Abstract
Purpose
To determine the incidence and microbiological profile of mycotic keratitis seen at a tertiary care eye hospital.
Materials and methods
A retrospective review of microbiology records of patients presenting with suspected microbial keratitis seen between January 2006 and December 2009 was performed. Patients with positive fungal cultures were further analyzed for the type of fungus isolated and associated bacterial pathogens.
Results
Microbiology records of 2300 patients with suspected microbial keratitis were reviewed. A microbiological diagnosis of mycotic keratitis was established in 87 (3.8%) patients over a four year period based on positive fungal cultures. The yearly incidence of mycotic keratitis was 3.2% (2006), 4.9% (2007), 3.3% (2008) and 3.6% (2009). Filamentous fungi were isolated more often than yeasts. Aspergillus species followed by Fusarium species and Trichophyton species were the commonest filamentous fungi isolated while Candida albicans was the most frequently encountered yeast. Mixed infections due to fungal and bacterial pathogens were seen in 25/87 (28.7%) patients.
Conclusion
Cumulative incidence of mycotic keratitis was 3.8% over a four year period. Aspergillus species and Candida albicans were the most frequent pathogenic organisms causing mycotic keratitis in this part of the world. Mixed infections were seen in 28.7% of the patients. Knowledge of the “local” etiology within a region may be valuable in the management of mycotic keratitis in instituting an empirical therapy, especially when facilities for microscopy, cultures and antifungal susceptibility are not readily available. The baseline information presented will also be helpful in the planning of a corneal ulcer management strategy and for future studies on mycotic keratitis.
Keywords: Mycotic keratitis, Incidence, Microbiological profile
Introduction
Microbial keratitis is one of the predominant causes of preventable form of blindness.1 Corneal morbidity caused by microbial keratitis is a major health problem worldwide.1,2 Several studies from different parts of the world have shown that this condition can be caused by several groups of bacteria, fungi, viruses and parasites.1–6 Although studies have shown that there could be distinct clinical features that can be ascribed to each one of these agent groups, a clinical diagnosis can often be difficult due to overlapping features.1 Further, the clinical features of mycotic keratitis caused by different fungal species itself can be very protean. Specific treatment requires prompt and accurate identification of the causative micro-organisms.7 The microbial causes of keratitis vary considerably between continents and countries and also within countries.2 Thus, it is essential to determine the local etiology within a given region when planning a corneal ulcer management strategy.
Corneal infection of fungal etiology is very common and forms a significant proportion of microbial keratitis as shown by several studies.1–3,8–13 Filamentous fungi are responsible for a larger proportion of these corneal infections in tropical climates than in temperate climates.
Individual case reports and a small case series have presented data on mycotic keratitis in this part of the world.14–21 However, there seems to be inadequate information about the incidence and microbiological profile of a large series of mycotic keratitis, especially in arid climates. It has therefore become increasingly important to gather sufficient data that would project the gravity of the problem of mycotic keratitis of a particular geographic region, for e.g.: Saudi Arabia, in terms of incidence and etiology, the documentation of which would serve as a useful guideline for practicing ophthalmologists.
This retrospective study aims to describe the incidence and microbiological profile of mycotic keratitis seen in this part of the world over a four year period.
Materials and methods
The study design was a retrospective analysis of data collected by the microbiology section of the Department of Pathology and Laboratory Medicine over a four year period (2006–2009). All patients, from various regions, who have been seen between January 2006 and December 2009 with a clinical diagnosis of microbial keratitis and who have been investigated for the evidence of etiological agents by microbiological cultures, were included in this study.
Microbiology procedures
As a part of the standard protocol, all patients were investigated for bacterial and fungal pathogens. A microbiological diagnosis of mycotic keratitis was established when fungal cultures were positive. All patients with purely bacterial keratitis (wherein only a bacterial pathogen was isolated) were excluded while mixed infections due to fungal and bacterial species were included in the analysis. Microbiological assays routinely performed for fungal keratitis included direct smears for fungal elements (Gram, Giemsa, Calcofluor white and Gomori’s methanamine silver (GMS stains) and fungal cultures using Saboraud’s Dextrose Agar (SDA). Positive fungal cultures are identified based on colony morphology, growth characteristics on various media and microscopic features. Yeasts were identified by employing VITEK 2 automated microbial identification system (Bio Merieux, France). Standard microbiological procedures and interpretative criteria were employed for processing the specimens for bacterial and fungal cultures as described previously.1
Data entry
Demographics and microbiology results of the patients entered into the VITEK 2 “bioLIAISON Data Trac” program were used to analyze the results of microbiological cultures. The bioLIAISON application is a data management system that integrates Vitek 2 (Microbiology identification system) test results and patient demographic information. The “BioLIAISON Data Trac” is a cumulative epidemiology reporting package that works with the BioLIAISON database. The program has a data collection form which is filled in by the technologists and saved. Patient demographics, specimen source, clinical diagnosis, bacterial and fungal pathogens isolated and comments can all be stored in the database for further analysis.
Results
Microbiology records of 2300 patients seen over a four year period (2006–2009) were reviewed. A microbiological diagnosis of mycotic keratitis was established in 87/2300 patients based on a positive fungal culture. Eighty eight fungal pathogens were isolated from 87 patients. One patient among them had a mixed infection due to two fungal pathogens. The cumulative incidence of mycotic keratitis over a four year period was 3.8%. The number of patients seen over a four year period and the yearly incidence is shown in Table 1.
Table 1.
Incidence of Mycotic keratitis over a period of four years.
| Year | Total no. of patients with microbial keratitis | Total no. of patients with mycotic keratitis (%) |
|---|---|---|
| 2006 | 595 | 19(3.2) |
| 2007 | 634 | 31(4.9) |
| 2008 | 521 | 17(3.3) |
| 2009 | 550 | 20(3.6) |
| Total | 2300 | 87(3.8) |
Note: figures in parentheses indicate percentage of incidence of mycotic keratitis.
The cumulative and yearly distribution of various fungi which were isolated is shown in Table 2. Eighty eight fungal pathogens were isolated from 87 patients. One patient had a mixed infection with Candida albicans and Rhodotorula species. Filamentous fungi were isolated from 62/87 (71.3%) patients while yeasts were seen in 25/87 (28.7%) patients with mycotic keratitis. Among the filamentous fungi, most were hyaline while dematiaceous fungi were isolated in 4 patients. Aspergillus flavus (n = 19) was the most frequent isolate followed by Fusarium species (n = 15) and Trichophyton species (n = 14). Among the yeasts, Candida albicans (n = 11) was the most frequent isolate.
Table 2.
Year wise distribution of various fungi isolated from patients with Mycotic keratitis (n = 87).
| Fungal isolates | 2006 | 2007 | 2008 | 2009 | Total |
|---|---|---|---|---|---|
| MOLDS | |||||
| Acremonium species | – | – | – | 1 | 1 |
| Alternaria species | – | – | – | 1 | 1 |
| Aspergillus flavus | 4 | 10 | 3 | 2 | 19 |
| Aspergillus fumigatus | – | 2 | – | – | 2 |
| Aspergillus niger | – | – | – | 1 | 1 |
| Aspergillus species | 1 | 1 | – | – | 2 |
| Bipolaris species | – | – | 1 | – | 1 |
| Dematiaceous fungi | 1 | 1 | – | – | 2 |
| Drechslera species | 1 | – | – | – | – |
| Fusarium species | 4 | 5 | 3 | 3 | 15 |
| Trichophyton schoenleinii | 2 | 2 | 1 | 1 | 6 |
| Trichophyton verrucosum | – | – | – | 1 | 1 |
| Trichophyton species | – | 4 | – | 3 | 7 |
| Trichoderma species | 1 | 1 | – | – | 2 |
| Unidentified fungi | – | – | 1 | – | 1 |
| Total | 14 | 26 | 9 | 13 | 62 |
| YEASTS | |||||
| Candida albicans | 3 | 1 | 3 | 4 | 11 |
| Candida dubliniensis | 1 | – | – | 1 | 2 |
| Candida glabrata | – | – | 1 | – | 1 |
| Candida parapsilosis | 1 | – | 2 | 2 | 5 |
| Candida pelliculosa | – | 1 | – | – | 1 |
| Candida rugosa | – | 1 | – | – | 1 |
| Candida tropicalis | – | 1 | 1 | – | 2 |
| Candida utilis | – | – | 1 | – | 1 |
| Geotrichum capitatum | – | 1 | – | – | 1 |
| Rhodotorula species | – | – | 1a | – | 1 |
| Total | 5 | 5 | 9 | 7 | 26 |
Candida albicans was also isolated from this patient.
Table 3 shows the bacterial pathogens which were isolated from patients with mycotic keratitis. Mixed infections due to various bacterial pathogens were seen in 25/87 patients with culture confirmed mycotic keratitis. Staphylococcus epidermidis and Propionibacterium acnes were the most frequent bacterial pathogens isolated from patients with mixed infections.
Table 3.
Bacterial pathogens isolated from patients with mycotic keratitis (Mixed infections) (n = 25/87 [28.7%]).
| Patient Sl. No. | Fungi isolated | Bacterial pathogens isolated |
|---|---|---|
| 1 | Acremonium species | a. Staphycoccus epidermidis |
| 2 | Trichophyton species | a. Staphycoccus epidermidis |
| 3 | Candida dubliniensis | a. Staphycoccus epidermidis |
| 4 | Alternaria species | a. Staphycoccus epidermidis, b. Propionibacterium acnes |
| 5 | Candida parapsilosis | a. Staphycoccus epidermidis |
| 6 | Aspergillus flavus | a. Staphycoccus epidermidis |
| 7 | Aspergillus flavus | a. Staphycoccus epidermidis, b. Viridans streptococci |
| 8 | Aspergillus flavus | a. Propionibacterium acnes |
| 9 | Trichophyton schoenleinii | a. Propionibacterium acnes |
| 10 | Aspergillus species | a. Staphycoccus epidermidis |
| 11 | Fusarium species | a. Staphycoccus hominis |
| 12 | Fusarium species | a. Staphycoccus epidermidis |
| 13 | Trichophyton species | a. Propionibacterium acnes |
| 14 | Aspergillus fumigatus | a. Propionibacterium acnes |
| 15 | Aspergillus flavus | a. Staphycoccus warneri |
| 16 | Candida albicans | a. Staphycoccus epidermidis, b. Streptococcus mitis/oralis |
| 17 | Candida albicans | a. Corynebacterium urealyticum |
| 18 | Fusarium species | a. Propionibacterium acnes |
| 19 | Trichophyton verrucosum | a.Pseudomonas stutzeri, b. Staphylococcus epidermidis |
| 20 | Candida albicans | a. Staphycoccus aureus |
| 21 | Aspergillus niger | a. Streptococcus pneumoniae |
| 22 | Candida parapsilosis | a. Streptococcus pneumonia, b. Staphycoccus aureus |
| c. Corynebacterium species, d. Moraxella catarrhalis | ||
| 23 | Fusarium species | a. Klebsiella ozanae, b. Sphingomonas paucimobilis |
| 24 | Candida tropicalis | a. Enterococcus faecalis, b. Citrobacter freundii |
| 25 | Candida parapsilosis | a. Streptococcus pneumoniae |
Discussion
Microbial keratitis is one of the predominant causes of preventable corneal blindness and is seen worldwide.1–6 Mycotic keratitis forms an important component of this disease spectrum.1–3,8,21 A rising trend in the incidence of fungal keratitis has been observed possibly due to the indiscriminate use of broad spectrum antibiotics, immunosuppressive drugs and corticosteroids and improved microbiological techniques. Mycotic keratitis is expected to be seen more commonly in tropical and sub-tropical regions than in temperate regions.3
Prevalence of mycotic keratitis varies significantly from region to region. The incidence of mycotic keratitis in this region was 3.8% as shown in this study (Table 1). It is very low when compared to several other studies reported earlier.2,14 The incidence is similar to a study reported from Hong Kong 2 which lies on a latitude (22° 23′ 17″ N) which is almost similar to the location of Riyadh (24° 41′ 47″ N). However, such low prevalence’s have also been reported from UK, South Africa, Nepal and northern India 2 which all lies in different latitudes than Riyadh. The individual climatic conditions and urbanization may have a role to play in the prevalence of mycotic keratitis.
As shown by other studies, filamentous fungi were more commonly isolated than yeasts in our study (71.3% versus 28.7%) (Table 2). Aspergillus species (24/87) (27.6%) and Fusarium species (15/87) (17.2%) were the most frequently isolated filamentous fungal pathogens from cases of mycotic keratitis (Table 2) as has been shown by several studies.1–3,8,14,21,22 Our results correlate with this fact. An earlier study on a small number of patients from this region has also shown similar findings.15 However, Fusarium species was more commonly isolated as shown by studies from Florida, south India, Hong Kong, Nigeria, Paraguay, Tanzania and Singapore.2 The difference in the isolation rates of these fungal pathogens can be explained by the differences in the climate and the natural environment of individual regions. The higher incidence of mycotic keratitis due to Aspergillus species in dry climates, such as in this region, may be due to the fact that spores of Aspergillus species can tolerate hot, dry weather conditions.15 Aspergillus species are more ubiquitous and have been found almost everywhere on every conceivable type of substrate including soil and decaying organic debris while Fusarium species are common plant pathogens and are found in soil.2 This may be another reason that mycotic keratitis due to Aspergillus species are usually more common than Fusarium species.
An interesting finding of this study was the association of Trichophyton species with mycotic keratitis (Table 2). Mycotic keratitis due to Trichophyton species (Figs. 1 and 2) was seen in 14/87 (16.1%) patients. An earlier study from this region has shown that Trichophyton species is a rare cause of mycotic keratitis and may be associated with progressive keratolysis and perforation, scleral extension and endophthalmitis.19 Further studies on this pathogen are warranted to delineate the patient characteristics and the ultimate outcome of keratitis.
Figure 1.
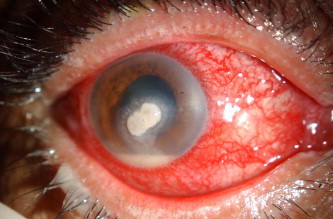
Diffuse illumination of the right eye with Trichophyton shoenleinii keratitis. Note the central thick infiltrate and the hypopyon.
Figure 2.
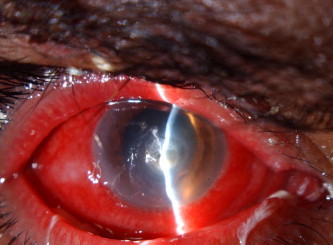
Slit lamp view of the right eye with Trichophyton shoenleinii keratitis after 2 months of therapy. Note the reduced infiltrate and the absence of hypopyon.
Candida species 24/87 (27.6%) was the most commonly isolated fungal pathogen among the yeasts causing mycotic keratitis (Table 2) (Figs. 3–5). Candida albicans was most common species isolated. Similar observations have been made by studies from Philadelphia and north India.8,23 Since Candida albicans is an ubiquitous commensal of mucous membranes in humans, with no geographic dominance, keratitis due to this organism tends to occur more frequently in areas where traumatic keratitis is uncommon but where other predisposing factors are important including systemic disorders or preexisting ocular abnormalities.22 Keratitis due to yeast-like and related fungi usually develops in eyes with preexisting epithelial or stromal ulceration (e.g. previous herpes simplex keratitis, contact lens induced corneal abrasions). Further analyses on these aspects are necessary since this study aimed to analyze only the mycological profile.
Figure 3.
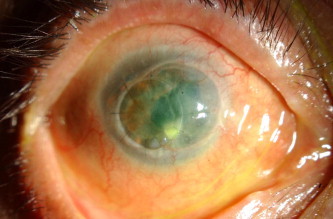
Diffuse illumination of the right eye with Candida utilis keratitis: Note the infiltrate along the suture.
Figure 4.
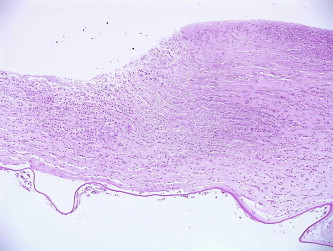
Histological appearance of the corneal tissue showing corneal stromal thinning with extensive inflammatory infiltrate, X 40, Hematoxylin & Eosin stain.
Figure 5.
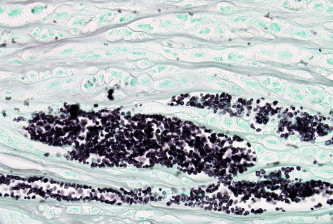
The appearance of yeast cells with special stain within the corneal stromal lamellae, X 2000, Gomori’s Methanamine Silver stain.
A single fungal agent was isolated as the only pathogen in 62/87 (71.3%) patients while mixed infections due to fungal and bacterial pathogens were seen in 25/87 patients (28.7%) (Table 3). One patient had a mixed infection due to Candida albicans and Rhodotorula species. Several bacterial species were isolated along with a fungal pathogen (Table 3). Mixed infections have been reported earlier by two studies with an incidence of 4% and 13.7% respectively.24,25 Mixed infections due to a fungal agent and bacterial pathogens may pose therapeutic challenges. Published data on mixed infections, as reported in this study, are sparse. Further analysis to reveal the progression and outcome of keratitis in such patients would be interesting.
In summary, medical therapy of mycotic keratitis consists of nonspecific measures and the use of specific antifungal agents. The antifungal ultimately selected as primary therapy necessarily depends on its easy availability and other criteria. If direct microscopic examination of corneal scrapes or corneal biopsy specimen yields unequivocal results that are consistent with clinical picture, treatment may be initiated: otherwise therapy may need to be withheld until culture reports become available.22 The treatment largely depends on the fungal species isolated in order to initiate appropriate antifungal therapy. The results of this study reveal the various fungal pathogens causing mycotic keratitis in this region which would help in better understanding of the infection and the course of the disease which depends on the etiological agent involved.
The results will certainly add to the clinical data, management of mycotic keratitis and selection of appropriate antifungal agents, especially in a setting where routine antifungal testing is not done. This baseline information will also be helpful in the planning of a corneal ulcer management strategy and for future studies on mycotic keratitis.
To the best of our knowledge, this is the largest case series study which delineates the incidence and describes the fungal species causing mycotic keratitis, in this region of the world.
Conflicts of interest
None declared.
Acknowledgment
The assistance provided by Ms. Najla Al Salama, Department of Ophthalmic Photography, is gratefully acknowledged.
References
- 1.Gopinathan U., Garg P., Fernandes M., Sharma S., Athmanathan S., Rao G.N. The epidemiological features and laboratory results of fungal keratitis: a 10-year review at a referral eye care center in South India. Cornea. 2002;21:555–559. doi: 10.1097/00003226-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Leck A.K., Thomas P.A., Hagan M., Kaliamurthy J., Ackuaku E., John M., Newman M.J., Codjoe F.S., Opintan J.A., Kalavathy C.M., Essuman V., Jesudasan C.A.N., Johnson G.J. Aetiology of suppurative corneal ulcers in Ghana and south India, and epidemiology of fungal keratitis. Br J Ophthalmol. 2002;86:1211–1215. doi: 10.1136/bjo.86.11.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bharathi M.J., Ramakrishnan R., Vasu S., Meenakshi R., Palaniappan R. Epidemiological characteristics and laboratory diagnosis of fungal keratitis. A three-year study. Indian J Ophthalmol. 2003;51:315–321. [PubMed] [Google Scholar]
- 4.Athmanathan S., Reddy S.B., Nutheti R., Rao G.N. Comparison of an immortalized human corneal epithelial cell line with Vero cells in the isolation of Herpes simplex virus-1 for the laboratory diagnosis of Herpes simplex keratitis. BMC Ophthalmol. 2002;30:2:3. doi: 10.1186/1471-2415-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma S., Garg P., Rao G.N. Patient characteristics, diagnosis, and treatment of non-contact lens related Acanthamoeba keratitis. Br J Ophthalmol. 2000;84:1103–1108. doi: 10.1136/bjo.84.10.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green M., Apel A., Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27:22–27. doi: 10.1097/ICO.0b013e318156caf2. [DOI] [PubMed] [Google Scholar]
- 7.Levey S.B., Katz H.R., Abrams D.A., Hirschbein M.J., Marsh M.J. The role of cultures in the management of ulcerative keratitis. Cornea. 1997;16:383–386. [PubMed] [Google Scholar]
- 8.Tanure M.A., Cohen E.J., Sudesh S., Rapuano C.J., Laibson P.R. Spectrum of fungal keratitis at Wills Eye Hospital, Philadelphia, Pennsylvania. Cornea. 2000;19:307–312. doi: 10.1097/00003226-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Miño de Kaspar H., Zoulek G., Paredes M.E., Alborno R., Medina D., Centurion de Morinigo M., Ortiz de Fresco M., Aguero F. Mycotic keratitis in Paraguay. Mycoses. 1991;34:251–254. doi: 10.1111/j.1439-0507.1991.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 10.Imwidthaya P. Mycotic keratitis in Thailand. J Med Vet Mycol. 1995;33:81–82. doi: 10.1080/02681219580000171. [DOI] [PubMed] [Google Scholar]
- 11.Gopinathan U., Sharma S., Garg P., Rao G.N. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: experience of over a decade. Indian J Ophthalmol. 2009;57:273–279. doi: 10.4103/0301-4738.53051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chander J., Singla N., Agnihotri N., Arya S.K., Deep A. Keratomycosis in and around Chandigarh: a five year study from a north Indian tertiary care hospital. Indian J Pathol Microbiol. 2008;51:304–306. doi: 10.4103/0377-4929.41700. [DOI] [PubMed] [Google Scholar]
- 13.Tilak R., Singh A., Maurya O.P., Chandra A., Tilak V., Gulati A.K. Mycotic keratitis in India: a five year retrospective study. J Infect Dev Ctries. 2010;4:171–174. doi: 10.3855/jidc.309. [DOI] [PubMed] [Google Scholar]
- 14.Idiculla T., Zachariah G., Keshav B., Basu S. A retrospective study of fungal corneal ulcers in the south Sharqiyah region in Oman. Sultan Qaboos Univ Med J. 2009;9:59–62. [PMC free article] [PubMed] [Google Scholar]
- 15.Khairallah S.H., Byrne K.A., Tabbara K.F. Fungal keratitis in Saudi Arabia. Doc Opthalmol. 1992;79:269–276. doi: 10.1007/BF00158257. [DOI] [PubMed] [Google Scholar]
- 16.Al- al-Hedaithy S.S., al-Kaff A.S. Exophiala jeanselmei keratitis. Mycoses. 1993;36:97–100. doi: 10.1111/j.1439-0507.1993.tb00695.x. [DOI] [PubMed] [Google Scholar]
- 17.Wagoner M.D., Badr I.A., Hidayat A.A. Chrysosporium parvum keratomycosis. Cornea. 1999;18:616–620. [PubMed] [Google Scholar]
- 18.Jradeh E.F., Al-Kharashi S.A., Tabbar K.F. Candida keratitis in a patient with candidiasis of the fingernails. Eur J Ophthalmol. 2001;1:380–382. doi: 10.1177/112067210101100412. [DOI] [PubMed] [Google Scholar]
- 19.Mohammad A., Al-Rajhi A., Wagoner M.D. Trichophyton funga Keratitis. Cornea. 2006;25:118–122. doi: 10.1097/01.ico.0000164834.77291.51. [DOI] [PubMed] [Google Scholar]
- 20.Al-Assiri A., Al-Jastaneiah S., Al-Khalaf A., Al-Fraikh H., Wagoner M.D. Late-onset donor-to-host transmission of Candida glabrata following corneal transplantation. Cornea. 2006;25:123–125. doi: 10.1097/01.ico.0000164777.80879.07. [DOI] [PubMed] [Google Scholar]
- 21.Shokohi T., Nowroozpoor-Dailami K., Moaddel-Haghighi T. Fungal keratitis in patients with corneal ulcer in Sari, Northern Iran. Arch Iran Med. 2006;9:222–227. 17. [PubMed] [Google Scholar]
- 22.Thomas P.A. Current perspectives in ophthalmic mycoses. Clin Mic Rev. 2003;16:730–797. doi: 10.1128/CMR.16.4.730-797.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saha R., das S. Mycological profile of infectious keratitis from Delhi. Indian J Med Res. 2006;123:159–164. [PubMed] [Google Scholar]
- 24.Hagan M., Wright E., Newman M., Dolin P., Johnson G. Causes of suppurative keratitis in Ghana. Br J Ophthalmol. 1995;79:1024–1028. doi: 10.1136/bjo.79.11.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capriotti JA, Pelletier JS, Shah M, Caivano DM, Turay P, Ritterband DC. The etiology of infectious corneal ulceration in Sierra Leone. Int Ophthalmol 2010 Jan 29 (Epub ahead of print). [DOI] [PubMed]


