Abstract
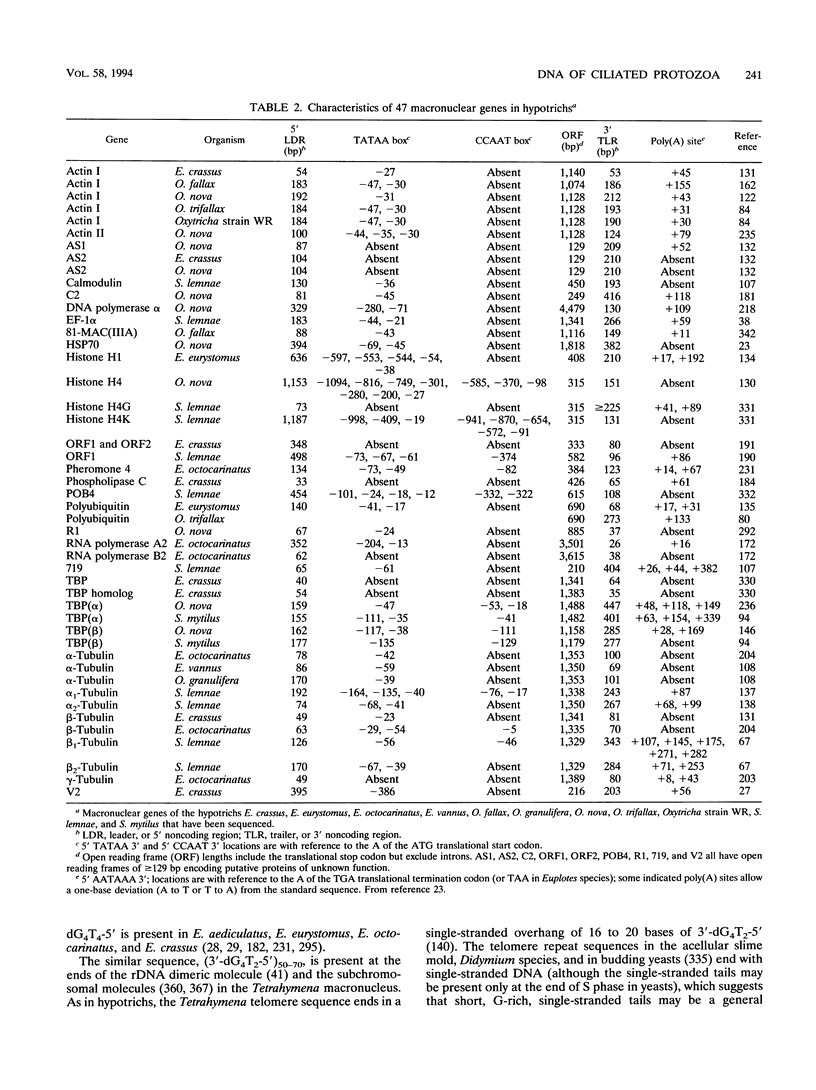
Ciliates contain two types of nuclei: a micronucleus and a macronucleus. The micronucleus serves as the germ line nucleus but does not express its genes. The macronucleus provides the nuclear RNA for vegetative growth. Mating cells exchange haploid micronuclei, and a new macronucleus develops from a new diploid micronucleus. The old macronucleus is destroyed. This conversion consists of amplification, elimination, fragmentation, and splicing of DNA sequences on a massive scale. Fragmentation produces subchromosomal molecules in Tetrahymena and Paramecium cells and much smaller, gene-sized molecules in hypotrichous ciliates to which telomere sequences are added. These molecules are then amplified, some to higher copy numbers than others. rDNA is differentially amplified to thousands of copies per macronucleus. Eliminated sequences include transposonlike elements and sequences called internal eliminated sequences that interrupt gene coding regions in the micronuclear genome. Some, perhaps all, of these are excised as circular molecules and destroyed. In at least some hypotrichs, segments of some micronuclear genes are scrambled in a nonfunctional order and are recorded during macronuclear development. Vegetatively growing ciliates appear to possess a mechanism for adjusting copy numbers of individual genes, which corrects gene imbalances resulting from random distribution of DNA molecules during amitosis of the macronucleus. Other distinctive features of ciliate DNA include an altered use of the conventional stop codons.
Full text
PDF





























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALONSO P., PEREZ SILVA J. GIANT CHROMOSOMES IN PROTOZOA. Nature. 1965 Jan 16;205:313–314. doi: 10.1038/205313a0. [DOI] [PubMed] [Google Scholar]
- Acevedo O. L., Dickinson L. A., Macke T. J., Thomas C. A., Jr The coherence of synthetic telomeres. Nucleic Acids Res. 1991 Jun 25;19(12):3409–3419. doi: 10.1093/nar/19.12.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. L., Kennel S. J., Cacheiro L., Olins A. L., Olins D. E. Examination of the macronuclear replication band in Euplotes eurystomus with monoclonal antibodies. J Cell Biol. 1986 Jan;102(1):131–136. doi: 10.1083/jcb.102.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. L., Olins A. L., Harp J. M., Olins D. E. Isolation and characterization of chromatin replication bands and macronuclei from Euplotes eurystomus. Eur J Cell Biol. 1985 Nov;39(1):217–223. [PubMed] [Google Scholar]
- Allen S. L., Weremiuk S. L. Defective micronuclei and genomic exclusion in selected C subclones of Tetrahymena. J Protozool. 1971 Aug;18(3):509–515. doi: 10.1111/j.1550-7408.1971.tb03364.x. [DOI] [PubMed] [Google Scholar]
- Allis C. D., Colavito-Shepanski M., Gorovsky M. A. Scheduled and unscheduled DNA synthesis during development in conjugating Tetrahymena. Dev Biol. 1987 Dec;124(2):469–480. doi: 10.1016/0012-1606(87)90500-8. [DOI] [PubMed] [Google Scholar]
- Allis C. D., Glover C. V., Gorovsky M. A. Micronuclei of Tetrahymena contain two types of histone H3. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4857–4861. doi: 10.1073/pnas.76.10.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuler M. I., Yao M. C. Macronuclear DNA of Tetrahymena thermophila exists as defined subchromosomal-sized molecules. Nucleic Acids Res. 1985 Aug 26;13(16):5817–5831. doi: 10.1093/nar/13.16.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin A. A., Pearlman R. E. Autonomously replicating sequences from the non transcribed spacers of Tetrahymena thermophila ribosomal DNA. Nucleic Acids Res. 1985 Apr 11;13(7):2647–2659. doi: 10.1093/nar/13.7.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin A. A., Pearlman R. E. In vitro deletion analysis of ARS elements spanning the replication origin in the 5' non-transcribed spacer of Tetrahymena thermophila ribosomal DNA. Nucleic Acids Res. 1986 Mar 25;14(6):2749–2762. doi: 10.1093/nar/14.6.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammermann D. Morphology and development of the macronuclei of the ciliates Stylonychia mytilus and Euplotes aediculatus. Chromosoma. 1971;33(2):209–238. doi: 10.1007/BF00285634. [DOI] [PubMed] [Google Scholar]
- Ammermann D., Muenz A. DNA and protein content of different hypotrich ciliates. Eur J Cell Biol. 1982 Apr;27(1):22–24. [PubMed] [Google Scholar]
- Ammermann D., Steinbrück G., von Berger L., Hennig W. The development of the macronucleus in the ciliated protozoan Stylonychia mytilus. Chromosoma. 1974 May 10;45(4):401–429. doi: 10.1007/BF00283386. [DOI] [PubMed] [Google Scholar]
- Ammermann D. The micronucleus of the ciliate Stylonychia mytilus; its nucleic acid synthesis and its function. Exp Cell Res. 1970 Jul;61(1):6–12. doi: 10.1016/0014-4827(70)90251-x. [DOI] [PubMed] [Google Scholar]
- Andersen H. A. Requirements for DNA replication preceding cell division in Tetrahymena pyriformis. Exp Cell Res. 1972 Nov;75(1):89–94. doi: 10.1016/0014-4827(72)90523-x. [DOI] [PubMed] [Google Scholar]
- Ashley T., Ward D. C. A "hot spot" of recombination coincides with an interstitial telomeric sequence in the Armenian hamster. Cytogenet Cell Genet. 1993;62(2-3):169–171. doi: 10.1159/000133464. [DOI] [PubMed] [Google Scholar]
- Austerberry C. F., Allis C. D., Yao M. C. Specific DNA rearrangements in synchronously developing nuclei of Tetrahymena. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7383–7387. doi: 10.1073/pnas.81.23.7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avides M. do C., Sunkel C. E., Moradas-Ferreira P., Rodrigues-Pousada C. Properties and partial characterization of the heat-shock factor from Tetrahymena pyriformis. Eur J Biochem. 1990 Dec 12;194(2):331–336. doi: 10.1111/j.1432-1033.1990.tb15621.x. [DOI] [PubMed] [Google Scholar]
- Baird S. E., Fino G. M., Tausta S. L., Klobutcher L. A. Micronuclear genome organization in Euplotes crassus: a transposonlike element is removed during macronuclear development. Mol Cell Biol. 1989 Sep;9(9):3793–3807. doi: 10.1128/mcb.9.9.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird S. E., Klobutcher L. A. Characterization of chromosome fragmentation in two protozoans and identification of a candidate fragmentation sequence in Euplotes crassus. Genes Dev. 1989 May;3(5):585–597. doi: 10.1101/gad.3.5.585. [DOI] [PubMed] [Google Scholar]
- Baird S. E., Klobutcher L. A. Differential DNA amplification and copy number control in the hypotrichous ciliate Euplotes crassus. J Protozool. 1991 Mar-Apr;38(2):136–140. doi: 10.1111/j.1550-7408.1991.tb06033.x. [DOI] [PubMed] [Google Scholar]
- Baird S. E., Klobutcher L. A. Genetic characterization and use of a restriction fragment length variant in the hypotrichous ciliate Euplotes crassus. J Protozool. 1988 Nov;35(4):459–465. doi: 10.1111/j.1550-7408.1988.tb04130.x. [DOI] [PubMed] [Google Scholar]
- Balagurumoorthy P., Brahmachari S. K., Mohanty D., Bansal M., Sasisekharan V. Hairpin and parallel quartet structures for telomeric sequences. Nucleic Acids Res. 1992 Aug 11;20(15):4061–4067. doi: 10.1093/nar/20.15.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon G. A., Bowen J. K., Yao M. C., Gorovsky M. A. Tetrahymena H4 genes: structure, evolution and organization in macro- and micronuclei. Nucleic Acids Res. 1984 Feb 24;12(4):1961–1975. doi: 10.1093/nar/12.4.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barahona I., Soares H., Cyrne L., Penque D., Denoulet P., Rodrigues-Pousada C. Sequence of one alpha- and two beta-tubulin genes of Tetrahymena pyriformis. Structural and functional relationships with other eukaryotic tubulin genes. J Mol Biol. 1988 Aug 5;202(3):365–382. doi: 10.1016/0022-2836(88)90271-9. [DOI] [PubMed] [Google Scholar]
- Baroin A., Prat A., Caron F. Telomeric site position heterogeneity in macronuclear DNA of Paramecium primaurelia. Nucleic Acids Res. 1987 Feb 25;15(4):1717–1728. doi: 10.1093/nar/15.4.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierbaum P., Dönhoff T., Klein A. Macronuclear and micronuclear configurations of a gene encoding the protein synthesis elongation factor EF 1 alpha in Stylonychia lemnae. Mol Microbiol. 1991 Jun;5(6):1567–1575. doi: 10.1111/j.1365-2958.1991.tb00804.x. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H., Gall J. G. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol. 1978 Mar 25;120(1):33–53. doi: 10.1016/0022-2836(78)90294-2. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Structure and function of telomeres. Nature. 1991 Apr 18;350(6319):569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Telomeres. Trends Biochem Sci. 1991 Oct;16(10):378–381. doi: 10.1016/0968-0004(91)90155-o. [DOI] [PubMed] [Google Scholar]
- Boswell R. E., Jahn C. L., Greslin A. F., Prescott D. M. Organization of gene and non-gene sequences in micronuclear DNA of Oxytricha nova. Nucleic Acids Res. 1983 Jun 11;11(11):3651–3663. doi: 10.1093/nar/11.11.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boswell R. E., Klobutcher L. A., Prescott D. M. Inverted terminal repeats are added to genes during macronuclear development in Oxytricha nova. Proc Natl Acad Sci U S A. 1982 May;79(10):3255–3259. doi: 10.1073/pnas.79.10.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgain F. M., Katinka M. D. Telomeres inhibit end to end fusion and enhance maintenance of linear DNA molecules injected into the Paramecium primaurelia macronucleus. Nucleic Acids Res. 1991 Apr 11;19(7):1541–1547. doi: 10.1093/nar/19.7.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk C. F., Conover R. K. Elimination of micronuclear specific DNA sequences early in anlagen development. Mol Cell Biol. 1985 Jan;5(1):93–98. doi: 10.1128/mcb.5.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk C. F., Navas P. A. Variable copy number of macronuclear DNA molecules in Tetrahymena. Dev Genet. 1992;13(2):111–117. doi: 10.1002/dvg.1020130204. [DOI] [PubMed] [Google Scholar]
- Brunk C. F., Sadler L. A. Characterization of the promoter region of Tetrahymena genes. Nucleic Acids Res. 1990 Jan 25;18(2):323–329. doi: 10.1093/nar/18.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk C. F., Tsao S. G., Diamond C. H., Ohashi P. S., Tsao N. N., Pearlman R. E. Reorganization of unique and repetitive sequences during nuclear development in Tetrahymena thermophila. Can J Biochem. 1982 Sep;60(9):847–853. doi: 10.1139/o82-107. [DOI] [PubMed] [Google Scholar]
- Brünen-Nieweler C., Schmidt H. J., Heckmann K. Two introns in the pheromone 3-encoding gene of Euplotes octocarinatus. Gene. 1991 Dec 30;109(2):233–237. doi: 10.1016/0378-1119(91)90613-g. [DOI] [PubMed] [Google Scholar]
- Butler A. P., Laughlin T. J., Cadilla C. L., Henry J. M., Olins D. E. Physical structure of gene-sized chromatin from the protozoan Oxytricha. Nucleic Acids Res. 1984 Apr 11;12(7):3201–3217. doi: 10.1093/nar/12.7.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R. C., Shalke G., Gorovsky M. A. Developmental rearrangements associated with a single type of expressed alpha-tubulin gene in Tetrahymena. Cell. 1984 Feb;36(2):441–445. doi: 10.1016/0092-8674(84)90237-x. [DOI] [PubMed] [Google Scholar]
- Caplan E. B. Histones and other basic nuclear proteins in genetically active and genetically inactive nuclei of the ciliate, Oxytricha sp. Biochim Biophys Acta. 1977 Nov 16;479(2):214–219. doi: 10.1016/0005-2787(77)90142-3. [DOI] [PubMed] [Google Scholar]
- Caron F. A high degree of macronuclear chromosome polymorphism is generated by variable DNA rearrangements in Paramecium primaurelia during macronuclear differentiation. J Mol Biol. 1992 Jun 5;225(3):661–678. doi: 10.1016/0022-2836(92)90393-x. [DOI] [PubMed] [Google Scholar]
- Caron F., Meyer E. Does Paramecium primaurelia use a different genetic code in its macronucleus? Nature. 1985 Mar 14;314(6007):185–188. doi: 10.1038/314185a0. [DOI] [PubMed] [Google Scholar]
- Cartinhour S. W., Herrick G. A. Three different macronuclear DNAs in Oxytricha fallax share a common sequence block. Mol Cell Biol. 1984 May;4(5):931–938. doi: 10.1128/mcb.4.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R., Brehm S. L. Replication of the extrachromosomal ribosomal RNA genes of Tetrahymena thermophilia. Nucleic Acids Res. 1981 Jul 24;9(14):3531–3543. doi: 10.1093/nar/9.14.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R., Rio D. C. Localization of transcribed regions on extrachromosomal ribosomal RNA genes of Tetrahymena thermophila by R-loop mapping. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5051–5055. doi: 10.1073/pnas.76.10.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry J. M., Blackburn E. H. The internally located telomeric sequences in the germ-line chromosomes of Tetrahymena are at the ends of transposon-like elements. Cell. 1985 Dec;43(3 Pt 2):747–758. doi: 10.1016/0092-8674(85)90248-x. [DOI] [PubMed] [Google Scholar]
- Cleffmann G. Amount of DNA produced during extra S phases in Tetrahymena. J Cell Biol. 1975 Jul;66(1):204–209. doi: 10.1083/jcb.66.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleffmann G. Chromatin elimination and the genetic organisation of the macronucleus in Tetrahymena thermophila. Chromosoma. 1980;78(3):313–325. doi: 10.1007/BF00327390. [DOI] [PubMed] [Google Scholar]
- Cleffmann G. Regulierung der DNS-Menge im Makronucleus von Tetrahymena. Exp Cell Res. 1968 Apr;50(1):193–207. doi: 10.1016/0014-4827(68)90407-2. [DOI] [PubMed] [Google Scholar]
- Colombo M. M., Swanton M. T., Donini P., Prescott D. M. Micronuclear DNA of Oxytricha nova contains sequences with autonomously replicating activity in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Sep;4(9):1725–1729. doi: 10.1128/mcb.4.9.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover R. K., Brunk C. F. Macronuclear DNA molecules of Tetrahymena thermophila. Mol Cell Biol. 1986 Mar;6(3):900–905. doi: 10.1128/mcb.6.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelmann K. K., Helftenbein E. Nucleotide sequence and expression of two beta-tubulin genes in Stylonychia lemnae. J Mol Biol. 1987 Dec 20;198(4):643–653. doi: 10.1016/0022-2836(87)90207-5. [DOI] [PubMed] [Google Scholar]
- Corliss J. O., Esser S. C. Comments on the role of the cyst in the life cycle and survival of free-living protozoa. Trans Am Microsc Soc. 1974 Oct;93(4):578–593. [PubMed] [Google Scholar]
- Csank C., Taylor F. M., Martindale D. W. Nuclear pre-mRNA introns: analysis and comparison of intron sequences from Tetrahymena thermophila and other eukaryotes. Nucleic Acids Res. 1990 Sep 11;18(17):5133–5141. doi: 10.1093/nar/18.17.5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J. Studies on Macronuclear DNA from Paramecium aurelia. Chromosoma. 1975 Dec 10;53(3):191–208. doi: 10.1007/BF00329171. [DOI] [PubMed] [Google Scholar]
- Cupples C. G., Pearlman R. E. Isolation and characterization of the actin gene from Tetrahymena thermophila. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5160–5164. doi: 10.1073/pnas.83.14.5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. C., Ward J. G., Herrick G., Allis C. D. Programmed nuclear death: apoptotic-like degradation of specific nuclei in conjugating Tetrahymena. Dev Biol. 1992 Dec;154(2):419–432. doi: 10.1016/0012-1606(92)90080-z. [DOI] [PubMed] [Google Scholar]
- Dawson D., Herrick G. Micronuclear DNA sequences of Oxytricha fallax homologous to the macronuclear inverted terminal repeat. Nucleic Acids Res. 1982 May 11;10(9):2911–2924. doi: 10.1093/nar/10.9.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson D., Herrick G. Rare internal C4A4 repeats in the micronuclear genome of Oxytricha fallax. Mol Cell Biol. 1984 Dec;4(12):2661–2667. doi: 10.1128/mcb.4.12.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson D., Herrick G. Telomeric properties of C4A4-homologous sequences in micronuclear DNA of Oxytricha fallax. Cell. 1984 Jan;36(1):171–177. doi: 10.1016/0092-8674(84)90086-2. [DOI] [PubMed] [Google Scholar]
- Din N., Engberg J. Extrachromosomal ribosomal RNA genes in Tetrahymena: structure and evolution. J Mol Biol. 1979 Nov 5;134(3):555–574. doi: 10.1016/0022-2836(79)90367-x. [DOI] [PubMed] [Google Scholar]
- Din N., Engberg J., Kaffenberger W., Eckert W. A. The intervening sequence in the 26S rRNA coding region of T. thermophila is transcribed within the largest stable precursor for rRNA. Cell. 1979 Oct;18(2):525–532. doi: 10.1016/0092-8674(79)90069-2. [DOI] [PubMed] [Google Scholar]
- Doerder F. P., Debault L. E. Cytofluorimetric analysis of nuclear DNA during meiosis, fertilization and macronuclear development in the ciliate Tetrahymena pyriformis, syngen 1. J Cell Sci. 1975 May;17(3):471–493. doi: 10.1242/jcs.17.3.471. [DOI] [PubMed] [Google Scholar]
- Dupuis P. The beta-tubulin genes of Paramecium are interrupted by two 27 bp introns. EMBO J. 1992 Oct;11(10):3713–3719. doi: 10.1002/j.1460-2075.1992.tb05456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwood H. J., Olsen G. J., Sogin M. L. The small-subunit ribosomal RNA gene sequences from the hypotrichous ciliates Oxytricha nova and Stylonychia pustulata. Mol Biol Evol. 1985 Sep;2(5):399–410. doi: 10.1093/oxfordjournals.molbev.a040362. [DOI] [PubMed] [Google Scholar]
- Engberg J., Nilsson J. R., Pearlman R. E., Leick V. Induction of nucleolar and mitochondrial DNA replication in Tetrahymena pyriformis. Proc Natl Acad Sci U S A. 1974 Mar;71(3):894–898. doi: 10.1073/pnas.71.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg J., Pearlman R. E. The amount of ribosomal RNA genes in Tetrahymena pyriformis in different physiological states. Eur J Biochem. 1972 Apr 11;26(3):393–400. doi: 10.1111/j.1432-1033.1972.tb01779.x. [DOI] [PubMed] [Google Scholar]
- Engberg J. Strong sequence conservation of a 38 bp region near the center of the extrachromosomal rDNA palindrome in different Tetrahymena species. Nucleic Acids Res. 1983 Jul 25;11(14):4939–4946. doi: 10.1093/nar/11.14.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein L. M., Forney J. D. Mendelian and non-mendelian mutations affecting surface antigen expression in Paramecium tetraurelia. Mol Cell Biol. 1984 Aug;4(8):1583–1590. doi: 10.1128/mcb.4.8.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenson D. P., Prescott D. M. Disruption of DNA synthesis in Euplotes by heat shock. Exp Cell Res. 1970 Dec;63(2):245–252. doi: 10.1016/0014-4827(70)90210-7. [DOI] [PubMed] [Google Scholar]
- Fang G. W., Cech T. R. Molecular cloning of telomere-binding protein genes from Stylonychia mytilis. Nucleic Acids Res. 1991 Oct 25;19(20):5515–5518. doi: 10.1093/nar/19.20.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang G., Cech T. R. Oxytricha telomere-binding protein: DNA-dependent dimerization of the alpha and beta subunits. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6056–6060. doi: 10.1073/pnas.90.13.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang G., Cech T. R. The beta subunit of Oxytricha telomere-binding protein promotes G-quartet formation by telomeric DNA. Cell. 1993 Sep 10;74(5):875–885. doi: 10.1016/0092-8674(93)90467-5. [DOI] [PubMed] [Google Scholar]
- Fang G., Gray J. T., Cech T. R. Oxytricha telomere-binding protein: separable DNA-binding and dimerization domains of the alpha-subunit. Genes Dev. 1993 May;7(5):870–882. doi: 10.1101/gad.7.5.870. [DOI] [PubMed] [Google Scholar]
- Findly R. C., Gall J. G. Free ribosomal RNA genes in Paramecium are tandemly repeated. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3312–3316. doi: 10.1073/pnas.75.7.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findly R. C., Gall J. G. Organization of ribosomal genes in Paramecium tetraurelia. J Cell Biol. 1980 Mar;84(3):547–559. doi: 10.1083/jcb.84.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink K., Zeuthen E. Heat shock proteins in Tetrahymena studied under growth conditions. Exp Cell Res. 1980 Jul;128(1):23–30. doi: 10.1016/0014-4827(80)90382-1. [DOI] [PubMed] [Google Scholar]
- Forney J. D., Blackburn E. H. Developmentally controlled telomere addition in wild-type and mutant paramecia. Mol Cell Biol. 1988 Jan;8(1):251–258. doi: 10.1128/mcb.8.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALL J. G. Macronuclear duplication in the ciliated protozoan Euplotes. J Biophys Biochem Cytol. 1959 Mar 25;5(2):295–308. doi: 10.1083/jcb.5.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galego L., Rodrigues-Pousada C. Regulation of gene expression in Tetrahymena pyriformis under heat-shock and during recovery. Eur J Biochem. 1985 Jun 18;149(3):571–578. doi: 10.1111/j.1432-1033.1985.tb08963.x. [DOI] [PubMed] [Google Scholar]
- Gall J. G. Free ribosomal RNA genes in the macronucleus of Tetrahymena. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3078–3081. doi: 10.1073/pnas.71.8.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaunitz C., Witte H., Gaunitz F. Primary structure of a gene-sized DNA encoding calmodulin from the hypotrichous ciliate Stylonychia lemnae. Gene. 1992 Oct 1;119(2):191–198. doi: 10.1016/0378-1119(92)90271-p. [DOI] [PubMed] [Google Scholar]
- Gilley D., Preer J. R., Jr, Aufderheide K. J., Polisky B. Autonomous replication and addition of telomerelike sequences to DNA microinjected into Paramecium tetraurelia macronuclei. Mol Cell Biol. 1988 Nov;8(11):4765–4772. doi: 10.1128/mcb.8.11.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri C. P., Gorovsky M. A. DNase I sensitivity of ribosomal genes in isolated nucleosome core particles. Nucleic Acids Res. 1980 Jan 11;8(1):197–214. doi: 10.1093/nar/8.1.197-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover C. V., Gorovsky M. A. Amino-acid sequence of Tetrahymena histone H4 differs from that of higher eukaryotes. Proc Natl Acad Sci U S A. 1979 Feb;76(2):585–589. doi: 10.1073/pnas.76.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godiska R., James C., Yao M. C. A distant 10-bp sequence specifies the boundaries of a programmed DNA deletion in Tetrahymena. Genes Dev. 1993 Dec;7(12A):2357–2365. doi: 10.1101/gad.7.12a.2357. [DOI] [PubMed] [Google Scholar]
- Godiska R., Yao M. C. A programmed site-specific DNA rearrangement in Tetrahymena thermophila requires flanking polypurine tracts. Cell. 1990 Jun 29;61(7):1237–1246. doi: 10.1016/0092-8674(90)90688-b. [DOI] [PubMed] [Google Scholar]
- Gorovsky M. A., Keevert J. B. Absence of histone F1 in a mitotically dividing, genetically inactive nucleus. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2672–2676. doi: 10.1073/pnas.72.7.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschling D. E., Cech T. R. Chromatin structure of the molecular ends of Oxytricha macronuclear DNA: phased nucleosomes and a telomeric complex. Cell. 1984 Sep;38(2):501–510. doi: 10.1016/0092-8674(84)90505-1. [DOI] [PubMed] [Google Scholar]
- Gottschling D. E., Zakian V. A. Telomere proteins: specific recognition and protection of the natural termini of Oxytricha macronuclear DNA. Cell. 1986 Oct 24;47(2):195–205. doi: 10.1016/0092-8674(86)90442-3. [DOI] [PubMed] [Google Scholar]
- Gray J. T., Celander D. W., Price C. M., Cech T. R. Cloning and expression of genes for the Oxytricha telomere-binding protein: specific subunit interactions in the telomeric complex. Cell. 1991 Nov 15;67(4):807–814. doi: 10.1016/0092-8674(91)90075-a. [DOI] [PubMed] [Google Scholar]
- Greenwood S. J., Schlegel M., Sogin M. L., Lynn D. H. Phylogenetic relationships of Blepharisma americanum and Colpoda inflata within the phylum ciliophora inferred from complete small subunit rRNA gene sequences. J Protozool. 1991 Jan-Feb;38(1):1–6. doi: 10.1111/j.1550-7408.1991.tb04783.x. [DOI] [PubMed] [Google Scholar]
- Greslin A. F., Loukin S. H., Oka Y., Prescott D. M. An analysis of the macronuclear actin genes of Oxytricha. DNA. 1988 Oct;7(8):529–536. doi: 10.1089/dna.1.1988.7.529. [DOI] [PubMed] [Google Scholar]
- Greslin A. F., Prescott D. M., Oka Y., Loukin S. H., Chappell J. C. Reordering of nine exons is necessary to form a functional actin gene in Oxytricha nova. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6264–6268. doi: 10.1073/pnas.86.16.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez J. C., Martin-Gonzalez A., Matsusaka T. Towards a generalized model of encystment (cryptobiosis) in ciliates: a review and a hypothesis. Biosystems. 1990;24(1):17–24. doi: 10.1016/0303-2647(90)90025-v. [DOI] [PubMed] [Google Scholar]
- Guttman S. D., Glover C. V., Allis C. D., Gorovsky M. A. Heat shock, deciliation and release from anoxia induce the synthesis of the same set of polypeptides in starved T. pyriformis. Cell. 1980 Nov;22(1 Pt 1):299–307. doi: 10.1016/0092-8674(80)90177-4. [DOI] [PubMed] [Google Scholar]
- Hallberg E. M., Fung P., Hallberg R. L. Genomic sequence encoding a heat shock-induced, RNA polymerase III-transcribed RNA from Tetrahymena thermophila. Nucleic Acids Res. 1992 Feb 25;20(4):912–912. doi: 10.1093/nar/20.4.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamana K., Iwai K. Fractionation and characterization of Tetrahymena histone in comparison with mammalian histones. J Biochem. 1971 Jun;69(6):1097–1111. doi: 10.1093/oxfordjournals.jbchem.a129563. [DOI] [PubMed] [Google Scholar]
- Hanyu N., Kuchino Y., Nishimura S., Beier H. Dramatic events in ciliate evolution: alteration of UAA and UAG termination codons to glutamine codons due to anticodon mutations in two Tetrahymena tRNAs. EMBO J. 1986 Jun;5(6):1307–1311. doi: 10.1002/j.1460-2075.1986.tb04360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper D. S., Jahn C. L. Actin, tubulin and H4 histone genes in three species of hypotrichous ciliated protozoa. Gene. 1989 Jan 30;75(1):93–107. doi: 10.1016/0378-1119(89)90386-7. [DOI] [PubMed] [Google Scholar]
- Harper D. S., Jahn C. L. Differential use of termination codons in ciliated protozoa. Proc Natl Acad Sci U S A. 1989 May;86(9):3252–3256. doi: 10.1073/pnas.86.9.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper D. S., Song K., Jahn C. L. Overamplification of macronuclear linear DNA molecules during prolonged vegetative growth of Oxytricha nova. Gene. 1991 Mar 1;99(1):55–61. doi: 10.1016/0378-1119(91)90033-8. [DOI] [PubMed] [Google Scholar]
- Harumoto T. Induced change in a non-mendelian determinant by transplantation of macronucleoplasm in Paramecium tetraurelia. Mol Cell Biol. 1986 Oct;6(10):3498–3501. doi: 10.1128/mcb.6.10.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser L. J., Roberson A. E., Olins D. E. Structure of the macronuclear polyubiquitin gene in Euplotes. Chromosoma. 1991 Jul;100(6):386–394. doi: 10.1007/BF00337517. [DOI] [PubMed] [Google Scholar]
- Hauser L. J., Treat M. L., Olins D. E. Cloning and analysis of the macronuclear gene for histone H1 from Euplotes eurystomus. Nucleic Acids Res. 1993 Jul 25;21(15):3586–3586. doi: 10.1093/nar/21.15.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helftenbein E., Müller E. Both alpha-tubulin genes are transcriptionally active in Stylonychia lemnae. Curr Genet. 1988 May;13(5):425–432. doi: 10.1007/BF00365664. [DOI] [PubMed] [Google Scholar]
- Helftenbein E. Nucleotide sequence of a macronuclear DNA molecule coding for alpha-tubulin from the ciliate Stylonychia lemnae. Special codon usage: TAA is not a translation termination codon. Nucleic Acids Res. 1985 Jan 25;13(2):415–433. doi: 10.1093/nar/13.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E. R., Blackburn E. H. An overhanging 3' terminus is a conserved feature of telomeres. Mol Cell Biol. 1989 Jan;9(1):345–348. doi: 10.1128/mcb.9.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E., Hardin C. C., Walk S. K., Tinoco I., Jr, Blackburn E. H. Telomeric DNA oligonucleotides form novel intramolecular structures containing guanine-guanine base pairs. Cell. 1987 Dec 24;51(6):899–908. doi: 10.1016/0092-8674(87)90577-0. [DOI] [PubMed] [Google Scholar]
- Herrick G., Cartinhour S. W., Williams K. R., Kotter K. P. Multiple sequence versions of the Oxytricha fallax 81-MAC alternate processing family. J Protozool. 1987 Nov;34(4):429–434. doi: 10.1111/j.1550-7408.1987.tb03207.x. [DOI] [PubMed] [Google Scholar]
- Herrick G., Cartinhour S., Dawson D., Ang D., Sheets R., Lee A., Williams K. Mobile elements bounded by C4A4 telomeric repeats in Oxytricha fallax. Cell. 1985 Dec;43(3 Pt 2):759–768. doi: 10.1016/0092-8674(85)90249-1. [DOI] [PubMed] [Google Scholar]
- Herrick G., Hunter D., Williams K., Kotter K. Alternative processing during development of a macronuclear chromosome family in Oxytricha fallax. Genes Dev. 1987 Dec;1(10):1047–1058. doi: 10.1101/gad.1.10.1047. [DOI] [PubMed] [Google Scholar]
- Herrick G. Non-coding DNA in macronuclear chromosomes of hypotrichous ciliates. J Protozool. 1992 Mar-Apr;39(2):309–312. doi: 10.1111/j.1550-7408.1992.tb01320.x. [DOI] [PubMed] [Google Scholar]
- Herrick G., Wesley R. D. Isolation and characterization of a highly repetitious inverted terminal repeat sequence from Oxytricha macronuclear DNA. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2626–2630. doi: 10.1073/pnas.75.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke B. J., Celander D. W., MacDonald G. H., Price C. M., Cech T. R. Two versions of the gene encoding the 41-kilodalton subunit of the telomere binding protein of Oxytricha nova. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1481–1485. doi: 10.1073/pnas.87.4.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono M., Endoh H., Okada N., Numata O., Watanabe Y. Tetrahymena actin. Cloning and sequencing of the Tetrahymena actin gene and identification of its gene product. J Mol Biol. 1987 Mar 20;194(2):181–192. doi: 10.1016/0022-2836(87)90367-6. [DOI] [PubMed] [Google Scholar]
- Horowitz S., Gorovsky M. A. An unusual genetic code in nuclear genes of Tetrahymena. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2452–2455. doi: 10.1073/pnas.82.8.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard E. A., Blackburn E. H. Reproducible and variable genomic rearrangements occur in the developing somatic nucleus of the ciliate Tetrahymena thermophila. Mol Cell Biol. 1985 Aug;5(8):2039–2050. doi: 10.1128/mcb.5.8.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter D. J., Williams K., Cartinhour S., Herrick G. Precise excision of telomere-bearing transposons during Oxytricha fallax macronuclear development. Genes Dev. 1989 Dec;3(12B):2101–2112. doi: 10.1101/gad.3.12b.2101. [DOI] [PubMed] [Google Scholar]
- Iwamura Y., Sakai M., Mita T., Muramatsu M. Unequal gene amplification and transcription in the macronucleus of Tetrahymena pyriformis. Biochemistry. 1979 Nov 27;18(24):5289–5294. doi: 10.1021/bi00591a004. [DOI] [PubMed] [Google Scholar]
- Jahn C. L. Bal31 sensitivity of micronuclear sequences homologous to C4A4/G4T4 repeats in Oxytricha nova. Exp Cell Res. 1988 Jul;177(1):162–175. doi: 10.1016/0014-4827(88)90034-1. [DOI] [PubMed] [Google Scholar]
- Jahn C. L., Doktor S. Z., Frels J. S., Jaraczewski J. W., Krikau M. F. Structures of the Euplotes crassus Tec1 and Tec2 elements: identification of putative transposase coding regions. Gene. 1993 Oct 29;133(1):71–78. doi: 10.1016/0378-1119(93)90226-s. [DOI] [PubMed] [Google Scholar]
- Jahn C. L., Krikau M. F., Shyman S. Developmentally coordinated en masse excision of a highly repetitive element in E. crassus. Cell. 1989 Dec 22;59(6):1009–1018. doi: 10.1016/0092-8674(89)90757-5. [DOI] [PubMed] [Google Scholar]
- Jahn C. L., Nilles L. A., Krikau M. F. Organization of the Euplotes crassus micronuclear genome. J Protozool. 1988 Nov;35(4):590–601. doi: 10.1111/j.1550-7408.1988.tb04157.x. [DOI] [PubMed] [Google Scholar]
- Jahn C. L., Prescott K. E., Waggener M. W. Organization of the micronuclear genome of oxytricha nova. Genetics. 1988 Sep;120(1):123–134. doi: 10.1093/genetics/120.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaraczewski J. W., Jahn C. L. Elimination of Tec elements involves a novel excision process. Genes Dev. 1993 Jan;7(1):95–105. doi: 10.1101/gad.7.1.95. [DOI] [PubMed] [Google Scholar]
- Jerka-Dziadosz M., Frankel J. The control of DNA synthesis in macronuclei and micronuclei of a hypotrich ciliate: a comparison of normal and regenerating cells. J Exp Zool. 1970 Jan;173(1):1–22. doi: 10.1002/jez.1401730102. [DOI] [PubMed] [Google Scholar]
- Johmann C. A., Gorovsky M. A. Purification and characterization of the histones associated with the macronucleus of Tetrahymena. Biochemistry. 1976 Mar 23;15(6):1249–1256. doi: 10.1021/bi00651a012. [DOI] [PubMed] [Google Scholar]
- KIMBALL R. F., BARKA T. Quantitative cytochemical studies on Paramecium aurelia. II. Feulgen microspectrophotometry of the macronucleus during exponential growth. Exp Cell Res. 1959 Apr;17(1):173–182. doi: 10.1016/0014-4827(59)90162-4. [DOI] [PubMed] [Google Scholar]
- Kaine B. P., Spear B. B. Nucleotide sequence of a macronuclear gene for actin in Oxytricha fallax. Nature. 1982 Feb 4;295(5848):430–432. doi: 10.1038/295430a0. [DOI] [PubMed] [Google Scholar]
- Kan N. C., Gall J. G. Sequence homology near the center of the extrachromosomal rDNA palindrome in Tetrahymena. J Mol Biol. 1981 Dec 25;153(4):1151–1155. doi: 10.1016/0022-2836(81)90472-1. [DOI] [PubMed] [Google Scholar]
- Kaney A. R., Speare V. J. An amicronucleate mutant of Tetrahymena thermophila. Exp Cell Res. 1983 Feb;143(2):461–467. doi: 10.1016/0014-4827(83)90074-5. [DOI] [PubMed] [Google Scholar]
- Kang C., Zhang X., Ratliff R., Moyzis R., Rich A. Crystal structure of four-stranded Oxytricha telomeric DNA. Nature. 1992 Mar 12;356(6365):126–131. doi: 10.1038/356126a0. [DOI] [PubMed] [Google Scholar]
- Kapler G. M., Blackburn E. H. A weak germ-line excision mutation blocks developmentally controlled amplification of the rDNA minichromosome of Tetrahymena thermophila. Genes Dev. 1994 Jan;8(1):84–95. doi: 10.1101/gad.8.1.84. [DOI] [PubMed] [Google Scholar]
- Karrer K. M., Gall J. G. The macronuclear ribosomal DNA of Tetrahymena pyriformis is a palindrome. J Mol Biol. 1976 Jun 25;104(2):421–453. doi: 10.1016/0022-2836(76)90280-1. [DOI] [PubMed] [Google Scholar]
- Karrer K. M. Germ line-specific DNA sequences are present on all five micronuclear chromosomes in Tetrahymena thermophila. Mol Cell Biol. 1983 Nov;3(11):1909–1919. doi: 10.1128/mcb.3.11.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrer K., Stein-Gavens S., Allitto B. A. Micronucleus-specific DNA sequences in an amicronucleate mutant of Tetrahymena. Dev Biol. 1984 Sep;105(1):121–129. doi: 10.1016/0012-1606(84)90267-7. [DOI] [PubMed] [Google Scholar]
- Katinka M. D., Bourgain F. M. Interstitial telomeres are hotspots for illegitimate recombination with DNA molecules injected into the macronucleus of Paramecium primaurelia. EMBO J. 1992 Feb;11(2):725–732. doi: 10.1002/j.1460-2075.1992.tb05105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzen A. L., Cann G. M., Blackburn E. H. Sequence-specific fragmentation of macronuclear DNA in a holotrichous ciliate. Cell. 1981 May;24(2):313–320. doi: 10.1016/0092-8674(81)90321-4. [DOI] [PubMed] [Google Scholar]
- Kaufmann J., Florian V., Klein A. TGA cysteine codons and intron sequences in conserved and nonconserved positions are found in macronuclear RNA polymerase genes of Euplotes octocarinatus. Nucleic Acids Res. 1992 Nov 25;20(22):5985–5989. doi: 10.1093/nar/20.22.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann J., Klein A. Gene dosage as a possible major determinant for equal expression levels of genes encoding RNA polymerase subunits in the hypotrichous ciliate Euplotes octocarinatus. Nucleic Acids Res. 1992 Sep 11;20(17):4445–4450. doi: 10.1093/nar/20.17.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B. O., Yao M. C. Tandemly repeated hexanucleotide at Tetrahymena rDNA free end is generated from a single copy during development. Cell. 1982 Nov;31(1):177–182. doi: 10.1016/0092-8674(82)90417-2. [DOI] [PubMed] [Google Scholar]
- Kiss G. B., Amin A. A., Pearlman R. E. Two separate regions of the extrachromosomal ribosomal deoxyribonucleic acid of Tetrahymena thermophila enable autonomous replication of plasmids in Saccharomyces cerevisiae. Mol Cell Biol. 1981 Jun;1(6):535–543. doi: 10.1128/mcb.1.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss G. B., Pearlman R. E. Extrachromosomal rDNA of Tetrahymena thermophila is not a perfect palindrome. Gene. 1981 Apr;13(3):281–287. doi: 10.1016/0378-1119(81)90032-9. [DOI] [PubMed] [Google Scholar]
- Klobutcher L. A., Huff M. E., Gonye G. E. Alternative use of chromosome fragmentation sites in the ciliated protozoan Oxytricha nova. Nucleic Acids Res. 1988 Jan 11;16(1):251–264. doi: 10.1093/nar/16.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobutcher L. A., Jahn C. L. Developmentally controlled genomic rearrangements in ciliated protozoa. Curr Opin Genet Dev. 1991 Oct;1(3):397–403. doi: 10.1016/s0959-437x(05)80306-5. [DOI] [PubMed] [Google Scholar]
- Klobutcher L. A., Jahn C. L., Prescott D. M. Internal sequences are eliminated from genes during macronuclear development in the ciliated protozoan Oxytricha nova. Cell. 1984 Apr;36(4):1045–1055. doi: 10.1016/0092-8674(84)90054-0. [DOI] [PubMed] [Google Scholar]
- Klobutcher L. A., Swanton M. T., Donini P., Prescott D. M. All gene-sized DNA molecules in four species of hypotrichs have the same terminal sequence and an unusual 3' terminus. Proc Natl Acad Sci U S A. 1981 May;78(5):3015–3019. doi: 10.1073/pnas.78.5.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobutcher L. A., Turner L. R., LaPlante J. Circular forms of developmentally excised DNA in Euplotes crassus have a heteroduplex junction. Genes Dev. 1993 Jan;7(1):84–94. doi: 10.1101/gad.7.1.84. [DOI] [PubMed] [Google Scholar]
- Klobutcher L. A., Turner L. R., Peralta M. E. Sequence of a Euplotes crassus macronuclear DNA molecule encoding a protein with homology to a rat form-I phosphoinositide-specific phospholipase C. J Protozool. 1991 Jul-Aug;38(4):425–427. doi: 10.1111/j.1550-7408.1991.tb01381.x. [DOI] [PubMed] [Google Scholar]
- Klobutcher L. A., Vailonis-Walsh A. M., Cahill K., Ribas-Aparicio R. M. Gene-sized macronuclear DNA molecules are clustered in micronuclear chromosomes of the ciliate Oxytricha nova. Mol Cell Biol. 1986 Nov;6(11):3606–3613. doi: 10.1128/mcb.6.11.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S., Kobayashi S. Microinjection of plasmid DNA encoding the A surface antigen of Paramecium tetraurelia restores the ability to regenerate a wild-type macronucleus. Mol Cell Biol. 1989 Oct;9(10):4398–4401. doi: 10.1128/mcb.9.10.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikau M. F., Jahn C. L. Tec2, a second transposon-like element demonstrating developmentally programmed excision in Euplotes crassus. Mol Cell Biol. 1991 Sep;11(9):4751–4759. doi: 10.1128/mcb.11.9.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchino Y., Hanyu N., Tashiro F., Nishimura S. Tetrahymena thermophila glutamine tRNA and its gene that corresponds to UAA termination codon. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4758–4762. doi: 10.1073/pnas.82.14.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazaki T., Hori H., Osawa S. Phylogeny of protozoa deduced from 5S rRNA sequences. J Mol Evol. 1983;19(6):411–419. doi: 10.1007/BF02102316. [DOI] [PubMed] [Google Scholar]
- Larson D. D., Blackburn E. H., Yaeger P. C., Orias E. Control of rDNA replication in Tetrahymena involves a cis-acting upstream repeat of a promoter element. Cell. 1986 Oct 24;47(2):229–240. doi: 10.1016/0092-8674(86)90445-9. [DOI] [PubMed] [Google Scholar]
- Larson D. D., Spangler E. A., Blackburn E. H. Dynamics of telomere length variation in Tetrahymena thermophila. Cell. 1987 Jul 31;50(3):477–483. doi: 10.1016/0092-8674(87)90501-0. [DOI] [PubMed] [Google Scholar]
- Larson D. D., Umthun A. R., Shaiu W. L. Copy number control in the Tetrahymena macronuclear genome. J Protozool. 1991 May-Jun;38(3):258–263. doi: 10.1111/j.1550-7408.1991.tb04439.x. [DOI] [PubMed] [Google Scholar]
- Lauth M. R., Spear B. B., Heumann J., Prescott D. M. DNA of ciliated protozoa: DNA sequence diminution during macronuclear development of Oxytricha. Cell. 1976 Jan;7(1):67–74. doi: 10.1016/0092-8674(76)90256-7. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Heumann J. M., Herrick G., Prescott D. M. The gene-size DNA molecules in Oxytricha. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):483–492. doi: 10.1101/sqb.1978.042.01.051. [DOI] [PubMed] [Google Scholar]
- Liang A., Heckmann K. Blepharisma uses UAA as a termination codon. Naturwissenschaften. 1993 May;80(5):225–226. doi: 10.1007/BF01175738. [DOI] [PubMed] [Google Scholar]
- Liang A., Heckmann K. The macronuclear gamma-tubulin-encoding gene of Euplotes octocarinatus contains two introns and an in-frame TGA. Gene. 1993 Dec 22;136(1-2):319–322. doi: 10.1016/0378-1119(93)90487-n. [DOI] [PubMed] [Google Scholar]
- Lin M., Prescott D. M. Electron microscope autoradiography of DNA synthesis in the replication band of two hypotrichous ciliates. J Protozool. 1985 Feb;32(1):144–149. doi: 10.1111/j.1550-7408.1985.tb03028.x. [DOI] [PubMed] [Google Scholar]
- Lipps H. J., Erhardt P. DNase I hypersensitivity of the terminal inverted repeat DNA sequences in the macronucleus of the ciliate Stylonychia mytilus. FEBS Lett. 1981 Apr 20;126(2):219–222. doi: 10.1016/0014-5793(81)80246-3. [DOI] [PubMed] [Google Scholar]
- Lipps H. J., Gruissem W., Prescott D. M. Higher order DNA structure in macronuclear chromatin of the hypotrichous ciliate Oxytricha nova. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2495–2499. doi: 10.1073/pnas.79.8.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipps H. J. In vitro aggregation of the gene-sized DNA molecules of the ciliate Stylonychia mytilus. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4104–4107. doi: 10.1073/pnas.77.7.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipps H. J., Morris N. R. Chromatin structure in the nuclei of the ciliate stylonychia mytilus. Biochem Biophys Res Commun. 1977 Jan 10;74(1):230–234. doi: 10.1016/0006-291x(77)91398-5. [DOI] [PubMed] [Google Scholar]
- Lipps H. J., Steinbrück G. Free genes for rRNAs in the macronuclear genome of the ciliate Stylonychia mytilus. Chromosoma. 1978 Oct 20;69(1):21–26. doi: 10.1007/BF00327378. [DOI] [PubMed] [Google Scholar]
- Liu Z., Frantz J. D., Gilbert W., Tye B. K. Identification and characterization of a nuclease activity specific for G4 tetrastranded DNA. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3157–3161. doi: 10.1073/pnas.90.8.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love H. D., Jr, Allen-Nash A., Zhao Q. A., Bannon G. A. mRNA stability plays a major role in regulating the temperature-specific expression of a Tetrahymena thermophila surface protein. Mol Cell Biol. 1988 Jan;8(1):427–432. doi: 10.1128/mcb.8.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn D. H., Sogin M. L. Assessment of phylogenetic relationships among ciliated protists using partial ribosomal RNA sequences derived from reverse transcripts. Biosystems. 1988;21(3-4):249–254. doi: 10.1016/0303-2647(88)90020-2. [DOI] [PubMed] [Google Scholar]
- Maercker C., Lipps H. J. Analysis of the subtelomeric regions of macronuclear gene-sized DNA molecules of the hypotrichous ciliate Stylonychia lemnae: implications for the DNA fragmentation process during macronuclear development? Dev Genet. 1993;14(5):378–384. doi: 10.1002/dvg.1020140507. [DOI] [PubMed] [Google Scholar]
- Martindale D. W., Allis C. D., Bruns P. J. RNA and protein synthesis during meiotic prophase in Tetrahymena thermophila. J Protozool. 1985 Nov;32(4):644–649. doi: 10.1111/j.1550-7408.1985.tb03094.x. [DOI] [PubMed] [Google Scholar]
- Martindale D. W., Bruns P. J. Cloning of abundant mRNA species present during conjugation of Tetrahymena thermophila: identification of mRNA species present exclusively during meiosis. Mol Cell Biol. 1983 Oct;3(10):1857–1865. doi: 10.1128/mcb.3.10.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale D. W. Codon usage in Tetrahymena and other ciliates. J Protozool. 1989 Jan-Feb;36(1):29–34. doi: 10.1111/j.1550-7408.1989.tb02679.x. [DOI] [PubMed] [Google Scholar]
- Martindale D. W., Gu Z. M., Csank C. Isolation and complete sequence of the yeast isoleucyl-tRNA synthetase gene (ILS1). Curr Genet. 1989 Feb;15(2):99–106. doi: 10.1007/BF00435455. [DOI] [PubMed] [Google Scholar]
- Martindale D. W., Martindale H. M., Bruns P. J. Tetrahymena conjugation-induced genes: structure and organization in macro- and micronuclei. Nucleic Acids Res. 1986 Feb 11;14(3):1341–1354. doi: 10.1093/nar/14.3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale D. W., Taylor F. M. Multiple introns in a conjugation-specific gene from Tetrahymena thermophila. Nucleic Acids Res. 1988 Mar 25;16(5):2189–2201. doi: 10.1093/nar/16.5.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo K. A., Orias E. Further evidence for lack of gene expression in the Tetrahymena micronucleus. Genetics. 1981 Aug;98(4):747–762. doi: 10.1093/genetics/98.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam E. V., Bruns P. J. Phenotypic assortment in Tetrahymena thermophila: assortment kinetics of antibiotic-resistance markers, tsA, death, and the highly amplified rDNA locus. Genetics. 1988 Oct;120(2):389–395. doi: 10.1093/genetics/120.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer E. Induction of specific macronuclear developmental mutations by microinjection of a cloned telomeric gene in Paramecium primaurelia. Genes Dev. 1992 Feb;6(2):211–222. doi: 10.1101/gad.6.2.211. [DOI] [PubMed] [Google Scholar]
- Meyer F., Schmidt H. J., Heckmann K. Pheromone 4 gene of Euplotes octocarinatus. Dev Genet. 1992;13(1):16–25. doi: 10.1002/dvg.1020130104. [DOI] [PubMed] [Google Scholar]
- Meyer F., Schmidt H. J., Plümper E., Hasilik A., Mersmann G., Meyer H. E., Engström A., Heckmann K. UGA is translated as cysteine in pheromone 3 of Euplotes octocarinatus. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3758–3761. doi: 10.1073/pnas.88.9.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami K., Kuhlmann H. W., Heckmann K. Is the initiation of macronuclear DNA synthesis in Euplotes dependent on micronuclear functions? Exp Cell Res. 1985 Dec;161(2):445–459. doi: 10.1016/0014-4827(85)90100-4. [DOI] [PubMed] [Google Scholar]
- Mitcham J. L., Lynn A. J., Prescott D. M. Analysis of a scrambled gene: the gene encoding alpha-telomere-binding protein in Oxytricha nova. Genes Dev. 1992 May;6(5):788–800. doi: 10.1101/gad.6.5.788. [DOI] [PubMed] [Google Scholar]
- Murti K. G. Electron-microscopic observations on the macronuclear development of Stylonychia mytilus and Tetrahymena pyriformis (Ciliophora-Protozoa). J Cell Sci. 1973 Sep;13(2):479–509. doi: 10.1242/jcs.13.2.479. [DOI] [PubMed] [Google Scholar]
- Murti K. G., Prescott D. M. Replication forms of the gene-sized DNA molecules of hypotrichous ciliates. Mol Cell Biol. 1983 Sep;3(9):1562–1566. doi: 10.1128/mcb.3.9.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanney D. L., McCoy J. W. Characterization of the species of the Tetrahymena pyriformis complex. Trans Am Microsc Soc. 1976 Oct;95(4):664–682. [PubMed] [Google Scholar]
- Narasimharao M. V., Prescott D. M. Micronuclear RNA synthesis in Paramecium caudatum. J Cell Biol. 1967 May;33(2):281–285. doi: 10.1083/jcb.33.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves A. M., Barahona I., Galego L., Rodrigues-Pousada C. Ubiquitin genes in Tetrahymena pyriformis and their expression during heat shock. Gene. 1988 Dec 15;73(1):87–96. doi: 10.1016/0378-1119(88)90315-0. [DOI] [PubMed] [Google Scholar]
- Nielsen H., Andreasen P. H., Dreisig H., Kristiansen K., Engberg J. An intron in a ribosomal protein gene from Tetrahymena. EMBO J. 1986 Oct;5(10):2711–2717. doi: 10.1002/j.1460-2075.1986.tb04555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomoto M., Imai N., Saiga H., Matsui T., Mita T. Characterization of two types of histone H2B genes from macronuclei of Tetrahymena thermophila. Nucleic Acids Res. 1987 Jul 24;15(14):5681–5697. doi: 10.1093/nar/15.14.5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomoto M., Matsui T., Saiga H., Mita T. Histone genes of ciliates. Oxf Surv Eukaryot Genes. 1988;5:251–278. [PubMed] [Google Scholar]
- Numata O., Tomiyoshi T., Kurasawa Y., Fu Z. X., Takahashi M., Kosaka T., Chiba J., Watanabe Y. Antibodies against Tetrahymena 14-nm filament-forming protein recognize the replication band in Euplotes. Exp Cell Res. 1991 Mar;193(1):183–189. doi: 10.1016/0014-4827(91)90554-8. [DOI] [PubMed] [Google Scholar]
- Oakley C. E., Oakley B. R. Identification of gamma-tubulin, a new member of the tubulin superfamily encoded by mipA gene of Aspergillus nidulans. Nature. 1989 Apr 20;338(6217):662–664. doi: 10.1038/338662a0. [DOI] [PubMed] [Google Scholar]
- Oka Y., Shiota S., Nakai S., Nishida Y., Okubo S. Inverted terminal repeat sequence in the macronuclear DNA of Stylonychia pustulata. Gene. 1980 Sep;10(4):301–306. doi: 10.1016/0378-1119(80)90150-x. [DOI] [PubMed] [Google Scholar]
- Oka Y., Thomas C. A., Jr The cohering telomeres of Oxytricha. Nucleic Acids Res. 1987 Nov 11;15(21):8877–8898. doi: 10.1093/nar/15.21.8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olins A. L., Olins D. E., Franke W. W., Lipps H. J., Prescott D. M. Stereo-electron microscopy of nuclear structure and replication in ciliated protozoa (Hypotricha). Eur J Cell Biol. 1981 Aug;25(1):120–130. [PubMed] [Google Scholar]
- Olins D. E., Olins A. L., Cacheiro L. H., Tan E. M. Proliferating cell nuclear antigen/cyclin in the ciliate Euplotes eurystomus: localization in the replication band and in micronuclei. J Cell Biol. 1989 Oct;109(4 Pt 1):1399–1410. doi: 10.1083/jcb.109.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orias E., Flacks M. Macronuclear genetics of Tetrahymena. I. Random distribution of macronuclear genecopies in T. pyriformis, syngen 1. Genetics. 1975 Feb;79(2):187–206. doi: 10.1093/genetics/79.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRESCOTT D. M., KIMBALL R. F. Relation between RNA, DNA, and protein syntheses in the replicating nucleus of Euplotes. Proc Natl Acad Sci U S A. 1961 May 15;47:686–693. doi: 10.1073/pnas.47.5.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak J. Differential genic activity in Paramecium aurelia. J Exp Zool. 1967 Aug;165(3):395–417. doi: 10.1002/jez.1401650308. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Pelvat B., de Haller G. Macronuclear DNA in Stentor coeruleus: a first approach to its characterization. Genet Res. 1976 Apr;27(2):277–289. doi: 10.1017/s0016672300016463. [DOI] [PubMed] [Google Scholar]
- Pluta A. F., Kaine B. P., Spear B. B. The terminal organization of macronuclear DNA in Oxytricha fallax. Nucleic Acids Res. 1982 Dec 20;10(24):8145–8154. doi: 10.1093/nar/10.24.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta A. F., Spear B. B. Localization of genes for ribosomal RNA in the nuclei of Oxytricha fallax. Exp Cell Res. 1981 Oct;135(2):387–392. doi: 10.1016/0014-4827(81)90175-0. [DOI] [PubMed] [Google Scholar]
- Prat A., Katinka M., Caron F., Meyer E. Nucleotide sequence of the Paramecium primaurelia G surface protein. A huge protein with a highly periodic structure. J Mol Biol. 1986 May 5;189(1):47–60. doi: 10.1016/0022-2836(86)90380-3. [DOI] [PubMed] [Google Scholar]
- Preer J. R., Jr, Preer L. B., Rudman B. M., Barnett A. J. Deviation from the universal code shown by the gene for surface protein 51A in Paramecium. Nature. 1985 Mar 14;314(6007):188–190. doi: 10.1038/314188a0. [DOI] [PubMed] [Google Scholar]
- Preer J. R., Jr Quantitative predictions of random segregation models of the ciliate macronucleus. Genet Res. 1976 Apr;27(2):227–238. doi: 10.1017/s0016672300016426. [DOI] [PubMed] [Google Scholar]
- Prescott D. M., Greslin A. F. Scrambled actin I gene in the micronucleus of Oxytricha nova. Dev Genet. 1992;13(1):66–74. doi: 10.1002/dvg.1020130111. [DOI] [PubMed] [Google Scholar]
- Prescott D. M., Murti K. G., Bostock C. J. Genetic apparatus of Stylonychia sp. Nature. 1973 Apr 27;242(5400):576, 597-600. doi: 10.1038/242576a0. [DOI] [PubMed] [Google Scholar]
- Prescott D. M., Murti K. G. Chromosome structure in ciliated protozoans. Cold Spring Harb Symp Quant Biol. 1974;38:609–618. doi: 10.1101/sqb.1974.038.01.065. [DOI] [PubMed] [Google Scholar]
- Prescott D. M. Restructuring of DNA sequences in the germline genome of Oxytricha. Curr Opin Genet Dev. 1993 Oct;3(5):726–729. doi: 10.1016/s0959-437x(05)80090-5. [DOI] [PubMed] [Google Scholar]
- Prescott D. M. The syntheses of total macronuclear protein, histone, and DNA during the cell cycle in Euplotes eurystomus. J Cell Biol. 1966 Oct;31(1):1–9. doi: 10.1083/jcb.31.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott D. M. The unusual organization and processing of genomic DNA in hypotrichous ciliates. Trends Genet. 1992 Dec;8(12):439–445. doi: 10.1016/0168-9525(92)90328-2. [DOI] [PubMed] [Google Scholar]
- Price C. M., Cech T. R. Properties of the telomeric DNA-binding protein from Oxytricha nova. Biochemistry. 1989 Jan 24;28(2):769–774. doi: 10.1021/bi00428a053. [DOI] [PubMed] [Google Scholar]
- Price C. M., Cech T. R. Telomeric DNA-protein interactions of Oxytricha macronuclear DNA. Genes Dev. 1987 Oct;1(8):783–793. doi: 10.1101/gad.1.8.783. [DOI] [PubMed] [Google Scholar]
- Price C. M., Skopp R., Krueger J., Williams D. DNA recognition and binding by the Euplotes telomere protein. Biochemistry. 1992 Nov 10;31(44):10835–10843. doi: 10.1021/bi00159a026. [DOI] [PubMed] [Google Scholar]
- Pyne C. K. Electron microscopic studies on the macronuclear development in the ciliate Chilodonella uncinata. Cytobiologie. 1978 Oct;18(1):145–160. [PubMed] [Google Scholar]
- Pyne C. K., Ruch F., Leemann U., Schneider S. Development of the macronuclear anlage in the ciliate Chilodonella uncinate. I. Morphological and cytophotometric studies on the evolution of DNA. Chromosoma. 1974;48(3):225–238. doi: 10.1007/BF00326506. [DOI] [PubMed] [Google Scholar]
- Rae P. M., Spear B. B. Macronuclear DNA of the hypotrichous ciliate Oxytricha fallax. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4992–4996. doi: 10.1073/pnas.75.10.4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuraman M. K., Cech T. R. Assembly and self-association of oxytricha telomeric nucleoprotein complexes. Cell. 1989 Nov 17;59(4):719–728. doi: 10.1016/0092-8674(89)90018-4. [DOI] [PubMed] [Google Scholar]
- Rao M. V. Macronuclear development in Euplotes woodruffi following conjugation. Exp Cell Res. 1968 Feb;49(2):411–419. doi: 10.1016/0014-4827(68)90190-0. [DOI] [PubMed] [Google Scholar]
- Ribas-Aparicio R. M., Sparkowski J. J., Proulx A. E., Mitchell J. D., Klobutcher L. A. Nucleic acid splicing events occur frequently during macronuclear development in the protozoan Oxytricha nova and involve the elimination of unique DNA. Genes Dev. 1987 Jun;1(4):323–336. doi: 10.1101/gad.1.4.323. [DOI] [PubMed] [Google Scholar]
- Roth M., Lin M., Prescott D. M. Large scale synchronous mating and the study of macronuclear development in Euplotes crassus. J Cell Biol. 1985 Jul;101(1):79–84. doi: 10.1083/jcb.101.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M., Prescott D. M. DNA intermediates and telomere addition during genome reorganization in Euplotes crassus. Cell. 1985 Jun;41(2):411–417. doi: 10.1016/s0092-8674(85)80014-3. [DOI] [PubMed] [Google Scholar]
- Rudman B., Preer L. B., Polisky B., Preer J. R., Jr Mutants affecting processing of DNA in macronuclear development in paramecium. Genetics. 1991 Sep;129(1):47–56. doi: 10.1093/genetics/129.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STONE G. E., PRESCOTT D. M. CELL DIVISION AND DNA SYNTHESIS IN TETRAHYMENA PYRIFORMIS DEPRIVED OF ESSENTIAL AMINO ACIDS. J Cell Biol. 1964 May;21:275–281. doi: 10.1083/jcb.21.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. M., Mikami K., Leeck C. L., Forney J. D. Non-Mendelian inheritance of macronuclear mutations is gene specific in Paramecium tetraurelia. Mol Cell Biol. 1994 Apr;14(4):2479–2484. doi: 10.1128/mcb.14.4.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen D., Gilbert W. Formation of parallel four-stranded complexes by guanine-rich motifs in DNA and its implications for meiosis. Nature. 1988 Jul 28;334(6180):364–366. doi: 10.1038/334364a0. [DOI] [PubMed] [Google Scholar]
- Seyfert H. M., Cleffmann G. Mean macronuclear DNA contents are variable in the ciliate Tetrahymena. J Cell Sci. 1982 Dec;58:211–223. doi: 10.1242/jcs.58.1.211. [DOI] [PubMed] [Google Scholar]
- Seyfert H. M., Preparata R. M. The regulation of amounts and proportions of genetic elements in the macronuclei of Tetrahymena thermophila strains of diverse karyotype. J Cell Sci. 1979 Dec;40:111–123. doi: 10.1242/jcs.40.1.111. [DOI] [PubMed] [Google Scholar]
- Smith F. W., Feigon J. Quadruplex structure of Oxytricha telomeric DNA oligonucleotides. Nature. 1992 Mar 12;356(6365):164–168. doi: 10.1038/356164a0. [DOI] [PubMed] [Google Scholar]
- Soares H., Cyrne L., Barahona I., Rodrigues-Pousada C. Different patterns of expression of beta-tubulin genes in Tetrahymena pyriformis during reciliation. Eur J Biochem. 1991 Apr 23;197(2):291–299. doi: 10.1111/j.1432-1033.1991.tb15910.x. [DOI] [PubMed] [Google Scholar]
- Soldo A. T., Godoy G. A. The kinetic complexity of Paramecium macronuclear deoxyribonucleic acid. J Protozool. 1972 Nov;19(4):673–678. doi: 10.1111/j.1550-7408.1972.tb03558.x. [DOI] [PubMed] [Google Scholar]
- Spear B. B. Isolation and mapping of the rRNA genes in the macronucleus of Oxytricha fallax. Chromosoma. 1980;77(2):193–202. doi: 10.1007/BF00329544. [DOI] [PubMed] [Google Scholar]
- Spear B. B., Lauth M. R. Polytene chromosomes of Oxytricha: biochemical and morphological changes during macronuclear development in a ciliated protozoan. Chromosoma. 1976 Jan 27;54(1):1–13. doi: 10.1007/BF00331828. [DOI] [PubMed] [Google Scholar]
- Stargell L. A., Bowen J., Dadd C. A., Dedon P. C., Davis M., Cook R. G., Allis C. D., Gorovsky M. A. Temporal and spatial association of histone H2A variant hv1 with transcriptionally competent chromatin during nuclear development in Tetrahymena thermophila. Genes Dev. 1993 Dec;7(12B):2641–2651. doi: 10.1101/gad.7.12b.2641. [DOI] [PubMed] [Google Scholar]
- Stargell L. A., Gorovsky M. A. TATA-binding protein and nuclear differentiation in Tetrahymena thermophila. Mol Cell Biol. 1994 Jan;14(1):723–734. doi: 10.1128/mcb.14.1.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stargell L. A., Karrer K. M., Gorovsky M. A. Transcriptional regulation of gene expression in Tetrahymena thermophila. Nucleic Acids Res. 1990 Nov 25;18(22):6637–6639. doi: 10.1093/nar/18.22.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele C. J., Barkocy-Gallagher G. A., Preer L. B., Preer J. R., Jr Developmentally excised sequences in micronuclear DNA of Paramecium. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2255–2259. doi: 10.1073/pnas.91.6.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrück G., Haas I., Hellmer K. H., Ammermann D. Characterization of macronuclear DNA in five species of ciliates. Chromosoma. 1981;83(2):199–208. doi: 10.1007/BF00286789. [DOI] [PubMed] [Google Scholar]
- Steinbrück G. Molecular reorganization during nuclear differentiation in ciliates. Results Probl Cell Differ. 1986;13:105–174. doi: 10.1007/978-3-540-39838-7_3. [DOI] [PubMed] [Google Scholar]
- Stoll S., Zirlik T., Maercker C., Lipps H. J. The organization of internal telomeric repeats in the polytene chromosomes of the hypotrichous ciliate Stylonychia lemnae. Nucleic Acids Res. 1993 Apr 25;21(8):1783–1788. doi: 10.1093/nar/21.8.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundquist W. I., Klug A. Telomeric DNA dimerizes by formation of guanine tetrads between hairpin loops. Nature. 1989 Dec 14;342(6251):825–829. doi: 10.1038/342825a0. [DOI] [PubMed] [Google Scholar]
- Swanton M. T., Greslin A. F., Prescott D. M. Arrangement of coding and non-coding sequences in the DNA molecules coding for rRNAs in Oxytricha sp. DNA of ciliated protozoa. VII. Chromosoma. 1980;77(2):203–215. doi: 10.1007/BF00329545. [DOI] [PubMed] [Google Scholar]
- Swanton M. T., Heumann J. M., Prescott D. M. Gene-sized DNA molecules of the macronuclei in three species of hypotrichs: size distributions and absence of nicks. DNA of ciliated protozoa. VIII. Chromosoma. 1980;77(2):217–227. doi: 10.1007/BF00329546. [DOI] [PubMed] [Google Scholar]
- Takagi Y., Kanazawa N. Age-associated change in macronuclear DNA content in Paramecium caudatum. J Cell Sci. 1982 Apr;54:137–147. doi: 10.1242/jcs.54.1.137. [DOI] [PubMed] [Google Scholar]
- Takemasa T., Ohnishi K., Kobayashi T., Takagi T., Konishi K., Watanabe Y. Cloning and sequencing of the gene for Tetrahymena calcium-binding 25-kDa protein (TCBP-25). J Biol Chem. 1989 Nov 15;264(32):19293–19301. [PubMed] [Google Scholar]
- Tanguay R. M. Transcriptional activation of heat-shock genes in eukaryotes. Biochem Cell Biol. 1988 Jun;66(6):584–593. doi: 10.1139/o88-069. [DOI] [PubMed] [Google Scholar]
- Tausta S. L., Klobutcher L. A. Detection of circular forms of eliminated DNA during macronuclear development in E. crassus. Cell. 1989 Dec 22;59(6):1019–1026. doi: 10.1016/0092-8674(89)90758-7. [DOI] [PubMed] [Google Scholar]
- Tausta S. L., Klobutcher L. A. Internal eliminated sequences are removed prior to chromosome fragmentation during development in Euplotes crassus. Nucleic Acids Res. 1990 Feb 25;18(4):845–853. doi: 10.1093/nar/18.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tausta S. L., Turner L. R., Buckley L. K., Klobutcher L. A. High fidelity developmental excision of Tec1 transposons and internal eliminated sequences in Euplotes crassus. Nucleic Acids Res. 1991 Jun 25;19(12):3229–3236. doi: 10.1093/nar/19.12.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truett M. A., Gall J. G. The replication of ribosomal DNA in the macronucleus of Tetrahymena. Chromosoma. 1977 Dec 6;64(4):295–303. doi: 10.1007/BF00294937. [DOI] [PubMed] [Google Scholar]
- Vermeesch J. R., Price C. M. Telomeric DNA sequence and structure following de novo telomere synthesis in Euplotes crassus. Mol Cell Biol. 1994 Jan;14(1):554–566. doi: 10.1128/mcb.14.1.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeesch J. R., Williams D., Price C. M. Telomere processing in Euplotes. Nucleic Acids Res. 1993 Nov 25;21(23):5366–5371. doi: 10.1093/nar/21.23.5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODARD J., GELBER B., SWIFT H. Nucleoprotein changes during the mitotic cycle in Paramecium aurelia. Exp Cell Res. 1961 Mar;23:258–264. doi: 10.1016/0014-4827(61)90036-2. [DOI] [PubMed] [Google Scholar]
- Wang W., Skopp R., Scofield M., Price C. Euplotes crassus has genes encoding telomere-binding proteins and telomere-binding protein homologs. Nucleic Acids Res. 1992 Dec 25;20(24):6621–6629. doi: 10.1093/nar/20.24.6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefes I., Lipps H. J. The two macronuclear histone H4 genes of the hypotrichous ciliate Stylonychia lemnae. DNA Seq. 1990;1(1):25–32. doi: 10.3109/10425179009041344. [DOI] [PubMed] [Google Scholar]
- Wegner M., Helftenbein E., Müller F., Meinecke M., Müller S., Grummt F. Identification of an amplification promoting DNA sequence from the hypotrichous ciliate Stylonychia lemnae. Nucleic Acids Res. 1989 Nov 11;17(21):8783–8802. doi: 10.1093/nar/17.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R. H., Doerder F. P. Age-dependent micronuclear deterioration in Tetrahymena pyriformis, syngen 1. Mech Ageing Dev. 1975 May-Aug;4(3-4):263–279. doi: 10.1016/0047-6374(75)90028-7. [DOI] [PubMed] [Google Scholar]
- Weisman-Shomer P., Fry M. QUAD, a protein from hepatocyte chromatin that binds selectively to guanine-rich quadruplex DNA. J Biol Chem. 1993 Feb 15;268(5):3306–3312. [PubMed] [Google Scholar]
- Wellinger R. J., Wolf A. J., Zakian V. A. Saccharomyces telomeres acquire single-strand TG1-3 tails late in S phase. Cell. 1993 Jan 15;72(1):51–60. doi: 10.1016/0092-8674(93)90049-v. [DOI] [PubMed] [Google Scholar]
- Wenkert D., Allis C. D. Timing of the appearance of macronuclear-specific histone variant hv1 and gene expression in developing new macronuclei of Tetrahymena thermophila. J Cell Biol. 1984 Jun;98(6):2107–2117. doi: 10.1083/jcb.98.6.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley R. D. Inverted repetitious sequences in the macronuclear DNA of hypotrichous ciliates. Proc Natl Acad Sci U S A. 1975 Feb;72(2):678–682. doi: 10.1073/pnas.72.2.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. C., Allen S. L. Alternative processing of sequences during macronuclear development in Tetrahymena thermophila. J Protozool. 1986 Feb;33(1):30–38. doi: 10.1111/j.1550-7408.1986.tb05551.x. [DOI] [PubMed] [Google Scholar]
- White T. C., el-Gewely M. R., Allen S. L. Eliminated sequences with different copy numbers clustered in the micronuclear genome of Tetrahymena thermophila. Mol Gen Genet. 1985;201(1):65–75. doi: 10.1007/BF00397988. [DOI] [PubMed] [Google Scholar]
- Wild M. A., Gall J. G. An intervening sequence in the gene coding for 25S ribosomal RNA of Tetrahymena pigmentosa. Cell. 1979 Mar;16(3):565–573. doi: 10.1016/0092-8674(79)90030-8. [DOI] [PubMed] [Google Scholar]
- Wiley S. R., Kraus R. J., Mertz J. E. Functional binding of the "TATA" box binding component of transcription factor TFIID to the -30 region of TATA-less promoters. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5814–5818. doi: 10.1073/pnas.89.13.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. R., Herrick G. Expression of the gene encoded by a family of macronuclear chromosomes generated by alternative DNA processing in Oxytricha fallax. Nucleic Acids Res. 1991 Sep 11;19(17):4717–4724. doi: 10.1093/nar/19.17.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R., Raghuraman M. K., Cech T. R. Monovalent cation-induced structure of telomeric DNA: the G-quartet model. Cell. 1989 Dec 1;59(5):871–880. doi: 10.1016/0092-8674(89)90610-7. [DOI] [PubMed] [Google Scholar]
- Witt P. L. Unequal distribution of DNA in the macronuclear division of the ciliate Euplotes eurystomus. Chromosoma. 1977 Mar 7;60(1):59–67. doi: 10.1007/BF00330411. [DOI] [PubMed] [Google Scholar]
- Woodard J., Kaneshiro E., Gorovsky M. A. Cytochemical studies on the problem of macronuclear subnuclei in tetrahymena. Genetics. 1972 Feb;70(2):251–260. doi: 10.1093/genetics/70.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard J., Woodard M., Gelber B., Swift H. Cytochemical studies of conjugation in Paramecium aurelia. Exp Cell Res. 1966 Jan;41(1):55–63. doi: 10.1016/0014-4827(66)90546-5. [DOI] [PubMed] [Google Scholar]
- Wu M., Allis C. D., Richman R., Cook R. G., Gorovsky M. A. An intervening sequence in an unusual histone H1 gene of Tetrahymena thermophila. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8674–8678. doi: 10.1073/pnas.83.22.8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wünning I. U., Lipps H. J. A transformation system for the hypotrichous ciliate Stylonychia mytilus. EMBO J. 1983;2(10):1753–1757. doi: 10.1002/j.1460-2075.1983.tb01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi K., Ochiai T., Usuki I. The unique structure of the Paramecium caudatum hemoglobin gene: the presence of one intron in the middle of the coding region. Biochim Biophys Acta. 1992 Nov 15;1171(1):81–87. doi: 10.1016/0167-4781(92)90142-m. [DOI] [PubMed] [Google Scholar]
- Yao M. C., Choi J., Yokoyama S., Austerberry C. F., Yao C. H. DNA elimination in Tetrahymena: a developmental process involving extensive breakage and rejoining of DNA at defined sites. Cell. 1984 Feb;36(2):433–440. doi: 10.1016/0092-8674(84)90236-8. [DOI] [PubMed] [Google Scholar]
- Yao M. C. Elimination of specific DNA sequences from the somatic nucleus of the ciliate Tetrahymena. J Cell Biol. 1982 Mar;92(3):783–789. doi: 10.1083/jcb.92.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. C., Gall J. G. A single integrated gene for ribosomal RNA in a eucaryote, Tetrahymena pyriformis. Cell. 1977 Sep;12(1):121–132. doi: 10.1016/0092-8674(77)90190-8. [DOI] [PubMed] [Google Scholar]
- Yao M. C., Gorovsky M. A. Comparison of the sequences of macro- and micronuclear DNA of Tetrahymena pyriformis. Chromosoma. 1974;48(1):1–18. doi: 10.1007/BF00284863. [DOI] [PubMed] [Google Scholar]
- Yao M. C., Kimmel A. R., Gorovsky M. A. A small number of cistrons for ribosomal RNA in the germinal nucleus of a eukaryote, Tetrahymena pyriformis. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3082–3086. doi: 10.1073/pnas.71.8.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. C. Ribosomal RNA gene amplification in Tetrahymena may be associated with chromosome breakage and DNA elimination. Cell. 1981 Jun;24(3):765–774. doi: 10.1016/0092-8674(81)90102-1. [DOI] [PubMed] [Google Scholar]
- Yao M. C., Yao C. H., Monks B. The controlling sequence for site-specific chromosome breakage in Tetrahymena. Cell. 1990 Nov 16;63(4):763–772. doi: 10.1016/0092-8674(90)90142-2. [DOI] [PubMed] [Google Scholar]
- Yao M. C., Yao C. H. Repeated hexanucleotide C-C-C-C-A-A is present near free ends of macronuclear DNA of Tetrahymena. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7436–7439. doi: 10.1073/pnas.78.12.7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. C., Zheng K., Yao C. H. A conserved nucleotide sequence at the sites of developmentally regulated chromosomal breakage in Tetrahymena. Cell. 1987 Mar 13;48(5):779–788. doi: 10.1016/0092-8674(87)90075-4. [DOI] [PubMed] [Google Scholar]
- Yao M. C., Zhu S. G., Yao C. H. Gene amplification in Tetrahymena thermophila: formation of extrachromosomal palindromic genes coding for rRNA. Mol Cell Biol. 1985 Jun;5(6):1260–1267. doi: 10.1128/mcb.5.6.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda L. F., Yao M. C. Short inverted repeats at a free end signal large palindromic DNA formation in Tetrahymena. Cell. 1991 Nov 1;67(3):505–516. doi: 10.1016/0092-8674(91)90525-4. [DOI] [PubMed] [Google Scholar]
- Yokoyama R. W., Yao M. C. Elimination of DNA sequences during macronuclear differentiation in Tetrahymena thermophila, as detected by in situ hybridization. Chromosoma. 1982;85(1):11–22. doi: 10.1007/BF00344591. [DOI] [PubMed] [Google Scholar]
- Yokoyama R., Yao M. C. Internal micronuclear DNA regions which include sequences homologous to macronuclear telomeres are deleted during development in Tetrahymena. Nucleic Acids Res. 1984 Aug 10;12(15):6103–6116. doi: 10.1093/nar/12.15.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama R., Yao M. C. Sequence characterization of Tetrahymena macronuclear DNA ends. Nucleic Acids Res. 1986 Mar 11;14(5):2109–2122. doi: 10.1093/nar/14.5.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Y., Aufderheide K., Morand J., Rodkey K., Forney J. Macronuclear transformation with specific DNA fragments controls the content of the new macronuclear genome in Paramecium tetraurelia. Mol Cell Biol. 1991 Feb;11(2):1133–1137. doi: 10.1128/mcb.11.2.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G. L., Blackburn E. H. Amplification of tandemly repeated origin control sequences confers a replication advantage on rDNA replicons in Tetrahymena thermophila. Mol Cell Biol. 1990 May;10(5):2070–2080. doi: 10.1128/mcb.10.5.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G. L., Blackburn E. H. Developmentally programmed healing of chromosomes by telomerase in Tetrahymena. Cell. 1991 Nov 15;67(4):823–832. doi: 10.1016/0092-8674(91)90077-c. [DOI] [PubMed] [Google Scholar]
- Zahler A. M., Prescott D. M. DNA primase and the replication of the telomeres in Oxytricha nova. Nucleic Acids Res. 1989 Aug 11;17(15):6299–6317. doi: 10.1093/nar/17.15.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahler A. M., Prescott D. M. Telomere terminal transferase activity in the hypotrichous ciliate Oxytricha nova and a model for replication of the ends of linear DNA molecules. Nucleic Acids Res. 1988 Jul 25;16(14B):6953–6972. doi: 10.1093/nar/16.14.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahler A. M., Williamson J. R., Cech T. R., Prescott D. M. Inhibition of telomerase by G-quartet DNA structures. Nature. 1991 Apr 25;350(6320):718–720. doi: 10.1038/350718a0. [DOI] [PubMed] [Google Scholar]
- Zakian V. A. Structure and function of telomeres. Annu Rev Genet. 1989;23:579–604. doi: 10.1146/annurev.ge.23.120189.003051. [DOI] [PubMed] [Google Scholar]