Abstract
Lacrimal gland neoplasms are rare and much of our knowledge of the behavior and molecular pathogenesis of these tumors comes from study of the similar, but more numerous salivary gland neoplasms. After briefly discussing the classification of lacrimal gland neoplasms, I review three areas of emerging knowledge in the pathogenesis of these neoplasms: (1) the concept of adenoid cystic carcinoma with high-grade transformation and the associated cytogenetic changes; (2) recent analysis of the MYB-NFIB gene fusion in adenoid cystic carcinoma, and; (3) overexpression of HER2 in malignant salivary and lacrimal neoplasms.
Keywords: Lacrimal gland neoplasms, Molecular pathogenesis
Introduction
The pathology of salivary and lacrimal gland neoplasms is complex with more than 30 entities recognized based on the 2005 WHO Classification of Tumours: Pathology and Genetics of Head and Neck Tumors. We recently completed a review of 118 lacrimal gland neoplasms obtained from four centres.1 Most numerous were 57 pleomorphic adenomas (PA). Of the 59 malignant neoplasms, the most common was adenoid cystic carcinoma (ACC) (38), followed by carcinoma ex pleomorphic adenoma (CaPA) (9), adenocarcinoma NOS (3) and others (9; 1 or 2 cases each). This is one of the largest reviews of epithelial neoplasms of the lacrimal gland and highlights one of the problems of learning about lacrimal gland tumors, the small numbers of cases seen at one institution.
A note on salivary versus lacrimal
Most of the molecular information about epithelial lacrimal neoplasms comes from the study of salivary gland neoplasms, just by virtue of their greater numbers. In this presentation I have assumed that molecular findings of epithelial neoplasms of the salivary glands can be applied to lacrimal neoplasms of the same histological type. However, there is one piece of information that corroborates this assumption.
In 1994 Hrynchak, White, Berean, Horsman reported the cytogenetic findings of 7 lacrimal gland neoplasms: 4 PAs, 1 CaPA, 2 ACCs.2 The cytogenetic findings were similar to those in salivary gland neoplasms. The PAs had breakpoints in 3p/3q, 8p/8q, 12q, similar to those in the salivary glands. One ACC had a t(6;9)(q23;p22), a translocation seen in a minority of salivary ACCs, but one that has become highly important in understanding the pathogenesis of these neoplasms. The other ACC had a complex karyotype including trisomy 5 and 7.
ACC with high grade transformation (dedifferentiation)
The molecular changes in ACC are not well understood; from array CGH most common losses are in 1p32-36, 6q23-27 and 12q12-14; most common gains are of 8q24 and 22q13. Deletions in 1p32-26 are associated with an aggressive course, but not proven to be an independent factor yet.3
ACC with dedifferentiation is now known as ACC with high-grade transformation (HGT). This situation occurs when a conventional ACC develops a histologic pattern characterized by areas of pleomorphic, mitotically active high-grade carcinoma in juxtaposition to conventional ACC (Fig. 1). HGT is a monophasic tumor composed of phenotypically ductal cells only, with loss of some or all of the myoepithelial layers seen in conventional ACCs. The transition between the two may be gradual. CGH of these cases has demonstrated gains in 8q24, 17q11, 17q23 and 15q11; and losses in 9q34, 4p, 1p36 and 11q22. This portends a poor median survival of 12–36 mos. The gains in 8q are associated with c-myc amplification and p53 is often overexpressed.4 In our recent review of 118 lacrimal gland neoplasms, 2/38 ACC had HGT.1
Figure 1.
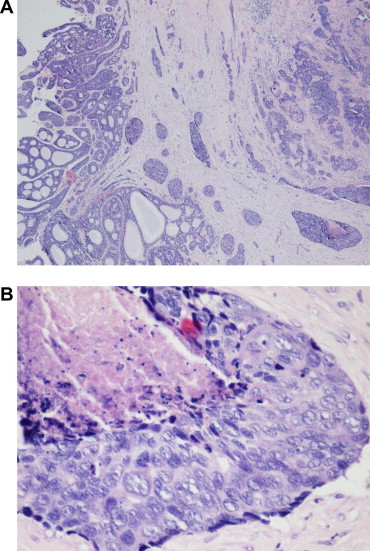
Adenoid cystic carcinoma with high-grade transformation. (A) Low power H&E photomicrograph of adenoid cystic carcinoma: cribriform pattern on left with high-grade transformation on right. (B) High power of high-grade transformation showing necrosis, enlarged pleomorphic nuclei and mitoses.
t(6;9) and MYB–NFIB gene fusion
There is a frequent loss of 6q associated with translocations in ACC. The t(6;9) is found in 14%, some as the sole change as in our lacrimal ACC. In 43%, 9p is the partner. In cases with this translocation a MYB–NFIB fusion gene has been identified. This results in a fusion transcript with the loss of the MYB 3′ untranslated region, leading to high MYB expression.5 Mitani et al.6 studied 123 salivary neoplasms and found the MYB–NFIB fusion in 20/72 (28%) primary ACCs, 6/17 (35%) metastatic ACCs and 0/34 non-ACCs and normal gland tissue. Fourteen variants of the fusion were present.
They also studied MYB expression by qRT-PCR and found increased MYB expression levels in fusion positive, and unexpectedly in 60% fusion negative tumors, implying a different mechanism for MYB overexpression in the latter tumors. There was minimal expression in non-ACCs.
They also demonstrated strong nuclear staining for MYB protein in 17/20 (85%) fusion positive and 25/41 (61%) fusion negative tumors. MYB expression was limited to myoepithelial cells in tubular and cribriform pattern tumors. MYB expression was not present in tumors with alternate translocations. There was no clinicopathologic correlation except with age. The importance of this finding is that it implies that multiple genetic events including loss and translocation involving 6q are associated with the pathogenesis of ACC. The major consequence of fusion is a dramatic increase in the expression of MYB protein that is attributed to the loss of MYB sequences containing regulatory binding sites for miRNA. These findings have been corroborated by West et al.7
Her2 and salivary/lacrimal neoplasms
The Her signaling network consists of four receptors and their ligands, of which Her2 and EGFR (Her1) are the most widely studied.8 Vidal et al.9 studied ACC and non-ACC patients who had been treated with Lapatinib, a blocker of Her1 and Her2. Of the 20 ACC cases there was no HER2 amplification, although 1/20 was 2+ pos on IHC. Of the 19 non-ACC cases 3/17 were amplified for HER2 and 3+ on IHC and a total of 8/19 ⩾ 2+ on IHC. (Fig. 2) 2 of 3 with Her2 amplification had longer times to progression of disease. They found that overall, patients with low and high HER2 ratios had a longer time to progression than those with a moderate ratio. No tumors had EGFR amplification even though several had ⩾2+ on IHC. Many of the positive tumors were ductal adenocarcinomas of the salivary gland.10
Figure 2.
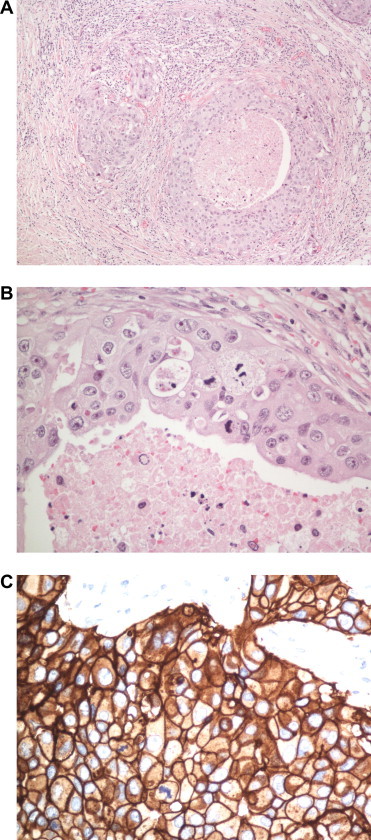
Ductal adenocarcinoma. (A) Lower power H&E photomicrograph of ductal adenocarcinoma show invasive pattern. (B) High power shows comedo-type necrosis, pleomorphic nuclei and mitotic figures. (C) High power strong, membranous staining for Her2. (Immunoperoxidase with anti-Her2 antibody, hematoxylin counterstain.).
Simpson and Di Palma11 have advocated creating a sub-classification of ductal adenocarcinoma based on IHC staining similar to that in the breast, which may help to dictate treatment:
Luminal (70%),
HER2 amplified (15%),
Basal (3%),
Unclassified (11%).
4 cm Rule
Although there are many possible new prognostic and predictive markers being studied for salivary neoplasms, none has attained universal validation and usage. Speight and Barrett12 reviewed the conventional prognostic factors of grade and histological type, stage, perineural involvement, site of neoplasm and status of margins and distilled the information down to a simple 4 cm rule, thus giving credence to the TNM classification. Those that are <4 cm do well regardless of histological type and grade and those that are >4 cm do poorly, and usually require radiotherapy.
References
- 1.Weis E., Rootman J., Joly T.J., Berean K.W., Al-Katan H.M., Pasternak S. Epithelial lacrimal gland tumors. Arch Ophthalmol. 2009;127:1016–1028. doi: 10.1001/archophthalmol.2009.209. [DOI] [PubMed] [Google Scholar]
- 2.Hrynchak M., White V., Berean K., Horsman D. Cytogenetic findings in seven lacrimal gland neoplasms. Cancer Genet Cytogenet. 1994;75:133–138. doi: 10.1016/0165-4608(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 3.Seethala R.R. Histologic grading and prognostic biomarkers in salivary gland carcinomas. Adv Anat Pathol. 2011;18:29–45. doi: 10.1097/PAP.0b013e318202645a. [DOI] [PubMed] [Google Scholar]
- 4.Seethala R.R., Cieply K., Barnes E.L., Dacic S. Progressive genetic alterations of adenoid cystic carcinoma with high-grade transformation. Arch Pathol Lab Med. 2011;135:123–130. doi: 10.5858/2010-0048-OAR.1. [DOI] [PubMed] [Google Scholar]
- 5.Persson M., Andren Y., Mark J., Horlings H.M., Persson F., Stenman G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. PNAS. 2009;106:18740–18744. doi: 10.1073/pnas.0909114106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitani Y., Li J., Rao P.H., Zhao Y-J., Bell D., Lippman S.M. Comprehensive analysis of the MYB–NFIB gene fusion in salivary adenoid cystic carcinoma: incidence, variability and clinicopathologic significance. Clin Cancer Res. 2010;16:4722–4731. doi: 10.1158/1078-0432.CCR-10-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.West R.B., Kong C., Clarke N., Gilks T., Lipsick J.S., Cao H. MYB expression and translocation in adenoid cystic carcinomas and other salivary gland tumors with clinicopathologic correlation. Am J Surg Path. 2011;35:92–99. doi: 10.1097/PAS.0b013e3182002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutierrez C., Schiff R. Her2: biology, detection and clinical implications. Arch Pathol Lab Med. 2011;135:55–62. doi: 10.1043/2010-0454-RAR.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vidal L., Tsao M.S., Pond G.R., Cohen E.E.W., Cohen R.B., Chen E.X. Fluorescent in situ hybridization gene amplification analysis of EGFR and Her2 in patients with malignant salivary gland tumors treated with Lapatinib. Head Neck. 2009;31:1006–1012. doi: 10.1002/hed.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams M.D., Roberts D.B., Kies M.S., Mao L., Weber R.S., El-Naggar A.K. Genetic and expression analysis of HER-2 and EGFR genes in salivary duct carcinoma: Empirical and therapeutic significance. Clin Cancer Res. 2010;16:2266–2274. doi: 10.1158/1078-0432.CCR-09-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson R.H.W., Di Palma S. Selected recent advances in the pathology of salivary neoplasms. Diag Histopathol. 2010;16:276–286. [Google Scholar]
- 12.Speight P.M., Barrett A.W. Prognostic factors in malignant tumors of the salivary glands. Br J Oral Maxillofac Surg. 2009;47:587–593. doi: 10.1016/j.bjoms.2009.03.017. [DOI] [PubMed] [Google Scholar]


