the prophage-encoded adjacent lukS and lukF genes,which produce the two toxin parts, LukS and LukF. Both are needed for the cytolytic activity of the toxin. Already in 1932, Panton and Valentine noted an association between PVL production and abscess formation(Panton and Valentine, 1932), but interest in PVL increased enormously when an epidemiological association between presence of the lukSF genes and CA-MRSA was detected (Vandenesch et al., 2003). Most CA-MRSA strains have lukSF genes, while their frequency in MSSA is much lower, and they are absent from predominant HA-MRSA clones.
PVL is lytic to human neutrophils at concentrations between 0.3 and 2 µg/ml (Genestier et al., 2005). Concentrations of PVL reaching or exceeding that range were demonstrated in human skin abscesses and in some clinical specimens from different infection types (Badiou et al., 2008; Badiou et al., 2010). In laboratory experiments, the contribution of PVL to the lytic activity toward human neutrophils when assayed with CA-MRSA culture filtrates proved strongly dependent on the type of growth media used(Graves et al., 2010). For that reason, such studies are barely conclusive when judging the role of PVL in CA-MRSA virulence. When assayed with live bacteria, PVL did not have a significant contribution to neutrophil killing by strains LAC and MW2 (Voyich et al., 2006). Quite unexpectedly, these laboratory experiments thus indicated that the contribution of PVL to the neutrophil killing capacity of important CA-MRSA strains is minor.
Accordingly, several skin and lung infection studies in mice with isogenic deletion mutants of the lukSF genes in strains LAC (USA300) and MW2 (USA400) did not reveal differences in virulence (Bubeck Wardenburg et al., 2007; Bubeck Wardenburg et al., 2008; Voyich et al., 2006). However, some other mouse studies showed differences (Brown et al., 2008; Labandeira-Rey et al., 2007), although in part these results may have been caused by unintended secondary mutations (Villaruz et al., 2009). Notably, it was found later that mouse neutrophils are much less sensitive to the cytolytic activity of PVL than those of humans (Hongo et al., 2009). Mechanistically, this difference was recently explained by the discovery of species-specific interaction of PVL with C5a receptors (Spaan et al., 2012), explaining the negative results of the earlier mouse infection studies regarding the contribution of PVL to CA-MRSA virulence. These findings lay to rest the intense scientific discussion about the initial controversial findings of the mouse infection studies, which now have no more than historical importance.
The sensitivity of rabbit neutrophils to PVL is in the range of those of humans (Loffler et al., 2010; Szmigielski et al., 1999); and therefore, more recent infection studies were performed in rabbits. In a model of severe pneumonia, rabbits showed increased morbidity and mortality when infected with PVL-containing USA300 as compared to an isogenic lukSF mutant, indicating a significant role of PVL in the development of severe CA-MRSA pneumonia (Diep et al., 2010). Notably, in this study the contribution of PVL to pathogenesis could be linked entirely to its interaction with neutrophils. Furthermore, PVL had a significant effect in experimental CA-MRSA (USA300) osteomyelitis when assayed in rabbits (Cremieux et al., 2009). However, results in rabbit skin infection models with USA300 were controversial. Lipinska et al. demonstrated a moderate yet significant impact of PVL on rabbit skin infection (Lipinska et al., 2011), whereas Kobayashi et al. failed to detect such an effect (Kobayashi et al., 2011). Notably, in the Kobayashi study, lukSF mutants were also compared to mutants in hla (alpha-toxin) and psmα (phenol-soluble modulinα operon), both of which showed a strong impact on the development of skin infection. Furthermore, Li et al. compared the virulence of different globally occurring PVL-positive and -negative CA-MRSA clones and did not find a correlation between presence or expression of the lukSF genes and virulence, while they found such a correlation for expression of the psmα and hla genes (Li et al., 2010). The reason why divergent results were obtained in these rabbit skin infection studies is unclear. Possibly, intradermal injection as used in the Lipinska et al. and Li et al. studies may better reflect the natural infection scenario leading to the types of skin infection that are typical for PVL-positive strains, such as furunculosis or folliculitis (Del Giudice et al., 2011). However, while using the same mode of injection, the Lipinska et al. and Li et al. studies reached contrasting conclusions on the role of PVL in skin infection.
In addition to causing lysis of neutrophils, PVL has substantial pro-inflammatory effects that can be observed at concentrations as low as 5 ng/ml (Konig et al., 1995). As with many toxins, it is not clear whether such pro-inflammatory effects are of benefit to the bacteria, as they may cause excessive phagocyte infiltration and tissue damage, or whether they serve the human innate immune system to recognize infiltrating bacteria and launch a defensive response. Most likely, both mechanisms contribute to infection outcome and it depends on the specific scenario, which has a stronger impact. Diep et al. provided evidence in a rabbit pneumonia infection model suggesting that the pro-inflammatory effects of PVL contribute to increased infectivity (Diep et al., 2010). In contrast, Yoong et al. showed that presence of PVL stimulates the immune system, leading to better clearance of the bacteria (Yoong and Pier, 2010).
Finally, it needs to be noted that an increasing number of CA-MRSA clones are found that do not contain lukSF genes (Otto, 2010), for example in Korea (Lee et al., 2010) and the United Kingdom (Otter and French, 2008). Li et al. showed in rabbit skin infection studies that these clones are in average not less virulent than lukSF-containing CA-MRSA clones (Li et al., 2010). Furthermore, there was no difference in virulence between USA500, the PVL-negative progenitor of USA300, and USA300 itself. Altogether, the intensive recent research on PVL and its role in CA-MRSA infection has shown that the importance of PVL as a determinant of CA-MRSA virulence was initially overestimated. PVL certainly is a virulence factor, but only in specific infection types and scenarios, and only in some strains, does it appear to significantly contribute to the severity of disease caused by CA-MRSA.
Phenol-soluble modulins
Phenol-soluble modulins (PSMs) are a family of amphipathic, α-helical peptide produced by staphylococci (Wang et al., 2007). Many members of the PSM family have pronounced cytolytic activity toward a variety of human cells, including neutrophils and erythrocytes (Cheung et al., 2012; Cheung et al., 2010; Wang et al., 2007). In addition, PSMs trigger inflammatory responses by interaction with the formyl peptide receptor 2 (FPR2) (Kretschmer et al., 2010). PSMs also contribute to staphylococcal biofilm formation by forming fibril-like structures (Schwartz et al., 2012), structure biofilms, and cause biofilm detachment, resulting in the dissemination of biofilm-associated infection (Periasamy et al., 2012; Wang et al., 2011).
While the ~ 40–45 amino acid long β-type PSMs are barely cytolytic, PSMs of the α-type, ~ 20–25 amino acids in length, may have strong cytolytic activity (Cheung et al., 2012; Cheung et al., 2010; Wang et al., 2007). In particular, the PSMα peptides of S. aureus, PSMα1 – PSMα4, which are encoded in the psmα operon, contain the potent cytolysin PSMα3. S. aureus delta-toxin, a member of the α-type PSMs, is a moderately potent yet often strongly expressed cytolysin.
PSMs are produced in high amounts in CA-MRSA strains, whereas production is in average lower in typical HA-MRSA strains such as USA100 and USA200 strains (Li et al., 2009; Wang et al., 2007). This may at least in part be due to the facts that (i) the accessory gene regulator (Agr) virulence regulator exerts an exceptionally strict control over PSM expression (Queck et al., 2008), and (ii) HA-MRSA strains often show low, while CA-MRSA strains commonly have high Agr activity (Li et al., 2009).
The PSMα peptides have a significant impact on CA-MRSA virulence in experimental skin infection using mice or rabbits and bacteremia in mice (Kobayashi et al., 2011; Wang et al., 2007). Notably, PSMα peptides, above all PSMα3, are responsible for the increased neutrophil killing capacity that distinguishes CA- from HA-MRSA strains (Wang et al., 2007). Recent findings suggest that PSMs are expressed after neutrophil ingestion of the bacteria in the neutrophil phagosome as a result of Agr induction, identifying PSMs as the main mediator of quorum- (or diffusion-) sensing-induced neutrophil killing after S. aureus ingestion (Surewaard et al., 2012).
Alpha-toxin
Alpha-toxin is a cytolysin that is produced by most S. aureus strains (Bhakdi and Tranum-Jensen, 1991). While it is not lytic to human neutrophils (Valeva et al., 1997), it lyses a series of other cell types including macrophages and erythrocytes. The cytolytic activity of alpha-toxin is dependent on the interaction with the ADAM10 receptor (Wilke and Bubeck Wardenburg, 2010); and ADAM10-deficient mice are protected from lethal pneumonia and severe S. aureus skin infection (Inoshima et al., 2011; Inoshima et al., 2012). Alpha-toxin also leads to neutrophil chemotaxis (Bartlett et al., 2008)and has pro-inflammatory effects, including induction of the inflammasome (Craven et al., 2009) and generation of highly pro-inflammatory cytokines such as IL-1β and IL-18 (Craven et al., 2009). Finally, alpha-toxin has been shown to contribute to the penetration of the epithelial barrier during skin infection with USA300 (Soong et al., 2012).
Alpha-toxin has a demonstrated impact on virulence in many infection models. In CA-MRSA (USA300) pneumonia, alpha-toxin has a significant effect on morbidity and mortality(Bubeck Wardenburg et al., 2007). Immunization of mice with the mutant, non-toxic H35L derivative of alpha-toxin or passive immunization with anti-alpha-toxin antibodies protects from lethal pulmonary challenge(Bubeck Wardenburg and Schneewind, 2008). Furthermore, alpha-toxin has a strong impact on the development of USA300 skin infection (Kobayashi et al., 2011), which is also prevented by passive or active immunization (Kennedy et al., 2010).
Gene expression versus gene acquisition
The evolution of virulence in S. aureus clones and lineages is frequently only analyzed in terms of acquisition or loss of virulence-associated genes. However, significant changes in virulence may arise from minute changes in the genome, for example in regulatory loci such as Agr (DeLeo et al., 2011; Kennedy et al., 2008),which may remain unrecognized in such analyses. PSMs and alpha-toxin are expressed at high levels in CA- compared to HA-MRSA strains (Li et al., 2009), in accordance with the notion that gene expression changes contributed to the evolution of highly virulent CA-MRSA. This is likely at least in part due to the high activity in CA-MRSA of Agr, which controls expression of most S. aureus toxins and has a strong impact on CA-MRSA virulence(Cheung et al., 2011). Interestingly, it was reported that Agr controls genes in CA-MRSA strains in a fashion that is somewhat different from other strains (Cheung et al., 2011). However, the specific genetic events that led to these gene expression changes are still unknown.
Notably, explaining the evolution of virulence of CA-MRSA based on the core genomeencoded alpha-toxin and PSMs or the MGE-encoded PVL is thus categorically different in terms of recognizing the contribution of gene expression versus only that of gene acquisition or presence. However, these two mechanisms are not mutually exclusive and it is likely that they both contributed to the evolution of CA-MRSA virulence. Furthermore, because PVL is also under control of Agr (Queck et al., 2008), both gene acquisition and enhanced expression mechanisms may have contributed to the evolution of the part of CA-MRSA virulence that is based on PVL.
Fitness
The epidemiological success of pathogenic S. aureus is not solely due to virulence factors in the stricter sense, such as toxins. It is also dependent on factors enhancing what can be called “fitness”, i.e. the capacity to grow and persist in the human host. S. aureus is a colonizer at least in parts of the human population (Wertheim et al., 2005)and infections commonly originate from colonizing strains(von Eiff et al., 2001). Whether this is always true for CA-MRSA infection is not entirely clear, as direct infection from patients with S. aureus skin infection or from infected fomites appears possible. Nevertheless, factors that enhance fitness of CA-MRSA during colonization likely contribute to the spread of CA-MRSA in the population, by increasing the reservoir for subsequent infection and thus the epidemiological success of CA-MRSA as pathogens. Furthermore, genes that promote growth and persistence during infection also ultimately increase CA-MRSA virulence. The investigation of such factors has lacked behind the analysis of CA-MRSA toxins. However, as mentioned above, certain SCCmec elements are characteristic for CA-MRSA. In-vitro results indicate that SCCmec type IV has a role in enhancing CA-MRSA fitness(Lee et al., 2007), but in an in-vivo model of rabbit bacteremia, no impact on competitive fitness was detected (Diep et al., 2008b). Nevertheless, the fact that all CA-MRSA strains detected so far harbor one type of the novel, short SCCmec types IV or V indicates that they played a crucial role in the evolution of CA-MRSA.
Enhanced success of the USA300 CA-MRSA strain in particular has recently been linked to genes present in the arginine catabolic mobile element (ACME), which among CA-MRSA clones is limited to strain USA300, and was most likely acquired from S. epidermidis (Diep et al., 2006; Diep et al., 2008b). The most predominant features of ACME are an arginine deiminase and an oligopeptide transport gene cluster. These were frequently speculated to contribute to the fitness of strain USA300, such as by increasing survival in an acidic environment as found on the human skin or enhancing nutrient uptake, respectively (Diep et al., 2006; Diep and Otto, 2008). However, while ACME overall appeared to increase pathogen survival, no particular role could be attributed to either of these two clusters. Recently, Joshi et al. demonstrated that a decisive function in fitness might be due to another gene present on ACME, namely a spermidin Nacetyltransferase encoded by the speG gene (Joshi et al., 2011). While S. aureus has a unique sensitivity among bacteria to polyamines such as spermidin, SpeG detoxifies polyamines and may thus enhance survival of USA300 on the human skin.
Conclusions
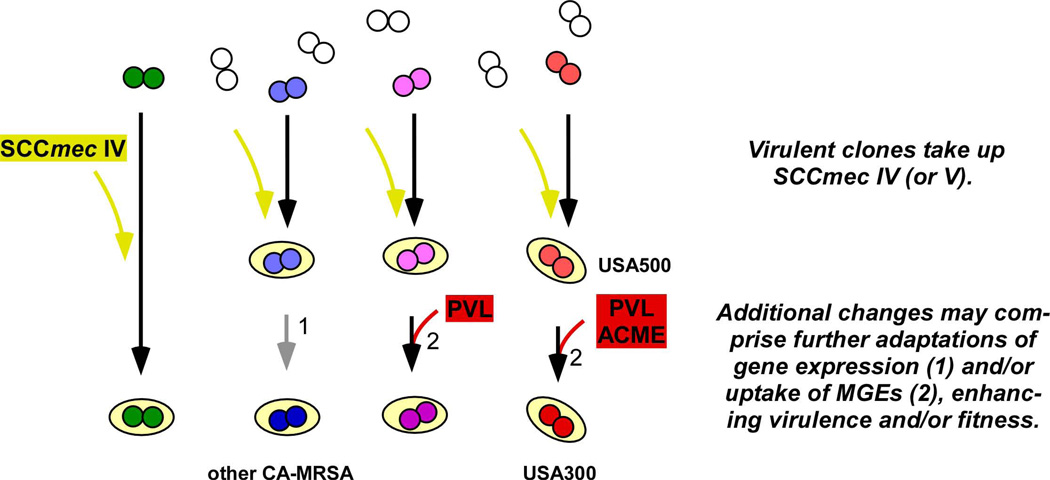
Recent research revealed that CA-MRSA strains have increased virulence and fitness properties compared to traditional HA-MRSA strains. Mechanistically, this is likely due to a combination of (i) the acquisition of novel genes on MGEs, such as novel SCCmec elements, the PVL-encoding phage or, in case of USA300, ACME, and (ii) adaptations in gene expression, most importantly resulting in enhanced toxin expression (Fig. 1).
Fig. 1. Evolution of CA-MRSA.
The acquisition of SCCmec type IV (or V) by virulent strains appears to have been a common first step in the evolution of CA-MRSA strains. In some strains, as shown on the left, this combination may have been sufficient to produce highly virulent, methicillin-resistant strains with the capacity to infect healthy people, while in others, additional steps were required. These additional steps may have involved adaptations of gene expression, such as to enhance toxin production, and/or the uptake of MGEs, including or not including the acquisition of PVL-encoding genes. On the right, the assumed scenario for the evolution of USA300 is shown. Uptake of SCCmec type IV by a virulent ST8 strain resulted in a virulent MRSA strain (USA500), whose fitness was further improved by uptake of ACME. Acquisition of PVL-encoding genes appears to have increased virulence at least in some infection types.
The development of high virulence and the acquisition of methicillin resistance cannot be separated when analyzing the evolution of CA-MRSA. The acquisition of the low fitness-cost SCCmec elements appears to have been a necessary step in that evolution. In addition, results obtained so far indicate that there is a trade-off between the expression of methicillin resistance and that of important virulence determinants in MRSA. In support of that idea, CA-MRSA have lower methicillin resistance than HA-MRSA and HA-MRSA express toxins and other virulence genes at lower levels. The effective combination of methicillin resistance with high toxin expression likely required a specific strain background in which the acquisition of SCCmec did not lead to significant fitness and toxin expression decreases, or genetic adaptations subsequent to the acquisition of methicillin resistance. The precise nature of these genetic changes is largely unknown. Interestingly, recent findings indicate that there are regulatory components on SCCmec elements, including the methicillin resistance genes themselves, which control expression of genome-encoded genes (Pozzi et al., 2012). Furthermore, the genome-encoded virulence regulator Agr regulates expression of mecA in USA300 (Cheung et al., 2011). The “trade-off” between methicillin resistance and virulence gene expression may thus happen in a fashion that is more direct than previously thought.
The contribution of toxins to CA-MRSA virulence has received most attention, in part because these may represent promising targets for therapeutic intervention. For such endeavors, it is of utmost importance to analyze and rank their relative importance for CA-MRSA pathogenesis. The key players that have been linked to CA-MRSA virulence, alpha-toxin, PSMα peptides, and PVL, were analyzed in a comparative fashion in rabbit pneumonia and skin infection models. Alpha-toxin and PSMα turned out to strongly impact skin infection, but PVL had no effect in that study (Kobayashi et al., 2011). All three toxins significantly affected the progression of severe pneumonia, with PVL showing the greatest impact (Diep, 2010). These findings reflect that different types of CA-MRSA disease may require different toxins and thus, different therapeutic approaches. Notably, these studies were performed with USA300, while we do not yet understand which factors mainly contribute to disease progression in CA-MRSA other than USA300, particularly in PVL-negative CA-MRSA.
In conclusion, there are certain factors that CA-MRSA have in common and distinguish them from other MRSA (Table 1). These comprise the presence of low-fitness cost SCCmec elements and increased virulence characteristics. However, different CA-MRSA clones appear to have achieved the goal of simultaneous expression of sufficiently high methicillin resistance and aggressive virulence characteristics using different approaches in convergent evolution (Fig. 1). Most appear to have up-regulated toxin expression. Some, but not all, relied on acquisition of additional toxins such as PVL. Specific ones, such as USA300, relied on yet other factors such as ACME to increase resistance to host-derived toxic agents and thus survival in the human host.
Table 1.
Characteristic virulence- and fitness-associated features of CA-MRSA.
| Factor | Role | Presence | Remarks |
|---|---|---|---|
| Virulence | |||
| Alpha-toxin | Cytolysin, pro- Inflammatory |
All CA-MRSA Increased Expression compared to HA- MRSA |
Affects outcome of CA-MRSA pneumonia, skin Infection |
| PSMα peptides (PSMα1 - PSMα4) |
Cytolysins, leukocidins, pro- Inflammatory |
All CA-MRSA Increased Expression compared to HA- MRSA |
Affects outcome of CA-MRSA pneumonia, skin Infection |
| Panton-Valentine leukocidin (PVL) |
Leukocidin, pro- Inflammatory |
Some CA-MRSA, including USA300 Located on prophage, absent from HA-MRSA |
Affects outcome of CA-MRSA pneumonia, controversial reports on skin infection |
| Agr | Virulence regulator | All CA-MRSA Increased Expression compared to HA- MRSA |
Controls expression of alpha-toxin, PSMα, PVL and PSMα, PVL and other toxins |
| Fitness | |||
| SCCmec types IV or V |
Carry methicillin resistance genes |
All CA-MRSA Absent from HA- MRSA |
Less fitness cost compared to other SCCmec types? |
| SpeG | N-spermidin- acetyltransferase |
On ACME (only in USA300) |
Abrogates S. aureus sensitivity to host- derived toxic polyamines |
Acknowledgements
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badiou C, Dumitrescu O, Croze M, Gillet Y, Dohin B, Slayman DH, Allaouchiche B, Etienne J, Vandenesch F, Lina G. Panton-Valentine leukocidin is expressed at toxic levels in human skin abscesses. Clin Microbiol Infect. 2008;14:1180–1183. doi: 10.1111/j.1469-0691.2008.02105.x. [DOI] [PubMed] [Google Scholar]
- Badiou C, Dumitrescu O, George N, Forbes AR, Drougka E, Chan KS, Ramdani-Bouguessa N, Meugnier H, Bes M, Vandenesch F, Etienne J, Hsu LY, Tazir M, Spiliopoulou I, Nimmo GR, Hulten KG, Lina G. Rapid detection of Staphylococcus aureus Panton-Valentine leukocidin in clinical specimens by enzyme-linked immunosorbent assay and immunochromatographic tests. J Clin Microbiol. 2010;48:1384–1390. doi: 10.1128/JCM.02274-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber M. Methicillin-resistant staphylococci. J Clin Pathol. 1961;14:385–393. doi: 10.1136/jcp.14.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber M, Rozwadowska-Dowzenko M. Infection by penicillin-resistant staphylococci. Lancet. 1948;2:641–644. doi: 10.1016/s0140-6736(48)92166-7. [DOI] [PubMed] [Google Scholar]
- Bartlett AH, Foster TJ, Hayashida A, Park PW. Alpha-toxin facilitates the generation of CXC chemokine gradients and stimulates neutrophil homing in Staphylococcus aureus pneumonia. J Infect Dis. 2008;198:1529–1535. doi: 10.1086/592758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus . Microbiol Rev. 1991;55:733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EL, Dumitrescu O, Thomas D, Badiou C, Koers EM, Choudhury P, Vazquez V, Etienne J, Lina G, Vandenesch F, Bowden MG. The Panton-Valentine leukocidin vaccine protects mice against lung and skin infections caused by Staphylococcus aureus USA300. Clin Microbiol Infect. 2009;1:156–164. doi: 10.1111/j.1469-0691.2008.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubeck Wardenburg J, Bae T, Otto M, DeLeo FR, Schneewind O. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med. 2007;13:1405–1406. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- Bubeck Wardenburg J, Palazzolo-Ballance AM, Otto M, Schneewind O, DeLeo FR. Panton-Valentine leukocidin is not a virulence determinant in murine models of community-associated methicillin-resistant Staphylococcus aureus disease. J Infect Dis. 2008;198:1166–1170. doi: 10.1086/592053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubeck Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med. 2008;205:287–294. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. From the Centers for Disease Control and Prevention. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus--Minnesota and North Dakota, 1997–1999. Jama. 1999;282:1123–1125. [PubMed] [Google Scholar]
- Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001;7:178–182. doi: 10.3201/eid0702.010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GY, Duong AC, Otto M. Direct and synergistic hemolysis caused by Staphylococcus phenol-soluble modulins: implications for diagnosis and pathogenesis. Microbes Infect. 2012;14:380–386. doi: 10.1016/j.micinf.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GY, Rigby K, Wang R, Queck SY, Braughton KR, Whitney AR, Teintze M, DeLeo FR, Otto M. Staphylococcus epidermidis strategies to avoid killing by human neutrophils. PLoS Pathog. 2010;6:e1001133. doi: 10.1371/journal.ppat.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung GY, Wang R, Khan BA, Sturdevant DE, Otto M. Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect Immun. 2011;79:1927–1935. doi: 10.1128/IAI.00046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven RR, Gao X, Allen IC, Gris D, Bubeck Wardenburg J, McElvania-Tekippe E, Ting JP, Duncan JA. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS ONE. 2009;4:e7446. doi: 10.1371/journal.pone.0007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremieux AC, Dumitrescu O, Lina G, Vallee C, Cote JF, Muffat-Joly M, Lilin T, Etienne J, Vandenesch F, Saleh-Mghir A. Panton-valentine leukocidin enhances the severity of community-associated methicillin-resistant Staphylococcus aureus rabbit osteomyelitis. PLoS One. 2009;4:e7204. doi: 10.1371/journal.pone.0007204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum RS, Ito T, Hiramatsu K, Hussain F, Mongkolrattanothai K, Jamklang M, BoyleVavra S. A novel methicillin-resistance cassette in community-acquired methicillin-resistant Staphylococcus aureus isolates of diverse genetic backgrounds. J Infect Dis. 2002;186:1344–1347. doi: 10.1086/344326. [DOI] [PubMed] [Google Scholar]
- David MZ, Rudolph KM, Hennessy TW, Boyle-Vavra S, Daum RS. Molecular epidemiology of methicillin-resistant Staphylococcus aureus, rural southwestern Alaska. Emerg Infect Dis. 2008;14:1693–1699. doi: 10.3201/eid1411.080381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice P, Bes M, Hubiche T, Blanc V, Roudiere L, Lina G, Vandenesch F, Etienne J. Panton-Valentine leukocidin-positive Staphylococcus aureus strains are associated with follicular skin infections. Dermatology. 2011;222:167–170. doi: 10.1159/000324044. [DOI] [PubMed] [Google Scholar]
- DeLeo FR, Kennedy AD, Chen L, Bubeck Wardenburg J, Kobayashi SD, Mathema B, Braughton KR, Whitney AR, Villaruz AE, Martens CA, Porcella SF, McGavin MJ, Otto M, Musser JM, Kreiswirth BN. Molecular differentiation of historic phage-type 80/81 and contemporary epidemic Staphylococcus aureus . Proc Natl Acad Sci U S A. 2011;108:18091–18096. doi: 10.1073/pnas.1111084108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, Jones RN, Beach M. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin Infect Dis. 2001;32(Suppl 2):S114–S132. doi: 10.1086/320184. [DOI] [PubMed] [Google Scholar]
- Diep B. Relative effects of Panton Valentine Leukocidin, alpha-hemolysin and alpha-type phenol-soluble modulins in pathogenesis of community-associated MRSA necrotizing pneumonia; International Conference on Antimicrobial Agents and Chemotherapy; Boston. 2010. [Google Scholar]
- Diep BA, Chambers HF, Graber CJ, Szumowski JD, Miller G, Han LL, Chen JH, Lin F, Lin J, Phan TH, Carleton HA, McDougal LK, Tenover FC, Cohen DE, Mayer KH, Sensabaugh GF, Perdreau-Remington F. Emergence of multidrug-resistant, community-associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with men. Ann Intern Med. 2008a;148:249–257. doi: 10.7326/0003-4819-148-4-200802190-00204. [DOI] [PubMed] [Google Scholar]
- Diep BA, Chan L, Tattevin P, Kajikawa O, Martin TR, Basuino L, Mai TT, Marbach H, Braughton KR, Whitney AR, Gardner DJ, Fan X, Tseng CW, Liu GY, Badiou C, Etienne J, Lina G, Matthay MA, DeLeo FR, Chambers HF. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc Natl Acad Sci U S A. 2010;107:5587–5592. doi: 10.1073/pnas.0912403107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, Sensabaugh GF, Perdreau-Remington F. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus . Lancet. 2006;367:731–739. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- Diep BA, Otto M. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol. 2008;16:361–369. doi: 10.1016/j.tim.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep BA, Stone GG, Basuino L, Graber CJ, Miller A, des Etages SA, Jones A, Palazzolo-Ballance AM, Perdreau-Remington F, Sensabaugh GF, DeLeo FR, Chambers HF. The arginine catabolic mobile element and staphylococcal chromosomal cassette mec linkage: convergence of virulence and resistance in the USA300 clone of methicillin-resistant Staphylococcus aureus . J Infect Dis. 2008b;197:1523–1530. doi: 10.1086/587907. [DOI] [PubMed] [Google Scholar]
- Fridkin SK, Hageman JC, Morrison M, Sanza LT, Como-Sabetti K, Jernigan JA, Harriman K, Harrison LH, Lynfield R, Farley MM. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436–1444. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- Genestier AL, Michallet MC, Prevost G, Bellot G, Chalabreysse L, Peyrol S, Thivolet F, Etienne J, Lina G, Vallette FM, Vandenesch F, Genestier L. Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J Clin Invest. 2005;115:3117–3127. doi: 10.1172/JCI22684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves SF, Kobayashi SD, Braughton KR, Diep BA, Chambers HF, Otto M, DeLeo FR. Relative contribution of Panton-Valentine leukocidin to PMN plasma membrane permeability and lysis caused by USA300 and USA400 culture supernatants. Microbes Infect. 2010;12:446–456. doi: 10.1016/j.micinf.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu K, Cui L, Kuroda M, Ito T. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2001;9:486–493. doi: 10.1016/s0966-842x(01)02175-8. [DOI] [PubMed] [Google Scholar]
- Hongo I, Baba T, Oishi K, Morimoto Y, Ito T, Hiramatsu K. Phenol-soluble modulin alpha 3 enhances the human neutrophil lysis mediated by Panton-Valentine leukocidin. J Infect Dis. 2009;200:715–723. doi: 10.1086/605332. [DOI] [PubMed] [Google Scholar]
- Inoshima I, Inoshima N, Wilke GA, Powers ME, Frank KM, Wang Y, Bubeck Wardenburg J. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat Med. 2011;17:1310–1314. doi: 10.1038/nm.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoshima N, Wang Y, Bubeck Wardenburg J. Genetic requirement for ADAM10 in severe Staphylococcus aureus skin infection. J Invest Dermatol. 2012;132:1513–1516. doi: 10.1038/jid.2011.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi GS, Spontak JS, Klapper DG, Richardson AR. Arginine catabolic mobile element encoded speG abrogates the unique hypersensitivity of Staphylococcus aureus to exogenous polyamines. Mol Microbiol. 2011;82:9–20. doi: 10.1111/j.1365-2958.2011.07809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, Braughton KR, Schneewind O, DeLeo FR. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis. 2010;202:1050–1058. doi: 10.1086/656043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AD, Otto M, Braughton KR, Whitney AR, Chen L, Mathema B, Mediavilla JR, Byrne KA, Parkins LD, Tenover FC, Kreiswirth BN, Musser JM, DeLeo FR. Epidemic community-associated methicillin-resistant Staphylococcus aureus: recent clonal expansion and diversification. Proc Natl Acad Sci U S A. 2008;105:1327–1332. doi: 10.1073/pnas.0710217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby WM. Extraction of a highly potent penicillin inactivator from penicillin resistant staphylococci. Science. 1944;99:452–453. doi: 10.1126/science.99.2579.452. [DOI] [PubMed] [Google Scholar]
- Kobayashi SD, Malachowa N, Whitney AR, Braughton KR, Gardner DJ, Long D, Bubeck Wardenburg J, Schneewind O, Otto M, DeLeo FR. Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J Infect Dis. 2011;204:937–941. doi: 10.1093/infdis/jir441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig B, Prevost G, Piemont Y, Konig W. Effects of Staphylococcus aureus leukocidins on inflammatory mediator release from human granulocytes. J Infect Dis. 1995;171:607–613. doi: 10.1093/infdis/171.3.607. [DOI] [PubMed] [Google Scholar]
- Kretschmer D, Gleske AK, Rautenberg M, Wang R, Koberle M, Bohn E, Schoneberg T, Rabiet MJ, Boulay F, Klebanoff SJ, van Kessel KA, van Strijp JA, Otto M, Peschel A. Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus . Cell Host Microbe. 2010;7:463–473. doi: 10.1016/j.chom.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labandeira-Rey M, Couzon F, Boisset S, Brown EL, Bes M, Benito Y, Barbu EM, Vazquez V, Hook M, Etienne J, Vandenesch F, Bowden MG. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science. 2007;315:1130–1133. doi: 10.1126/science.1137165. [DOI] [PubMed] [Google Scholar]
- Lee SM, Ender M, Adhikari R, Smith JM, Berger-Bachi B, Cook GM. Fitness cost of staphylococcal cassette chromosome mec in methicillin-resistant Staphylococcus aureus by way of continuous culture. Antimicrob Agents Chemother. 2007;51:1497–1499. doi: 10.1128/AAC.01239-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Kim YJ, Chung DR, Jung KS, Kim JS. Invasive infection caused by a community-associated methicillin-resistant Staphylococcus aureus strain not carrying Panton-Valentine leukocidin in South Korea. J Clin Microbiol. 2010;48:311–313. doi: 10.1128/JCM.00297-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Cheung GY, Hu J, Wang D, Joo HS, Deleo FR, Otto M. Comparative analysis of virulence and toxin expression of global community-associated methicillin-resistant Staphylococcus aureus strains. J Infect Dis. 2010;202:1866–1876. doi: 10.1086/657419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Diep BA, Villaruz AE, Braughton KR, Jiang X, DeLeo FR, Chambers HF, Lu Y, Otto M. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus . Proc Natl Acad Sci U S A. 2009;106:5883–5888. doi: 10.1073/pnas.0900743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinska U, Hermans K, Meulemans L, Dumitrescu O, Badiou C, Duchateau L, Haesebrouck F, Etienne J, Lina G. Panton-Valentine leukocidin does play a role in the early stage of Staphylococcus aureus skin infections: a rabbit model. PLoS ONE. 2011;6:e22864. doi: 10.1371/journal.pone.0022864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler B, Hussain M, Grundmeier M, Bruck M, Holzinger D, Varga G, Roth J, Kahl BC, Proctor RA, Peters G. Staphylococcus aureus Panton-Valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 2010;6:e1000715. doi: 10.1371/journal.ppat.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus . J Clin Invest. 2003;111:1265–1273. doi: 10.1172/JCI18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mediavilla JR, Chen L, Mathema B, Kreiswirth BN. Global epidemiology of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA) Curr Opin Microbiol. 2012;15:588–595. doi: 10.1016/j.mib.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Miller LG, Perdreau-Remington F, Rieg G, Mehdi S, Perlroth J, Bayer AS, Tang AW, Phung TO, Spellberg B. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445–1453. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- Otter JA, French GL. The emergence of community-associated methicillin-resistant Staphylococcus aureus at a London teaching hospital, 2000–2006. Clin Microbiol Infect. 2008;14:670–676. doi: 10.1111/j.1469-0691.2008.02017.x. [DOI] [PubMed] [Google Scholar]
- Otto M. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus . Annu Rev Microbiol. 2010;64:143–162. doi: 10.1146/annurev.micro.112408.134309. [DOI] [PubMed] [Google Scholar]
- Panton PN, Valentine FCO. Staphylococcal toxin. Lancet. 1932;1:506–508. [Google Scholar]
- Periasamy S, Joo HS, Duong AC, Bach TH, Tan VY, Chatterjee SS, Cheung GY, Otto M. How Staphylococcus aureus biofilms develop their characteristic structure. Proc Natl Acad Sci U S A. 2012;109:1281–1286. doi: 10.1073/pnas.1115006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi C, Waters EM, Rudkin JK, Schaeffer CR, Lohan AJ, Tong P, Loftus BJ, Pier GB, Fey PD, Massey RC, O'Gara JP. Methicillin resistance alters the biofilm phenotype and attenuates virulence in Staphylococcus aureus device-associated infections. PLoS Pathog. 2012;8:e1002626. doi: 10.1371/journal.ppat.1002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queck SY, Jameson-Lee M, Villaruz AE, Bach TH, Khan BA, Sturdevant DE, Ricklefs SM, Li M, Otto M. RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus . Mol Cell. 2008;32:150–158. doi: 10.1016/j.molcel.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz K, Syed AK, Stephenson RE, Rickard AH, Boles BR. Functional amyloids composed of phenol soluble modulins stabilize Staphylococcus aureus biofilms. PLoS Pathog. 2012;8:e1002744. doi: 10.1371/journal.ppat.1002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong G, Chun J, Parker D, Prince A. Staphylococcus aureus activation of caspase 1/calpain signaling mediates invasion through human keratinocytes. J Infect Dis. 2012;205:1571–1579. doi: 10.1093/infdis/jis244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaan AN, Henry T, Thorburn AN, van Rooijen WJM, Badiou C, Aerts P, de Haas CJ, van Kessel KPM, Mackay CR, Vandenesch F, Lina G, van Strijp JA. Panton-Valentine leukocidin receptors. International Symposium on Staphylococci and Staphylococcal Infections; Lyon. 2012. [Google Scholar]
- Surewaard BG, Nijland R, Spaan AN, Kruijtzer JA, de Haas CJ, van Strijp JA. Inactivation of staphylococcal phenol soluble modulins by serum lipoprotein particles. PLoS Pathog. 2012;8:e1002606. doi: 10.1371/journal.ppat.1002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmigielski S, Prevost G, Monteil H, Colin DA, Jeljaszewicz J. Leukocidal toxins of staphylococci. Zentralbl Bakteriol. 1999;289:185–201. doi: 10.1016/s0934-8840(99)80105-4. [DOI] [PubMed] [Google Scholar]
- Valeva A, Walev I, Pinkernell M, Walker B, Bayley H, Palmer M, Bhakdi S. Transmembrane beta-barrel of staphylococcal alpha-toxin forms in sensitive but not in resistant cells. Proc Natl Acad Sci U S A. 1997;94:11607–11611. doi: 10.1073/pnas.94.21.11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, Heffernan H, Liassine N, Bes M, Greenland T, Reverdy ME, Etienne J. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis. 2003;9:978–984. doi: 10.3201/eid0908.030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaruz AE, Bubeck Wardenburg J, Khan BA, Whitney AR, Sturdevant DE, Gardner DJ, DeLeo FR, Otto M. A point mutation in the agr locus rather than expression of the Panton-Valentine leukocidin caused previously reported phenotypes in Staphylococcus aureus pneumonia and gene regulation. J Infect Dis. 2009;200:724–734. doi: 10.1086/604728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med. 2001;344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- Voyich JM, Braughton KR, Sturdevant DE, Whitney AR, Said-Salim B, Porcella SF, Long RD, Dorward DW, Gardner DJ, Kreiswirth BN, Musser JM, DeLeo FR. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J Immunol. 2005;175:3907–3919. doi: 10.4049/jimmunol.175.6.3907. [DOI] [PubMed] [Google Scholar]
- Voyich JM, Otto M, Mathema B, Braughton KR, Whitney AR, Welty D, Long RD, Dorward DW, Gardner DJ, Lina G, Kreiswirth BN, DeLeo FR. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis. 2006;194:1761–1770. doi: 10.1086/509506. [DOI] [PubMed] [Google Scholar]
- Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, DeLeo FR, Otto M. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- Wang R, Khan BA, Cheung GY, Bach TH, Jameson-Lee M, Kong KF, Queck SY, Otto M. Staphylococcus epidermidis surfactant peptides promote biofilm maturation and dissemination of biofilm-associated infection in mice. J Clin Invest. 2011;121:238–248. doi: 10.1172/JCI42520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- Wilke GA, Bubeck Wardenburg J. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc Natl Acad Sci U S A. 2010;107:13473–13478. doi: 10.1073/pnas.1001815107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoong P, Pier GB. Antibody-mediated enhancement of community-acquired methicillin-resistant Staphylococcus aureus infection. Proc Natl Acad Sci U S A. 2010;107:2241–2246. doi: 10.1073/pnas.0910344107. [DOI] [PMC free article] [PubMed] [Google Scholar]