Abstract
Posterior polar cataract is a rare form of congenital cataract. It is usually inherited as an autosomal dominant disease, yet it can be sporadic. Five genes have been attributed to the formation of this disease. It is highly associated with complications during surgery, such as posterior capsule rupture and nucleus drop. The reason for this high complication rate is the strong adherence of the opacity to the weak posterior capsule. Different surgical strategies were described for the handling of this challenging entity, most of which emphasized the need for gentle maneuvering in dealing with these cases. It has a unique clinical appearance that should not be missed in order to anticipate, avoid, and minimize the impact of the complications associated with it.
Keywords: Posterior polar cataract, Congenital cataract, Posterior capsular plaque, Posterior capsule rupture, Posterior capsule tear, Posterior capsule rent
Introduction
Lens opacities can have a wide variety of presentations ranging from a white dot in the anterior lens capsule to dense, total opacity involving all the lens structures. It can be zonular, nuclear, subcapsular, polar, sutural, total, and membranous. One of the important morphologies of lens opacity is the posterior polar cataract. A posterior polar cataract is a rare form of congenital cataract with incidence ranging from 3 to 5 in 1000.1–3 It was found to be bilateral in 65–80% of the cases.4,5 There is no sex predilection in general. The importance of posterior polar cataract lies in its higher risk of complications – posterior capsular tear and possible nucleus drop during surgery.
This article reviews the etiology, clinical presentation, different surgical techniques, and the management of posterior polar cataract.
Pathogenesis
The position of lens opacity is largely determined by the anatomy of the lens and the timing and nature of the insult that caused the abnormality by altering the embryogenesis. The developing lens requires nutrition that is obtained through the tunica vasculosa lentis (TVL), which is a vascular network, supplied posteriorly by the hyaloid artery, a branch of the primary dorsal ophthalmic artery, and anteriorly from an anastomosis with vessels in the pupillary membrane. It has been suggested that posterior polar cataracts are caused by persistence of the hyaloid artery6,7 or invasion of the lens by mesoblastic tissue.8,9 It appears that posterior polar cataract forms during embryonic life or early in infancy and usually becomes symptomatic 30–50 years later. The exact pathogenesis of posterior polar cataract is still unknown. However, it has been noted to occur as a result of gene mutation.10 The proximal cause of the abnormality is expressed as an abnormality in lens development, specifically in the lens fibers that fail to develop normally and form an opacity close to and sometimes adherent to the posterior capsule.
A posterior polar cataract consists of dysplastic lens fibers, which, in their migration posteriorly from the lens equator, exhibit progressive lens opacity, increased degenerative changes, with the formation of a characteristic discoid posterior polar plaque-like cataract and the accumulation of extracellular material.11–13 There is extreme thinness and fragility of the posterior capsule (or perhaps even absent) with adherence of the acellular opacity to the capsule.14 The capsular thinning was demonstrated histologically in one study15 while another study did not find it.16 In approximately 20% of the cases, an association with a congenital defect in the posterior capsule has been reported.4
It seems that the high incidence of posterior capsule rupture during surgery for those patients might be because of two reasons. First, there might be tight adherence of the plaque to an otherwise normal capsule. Second, the posterior capsule itself underlying the plaque is exceptionally thin that ruptures to minimal trauma.
Inheritance and genetics
It has been recognized that posterior polar cataracts seemed to follow an autosomal dominant inheritance pattern,17,18 although it is occasionally sporadic. Positive family history was found in 40–55% of the patients.4,5 Molecular genetic analyses have demonstrated that an autosomal-dominant posterior polar cataract is a genetically heterogenous disease.17,19 The direct cause of the lenticular fiber malformation during lens development has not been well understood.20–22 There are five genes attributed to posterior polar cataract (CTTP) that have been identified. CTPP1 (OMIM 116600) has been mapped to 1p36.19 CTPP2 has been associated with CRYAB on 11q22-q22.3, and a Pro20Ser mutation and a deletion mutation (450delA) have also been highlighted.23,24 The CHMP4B gene on chromosome 20p12-q12 is responsible for CTPP3 (OMIM 605387). Three mutations of PITX3 gene on chromosome 10q25, 38G > A mutation, 17-bp insertion, and 650delG have been reported to cause CTPP4 (OMIM 610623).10,25–27 Two loci with unknown genes have similarly been reported, 14q22-q23 for CTPP5 (OMIM 610634)28 and 16q22.29
Clinical presentation
Posterior polar cataract presents as a distinctive discoid lens opacity situated posteriorly, adjacent to the posterior capsule. It is usually associated with remnant of the hyaloid system.30 Duke-Elder mentioned that it can be stationary as well as progressive cataracts.31 The stationary-type, which is more common (about 65%),5 is a well-circumscribed circular opacity localized on the central posterior capsule. The concentric thickened rings around the central plaque opacity give an appearance of a Bull’s-eye. Sometimes, the opacity is camouflaged by nuclear sclerosis. Sometimes there is a smaller satellite rosette lesion adjacent to the central opacity. Progression may begin in any decade. In the progressive type, whitish opacification changes take place in the posterior cortex in the form of radiating rider opacity. It has feathery and scalloped edges but they do not involve the nucleus and does not extend as far anteriorly as the original opacity. Both stationary and progressive posterior polar cataract may become symptomatic. The lens may have evidence of small opacity at birth, but there are cataractous changes later in life, usually at 30–50 years of age. The appearance of symptoms might be due to the vacuole like changes in the vicinity of the central opacity. The typical symptoms are increasing glare while driving at night and difficulty in reading fine prints. Other symptoms include intolerance to light. The cause of glare, reduced contrast sensitivity, and decreased visual acuity is forward light scattering (light scattering toward the retina). The reasons for delayed presentation might be increasing density of the opacity, age-related papillary miosis, or increased functional needs or visual expectations. If it is visually significant since childhood, it might present with strabismus indicating poor visual function in that eye.
The diagnosis of a posterior polar cataract is self-evident on slit-lamp examination and does not require special diagnostic procedures beyond a full ophthalmic examination. Slit-lamp examination and pupillary retroillumination allow a good evaluation of the visual significance of the opacity. Posterior polar cataract is easily identified by the slit lamp and often clearly delineated. In contrast to anterior polar cataract, the posterior form of moderate degree is rarely discovered within the first month of life. When the posterior polar cataract is fully formed, it presents as a dense, circular plaque in the central posterior part of the lens giving rise to what was described by bull’s-eye appearance (concentric rings around the central opacity). It can be surrounded by vacuoles and smaller areas of degenerated lens material. Often, the only benefit to the surgeon is that the defect is so clearly visible (Figs. 1 and 2). Examination of the anterior vitreous may reveal oil-like droplets or particles.6,9 The presence of such finding should raise the possibility of pre-existing capsular opening. Mean lens thickness in posterior polar cataract was found to be lower than that found in eyes with senile cataract.5 In that study, the affected individuals were relatively younger than patients with other forms of senile cataract. Yet, they found an insignificant correlation between lens thickness and patient age. This entity is rare and can be seen to progress from its initial discovery. It can be associated with other ocular features like microphthalmia, microcornea, anterior polar cataract, psychosomatic disorders.8,32,33 In addition, it was associated with ectodermal dysplasia, Rothmund disease, scleroderma, incontinentia pigmenti, congenital dyskeratosis, congenital ichthyosis, and congenital atrophy of the skin.31
Figure 1.
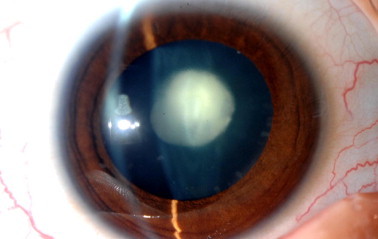
The clinical appearance of posterior polar cataract. Note the discoid shape opacity in the center of the posterior capsule.
Figure 2.
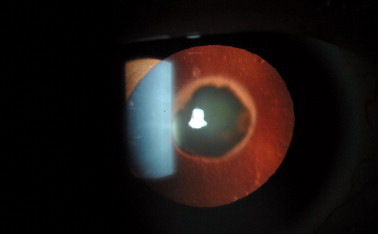
Retroillumination view of posterior polar cataract.
Classification
The main hindrance in fully adopting a functional grading system for posterior polar cataracts appears to be the small sample sizes available in the studies. This is because the entity is rare.
Singh classified posterior polar cataract into2:
-
–
Type 1: the posterior polar opacity is associated with posterior subcapsular cataract.
-
–
Type 2: sharply defined round or oval opacity with ringed appearance like an onion with or without grayish spots at the edge.
-
–
Type 3: sharply defined round or oval white opacity with dense white spots at the edge often associated with thin or absent posterior capsule. These dense white spots are a diagnostic sign (Daljit Singh sign) of posterior capsule leakage with or without repair and extreme fragility34 the incidence of this type in Indian adult cataract patient population was found to be about one in 300.
-
–
Type 4: Combination of the above 3 types with nuclear sclerosis.
Singh observed the possibility of conversion of posterior polar cataract from type two to type three over following patients who for many years were reluctant to have surgery. He recommended not to delay surgery unnecessarily in these cases.
Schroeder35 on the other hand graded posterior polar cataract in his pediatric patients according to its effect in pupillary obstruction in the red reflex testing as follows:
-
•
Grade 1: a small opacity without any effect on the optical quality of the clear part of the lens.
-
•
Grade 2: a two-thirds obstruction without other effect.
-
•
Grade 3: the disc-like opacity in the posterior capsule is surrounded by an area of further optical distortion. Only the dilated pupil shows a clear red reflex surrounding this zone.
-
•
Grade 4: the opacity is totally occlusive; no sufficient red reflex is obtained by dilation of the pupil.
-
•
He advocated for patching before surgery as it can play a diagnostic role apart from its importance for the postoperative management. If patching works well, surgery is not urgent; if not, it should be done soon. His retinoscopic grading that was mentioned above would help in timely planning of surgery. The higher the grade of papillary obstruction the earlier the surgery should be carried out. The first step in managing grade 1 should be patching while in grade 2, it should be patching with mydriasis and bifocal glasses. In grades 3 and 4, patients should be patched with early surgical intervention as soon as possible for better outcome. However, grade 4 posterior polar cataract, pre-existing strabismus and refusal of therapy are poor prognostic factors.
Indications for surgery
Difficulty in carrying out daily activities is the main indication for surgery. An important point of consideration in these patients is the lower surgical complications and easier technique if surgery is done while the nucleus is soft. Another point is the possibility of the formation of capsular defects over time in patients with initially intact capsules.2 Furthermore, when it is visually significant in childhood, it is considered amblyogenic. All these reasons emphasize the importance of early intervention in these cases.
Counseling
During the preoperative examination, the physician should inform the patient of the possibility of the nucleus’ dropping intraoperatively due to a posterior capsular rupture, a relatively long operative time, secondary posterior segment intervention, and a delayed visual recovery. In addition, the surgeon should discuss Nd:YAG capsulotomy for residual plaque4,5,36 and emphasize the possibility of preexisting amblyopia, especially in cases of unilateral posterior polar cataract.36
Because the understanding of posterior polar cataract, an autosomal dominant condition, is continually expanding, genetic counseling for the parents in addition to screening of family members is important.
Choosing the surgical techniques
While Osher et al.4 found no difference in the rate of posterior capsule rupture between phacoemulsification and extracapsular cataract extraction (ECCE), Das et al. in a retrospective analysis concluded that phacoemulsification is preferred to ECCE in posterior polar cataract as they found higher rates of complications with ECCE.37 However, they recommended ECCE in harder cataract and dense central plaques. They found out that posterior capsule rupture occurs most commonly during emulsification of the nucleus in phacoemulsification and during nucleus expression in ECCE cases. Vasavada and Singh found that rupture occurs most commonly during epinucleus removal in phacoemulsification,5 while Osher et al. found it to happen during removal of the posterior polar opacity or during cleaning of the posterior capsule after plaque removal.4
Hayashi et al.36 recommended for phacoemulsification if the opacity was <4 mm and the nucleus was soft. If the opacity is larger than 4 mm and the nucleus was soft, pars plana lensectomy and vitrectomy are advised. In case of larger opacity than 4 mm and dense nucleus, they recommended intracapsular cataract extraction.
Anesthesia
Local and topical anesthesia can be utilized for operating cases with posterior polar cataract. Vasavada and Singh5 preferred peribulbar anesthesia as it provides prolonged action and reduces positive pressure on the vitreous. This is in contrast to topical anesthesia, in which squeezing the lids with a speculum can distort the globe. Increased eye movement and lack of hypotony would increase the forward movement of the posterior capsule. The preoperative gentle oculopressure was advised by Osher et al. to diminish the intraoperative posterior pressure.4 If the patient is young or did not seem cooperative, general anesthesia should be considered.
Phacoemulsification
The incision
Starting with the side port incision followed by the injection of viscoelastic material might be better than staring with the main incision. This will avoid the possible chamber collapse that might predispose to premature rupture of the capsule. The incision of the phacoemulsification surgery can be a usual coaxial one, whether corneal or scleral, or it can be microincision for bimanual technique.
Haripriya et al.38 used a bimanual microphacoemulsification technique with separate infusion and aspiration instruments placed through watertight incisions 1.4 mm in width. This enhanced the control during phacoemulsification with less risk of posterior capsule tear. The average fluid flow through the irrigating chopper is approximately 45–50 mL/min comparing to A high-flow system is present in the anterior chamber during coaxial phacoemulsification, with an average irrigation of 90 mL/min when the bottle height is at 100 cm which can cause a pressure increase within the capsular bag and increase the risk for capsule rupture and vitreous disturbance early in the procedure. Beside having a controlled operating environment for slow motion phacoemulsification, the advantages of this technique lie primarily in the following: (1) allowing withdrawal of the phaco-needle first while maintaining the anterior chamber with infusion from the separate irrigating chopper, and (2) easing injection of viscoelastic into the anterior chamber before final withdrawal of the irrigating chopper.
The capsulorhexis
It is important to avoid overpressurizing the anterior chamber with viscoelastic material. The use of a heavy viscoelastic might be advantageous in the young patients whose scleral elasticity contributes to the tendency toward anterior chamber shallowing. Starting the rhexis should be by pinching the capsule by the forceps or by the cystotome rather by downward movement of the forceps which might lead to posterior capsule rupture or enlarging an existing one.
Specially, with harder nuclei, a moderate to large capsulorhexis (⩾5 mm) is beneficial for several reasons. During hydrodelineation, excess fluid is released into the anterior chamber, reducing intralenticular pressure. Additionally, if a posterior capsule rupture occurs as a result of accidental hydrodissection, the nucleus will be more likely to prolapse into the anterior chamber than drop into the vitreous. Finally, if vitreous loss occurs during phacoemulsification, it is easier to manually prolapse the nucleus into the anterior chamber in the presence of a large capsulorhexis without having to further enlarge it.39
However, it should not be too large so that if the posterior capsule is compromised, enough rim would be present to implant the lens in the ciliary sulcus.
Hydrodissection and hydrodelineation
While Fine et al.40 did hydrodissection in multiple quadrants with tiny amount of fluid without allowing the wave to transmit across the posterior capsule, cortical cleaving hydrodissection is considered a contraindication in eyes with posterior polar cataract. A weak point can produce hydraulic posterior capsule rupture during hydrodissection. Meanwhile, hydrodelineation, which is the separation between the nucleus and the epinucleus, is mandatory. It is worth mentioning that the surgeon should avoid vigorous decompression of the capsular bag after the delineation. In addition, nuclear rotation is contraindicated as it can act as a trephine to the posterior capsule.
Vasavada and Raj described a technique that was described for dense and posterior polar cataract called inside-out delineation.41 In this technique, a trench is first sculpted and a right-angled cannula is used to subsequently direct fluid perpendicularly to the lens fibers in the desired plane through one wall of the trench. This would avoid the possibility of inadvertent subcapsular injection and overcome the difficulty of introducing cannula to a significant depth in a dense cataract.
Parameters of the phacoemulsification machine
It was agreed in all studies that a slow motion phacoemulsification with low parameters should be used in cases with posterior polar cataract the power should be 60%, bottle height 55–70 cm, aspiration rate 15–25 mL/min, vacuum 30–100 mm Hg.4,5 The low vacuum and aspiration rates maintain a very stable chamber and the reduced infusion drives less fluid around the lens.
Nucleotomy techniques
To reduce the risk of posterior capsule rupture and vitreous loss, the posterior capsule should be presumed to be absent beneath the area of the posterior polar cataract. If the nucleus is soft, it can be aspirated by phacoaspiration technique. With harder ones phaco chop and other techniques, that will be described later, were recommended as they exert minimal stress on the capsular bag. During the procedure, it is important to remember to avoid collapse of the anterior chamber as this might cause the anterior tenting of the posterior capsule and can lead to spontaneous rupture. This can be done by judicious injection of a dispersive viscoelastic through the side port incision before withdrawal of the phacoemulsification tip. In addition, nuclear rotation and aggressive nuclear cracking techniques with wide separation of fragments should be avoided.
Lee and Lee1 sculpted the nucleus in the shape of the Greek letter lambda “λ technique”, then cracking along both arms and removing the distal central piece. The advantage of this is its gentleness in not stretching the capsule while removing the quadrants, especially the first one.
Salahuddin42 described a technique called “inverse horse-shoe” in which after sculpting, he divides the distal end of the nucleus. After that, viscoelastic is injected to lift up the two limbs of the nucleus forming a visco shell around the nucleus. The nucleus could be divided into two halves without causing undue stretching on the posterior capsule. Then, the two segments can be engaged, brought to the center, chopped, and emulsified separately.
The presence of dense nuclear component in posterior polar cataract is not common. But if present, the hydrodelineation would be difficult to achieve. Lim and Goh43 developed a technique where they pre-chop the epinucleus in a piecemeal in situ without getting the chopper to reach all the way down to the depth of the posterior epinucleus. This would facilitate mobilizing the dense endonucleus from the anterior epinucleus shell.
Chee44 devised a technique for hard posterior polar cataracts in which she cracks the nucleus in the periphery (partially) avoiding the posterior polar opacity and then chops it into quadrants without rotation; then with the phaco tip she engages the core of the quadrant while cleaving along the lenticular lamellae, using the chopper to a depth that leaves a nuclear shell, sparing the polar cataract. Finally, the nucleus is peeled away from the outer nuclear shell, which is kept in place by the phaco chopper.
Epinucleus removal
If there is a pre-existing capsular rent, evident by the presence of iridescent refractile lens particles in vitreous with onion skinning of central dense plaque, it can be managed by layer-by-layer phacoemulsification technique described by Vajpayee et al.45 After hydrodelineation, the nucleus is sculpted and cracked into halves gently which is then aspirated with low parameter. Then epinucleus is aspirated layer after layer by automated bimanual irrigation and aspiration cannula. The penultimate layer was carefully aspirated leaving thin layer of cortex adherent to the capsule. The most posterior layer along with the plaque was then viscodissected and aspirated using bimanual irrigation and aspiration cannula. It is important to remember to leave the central area attached until the last stage of cortical aspiration.2
Fine et al.40 used minimal hydrodissection and hydrodelinealtion, nuclear emulsification from within the epinuclear shell and gentle viscodissection of the epinucleus and cortex to avoid unnecessary pressure on the posterior capsule and to protect the region of the greatest potential weakness throughout the procedure.
Allen and Wood46 avoided rotation of the nucleus and used gentle viscodissection by injecting viscoelastic between the capsule and the cortex, and employed low power and low vacuum settings in order to reduce the stress on the posterior capsule.
Taskapili et al.47 compared retrospectively outcome of surgery for posterior polar cataract with and without cortical viscodissection. In the viscodissection group and after aspiration of the nucleus, the epinucleus is viscodissected by sodium hyaluronate 1.4% in four quadrant and aspirated. Then the plaque aspiration is also helped by viscodissection of the plaque from the posterior capsule. In the non-viscodissection group, only slow motion phacoemulsification was carried out in the procedure grasping the epinucleus with the phacoemulsification tip at the end without viscodissection. They found a statistically significant higher rate of posterior capsule rupture in the non-viscodissection group.
Nagappa et al.48 described a technique in which only hydrolineation is done then with low parameters (bottle hight, vacuum, and aspiration), the nucleus is removed by phacoaspiration or chip-and-flip technique if it was soft, and direct-chop technique with minimal rotation for harder ones. Then the epinucleus in the quadrants opposite to the wound is removed or loosened by phacoaspiration. Then hydrodissection is performed to release the sub-incisional cortex which is then rotated and aspirated. By this sequence, the fluid wave would not generate adequate pressure to cleave through the weak posterior capsule and rather escape through the path of least resistance in the epinucleus-free area via the equatorial portion of the capsular bag. The authors considered this technique contraindicated if there is a preexisting posterior capsular defect that could be detected on slit-lamp examination or during initial steps of phacoemulsification.
Cortex aspiration
It should be done with low bottle height (15–25 cc/min). The usual pulling of the cortex should be minimized as possible. Instead, the aspiration tip should remain at the equatorial angle in the periphery, and the surgeon should wait until suction increases and the cortex is aspirated. Furthermore, it is better to pull the cortex tangentially rather than centrally to mobilize it. Vajpayee et al.45 described in their “layer-by-layer” technique aspirating a wedge-shaped cortical piece, pulling and separating it from approximately 3–4 mm outside the central area, with the aid of a blunt chopper. This maneuver of mechanical separation from the central plaque avoids traction or pull, which might otherwise be generated from attempting to directly aspirate the cortical matter. Alternatively, the posterior chamber can be filled with viscoelastic material and cortex removed using a “dry” (syringe stripping) technique. It is important to remember to avoid polishing the capsule in such patients as it is usually very thin and may rupture to minimal trauma.
Removal of the posterior polar opacity
It is important to try to leave the removal of the opacity till the last stage of cortical aspiration to avoid early rupture of the posterior capsule. It can be removed with viscodissection and aspirated by the phaco tip or the irrigation aspiration tip.47 Another way is to fill the eye with viscoelastic material and dislodge the opacity with a hook then to grab it and get it out by a forceps.2 This might give more control and avoid losing the opacity in the vitreous cavity with the irrigation flow. Although polishing of the capsule should be avoided in these cases, Osher used a technique termed “minimal residual aspiration” in which the foot pedal is depressed and released just as the irrigation aspiration tip contacts the posterior capsule. The vacuum that is created by the elasticity of the tubing will be enough to clean the capsule with minimal risking for tear. If the posterior capsular plaque is strongly adherent to the capsule that could not be peeled off even by viscodissection, the safest option is to leave the plaque untouched for later Nd-YAG laser capsulotomy.36
In case of the presence of a tear
If a tear is noticed during the procedure, viscoelastic material should be injected before withdrawal of the handpiece to preserve the anterior vitreous face. A dispersive rather than a cohesive viscoelastic is preferable as it is more adapted to maintaining space and stabilizing the anterior vitreous face. Then, the tear should be converted to continuous curvilinear capsulorhexis to put the intraocular lens in the bag without risking an extension of the capsular tear.
Anterior vitrectomy
Vasavada and Singh5 recommended 2 port vitrectomy With a high cutting rate and low vacuum and flow rates, vitrectomy can be safely performed even close to the torn capsule. The vitrector is never placed behind the peripheral posterior capsule. The infusion cannula is directed into the peripheral anterior chamber, and the fluid jet is directed toward the angle of the chamber, away from the defect. This reduces turbulence near the tip of the cutter and avoids enlarging the capsular tear. It also reduces hydration of the vitreous and forward movement of vitreous into the anterior chamber.
Intraocular lens (IOL) implantation
It depends on whether or not there is a capsular tear. If there is none or the size of the posterior capsular rupture is small or it could be converted to a round one, single piece IOL with low overall diameter can be implanted in the bag. It is advised to avoid touching the capsule while inserting the IOL. It might be safer to compress the trailing haptic rather than subjecting the capsular bag to rotational forces that might extend the tear. If the rupture is big or could not be converted to a round one, a multipiece IOL has to be placed in the ciliary sulcus with or without rhexis capture. The advantage of capturing the optic by the rhexis is to stabilize the IOL and to reduce the contact of optic with iris.
Mackool suggested the use of polymethyl methacrylate (PMMA) IOL since inserting a foldable IOL that opens within the capsular bag might stress the posterior capsule.2 He also described a technique in which he ties the two haptics of the IOL by 10–0 polypropylene suture thus compressing them. Then after the insertion, he would cut it and trim the ends.
In cases in which there is a big rupture with questionable zonular integrity, it would be safer to implant an anterior chamber IOL, suturing an IOL to the sclera or the iris, or even leave the patient aphakic for contact lens correction later.
Viscoelastic aspiration and wound closure
As the withdrawal of the irrigation aspiration tip after viscoelastic removal can sometimes be followed by chamber collapse, using AC maintainer or injecting fluid through the sideport incision while removing viscoelastic would keep the chamber formed till checking the integrity of the wounds. Suturing the wound would be advisable to avoid microleak and post operative hypotony that might lead to vitreous prolapse through the defect.
Extracapsular cataract extraction (ECCE)
The standard ECCE can be done paying attention to certain steps. First of all, the incision should be wide enough not to have tension transmitted to the capsule while delivering the nucleus. Delivering the nucleus is the most important step in this technique as it is the step associated with occurrence of posterior capsule rupture.37 One way of delivering the nucleus is described by Singh2 in which he delivers the nucleus by fluid pressure with an 18-gauge cannula connected to the saline bottle. Viscoexpression is another safe technique in such cases. Viscoelastic is injected between the nucleus and the epinucleus to elevate the superior pole of the nucleus followed by extracting the nucleus with a lens loop. The rest of the steps would be the same as previously described for phacoemulsification. The proponents of this technique argue that phacoemulsification produces uncontrolled pressure change and turbulence inside the capsular bag which may greatly increase the risk of posterior capsular tear, the loss of lens material into the vitreous cavity, and vitreous prolapse anteriorly.
Posterior segment approach
This approach might be indicated for large plaques (>4 mm) as recommended by Hayashi et al.36 Primary three ports pars plana vitrectomy was described for posterior polar cataract.36,49
Ghosh and Kirkby49 have done this technique in eight patients where they used a 19-gauge winged metal infusion canula (‘butterfly’) as an infusion line directly into the crystalline lens and either the vitreous cutter or fragmatome or both (depending on nuclear sclerosis) used to remove the lens. In all cases some lens fragments were dislocated posteriorly that were removed later by central vitrectomy. In the middle of the procedure, if anterior capsular opacified, the vitreous cutter in suction mode would be used to polish it from posteriorly with later central anterior capsulectomy by the vitreous cutter. Foldable sulcus intraocular lens is implanted.
In children after the anterior approach, a pars plicata entry can be created to access the posterior capsule for creation of a posterior vitectorhexis in pediatric patients as done by Mistr et al.50
Results
Comparing to 1.1% rate of posterior capsule rupture in cataract surgery.51 The rate of posterior capsule rupture in posterior polar cataract cases ranged from 0–40%.1,4,5,36,37,42,52,53 Das et al. found a higher incidence of posterior capsular rupture in younger patient (⩽40 years) (55% vs 22%, p = 0.005).37 On the other hand, Osher et al. did not find a relation between the capsular rupture and age, sex, or family history.4 Siatiri and Moghimi53 reported no posterior capsule tear in their study and attributed this to three factors: first, using a modified surgical hydrodissection free phacoemulsification technique; second, paying great deal of attention to the floppy posterior capsule; third, avoiding direct dissection of the polar opacity from the posterior capsule during surgery. Kumar et al.54 looked at the relationship of the size of lens opacity with the surgical outcome. In eyes with posterior polar opacity 4 mm or more, the incidence of posterior capsule rupture was 30.43% (7/23) whereas in eyes with less than 4 mm size the incidence was only 5.71% (2/35) with statistically significant difference (p = 0.039). This finding suggests that one should be more careful while operating larger polar opacities. Another option in this category is to go directly to posterior approach as suggested by Hayashi et al.36 Vasavada and Singh measured corneal pachymetry after the surgery and in all patients, corneal thickness returned to normal between 1st and 3rd month.5 In the same study, specular microscopy results revealed a mean percentage loss of 16.5% at one year after surgery, which is greater than in standard phacoemulsification.55 However, hexagonality and variance in cell size were not affected adversely.
Table 1 summarizes the results of different studies that were published in the management of posterior polar cataract.
Table 1.
Results of published articles in the management of posterior polar cataract.
| Author | Type of study | Year | Number of eyes | Mean follow up (months) | Preop VA | Post op VA | Mean age (years) | Rate of posterior capsule rupture | IOL placement | Anterior vitrectomy | Dropped nucleus | Comments | Technique | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Siatiri and Moghimi | Prospective | 2006 | 23 patients 38 eyes | 9.5 (2–20) | 20/76 | 20/25 | 33.5 (19–65) | None | In the bag | None | None | – | Leaving the plaque for later Yag | |||
| Haripriya et al. | Technique | 2006 | 8 eyes | – | – | – | 23–54 | 12.5% | In the bag if no complicated In the sulcus with optic capture if complicated |
None | None | Amblyopia 8 eyes (21%) Retinitis pigmentosa with macular degeneration 2 eyes (5.2%) Posterior capsule plaque left for later YAG in 7 eyes (18.4%) |
Bimanual microphaco | |||
| Schroeder | Retrospective | 2005 | 13 eyes | 48 | 20/125 | 20/34 | 4 (median) (0.1–12) | Intentionally done for all | In the bag except 2 newborns left aphakic | Intentionally done in all | None | Occlusion therapy preceded the surgery (2 weeks to 3 years) | Bimanual irrigation aspiration | |||
| Lee and Lee | Retrospective | 2003 | 31 patients 36 eyes | – | – | 94.4% 20/40 or better | – | 11.1% | Not mentioned | Bimanual vitrectomy | None | – | Lambda technique with dry aspiration | |||
| Hayashi et al. | Retrospective | 2003 | 28 eyes | Not mentioned | 20/133 | 20/22 | 49.6 ± 16.4 (23 to 74) | 7.1% | In the bag for small opacities (<4 mm) In the sulcus after PPL if >4 mm with soft nucleus Sclera sutured after ICCE if >4 mm with hard nucleus |
– | 1 eye with large polar opacity then PPV done but later developed RD | – | Phaco if opacity <4 mm and soft nucleus PPV, PPL if opacity >4 mm and soft nucleus ICCE and scleral fixated IOL if opacity >4 mm and hard nucleus |
|||
| Taskapili et al. | Retrospective | 2007 | 23 eyes | 11 cortical viscodissection | 35.4 ± 12.4 | 72% 20/80 or better | 100% 20/80 or better | 54.8 ± 14.5 (38–80) | 9.1% | Bag or sulcus | Done if complicated | None | Amblyopia 4 eyes (14.3%) Foveal dysfunction post RD 2 eyes (7.1%) Macular degeneration 1 eye (3.6%) 9 eyes (32.1%) had the plaque left for later YAG capsulotmy |
Viscodissection | ||
| 12 no cortical viscodissection | 34.3 ± 13.4 | 66.6% 20/80 or better | 100% 20/80 or better | 50.4 ± 7.4 (33–62) | 41.6% | No viscodissection | ||||||||||
| Ghosh and Kirkby (posterior approach) | Prospective | 2008 | 11 eyes 8 patients | 13 | 20/40 | 20/20 | 49.7% | Intentional for all | Sulcus | Posterior vitrectomy | – | – | 3 ports PPL, PPV | |||
| Vasavada and Singh | Prospective | 1999 | 25 eyes | 13.72 (7–11) | Not mentioned | 72% were 20/30 or better before YAG 96% had a final VA of 20/30 or better | 52 (17–75) | 36% | In the bag if no rupture In the sulcus if capsule ruptured (except one case in the bag) |
2 port vitrectomy | None | 1 ruptured capsule case developed ME 3 weeks post op 1 microcornea did not improve beyond 20/120 No IOP rise No retinal break |
Low parameters phaco | |||
| Osher et al. | Retrospective | 1990 | 31 eyes 22 patients | 7.2 (1 week to 32 months) | – | 96.7% 20/40 or better | 53 (22–81) | 26% | In the bag in 25 eyes In the sulcus in 6 eyes |
4 cases out of 8 complicated cases needed vitrectomy | None | 6 patients left with plaques 4 of which underwent later YAG ’2 eyes IOL decentration (1 reposited surgically) | Low parameters phaco | |||
| Nagappa et al. | Technique | 2011 | 17 eyes | – | – | 20/28 | 54.18 ± 10.54 | 6% | In the bag except 1 case in the sulcus | Done in 1 case | – | – | Modified epinucleus removal | |||
| Salahuddin | Prospective | 2010 | 28 eyes 22 patients | 8 (2–24) | 14.3% 20/40 or better | 85.7% 20/40 or better | 46 (25–71) | 7.1% | Al in the bag except 2 | None | 1 eye | Two patients left with plaque | Inverse horse-shoe technique | |||
| Kumar et al. | Retrospective | 2010 | 51 patients 58 eyes | 35 < 4 mm | 15.4 (12–40) | Not mentioned | 94.8% were 20/40 or better | 97.14% | 56.6 ± 2.4 | 15.51% | 5.71% | In the bag except 1 | – | None | Amblyopia 10.7% ARMD3.6% | Standard phacoemulsification |
| 23 > 4 mm | 91.3% | 30.43% | ||||||||||||||
| Das et al. | Retrospective | 2008 | 81 eyes 59 patients | 9 (2–70) | 22% 20/30 or better | 93.8% 20/30 or better | 46 (17–65) | 31% | 74% in the bag 26% in the sulcus | Not mentioned | 2 eyes | 2 eyes had YAG | Chip and flip for soft cataracts. Stop and chop for hard cataracts. | |||
| Vajpayee et al. | Interventional case series | 2008 | 8 eyes | Not mentioned | 20/140 | 20/26 | 43.75 ± 2.5 | 100% (pre-existing) | In the bag | None | None | 2 eyes PCO ’2 eyes CME, 1 eye amblyopia | Layer-by-layer phacoemulsification | |||
| Mistr et al. | Retrospective | 2008 | 30 eyes | More than 4 weeks | 12% 20/40 or better | 84% 20/40 or better | 6.36 ± 2.95 (0.82–15.96) | 89% intentionally 1 eye accidentally at IOL insertion | 96% in the bag | Not mentioned | None | 1 eye CME | Standard lens aspiration Pars plicata posterior vitectorhexis |
|||
Complications
Beside the high rate of posterior capsular rupture during surgery, there were reports of spontaneous rupture of posterior capsule in posterior polar cataract unilaterally and bilaterally.56,57 The patient in Ho et al. report had unilateral spontaneous rupture with posterior dislocation of the crystalline lens in the vitreous cavity. There was no history of trauma or other systemic diseases. They proposed that an increase in the size of the lens from nuclear sclerosis may cause increasing pressure on the posterior capsule with subsequent rupture.56 As their case did not have nuclear sclerosis, Ashraf et al. related the bilateral spontaneous rupture in their patient to the increase in the lens size due to the growth of crystalline lens that was shown to be linear throughout life58 posterior polar cataract is known to be predisposed to traumatic lens rupture. Skalka reported one instance of traumatic posterior capsule rupture with posterior polar cataract.59
Other reported complications of the surgery included retinal detachment, nucleus drop, macular edema.5,36,45,47,49,54
Macular edema was reported after surgery.5,45,47 it was noticed when there was a capsular defect5,45 although one case reported without the presence of complications.47
Amblyopia was blamed to be responsible for unsatisfactory visual improvement after surgery in 21%.53
Cases that underwent later YAG capsulotomy were not reported to experience any adverse events (e.g. retinal detachment, refractory uveitis, increased intraocular pressure).
Conclusion
Posterior polar cataract is a true challenge for cataract surgeons associated with a higher risk for surgical complication. Different techniques have been described to minimize this risk and improve its final outcome. All these techniques reflect that this type of cataract needs more gentle maneuvering with avoidance of chamber collapse or overinflation, low parameters, avoidance of hydrodissection, nucleus rotation, posterior capsule polishing, and excessive intraocular lens manipulation during surgery and should not be performed with same techniques as any standard cataract surgery. For larger opacities (>4 mm), one might consider going directly to posterior approach. Genetic studies have revealed the responsible genes in certain populations and this should guide the proper counseling of the patients in addition to screening the family members at risk.
References
- 1.Lee M.W., Lee Y.C. Phacoemulsification of posterior polar cataracts—a surgical challenge. Br J Ophthalmol. 2003;87:1426–1427. doi: 10.1136/bjo.87.11.1426-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masket S. Consultation section: Cataract surgical problem. J Cataract Refract Surg. 1997;23:819–824. [PubMed] [Google Scholar]
- 3.Vogt G., Horvath-Puho E., Czeizel E. A population-based case-control study of isolated congenital cataract. Orv Hetil. 2006;147(23):1077–1084. [PubMed] [Google Scholar]
- 4.Osher R.H., Yu B.C., Koch D.D. Posterior polar cataracts: a predisposition to intraoperative posterior capsular rupture. J Cataract Refract Surg. 1990;16:157–162. doi: 10.1016/s0886-3350(13)80724-9. [DOI] [PubMed] [Google Scholar]
- 5.Vasavada A.R., Singh R. Phacoemulsification with posterior polar cataract. J Cataract Refract Surg. 1999;25:238–245. doi: 10.1016/s0886-3350(99)80133-3. [DOI] [PubMed] [Google Scholar]
- 6.Gifford S.R. Congenital anomalies of the lens as seen with the slit lamp. Am J Ophthalmol. 1924;7:678–685. [Google Scholar]
- 7.Cordes FC. Types of congenital and juvenile cataracts. In GM, editor. Symposium on Diseases and Surgery of the lens. St. Louis, CV Mosby; 1957. p. 43–50.
- 8.Greeves R.A. Two cases of microphthalmia. Trans Ophthalmol Soc UK. 1914;34:289–300. [Google Scholar]
- 9.Szily A.V. The Doyne Memorial Lecture: the contribution of pathological examination to elucidation of the problems of cataract. Trans Ophthalmol Soc UK. 1938;58:595–660. [Google Scholar]
- 10.Addison P.K., Berry V., Ionides A.C., Francis P.J., Bhattacharya S.S., Moore A.T. Posterior polar cataract is the predominant consequence of a recurrent mutation in the PITX3 gene. Br J Ophthalmol. 2005;89(2):138–141. doi: 10.1136/bjo.2004.053413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eshaghian J., Streeten B.W. Human posterior subcapsular cataract; an ultrastructural study of the posterioly migrating cells. Arch Ophthalmol. 1980;98:134–143. doi: 10.1001/archopht.1980.01020030136016. [DOI] [PubMed] [Google Scholar]
- 12.Nagata M., Matsuura H., Fujinaga Y. Ultrastucture of posterior subcapsular cataract in the human lens. Ophthalmic Res. 1986;18:180–184. doi: 10.1159/000265430. [DOI] [PubMed] [Google Scholar]
- 13.Eshajian J. Human posterior subcapsular cataracts. Trans Ophthalmol Soc UK. 1982;102:364–368. [PubMed] [Google Scholar]
- 14.Hejtmancik JF, Datilles M. Congenital and inherited cataracts. In: Tasman W, Jaeger EA, editors. Duane’s clinical ophthalmology. CD ROM ed. Baltimore, Md.: Lippincott Williams & Wilkins; 2001; p. 1 [chapter 74].
- 15.Bernheimer S. Koennthis des angeborenen LinteronStares des Menschen. Arch Augen Leilkd. 1977;74:8–12. [Google Scholar]
- 16.Eshagian J. Human posterior subcapsular cataracts. Trans Ophthalmol Soc UK. 1982;102(Pt 3):364–368. [PubMed] [Google Scholar]
- 17.Yamada K., Tomita H.A., Kanazawa S., Mera A., Amemiya T., Niikawa N. Genetically distinct autosomal dominant posterior polar cataract in a four-generation Japanese family. Am J Ophthalmol. 2000;129(2):159–165. doi: 10.1016/s0002-9394(99)00313-x. [DOI] [PubMed] [Google Scholar]
- 18.Tulloh C.G. Hereditary posterior polar cataract with report of a pedigree. Br J Ophthalmol. 1955;39(6):374–379. doi: 10.1136/bjo.39.6.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ionides A.C., Berry V., Mackay D.S., Moore A.T., Bhattacharya S.S., Shiels A. A locus for autosomal dominant posterior polar cataract on chromosome 1p. Hum Mol Genet. 1997;6:47–51. doi: 10.1093/hmg/6.1.47. [DOI] [PubMed] [Google Scholar]
- 20.Bidinost C., Matsumoto M., Chung D. Heterozygous and homozygous mutations in PITX3 in a large Lebanese family with posterior polar cataracts and neurodevelopmental abnormalities. Invest Ophthalmol Vis Sci. 2006;47(4):1274–1280. doi: 10.1167/iovs.05-1095. [DOI] [PubMed] [Google Scholar]
- 21.Summers K.M., Withers S.J., Gole G.A., Piras S., Taylor P.J. Anterior segment mesenchymal dysgenesis in a large Australian family is associated with the recurrent 17 bp duplication in PITX3. Mol Vis. 2008;14:2010–2015. [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang T., Hua R., Xiao W. Mutations of the EPHA2 receptor tyrosine kinase gene cause autosomal dominant congenital cataract. Hum Mutat Mayn. 2009;30(5):E603–E611. doi: 10.1002/humu.20995. [DOI] [PubMed] [Google Scholar]
- 23.Berry V., Francis P., Reddy M.A., Collyer D., Vithana E., MacKay I., Dawson G., Carey A.H., Moore A., Bhattacharya S.S., Quinlan R.A. Alpha-B crystallin gene (CRYAB) mutation causes dominant congenital posterior polar cataract in humans. Am J Hum Genet. 2001;69:1141–1145. doi: 10.1086/324158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu M., Ke T., Wang Z., Yang Q., Chang W., Jiang F., Tang Z., Li H., Ren X., Wang X., Wang T., Li Q., Yang J., Liu J., Wang Q.K. Identification of a CRYAB mutation associated with autosomal dominant posterior polar cataract in a Chinese family. Invest Ophthalmol Vis Sci. 2006;47:3461–3466. doi: 10.1167/iovs.05-1438. [DOI] [PubMed] [Google Scholar]
- 25.Semina E.V., Ferrell R.E., Mintz-Hittner H.A., Bitoun P., Alward W.L., Reiter R.S., Funkhauser C., Daack-Hirsch S., Murray J.C. A novel homeobox gene PITX3 is mutated in families with autosomal-dominant cataracts and ASMD. Nat Genet. 1998;19:167–170. doi: 10.1038/527. [DOI] [PubMed] [Google Scholar]
- 26.Berry V., Yang Z., Addison P.K., Francis P.J., Ionides A., Karan G., Jiang L., Lin W., Hu J., Yang R., Moore A., Zhang K., Bhattacharya S.S. Recurrent 17 bp duplication in PITX3 is primarily associated with posterior polar cataract (CPP4) J Med Genet. 2004;41:e109. doi: 10.1136/jmg.2004.020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burdon K.P., McKay J.D., Wirth M.G. The PITX3 gene in posterior polar congenital cataract in Australia. Mol Vis. 2006;12:367–371. [PubMed] [Google Scholar]
- 28.Pras E., Mahler O., Kumar V., Frydman M., Gefen N., Pras E., Hejtmancik J.F. A new locus for autosomal dominant posterior polar cataract in Moroccan Jews maps to chromosome 14q22-23. J Med Genet. 2006;43:e50. doi: 10.1136/jmg.2005.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maumenee I.H. Classification of hereditary cataracts in children by linkage analysis. Ophthalmology. 1979;86:1554–1558. doi: 10.1016/s0161-6420(79)35365-9. [DOI] [PubMed] [Google Scholar]
- 30.Luntz MH. Clinical types of cataracts. Duane’s Ophthalmology. CD ROM. ed. Baltimore, M d.: Lippincott Williams & Wilkins; 2006.
- 31.Duke-Elder S. Congenital deformities. Part 2. Normal and Abnormal Development. System of Ophthalmology; vol. III. St. Louis: CV Mosby; 1964.
- 32.Primrose D.A. A slowly progressive degenerative condition characterized by mental deficiency, wasting of the limb musculature, and bone abnormalities, including ossification of the pinnae. J Ment Defic Res. 1982;26:101–106. doi: 10.1111/j.1365-2788.1982.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 33.Harman N.B. New pidegrees of cataract-posterior polar, anterior polar and microphthalmia, and lamellar. Trans Ophthalmol Soc UK. 1909;29:296–306. [Google Scholar]
- 34.Singh D., Worst J., Singh R., Singh I.R. Jaypee Brothers Medical Publishers; New Delhi, India: 1993. Cataract and IOL. p. 163–5. [Google Scholar]
- 35.Schroeder H.W. The management of posterior polar cataract: the role of patching and grading. Strabismus. 2005;13(4):153–156. doi: 10.1080/09273970500387753. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi K., Hayashi H., Nakao F. Outcomes of surgery for posterior polar cataract. J Cataract Refract Surg. 2003;29:45–49. doi: 10.1016/s0886-3350(02)01692-9. [DOI] [PubMed] [Google Scholar]
- 37.Das S., Khanna R., Mohiuddin S.M., Ramamurthy B. Surgical and visual outcomes for posterior polar cataract. Br J Ophthalmol. 2008;92(11):1476–1478. doi: 10.1136/bjo.2007.129403. [DOI] [PubMed] [Google Scholar]
- 38.Haripriya A., Aravind S., Vadi K., Natchiar G. Bimanual microphaco for posterior polar cataracts. J Cataract Refract Surg. 2006;32(6):914–917. doi: 10.1016/j.jcrs.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 39.Pong J., Lai J. Managing the hard posterior polar cataract. J Cat Refract Surg. 2008;34:530–531. doi: 10.1016/j.jcrs.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 40.Fine I.H., Packer M., Hoffman R.S. Management of posterior polar cataract. J Cataract Refract Surg. 2003;29:16–19. doi: 10.1016/s0886-3350(02)01616-4. [DOI] [PubMed] [Google Scholar]
- 41.Vasavada A.R., Raj S.M. Inside-out delineation. J Cataract Refract Surg. 2004;30:1167–1169. doi: 10.1016/j.jcrs.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 42.Salahuddin Inverse horse-shoe technique for the phacoemulsification of posterior polar cataract. Can J Ophthalmol. 2010;Apr 45(2):154–156. doi: 10.3129/i09-231. [DOI] [PubMed] [Google Scholar]
- 43.Lim Z., Goh J. Modified epinucleus pre-chop for the dense posterior polar cataract. Ophthalmic Surg Lasers Imaging. 2008;39(2):171–173. doi: 10.3928/15428877-20080301-11. [DOI] [PubMed] [Google Scholar]
- 44.Chee S.P. Management of the hard posterior polar cataract. J Cataract Refract Surg. 2007;33(9):1509–1514. doi: 10.1016/j.jcrs.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 45.Vajpayee R.B., Sinha R., Singhvi A., Sharma N., Titiyal J.S., Tandon R. ‘Layer by layer’ phacoemulsification in posterior polar cataract with pre-existing posterior capsular rent. Eye (Lond) 2008;22(8):1008–1010. doi: 10.1038/sj.eye.6702795. [DOI] [PubMed] [Google Scholar]
- 46.Allen D., Wood C. Minimizing risk to the capsule during surgery for posterior polar cataract. J Cataract Refract Surg. 2002;28:742–744. doi: 10.1016/s0886-3350(02)01244-0. [DOI] [PubMed] [Google Scholar]
- 47.Taskapili M., Gulkilik G., Kocabora M.S., Ozsutcu M. Phacoemulsification with viscodissection in posterior polar cataract: minimizing risk of posterior capsule tear. Ann Ophthalmol (Skokie) 2007;39(2):145–149. doi: 10.1007/s12009-007-0004-y. [DOI] [PubMed] [Google Scholar]
- 48.Nagappa S., Das S., Kurian M., Braganza A., Shetty R., Shetty B. Modified technique for epinucleus removal in posterior polar cataract. Ophthalmic Surg Lasers Imaging. 2011;42(1):78–80. doi: 10.3928/15428877-20101025-01. [DOI] [PubMed] [Google Scholar]
- 49.Kumar V., Ghosh B., Kaul U., Thakar M., Goel N. Posterior polar cataract surgery: a posterior segment approach. Eye (Lond) 2009;23(9):1879. doi: 10.1038/eye.2008.347. [DOI] [PubMed] [Google Scholar]
- 50.Mistr S.K., Trivedi R.H., Wilson M.E. Preoperative considerations and outcomes of primary intraocular lens implantation in children with posterior polar and posterior lentiglobus cataract. J AAPOS. 2008;12(1):58–61. doi: 10.1016/j.jaapos.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 51.Osher R.H., Cionni R.J. The torn posterior capsule: its intraoperative behavior, surgical management, and long-term consequences. J Cataract Refract Surg. 1990;16(4):490–494. doi: 10.1016/s0886-3350(13)80805-x. [DOI] [PubMed] [Google Scholar]
- 52.Gavriş M., Popa D., Cărăuş C., Gusho E., Clocoţan D., Horvath K., Ardelean A., Sângeorzan D. Phacoemulsification in posterior polar cataract. Oftalmologia. 2004;48(4):36–40. [PubMed] [Google Scholar]
- 53.Siatiri H., Moghimi S. Posterior polar cataract: minimizing risk of posterior capsule rupture. Eye (Lond) 2006;20(7):814–816. doi: 10.1038/sj.eye.6702023. Epub 2005 Oct 28. [DOI] [PubMed] [Google Scholar]
- 54.Kumar S., Ram J., Sukhija J., Severia S. Phacoemulsification in posterior polar cataract: does size of lens opacity affect surgical outcome? Clin Experiment Ophthalmol. 2010;38(9):857–861. doi: 10.1111/j.1442-9071.2010.02354.x. [DOI] [PubMed] [Google Scholar]
- 55.Hayashi K., Nakao F., Hayashi F. corneal endothelial cell loss after phacoemulsification using nuclear cracking procedure. J Cataract Refract Surg. 1994;20:44–47. doi: 10.1016/s0886-3350(13)80042-9. [DOI] [PubMed] [Google Scholar]
- 56.Ho S.F., Ahmed S., Zaman A.G. Spontaneous dislocation of posterior polar cataract. J Cataract Refract Surg. 2007;33(8):1471–1473. doi: 10.1016/j.jcrs.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 57.Ashraf H., Khalili M.R., Salouti R. Bilateral spontaneous rupture of posterior capsule in posterior polar cataract. Clin Experiment Ophthalmol. 2008;36(8):798–800. doi: 10.1111/j.1442-9071.2008.01889.x. [DOI] [PubMed] [Google Scholar]
- 58.Augusteyn R.C. Growth of the human eye lens. Mol Vis. 2007;13:252–257. [PMC free article] [PubMed] [Google Scholar]
- 59.Skalka H.W. Ultrasonic diagnosis of posterior lens opacity. Ophthalmic Surg. 1977;8(6):72–76. [PubMed] [Google Scholar]


