Abstract
Background
Peripheral nerve injury leads to changes in neuronal activity in the contralateral and ipsilateral primary somatosensory cortices (S1), which may lead to enduring sensory dysfunction and pain. Plasticity in the barrel and visual cortices has been shown to occur in a layer-specific manner. However, little is known about the layer specific changes associated with limb injury.
Objective
To determine the layer-specific changes in neuronal activity associated with short-term plasticity induced by peripheral nerve injury in the rat.
Methods
In vivo electrophysiology recordings (multiunit activity and local field potential) and high-resolution functional magnetic resonance imaging techniques were applied to characterize neuronal and hemodynamic responses across the depth of S1 contralateral and ipsilateral to the injury.
Results
Within 60 minutes following injury, atypical increases in neuronal and hemodynamic responses in the deprived S1, ipsilateral to the noninjured limb, were observed in response to stimulation of the noninjured limb. The most prominent increases in neuronal activity in the deprived S1 occurred in layer V.
Conclusion
Layer V neurons provide the major output of S1 and they send and receive transcallosal input. Thus, the immediate changes in neuronal firing patterns in layer V induced by the injury, can adversely affect the activity of subcortical regions and also interfere with normal cortical processing and interhemispheric communication. Therefore, a rehabilitation strategy that targets layer V neurons activity and starts immediately after the injury may benefit the functional outcome.
Keywords: plasticity, corpus callosum, fMRI, animal models, somatosensory cortex, sensory deprivation
Introduction
Peripheral nerve injury leads to significant changes in cortical and subcortical neuronal activity.1-4 Plasticity of central neuronal connections may be adaptive as this plasticity can compensate, if incompletely for the lack of peripheral sensory input. However, this plasticity may be maladaptive by potentiating neuropathic pain and dystonia.5,6 For example, about 80% of amputees in the United States suffer from phantom limb pain likely created by changes in cortical neuronal function.7,8
Plasticity in the primary somatosensory cortex (S1) associated with nerve injury was demonstrated to be composed of at least 2 time-dependent components: an extensive effect occurring rapidly (within minutes and hours) following denervation, which is usually attributed to modification of existing connections that are not normally functional,9-11 and a slower component emerging over days to weeks, which is thought to depend on the growth of new connections.1,12-15 Currently, there is no clear distinction between the transitions from short- to the long-term neuronal mechanisms that lead to neuronal changes following peripheral nerve injury.
In addition, little is known about the layer-specific changes associated with peripheral nerve injury in the limb. Plasticity in the barrel and visual cortices after sensory deprivation has been shown to take place in a layer specific manner.16-18 For example, removal of the rat's whiskers during the critical period leads to changes in synaptic transmission and plasticity in layers II/III and IV, pointing out that cortical reorganization has specific layer dependence. The plasticity mechanisms that are involved following limb injury may alter layer communication, interhemispheric communication and subcortical activity and could affect recovery. Synaptic modifications, levels of neurotransmitters and receptor synthesis, and neuronal architecture changes could all be involved in cortical reorganization, and hence, play a key role in the behavioral outcome for such processes,15,19,20 be they adaptive or maladaptive (eg, patients with peripheral nerve injury often develop significant pain).21 We suggest that identifying the anatomical location and the population of neurons that are primarily affected by the peripheral nerve injury, as well as the temporal dynamics of the changes in neuronal activity induced by the injury, are likely to shed light on developing appropriate neurorehabilitation strategies.
Recently, transcallosal inhibition mediated by cortical layer V was shown to play a pivotal role in maintaining intercortical communication and mediating cortical rivalry between the 2 hemispheres in healthy rats.22 In denervated rats, we previously demonstrated that in the weeks following the injury, stimulation of the noninjured limb resulted in increased neuronal responses in both healthy (contralateral to the noninjured limb) and deprived (ipsilateral to the noninjured limb) S1. Specifically, infragranular inhibitory interneurons had increased responses in the deprived S1.23 Ablation of the healthy S1 eliminated the neuronal activity in the deprived S1, suggesting that interhemispheric communication is involved in mediating these neuronal responses.4 Moreover, when we decreased transcallosal communication in denervated rats by optogenetics tools, we observed an immediate increase in excitatory neuronal function and functional magnetic resonance imaging (fMRI) responses in the infragranular layers of the deprived S1.24 Thus, it appears that long-term cortical plasticity mechanisms following peripheral nerve injury take place in the infragranular layers and rely heavily on transcallosal communication.
It is not clear whether modifications in the firing patterns of neurons located in the infragranular layers, which appear to be at least partly mediated by the transcallosal pathway, occur immediately following the injury or evolve with time. It will be useful to determine the temporal dynamics of the layer specific modifications associated with injury as some rehabilitation strategies such as constraint-induced therapy,25 transcranial magnetic stimulation26,27 and nerve blocking28 aim to target the transcallosal pathway.
Here we applied in vivo electrophysiology recording and fMRI techniques to identify the layer-specific changes induced immediately after the injury onset. Our results demonstrate that within 60 minutes of forepaw denervation, significant increases in neuronal activity and fMRI responses are observed in layer V of the deprived S1. These results suggest that rehabilitation strategies that target layer V neurons may be particularly useful in maximizing the functional outcome.
Methods
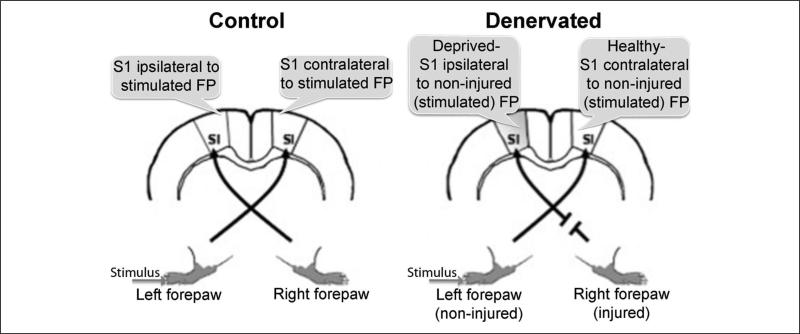
All animal procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Johns Hopkins University Animal Care and Use Committee. Twelve Sprague–Dawley rats (250 g) underwent right forepaw denervation. This surgery was immediately followed by in vivo electrophysiology (n = 7) and fMRI (n = 5) measurements. Twelve additional rats served as controls. The experimental design is illustrated in Figure 1.
Figure 1. Illustration of the experimental design.
Denervation procedures were performed on the right forepaw. Electrophysiology recordings were obtained from the primary somatosensory cortex (S1) forepaw (FP) representations ipsilateral to the noninjured forepaw (deprived S1) and contralateral to the noninjured FP (healthy S1).
Forepaw Denervation
An excision of the right forepaw radial, median, and ulnar nerves4 was carried out under 2% isoflurane anesthesia administrated via a nose cone. In the rat, these nerves contain sensory and motor fibers; thus severing them removed both efferent and afferent components. Measurements of neuronal responses to noninjured forepaw tactile stimulation in the healthy and the deprived S1 began 60 minutes following the forepaw denervation surgery. This time frame ensured that the effect the isoflurane anesthetic has on neuronal activity was largely diminished.
Craniotomy
Craniotomies above the right and the left S1 were performed on isoflurane-anesthetized rats prior to the forepaw denervation surgery. The rat's head was secured in a sterotaxic frame (Kopf Instruments, Tujunga, CA) positioned under a binocular microscope. A 1-inch long incision along the midline was made and the skull was exposed. Using a pneumatic drill, a 2-mm region of the skull centered at anteroposterior 0 mm, medial–lateral ±3.6 mm was removed. Dura was carefully removed before inserting the recording electrode into the cortex.
Forepaw Stimulation
Two needle electrodes were inserted into the left (noninjured) forepaw: one between digits 1 and 2, and the other between digits 3 and 4. Electrical stimulation was delivered using a stimulator (World Precision Instruments, Sarasota, FL) with current amplitude at 3 mA, pulse width at 300 μs, and stimulus repeated at 3 Hz for 10 s (electrophysiology) and 20 s (fMRI).
Electrophysiology
After craniotomy and denervation procedures, a bolus of dexmedetomidine (0.05 mg/kg subcutaneous), was delivered and was followed by continuous subcutaneous administration (0.1 mg/kg). An axial array microelectrode (FHC, Bowdoin, ME) with 12 sites spaced at 150 μm along the shank (diameter = 200 μm) was placed above the center of the right and left S1 forepaw representation (according to Paxinos and Watson29). The electrode was slowly inserted into the cortex until reaching the depth of 1800 μm below pia matter to cover the whole depth of the rat cortex (starting at 150 μm). Stimulation of the contralateral forepaw was initially performed to confirm large amplitude of the stimulation evoked responses and hence accurate location of the recording electrode. Multiunit activity (MUA) and local field potential (LFP) were sampled at 11 kHz and 1 kHz, respectively, and band-pass filtered between 500 Hz to 5 kHz and 0.1 to 500 Hz, respectively. Discriminated signals were collected with a CED interface and Spike2 data acquisition and analysis software (Cambridge Electronic Design [CED], Cambridge, UK). Signals recorded from all 12 contacts on the microelectrode were sampled simultaneously. Thirty single stimuli (total time 10 s) were obtained for each measurement. To identify MUA, the standard deviation (SD) of the MUA signals was calculated for 500 ms. MUA signals with amplitude greater than 4 times the SD (with an average of 6.60 ± 0.4) was defined as spiking activity. To define stimulus-evoked spiking activity, poststimulus time histogram analysis was performed. Poststimulus time histogram was obtained for MUA in each trial by event correlation analysis of the spiking with the stimulation using 5-ms bins. The SD of neuronal firing rates was calculated for the last 150 ms of the interstimulus interval of each trial. MUA that showed increased (>2 SD) activity in one 5-ms bin during the first 40 ms following the onset of the stimulation were considered to show stimulus-evoked response. LFP waveforms were averaged with respect to the stimulation trigger (90 single stimulations). The mean amplitude of the negative deflection was calculated for each train of stimuli. For group analysis, the amplitudes of the negative deflection were averaged across rats at the same cortical depth. Student's t tests were used to compare between the groups across the layers.
Functional Magnetic Resonance Imaging
Images were acquired on an 11.7 Tesla/16 cm horizontal bore small animal scanner (Bruker, Ettlingen, Germany). A 72-mm quadrature volume coil and a 15-mm diameter surface coil were used to transmit and receive MR signals, respectively. For blood oxygenation level–dependent (BOLD) fMRI, gradient echo, echo planar imaging was used with the following parameters: effective echo time (TE) = 11 ms, repetition time (TR) = 1000 ms, bandwidth = 250 kHz, field of view = 1.92 × 1.92 cm, and matrix size = 128 × 128. Five coronal slices with 1-mm thickness were acquired. T2-weighted RARE (rapid acquisition with relaxation enhancement) sequence was used to acquire high-resolution anatomical images with the following parameters: TE = 10 ms, TR = 5000 ms, bandwidth = 250 kHz, field of view = 1.92 × 1.92 cm, and matrix size = 256 × 256. During fMRI measurements, rats were anesthetized with dexmedetomidine in a similar manner that is described in the electrophysiology procedure section. The rat head was secured with ear bars and a bite bar in a water-heated animal cradle. Respiration rate, PO2, and heart rate were continuously monitored throughout all measurements (MouseOx, Starr Life Sciences, Oakmont, PA). The stimulation paradigm consisted of 20 scans during forepaw stimulation and 40 scans of rest intervals. The FMRIB Software Library (FSL 4.1.9) software was used for analysis.30 Activation maps were obtained using the general linear model. Z-score statistics were cluster-size thresholded for effective significance of P < .05. The activation threshold was set at 2.3. To characterize the layer BOLD fMRI activation, the surface of S1 forepaw representation (defined by a brain atlas) was defined by the user based on high-resolution (75 × 75 × 1000 μm) RARE images. The number of activated pixels in each of the automatic selected 3 regions of interest which represented the different layers was calculated.
Statistics
Two-tailed Student's t test was performed between the different conditions and groups. Results and figures show the mean ± standard error of the mean.
Results
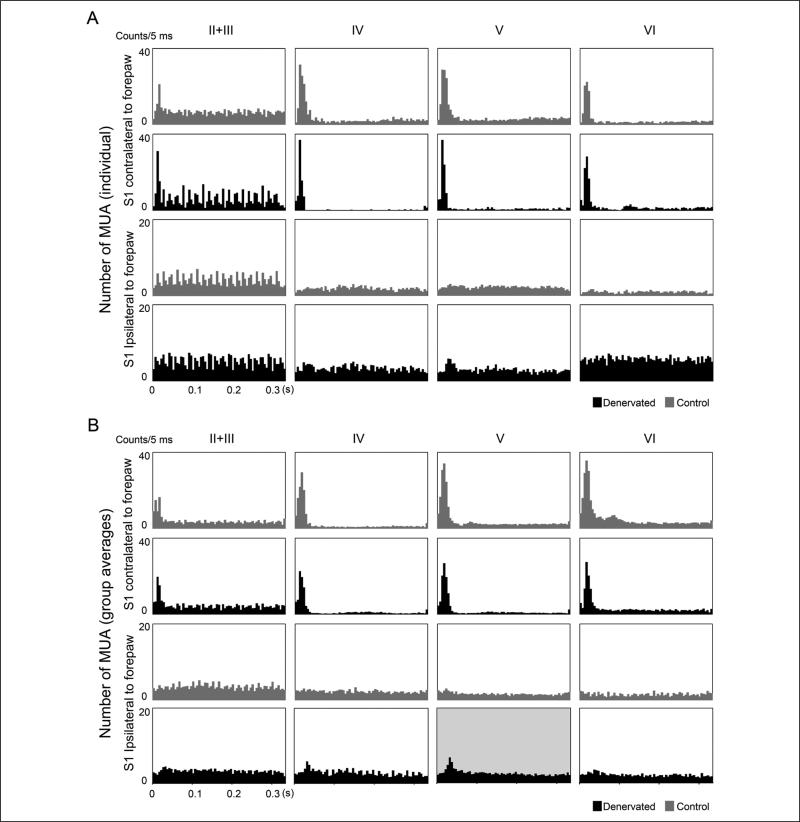
Measurements of neuronal responses to noninjured forepaw tactile stimulation in the healthy and the deprived S1 began 60 minutes following the forepaw denervation surgery. The number of evoked MUA responses, reflecting the spiking activity of neurons in the vicinity of the electrode, occurring within 40 ms from the onset of the forepaw stimulation was summed for each layer for every individual rat and averaged across the group. Figure 2 shows the representative and group average of poststimulus time histograms (accumulated number of evoked MUA responses as a function of time after stimulation onset) in the different cortical layers in the healthy and the deprived S1 of denervated and control rats. In control rats (n = 7), significant increases (P < .05) in neuronal responses across the cortical depth of S1 contralateral to forepaw stimulation were observed. As expected, no increases in neuronal responses were recorded in S1 ipsilateral to forepaw stimulation. In denervated rats (n = 7), increases in neuronal responses across the cortical depth of S1 contralateral to noninjured forepaw stimulation (healthy S1) were similar to those of controls. However, significant increases (P < .05) in neuronal responses were observed also in layer V (34.22 ± 9.1; accumulated number of evoked MUA responses) in S1 ipsilateral to noninjured forepaw stimulation (deprived S1).
Figure 2. Multiunit activity (MUA) responses in healthy and denervated rats.
Representative (A) and group (B) averages of poststimulus time histogram of control and denervated rats in response to forepaw stimulation. Accumulated evoked MUA responses from grouped neurons are depicted in the graphs as a function of time after stimulus onset. Sixty minutes following the forepaw denervation procedure, robust and significant increases in MUA were observed in layer V in S1 ipsilateral to noninjured forepaw stimulation (deprived S1, black) compared with controls (gray).
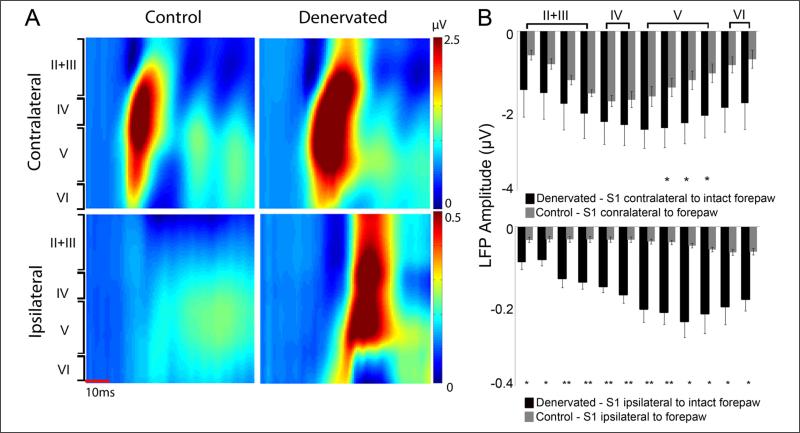
Local field potential responses, reflecting the averaged synaptic activity in the vicinity of the electrode were measured simultaneously with the MUA. Figure 3 shows the representative and group average LFP responses across the cortical depth in the healthy and the deprived cortex of denervated and control rats. In denervated rats, significant increases (P < .05) in LFP responses were observed in S1 layer V contralateral to noninjured forepaw stimulation (healthy S1) compared with controls. Significant increases in LFP responses were also observed across the cortical depth of S1 ipsilateral to noninjured forepaw stimulation (deprived S1) compared with controls. Thus, the in vivo electrophysiology measurements suggest that peripheral nerve injury immediately affects the function of neurons located in layers V of both the healthy and the deprived S1.
Figure 3. Local field potential (LFP) responses in healthy and denervated rats.
Representative (A) and group (B) averages of forepaw stimulus–induced LFP responses beginning 150 μm below the S1 cortical surface with LFP recordings performed at 150 μm increments of control and denervated rats. Sixty minutes following the forepaw denervation procedure, marked changes in LFP activity were observed in S1 contralateral (healthy S1) and ipsilateral (deprived S1) to noninjured forepaw stimulation (mean ± standard error of the mean).
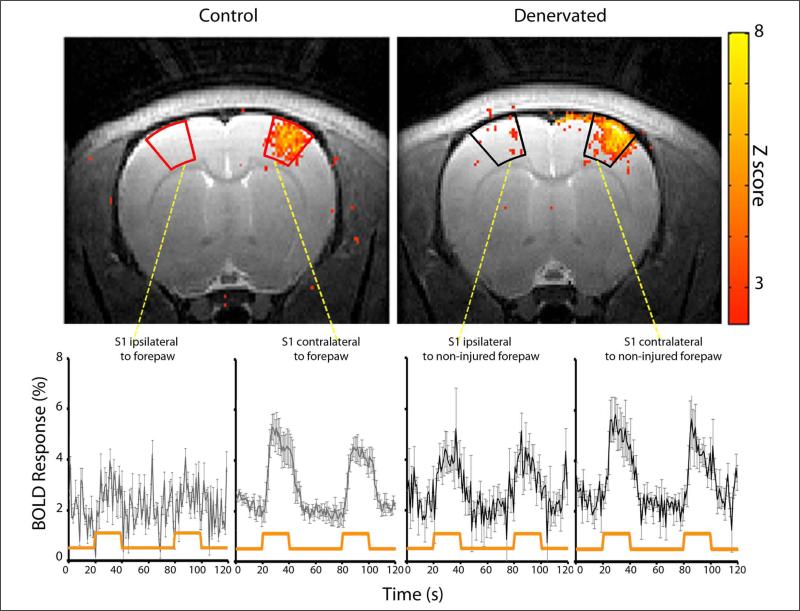
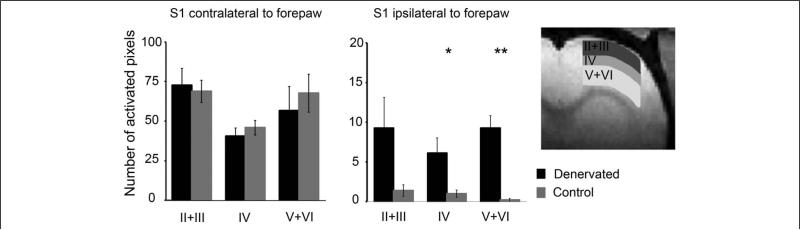
We then sought to determine if such changes in cortical layer activity could be visualized with noninvasive imaging modalities. BOLD fMRI responses were assessed in control (n = 5) and denervated (n = 5) rats in an 11.7 T horizontal animal scanner, permitting high spatial (150 × 150 × 1000 μm) and excellent temporal (1000 ms) resolution. Figure 4 shows BOLD fMRI activation Z-score map (P < .05) of individual control and denervated rats overlaid on high-resolution anatomical MRI images (Bregma 1.5 mm according to Paxinos and Watson29) and the corresponding BOLD time course within S1 area. Consistent with the electrophysiology measurements, in denervated rats, S1 ipsilateral to noninjured forepaw stimulation (deprived S1) showed significant increases in hemodynamic responses within 60 minutes following the injury compared with controls, as was determined by the number of active voxels (24.7 1 ± 5.7 in denervated rats; 2.60 ± 0.4 in control rats; P < .05). To characterize the layers’ BOLD responses, we defined 3 regions of interest: layers II + III (150-600 μm), layer IV (600-900 μm), and layers V + VI (900-1800 μm) as shown in Figure 5. The number of activated voxels was calculated for each region of interest. Increases in hemodynamic responses in S1 ipsilateral to noninjured forepaw stimulation (deprived S1) were detected in layer IV (6.14 ± 1.9; number of active voxels, P < .05) but were even more significant in layers V + VI (9.27 ± 1.5; P < .005) compared with controls (1.00 ± 0.4 and 0.20 ± 0.2, respectively). Thus, fMRI is capable of detecting immediate changes in neuronal activities associated with peripheral nerve injury with cortical layer resolution.
Figure 4. Functional magnetic resonance imaging (fMRI) responses in healthy and denervated rats.
fMRI Z-score maps overlaid on T2-weighted anatomical images and time courses of blood oxygenation level–dependent (BOLD) changes in response to forepaw stimulation of controls and denervated rats. Sixty minutes following the forepaw denervation procedure, robust and significant increases in S1 responses contralateral (healthy S1) and ipsilateral (deprived S1) to noninjured forepaw stimulation were observed. The time courses of BOLD signals from S1 were averaged across animals (mean ± standard error of the mean). The orange line indicates the stimulation paradigm.
Figure 5. Layer characteristics of the blood oxygenation level–dependent (BOLD) functional magnetic resonance imaging (fMRI) responses.
Layer characteristics of the BOLD fMRI signals were measured in S1 contralateral and ipsilateral to forepaw stimulation in control (gray) and denervated (black) rats. Three regions of interest (ROIs) representing layers II + III, IV, and V + VI were defined in S1 area as shown in the schematic. The average number of activated pixels was calculated for each ROI. Sixty minutes following the forepaw denervation procedure, significant increases in BOLD responses were observed in layers IV and V + VI in S1 ipsilateral to noninjured forepaw stimulation (deprived S1), with greater activity in layers V + VI (mean ± standard error of the mean).
Discussion
There has been great interest in exploring the cortical layer architecture in relation to cortical reorganization, especially in the barrel field and visual sensory cortices.17,18,31-34 However, little is known about the layer-specific changes induced by peripheral nerve injury to the limb and in the mature brain in particular. The MUA, LFP, and fMRI results demonstrate that within 60 minutes following the peripheral nerve injury, the greatest changes in neuronal activity, specifically increases in neuronal responses evoked by forepaw stimulation, are found in layer V of both the deprived and healthy S1.
Increased excitability of layer V pyramidal neurons in the mature brain was also demonstrated in the S1 barrel cortex after sensory deprivation,34 and in the motor cortex after peripheral facial nerve lesions.35 We have previously demonstrated that the increased responsiveness of the infragranular layer neurons persists for weeks after the peripheral nerve injury in adult rats.23,24 We used single unit recordings and juxtacellular labeling to show that the population of neurons with increased firing rates after the injury are inhibitory interneruons.23 This suggests that peripheral nerve injury induces long-term increases in inhibitory activity in the infragaranular layer of the deprived S1, which could be maladaptive. The identity of the layer V neurons that increase their responsiveness immediately after the injury is yet to be determined and the transition between short- and long-term plasticity mechanisms associated with limb injuries is not yet well understood. Future intracellular electrophysiology recordings may elucidate the exact neuronal mechanisms that play a role in this process.
Changes in the activity of neurons located in layer IV of the deprived S1 accompanied the changes observed in layer V but were less profound. A large percentage of neurons located in layer V send and receive transcallosal input whereas the thalamocortical projections mainly synapse on layer IV neurons. Thus, it appears that both the transcallosal and the thalamocortical projections play a significant role in shifting the balance between excitatory activity (mainly mediated by thalamocortical projections) and inhibitory activity (partially mediated by transcallosal projections) in the deprived S1 immediately following the injury. One potential mechanism may be via the decreased thalamocortical inputs leading to an “unmasking” of transcallosal synaptic inputs, which are typically silent in the healthy brain.
In addition to sending and receiving transcallosal input, neurons located in layer V of S1 send axons to the thalamus, dorsal column, trigeminal nuclei in the medulla, and other regions of the cortex. Thus, changes in firing rates of neurons in layer V induced by the injury have a substantial effect on intracortical, intercortical, and subcortical activity. Directly targeting layer V of S1 plasticity after limb injury by noninvasive brain stimulation techniques, such as transcranial magnetic stimulation and transcranial direct current stimulation, may be an effective rehabilitative approach to maximize the functional outcome. These techniques, applied over the sensorymotor cortex have been shown to be beneficial in treating chronic pain associated with injury.36,37
Another means of potentially manipulating layer V activity is through the corpus callosum. Indeed, human25,38-41 and animal studies suggest that the involvement of the interhemispheric connections in cortical plasticity following stroke and amputation may affect the degree of functional recovery of the affected limb. For example, rats that underwent unilateral cortical infracts were trained to use the nonparetic (ipsilateral to the cortical infract) or paretic (contralateral to the cortical infract) forepaw.42 Training of the nonparetic forepaw worsened subsequent performance of the paretic forepaw. However, transection of the transcallosal pathway improved the performance of the paretic forepaw. We have recently used optogenetics tools to decrease the transcallosal communication in the denervated rats. This led to immediate increase in excitatory neuronal function and fMRI responses in the infragranular layers of the deprived S1.24
The results suggest layer V as potential target for guided plasticity after limb injury in adults. The rapid changes in the neuronal firing pattern in layer V might be because of disinhibition and unmasking of existing inputs, and might be followed by changes in neuronal architecture and protein synthesis that maintain the altered sensory processing. Rehabilitative approaches that start immediately after the injury occurred are likely to be useful in downregulating layer V plasticity.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health Grant NIH R01NS072171.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Kaas JH, Florence SL, Jain N. Subcortical contributions to massive cortical reorganizations. Neuron. 1999;22:657–660. doi: 10.1016/s0896-6273(00)80725-4. [DOI] [PubMed] [Google Scholar]
- 2.Jones EG. Cortical and subcortical contributions to activity-dependent plasticity in primate somatosensory cortex. Annu Rev Neurosci. 2000;23:1–37. doi: 10.1146/annurev.neuro.23.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Wall JT, Xu J, Wang X. Human brain plasticity: an emerging view of the multiple substrates and mechanisms that cause cortical changes and related sensory dysfunctions after injuries of sensory inputs from the body. Brain Res Brain Res Rev. 2002;39:181–215. doi: 10.1016/s0165-0173(02)00192-3. [DOI] [PubMed] [Google Scholar]
- 4.Pelled G, Chuang KH, Dodd SJ, Koretsky AP. Functional MRI detection of bilateral cortical reorganization in the rodent brain following peripheral nerve deafferentation. Neuroimage. 2007;37:262–273. doi: 10.1016/j.neuroimage.2007.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lundborg G. A 25-year perspective of peripheral nerve surgery: evolving neuroscientific concepts and clinical significance. J Hand Surg Am. 2000;25:391–414. doi: 10.1053/jhsu.2000.4165. [DOI] [PubMed] [Google Scholar]
- 6.Navarro X, Vivo M, Valero-Cabre A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Ephraim PL, Wegener ST, MacKenzie EJ, Dillingham TR, Pezzin LE. Phantom pain, residual limb pain, and back pain in amputees: results of a national survey. Arch Phys Med Rehabil. 2005;86:1910–1919. doi: 10.1016/j.apmr.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 8.Flor H, Elbert T, Knecht S, et al. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375:482–484. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- 9.Calford MB. Dynamic representational plasticity in sensory cortex. Neuroscience. 2002;111:709–738. doi: 10.1016/s0306-4522(02)00022-2. [DOI] [PubMed] [Google Scholar]
- 10.Shin HC, Won CK, Jung SC, Oh S, Park S, Sohn JH. Interhemispheric modulation of sensory transmission in the primary somatosensory cortex of rats. Neurosci Lett. 1997;230:137–139. doi: 10.1016/s0304-3940(97)00486-2. [DOI] [PubMed] [Google Scholar]
- 11.Calford MB, Tweedale R. Interhemispheric transfer of plasticity in the cerebral cortex. Science. 1990;249:805–807. doi: 10.1126/science.2389146. [DOI] [PubMed] [Google Scholar]
- 12.Wall JT, Cusick CG. Cutaneous responsiveness in primary somatosensory (S-I) hindpaw cortex before and after partial hindpaw deafferentation in adult rats. J Neurosci. 1984;4:1499–1515. doi: 10.1523/JNEUROSCI.04-06-01499.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metzler J, Marks PS. Functional changes in cat somatic sensory-motor cortex during short-term reversible epidural blocks. Brain Res. 1979;177:379–383. doi: 10.1016/0006-8993(79)90790-x. [DOI] [PubMed] [Google Scholar]
- 14.Merzenich MM, Kaas JH, Wall JT, Sur M, Nelson RJ, Felleman DJ. Progression of change following median nerve section in the cortical representation of the hand in areas 3b and 1 in adult owl and squirrel monkeys. Neuroscience. 1983;10:639–665. doi: 10.1016/0306-4522(83)90208-7. [DOI] [PubMed] [Google Scholar]
- 15.Darian-Smith C, Gilbert CD. Axonal sprouting accompanies functional reorganization in adult cat striate cortex. Nature. 1994;368:737–740. doi: 10.1038/368737a0. [DOI] [PubMed] [Google Scholar]
- 16.Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- 17.Petersen CC, Brecht M, Hahn TT, Sakmann B. Synaptic changes in layer 2/3 underlying map plasticity of developing barrel cortex. Science. 2004;304:739–742. doi: 10.1126/science.1096750. [DOI] [PubMed] [Google Scholar]
- 18.Schierloh A, Eder M, Zieglgansberger W, Dodt HU. Effects of sensory deprivation on columnar organization of neuronal circuits in the rat barrel cortex. Eur J Neurosci. 2004;20:1118–1124. doi: 10.1111/j.1460-9568.2004.03557.x. [DOI] [PubMed] [Google Scholar]
- 19.Volkmar FR, Greenough WT. Rearing complexity affects branching of dendrites in the visual cortex of the rat. Science. 1972;176:1445–1447. doi: 10.1126/science.176.4042.1445. [DOI] [PubMed] [Google Scholar]
- 20.Florence SL, Taub HB, Kaas JH. Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science. 1998;282:1117–1121. doi: 10.1126/science.282.5391.1117. [DOI] [PubMed] [Google Scholar]
- 21.Lundborg G, Richard P. Bunge memorial lecture. Nerve injury and repair—a challenge to the plastic brain. J Peripher Nerv Syst. 2003;8:209–226. doi: 10.1111/j.1085-9489.2003.03027.x. [DOI] [PubMed] [Google Scholar]
- 22.Palmer LM, Schulz JM, Murphy SC, Ledergerber D, Murayama M, Larkum ME. The cellular basis of GABA(B)-mediated interhemispheric inhibition. Science. 2012;335:989–993. doi: 10.1126/science.1217276. [DOI] [PubMed] [Google Scholar]
- 23.Pelled G, Bergstrom DA, Tierney PL, et al. Ipsilateral cortical fMRI responses after peripheral nerve damage in rats reflect increased interneuron activity. Proc Natl Acad Sci U S A. 2009;106:14114–14119. doi: 10.1073/pnas.0903153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li N, Downey JE, Bar-Shir A, et al. Optogenetic-guided cortical plasticity after nerve injury. Proc Natl Acad Sci U S A. 2011;108:8838–8843. doi: 10.1073/pnas.1100815108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjorkman A, Rosen B, Lundborg G. Enhanced function in nerve-injured hands after contralateral deafferentation. Neuroreport. 2005;16:517–519. doi: 10.1097/00001756-200504040-00020. [DOI] [PubMed] [Google Scholar]
- 26.Werhahn KJ, Mortensen J, Kaelin-Lang A, Boroojerdi B, Cohen LG. Cortical excitability changes induced by deafferentation of the contralateral hemisphere. Brain. 2002;125(pt 6):1402–1413. doi: 10.1093/brain/awf140. [DOI] [PubMed] [Google Scholar]
- 27.Karl A, Birbaumer N, Lutzenberger W, Cohen LG, Flor H. Reorganization of motor and somatosensory cortex in upper extremity amputees with phantom limb pain. J Neurosci. 2001;21:3609–3618. doi: 10.1523/JNEUROSCI.21-10-03609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosén B, Björkman A, Lundborg G. Improved sensory relearning after nerve repair induced by selective temporary anaesthesia—a new concept in hand rehabilitation. J Hand Surg [Br] 2006;31:126–132. doi: 10.1016/j.jhsb.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd ed. Academic Press; Orlando, FL: 1986. [Google Scholar]
- 30.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 31.Diamond ME, Huang W, Ebner FF. Laminar comparison of somatosensory cortical plasticity. Science. 1994;265:1885–1888. doi: 10.1126/science.8091215. [DOI] [PubMed] [Google Scholar]
- 32.Schierloh A, Eder M, Zieglgansberger W, Dodt HU. Sensory deprivation changes the pattern of synaptic connectivity in rat barrel cortex. Neuroreport. 2003;14:1787–1791. doi: 10.1097/00001756-200310060-00006. [DOI] [PubMed] [Google Scholar]
- 33.Celikel T, Szostak VA, Feldman DE. Modulation of spike timing by sensory deprivation during induction of cortical map plasticity. Nat Neurosci. 2004;7:534–541. doi: 10.1038/nn1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breton JD, Stuart GJ. Loss of sensory input increases the intrinsic excitability of layer 5 pyramidal neurons in rat barrel cortex. J Physiol. 2009;587(pt 21):5107–5119. doi: 10.1113/jphysiol.2009.180943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munera A, Cuestas DM, Troncoso J. Peripheral facial nerve lesions induce changes in the firing properties of primary motor cortex layer 5 pyramidal cells. Neuroscience. 2012;223:140–151. doi: 10.1016/j.neuroscience.2012.07.063. [DOI] [PubMed] [Google Scholar]
- 36.Song S, Sandrini M, Cohen LG. Modifying somatosensory processing with non-invasive brain stimulation. Restor Neurol Neurosci. 2011;29:427–437. doi: 10.3233/RNN-2011-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lefaucheur JP, Antal A, Ahdab R, et al. The use of repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) to relieve pain. Brain Stimul. 2008;1:337–344. doi: 10.1016/j.brs.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Bjorkman A, Rosen B, Lundborg G. Anaesthesia of the axillary plexus induces rapid improvement of sensory function in the contralateral hand: an effect of interhemispheric plasticity. Scand J Plast Reconstr Surg Hand Surg. 2005;39:234–237. doi: 10.1080/0284431051006493. [DOI] [PubMed] [Google Scholar]
- 39.Floel A, Nagorsen U, Werhahn KJ, et al. Influence of somatosensory input on motor function in patients with chronic stroke. Ann Neurol. 2004;56:206–212. doi: 10.1002/ana.20170. [DOI] [PubMed] [Google Scholar]
- 40.Voller B, Floel A, Werhahn KJ, Ravindran S, Wu CW, Cohen LG. Contralateral hand anesthesia transiently improves poststroke sensory deficits. Ann Neurol. 2006;59:385–388. doi: 10.1002/ana.20689. [DOI] [PubMed] [Google Scholar]
- 41.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 42.Allred RP, Cappellini CH, Jones TA. The “good” limb makes the “bad” limb worse: experience-dependent interhemispheric disruption of functional outcome after cortical infarcts in rats. Behav Neurosci. 2010;124:124–132. doi: 10.1037/a0018457. [DOI] [PMC free article] [PubMed] [Google Scholar]