Abstract
Purpose
To analyze the anatomical and functional outcome of glued intra ocular lens (IOL) implantation technique and its modifications.
Design
Retrospective observational case series.
Methods
This is a retrospective analysis of the patients who underwent glued intra ocular lens implantation from December 2007 to August 2010. Post operative uncorrected vision, best corrected visual acuity (BCVA), intra ocular pressure, IOL position, endothelial cells and anterior chamber reaction at their last follow up are analyzed from their concerned case sheets documentation. Subjective changes are analyzed via individual questionnaire. Immediate and late postoperative complications are also evaluated. Surgical modifications and the difference in the operated eyes are analyzed.
Results
Total 210 glued IOL eyes have been reviewed. Out of this 210, there are 152 (PMMA glued IOL), 21 (three piece foldable IOL), 5 (multifocal foldable IOL), 12 (pediatric glued IOL), 5 (20G sutureless vitrectomy), 2 (glued iris prosthesis) and 3 (transposition of posterior chamber IOL into anterior chamber). In combined surgeries there are, 5 (optical penetrating keratoplasty), 2 (descemet’s stripping endothelial keratoplasty) and 3 (iridoplasty). The modifications in glued IOL are handshake technique, injectable or foldable glued IOL, Multifocal glued IOL and intraoperative 23/25G trocar cannula infusion. Decentration (1.97%), macular edema (1.97%) and optic capture (2.63%) are the post operative complications encountered in rigid glued IOL. Good patient satisfaction is seen in the eyes with IOL repositioning, IOL exchange and multifocal glued IOL. There is significant improvement in BCVA in PMMA IOL (p = 1.35 × 10−5) and foldable IOL (p = 0.000).
Conclusion
Glued IOL seems to be a good alternative in IOL implantation in eyes with deficient capsules. The modifications in the existing technique decrease the learning time and risk for complications.
Keywords: Glued IOL, Sutureless IOL implantation, Injectable glued IOL, Handshake technique, Foldable glued IOL, Injectable glued IOL, 20G Sutureless vitrectomy with IOL repositioning
1. Introduction
Intraocular lens implantation (IOL) in the eyes that lack posterior capsular support is a problem for cataract surgeons for a long time. It is not only due to the visual outcome but also to the related complications they face in the post operative period. The usual modalities accomplished in the past are the iris fixated IOL (Zeh and Price, 2000; Lorencova et al., 2007), anterior chamber (AC) IOL Bergman and Laatikainen, 1996, 1997; Rattigan et al., 1996; Collins et al., 2003; Donaldson et al., 2005, sutured and sutureless trans-scleral fixated IOL (Solomon et al., 1993; Asadi and Kheirkhah, 2008; Parekh et al., 2007; Gabor and Pavilidis, 2007; Maggi and Maggi, 1997) Surgical expertise, prolonged surgical time, suture induced inflammation, suture degradation and delayed IOL subluxation or dislocation due to broken suture are some of the limitations in the sutured scleral fixated intraocular lenses (SFIOL) Price et al., 2005; Jongebloed and Worst, 1986; Hayashi et al., 1999; Biro, 1993; Heilskov et al., 1989. Though these complications are not encountered with AC IOL’s, endothelial loss, corneal decompensation and recurrent uveitis are often seen (Numa et al., 1993; Bergman et al., 1993). In 2007, we introduced the new technique (Glued IOL) (Agarwal et al., 2008, 2009) of placing a posterior chamber IOL in the eyes with deficient posterior capsule using a quick acting surgical fibrin sealant. In this article we have reviewed the surgical results (anatomical and functional), modifications, indications, limitations, complications and results of the glued IOL technique (Kumar et al., 2010a,b,c,d; Prakash et al., 2009a,b; Nair et al., 2009; Prakash et al., 2010a; Agarwal et al., 2010; Jacob et al., 2010; Prakash et al., 2010b).
2. Materials and methods
This is a retrospective analysis of the patients who underwent glued intra ocular lens implantation for various indications from December 2007 to August 2010. All patients have been explained about the retrospective analysis and informed consent has been taken. The study has been approved by our Institutional Review Board (IRB). Patients with glued IOL, with regular follow up are included and patients with irregular follow up have been excluded. Post operative uncorrected vision, best corrected visual acuity (BCVA) in Snellen visual acuity charts, Intra ocular pressure (non contact tonometer), IOL position (ultra sound biomicroscopy and anterior segment optical coherence tomography), endothelial cells (Topcon specular microscopy) and anterior chamber reaction (slit lamp microscopy) at their last follow up are analyzed from their concerned case sheets documentation. Subjective changes are analyzed via individual questionnaire. Immediate and late postoperative complications are also evaluated. Surgical modifications and the difference in the operated eyes are analyzed.
2.1. Surgical technique
Under peribulbar anesthesia, localized peritomy at the site of exit of the IOL haptics is done. Infusion cannula or anterior chamber (AC) maintainer is inserted. Positioning of the infusion cannula should be in the pars plana about 3 mm from the limbus. Anterior segment surgeons can use an AC maintainer or 23G trocar cannula infusion. Two partial thickness limbal based scleral flaps about 2.5 mm × 2.5 mm are created exactly 180° diagonally (use RK marker & pen) apart and about 1–1.5 mm from the limbus. This is followed by vitrectomy via pars plana or anterior route to remove all vitreous traction. Two straight sclerotomies with a 20G needle are made about 1 mm from the limbus under the existing scleral flaps. A corneo-scleral tunnel incision is then prepared for introducing the IOL in the case of PMMA non foldable IOL or corneal incision with keratome in the case of injectable three piece foldable IOL. While the IOL is being introduced with one hand, an end gripping 23G micro rhexis forceps (Micro Surgical Technology, USA) is passed through the opposite sclerotomy with the other hand. The tip of the leading haptic is then grasped with the micro rhexis forceps, pulled through the sclerotomy following the curve of the haptic and is externalized under the scleral flap. Similarly, the trailing haptic is also externalized through the other sclerotomy under the scleral flap.
The haptic tips are tucked into the intralamellar scleral tunnel made with a 26G needle at the point of externalization of the haptics on either side. Then, the reconstituted fibrin glue prepared is injected through the cannula of the syringe delivery system under the scleral flaps. Local pressure is given over the flaps for about 10–20 s for the formation of fibrin polypeptides. Corneo scleral wound is closed with 10–0 monofilament nylon in PMMA IOL and in the case of foldable IOL corneal incision is closed with fibrin glue. The anterior chamber maintainer or the infusion cannula is removed. Conjunctiva is closed with the fibrin glue in all eyes irrespective of the type of IOL.
3. Modifications
3.1. Scleral tucking
Scleral tucking has increased the stability of the IOL tremendously. Although complete scleral wound healing with collagen fibrils may take up to 3 months, since the haptic is snugly placed inside a scleral pocket, the IOL remains stable (Lee et al., 1995; Agarwal et al., 2009). The longitudinal and the transverse mobilities are limited with tucking. A 26G needle is used for making the intrascleral intralamellar tunnel and the IOL haptic tip is gently inserted along the tunnel without undue pressure. The length of the tucking depends upon the overall diameter of the IOL. Moreover, scleral tucking helps in manipulating the centeration of the IOL.
3.2. Foldable glued IOL
Though in the initial cases the technique was performed with PMMA IOL’s, subsequently we have moved into foldable or injectable glued IOL procedures. The IOL is injected through the corneal wound, the end opening forceps (Fig. 1A–C) is passed through the initial sclerotomy and the leading haptic is caught. The available injectors for the three piece IOL’s have either a pushing or a screwing method for unfolding the IOL. In an injector with pushing mechanism, one hand pushes the injector plunger while the other hand holds the end opening forceps ready to grab the tip of the haptic as it comes out of the injector. This prevents the IOL from falling into the vitreous cavity. One should always remember that in a three piece IOL it is better not to do wound assisted injection. Rather one should pass the cartridge into the anterior chamber and inject as that way the IOL cannot break.
Figure 1.
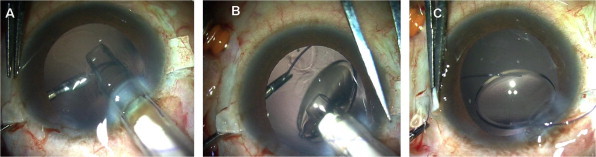
Injectable glued IOL technique: (A) IOL is injected via the corneal wound, (B) Leading haptic tip is received by the forceps, (C) Haptic tip is externalized under the scleral flaps.
3.3. Multifocal glued IOL
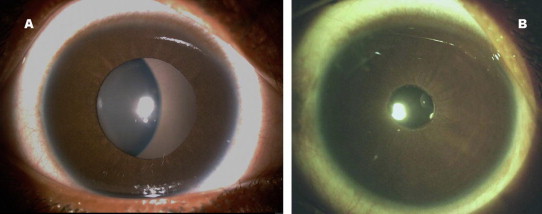
Glued IOL procedure can be performed with multifocal (diffractive or refractive) IOL’s. It is well known that good centeration is mandatory in multiple optics IOL’s to provide the best corrected vision without optical disturbances. Serial digital slit lamp images of the eye with full pupillary dilatation showed good IOL centeration (Fig. 2). An image processing with Matlab version 7.1 (Mathworks, Inc) is done to quantify decentration. Ultrasound biomicrocopy can also be used to note the position or tilt of IOL on each visit.
Figure 2.
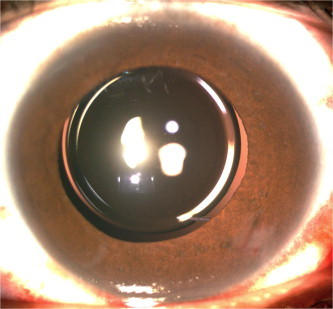
Clinical picture of a well centered multifocal IOL.
The steps to follow in a multifocal IOL:
-
1.
Scleral flaps should be exactly 180° (use RK marker & pen).
-
2.
Scleral tucking should be manipulated to bring perfect centeration.
-
3.
Proper white to white diameter measurement on table for optic diameter.
-
4.
Preoperative angle kappa can be taken into consideration.
3.4. 23/25G trocar cannula infusion
The trocar infusion kit (Optikon, Italy) is separately available (Kumar et al., 2010c) and can be used by anterior segment surgeons in special situations. It contains a scleral guide, inserter and an infusion cannula. The scleral guide is inserted into the pars plana about 3 mm from the limbus with the help of the inserter and the inserter is removed. Then the infusion cannula connected to the infusion bottle is inserted. Similarly while removal, the infusion is switched off and the scleral guide removed. No suture is applied in the sclerotomy site. It was noted that the surgical time taken for fixing the infusion cannula is reduced with 23G infusion. It is also safe and easy in the hands of the anterior segment surgeons as one need to just insert the trocar and fix the infusion cannula. One should always check if the tip of the infusion cannula is in the vitreous cavity before the infusion is started. If the pupil is miotic then an iris retractor can be used to retract the iris to check if the infusion cannula is in the vitreous cavity. The direct visualization of the cannula during entrance and exit decreases the risk of complications.
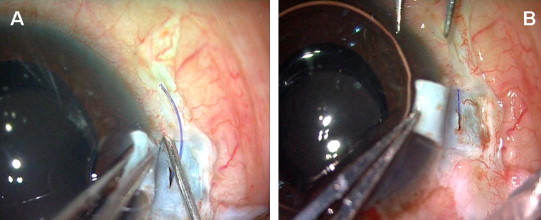
3.5. Handshake technique
In the “Handshake” technique (Fig. 3), the end opening forceps is passed through the opposite sclerotomy site while another forceps is in the pupillary plane ready to receive the haptic. Any portion of the leading haptic is initially grasped with the forceps. Now the other forceps starts grabbing the haptic like a hand shake and this process is continued till the tip of the haptic is held by forceps. The haptic is then pulled under the scleral flap. Similarly the trailing haptic is externalized. Thus both the haptics are externalized under the scleral flaps. Difficulty occurs if the screwing mechanism injector is used, where both hands are used to inject the IOL. In such a case one should follow the “Handshake” technique. Also in case of difficult situation where the leading haptic tip is not visualized in the pupillary plane or in a non dilating pupil where the iris overlies the haptic, this “Handshake” technique is performed.
Figure 3.
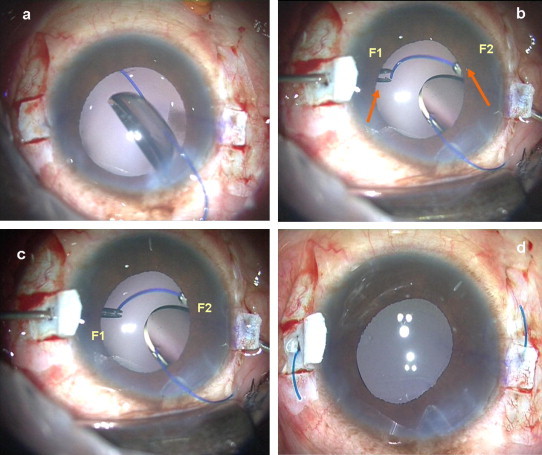
Handshake technique. (a) Foldable IOL haptic is below the iris, (b) One end opening forceps (F2) is passed through the opposite sclerotomy site while other forceps (F1) is ready to receive the haptic, (c) The leading haptic is grasped with forceps (F2) and the haptic tip is fed into another forceps (F1), (d) Both the haptics externalized under the scleral flaps.
3.6. 20G sutureless vitrectomy for dislocated IOL (Kumar et al., 2010b)
Localized peritomy and wet cautery of the sclera at 3, 9 and 7 o’clock is performed. Two partial thickness limbal based scleral flaps (f1, f2) 2.5 mm × 2.5 mm are created exactly 180° diagonally apart and 1 mm from the limbus. A third scleral flap (f3) is made about 2 mm from the limbus. A pars plana sclerotomy about 3 mm from the limbus is made with a 20G needle under the scleral flap f3. A polyglactil 6–0 suture is placed and a 4 mm infusion cannula connected to 500 ml bottle of balanced salt solution is inserted through the sclerotomy. Infusion cannula with a halogen light source (Chandelier illumination) can also be used. The fluid flow is started after the visualization of the tip of infusion cannula in the vitreous cavity. Two straight sclerotomies with a 20G needle are made about 1 mm from the limbus under the existing scleral flaps (f1, f2). Accurus® 400 VS (Alcon Laboratories, Inc., Fort Worth, TX) vitrectomy system is used for posterior vitrectomy. The posterior vitreous detachment is induced mechanically using suction of the 20 gauge (20G) vitrectomy probe. A thorough vitrectomy to free all the IOL attachments is done with 20G vitrectomy probe and endoilluminator. When the vitreous tractions are released, a diamond coated 20G intravitreal forceps (Grieshaber, Alcon, Fort Worth, Tex., USA) is used to hold the haptic tip. The IOL is gently lifted up to bring it at the level of the sclerotomy sites. The intravitreal forceps (holding the haptic) is then withdrawn from the sclerotomy site (f1), externalizing the haptic in the process. With the assistant holding the tip of the externalized haptic, the other haptic is pulled through the other sclerotomy (f2) using intravitreal forceps. The tips of the haptic are then tucked through an intralamellar scleral tunnel made with a 26G needle at the point of externalization. The scleral flaps (f1, f2) are then closed with fibrin glue (Tisseel, Baxter, USA). The polyglactil suture and the infusion cannula are subsequently removed and the third scleral flap (f3) is also sealed with the glue. The conjunctiva is also apposed at the peritomy sites with the tissue glue.
3.7. Glued iris prosthesis
The glued iris prosthesis (Kumar et al., 2010d) that we used is a PMMA aniridia IOL of OV lens Style ANI5 (Intra Ocular Care, Gujarat, India). The optic has a central clear zone (clear optic zone) with a peripheral opaque or pigmented annulus and an ‘A’ constant of 118.2. The haptics are also made of PMMA with acute angulations and they have an eyelet for prolene suture placement during trans-scleral fixation. After making the flaps, the haptics are externalized as in regular glued IOL technique and the tips are then inserted in the scleral tunnel, followed by fibrin glue application on the scleral flap bed.
3.8. Repositioning the decentered IOL
Glued IOL technique can be used for recenteration of decentered IOL (Fig. 4). The luxated IOL, decentered sulcus fixated IOL or suture scleral fixated IOL are usually repositioned. In all these conditions the same IOL can be repositioned by glued IOL method. The IOL explantation is prevented, thereby the surgical risk of inflammation, suture induced astigmatism and infection is decreased.
Figure 4.
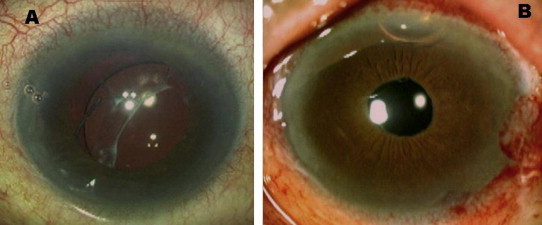
Preoperative picture of decentered IOL (A) and post operative picture (B) showing IOL centeration without explantation.
3.9. Pediatric glued IOL
Nowadays most of the pediatric cataract surgeries are combined with IOL implantation. Ectopia lentis, congenital cataract with luxation or traumatic cataract are often associated with zonular weakness. Pediatric glued IOL in these eyes have shown promising results (Fig. 5). Here in the pediatric eyes, the two straight sclerotomies with a 22G needle are made under the existing scleral flaps about 1 from the limbus and the flaps size is reduced to 2–2.5 mm.
Figure 5.
(A) Ectopia lentis in pediatric eye, (B) one year post operative picture.
3.10. Combined surgeries
Glued IOL procedure can be combined with trabeculectomy, penetrating keratoplasty (PK) (Prakash et al., 2009b), Descemet’s stripping endothelial keratoplasty (DSEK) (Prakash et al., 2010), iridoplasty, anterior segmentation transplantation (AST) (Jacob et al., 2010) and strabismus surgery. Posterior chamber IOL which was malpositioned in the anterior chamber can be repositioned posteriorly with glued IOL technique without explantation (Prakash et al., 2010).
4. Surgical results
Total 210 eyes have been reviewed. Out of this 152 eyes had single piece rigid Glued IOL, 21 eyes had monofocal three piece foldable IOL and 5 eyes had multifocal foldable IOL. There are 12 eyes with pediatric glued IOL, 5 eyes with 20G sutureless vitrectomy, 2 eyes with glued iris prosthesis and 3 eyes with transposition of posterior chamber IOL into anterior chamber. In the combined surgeries, 5 eyes had optical penetrating keratoplasty, 2 eyes had descemet’s stripping and endothelial keratoplasty and 3 eyes had iridoplasty. The mean follow up of 152 eyes with PMMA glued IOL is 9.7 ± 3.2 months. Fifty-nine (38.8%) out of 152 eyes completed ⩾12 months follow up. Hundred and sixteen (76.3%) and 36 (23.68%) eyes out of 152 eyes underwent the surgery as primary and secondary procedure, respectively. The most common indication is posterior capsular rupture with no sulcus support (56%) followed by subluxated cataract (21%). Eight out of 152 eyes underwent IOL exchange, out of which 6 eyes had AC IOL and 2 eyes for sutured scleral fixated IOL. The most common indication for IOL exchange is uveitis and significant improvement noted in the post operative period with subjective questionnaire (Table 1). Single piece PMMA IOL with optic size 6.5 mm and overall diameter of 13 mm is implanted in all the eyes. The mean preoperative UCVA in decimal equivalents is 0.024 ± 0.02 and the mean postoperative UCVA at the last follow up is 0.53 ± 0.26. There is significant improvement in UCVA (Paired t-test p = 1.00 × 10−51). Mean BCVA preoperatively is 0.71 ± 0.26 and is 0.80 ± 0.21 postoperatively (p = 1.35 × 10−5). Seventy-nine out of 152 eyes (52%) gained 20/20 visual acuity at one month postoperatively.
Table 1.
Symptomatic improvement in eyes with IOL exchange over a period follow up (9.7 ± 2.7 months).
| Category score | Symptom scale | Patient history | Pre operative n = 6 | Post operative n = 6 |
|---|---|---|---|---|
| 0 | Asymptomatic | No symptoms | 0 | 5 |
| 1 | Mild | Occasional uveitis responding to medications | 2 | 0 |
| 2 | Moderate | Recurrent uveitis (repeated episode of uveitis separated by periods of inactivity without treatment, in which these periods of inactivity without treatment are at least 3 months in duration) | 2 | 0 |
| 3 | Severe | Persistent uveitis characterized by prompt relapse (in less than 3 months) after discontinuation of therapy. | 2 | 0 |
Multipiece foldable IOL are implanted in 58 eyes, however, results are analyzed only in 21 eyes that finished minimum 6 months follow up. The mean post operative UCVA is 0.26 ± 0.15 and BCVA is 0.51 ± 0.25. There is significant change in both postoperative BCVA and UCVA (Friedman test, p = 0.000). The mean preoperative and one year post operative endothelial densities are 2872.7 ± 216.3 cells/mm2 and 2750.2 ± 194.3 cells/mm2, respectively.
Five eyes of 5 patients underwent sutureless 20G vitrectomy with fibrin glue assisted PC IOL implantation without explantation. The mean preoperative UCVA is 0.08 ± 0.07 and post operative mean UCVA is 0.53 ± 0.13. There is significant difference in the UCVA (p = 0.043).The mean post operative best corrected visual acuity (BCVA) is 0.76 ± 0.22. The mean post operative central foveal thickness measured in spectral domain Optical coherence tomography (OCT) (Cirrus OCT, Carl Zeiss Meditec, Dublin, California, USA) is 281 μ at one year follow up. Ultra sound biomicroscopy with 50 MHz frequency and 50 μ resolution showed no vitreous traction or uveal incarceration in pars plana ports in the post operative period. The serial images taken at the pars plicata region showed good wound closure.
Five eyes of 5 patients underwent multifocal (three refractive and two diffractive) glued IOL procedure. The indication is aphakia in all the 5 eyes. The mean preoperative spectacle BCVA is 0.60 ± 0.25 and the mean postoperative BCVA is 0.77 ± 0.34. There is no significant difference in BCVA (p = 1.000). The postoperative mean additional add for best near corrected vision is 0.5 ± 1.1 D. There is significant decrease in the near addition (p = 0.000). The mean postoperative intra ocular pressure (IOP) as noted with non contact tonometry is 12 ± 1.7 mmHg. There is no significant change in IOP (p = 0.083). The mean postoperative specular count is 2355 ± 277 cells/mm2. Good patient satisfaction is seen as the subjective dysphotopic phenomenon like glare or ghost images are observed to be less.
Twelve eyes of 7 children underwent the glued IOL implantation (Fig. 5A and B). The mean follow up is 12 ± 6.4 months. The mean age is 9.08 ± 4.4. The mean preoperative UCVA is 0.17 ± 0.11. The mean postoperative UCVA is 0.25 ± 0.15. There is significant difference in the UCVA (Friedman test, p = 0.008). The post operative mean best spectacle corrected visual acuity is 0.67 ± 0.27 and there is significant change in BCVA (p = 0.007). There is no correlation between postoperative UCVA and BCVA with age. Amblyopia is diagnosed in 8 out of 12 eyes due to long standing visual deprivation; Occlusion treatment (partial occlusion, minimum 6hours per day of the better eye) has been started in eyes with amblyopia.
5. Complications
Table. 2 gives the complication profile in PMMA IOL’s at one year follow up. In foldable glued IOL, sub conjunctival haptic was seen in 4 eyes. Decentration was seen in one eye and repositioning was done for the same. Resolving vitreous hemorrhage was seen in 3 eyes. No endophthalmitis was seen in any of the eyes. Recurrent uveitis was noted in 4 out of 58 eyes with foldable IOL. Optic capture was observed in 4 PMMA IOL and 3 foldable glued IOL. Chronic macular edema was seen in 3 eyes with PMMA IOL. There was no post operative secondary glaucoma in any of the eyes.
Table 2.
Intra and post operative complications analysis.
| Number of eyes (%) (out of 152) | |
|---|---|
| Mean follow up = 9.7 ± 2.7 months | |
| Intra operative | |
| Hyphema | n = 2 (1.31%) |
| Post operative | |
| Early(<1 month) | |
| Grade 2 anterior uveitis | n = 0 (0%) |
| Decentration | n = 3 (1.97%) |
| Macular edema | n = 3 (1.97%) |
| Late (>1 month) | |
| Optic capture | n = 4 (2.63%) |
| Pigment dispersion | n = 3 (1.97%) |
| Endophthalmitis | n = 0 (0%) |
| Chronic macular edema | n = 3 (1.97%) |
6. Discussion
6.1. Tissue glue
Fibrin glue has been used previously in various medical specialties as a hemostatic agent to arrest bleeding, seal tissues and as an adjunct to wound healing. The commercially available fibrin glue is virus inactivated and is checked for viral antigen and antibodies with polymerase chain reaction; hence the chances of transmission of infection are very low. But with tissue derivatives, there is always a theoretical possibility of transmission of viral infections; therefore, it is mandatory to get informed consent from the patient before the procedure. Though the use of fibrin glue in ophthalmology (Lagoutte et al., 1989; Tilanus et al., 1994, 1995; Grewing and Mester, 1997) is considered off-label, it has been successfully used to repair lacerated canaliculi, to seal full thickness macular holes, to seal cataract incisions, and corneal perforations. It has also been used for temporary closure of scleral flaps after trabeculectomy in eyes with hypotony, conjunctival fistula closure, conjunctival autografts and amniotic membrane transplantation. The commercially available tissue glue we used is Tisseel (Baxter, Deerfield, IL). Tisseel is plasma derived. The commercial product is a two component system. The first component contains highly concentrated fibrinogen, factor XIII and fibronectin. The second component contains thrombin, calcium chloride, and aprotinin. Mixing of the two components promotes the formation and cross-linking of fibrin. Fibrin glue has been shown to provide airtight closure and by the time the fibrin starts degrading, surgical adhesions would have already occurred in the scleral bed. Although complete scleral wound healing with collagen fibrils may take up to 3 months (Ferguson and Malik, 2003; Teichmann and Teichmann, 1997), since the haptic is snugly placed inside a scleral pocket, the IOL remains stable.
6.2. Biophysics of IOL stability
The change in the properties of the biomaterial when the IOL is placed in a stretched position is a major concern. There are 2 factors that contribute to the ability of IOL loops to maintain their original symmetrical configuration. One is the loop rigidity (the resistance of the haptic to external forces that bend the loops centrally) and the other is the loop memory (the ability of the loops to reexpand laterally to their original size and configuration). These 2 factors can be demonstrated by compressing or stretching the haptics in vivo. In vivo, the centrifugal force vector due to resistance to compression by the capsular bag keeps the IOL stable. Similarly, with glued IOL, the stretch creates a centripetal resistance force. Along with the intralamellar scleral tuck, this stabilizes the IOL. Spontaneous IOL dislocation is one of the main problems associated with transcleral fixation of suture-fixated IOLs. However, this is known to occur due to suture degradation or disintegration. In the glued IOL since the haptics are snugly tucked into the scleral tunnel (Fig. 6) the chance is reduced. When the eye moves, it acquires kinetic energy from its muscles and attachments and the energy is dissipated to the internal fluids as it stops. Thus pseudophacodonesis is the result of oscillations of the fluids in the anterior and posterior segment of the eye. These oscillations, initiated by movement of the eye, result in shearing forces on the corneal endothelium as well as vitreous motion leading to permanent damage. Although complete scleral wound healing with collagen fibrils may take up to 3 months (Lee et al., 1995; Agarwal et al., 2009), since the haptic is snugly placed inside a scleral pocket, the IOL remains stable. There are no clinical pseudophacodonesis observed in glued IOL’s due to good stability of the IOL.
Figure 6.
Intralamellar scleral tunnel: (A) made with 26 G needle and (B) Scleral tucking of the IOL haptic.
6.3. Glued IOL for various indications
Ectopia lentis or subluxated cataract is often associated with zonular weakness, which makes surgery with lens extraction and implantation of an intraocular lens (IOL) challenging. Moreover posterior capsule rupture can prevent normal IOL implantation in the capsular bag. Capsular tension rings (CTR) have been used in some cases of luxated lens where zonular weakness is identified. However in total loss of capsule or aniridia CTR cannot be placed. Similarly iris claw lenses cannot be placed in aniridia. Anterior chamber IOL may not be placed in eyes with low cell count or aniridia and recurrent uveitis patients are not ideal for ACIOL’s. When PCR is small, routinely the IOL’s have been placed in the ciliary sulcus in the past. However this also is not free from complications. Recurrent uveitis, pseudophacodonesis, IOL tilt and induced aberrations are known to occur even in sulcus fixated IOLs. Ferguson and Malik (2003) observed pseudophakic posterior iris chaffing syndrome which results from the haptics of the sulcus-fixated IOL in direct contact with the posterior surface of the overlying iris causing focal iris atrophy and pigment dispersion. Microhyphaemas, intermittent spikes in intra-ocular pressure or pigment dispersion on a long term leads to UGH syndrome. Recurrent redness and pain is the common presentation in these eyes with UGH syndrome. IOL rotation and recurrent irritation of iris are known to cause late UGH syndrome. Moreover, rubbing between the IOL optic and iris seems to contribute to the high flare counts in eyes with a sulcus-to-sulcus IOL fixation. In our series, consistent vault was maintained as seen in UBM (Fig. 7) between the iris and the IOL which is one factor for less post operative uveitis and pigment dispersion.
Figure 7.
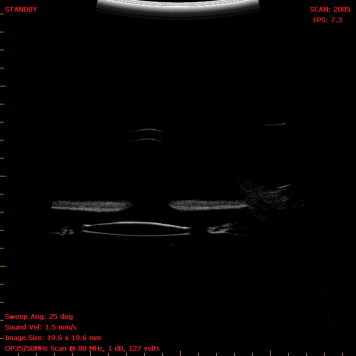
Ultrasound biomicroscopy image of Glued IOL.
Especially in pediatric eyes with deficient capsule, where there are very few options left; early visual recovery is mandatory to prevent sensory deprivation. None of the pediatric patients in our group developed post operative strabismus, retinal detachment, IOL dislocation, endophthalmitis or glaucoma. Moreover continuous occlusion treatment has shown good functional improvement in the operated eyes.
It has been shown that the overall length (12.5–14.0 mm) of the IOL helps ensure firm, stable fixation at the posterior chamber behind the iris, where the average diameter in emmetropic eyes is approximately 13.0 mm. In addition, the large optics lowers the risk of clinically significant postoperative decentration. Since the overall diameter of the routine IOL is about 12–13 mm, with the haptic being placed in its normal curved configuration and without any traction, there is no distortion or change in shape of the IOL optic. The mean shift from the geometric centre of IOL with respect to limbus was 0.06 mm ± 1.7 in one of our series. Hence good centeration was possible with the technique over the time period. Externalization of the greater part of the haptics into the scleral tunnel along its curvature stabilizes the axial positioning of the IOL and thereby prevents any IOL tilt (Teichmann and Teichmann, 1997). This is well shown by our one year follow up results with no haptic extrusion and good flap apposition. The intralamellar scleral tuck provides greater stability and safety unlike the ‘sutures’ in the sutured scleral fixated IOL as demonstrated ex vivo (Fig. 8).
Figure 8.
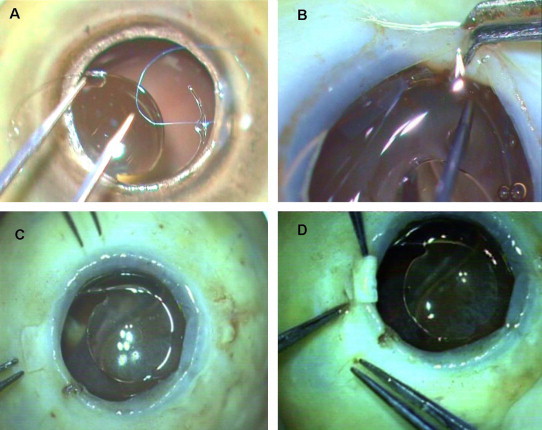
(A) Sutured scleral fixated (SF) IOL, (B) SF IOL showing iris shaffing, (C) Glued IOL in cadaver eye D: Haptic is externalized and tucked with less chance of uveal irritation.
The glued IOL technique has evolved over time, with one of the greatest changes being the use of foldable IOLs. This makes it possible to perform the entire procedure through small self-sealing incisions. This has the intraoperative advantage of having a well-formed globe throughout the surgery. It eliminates iris prolapse during IOL insertion and wound suturing and significantly decreases surgical time. This foldable glued IOL has postoperative advantages of having fewer complications associated with larger wounds, such as postoperative wound leak and shallow anterior chamber, as well as decreases the astigmatism. Handshake technique helps in early learning curve for easy haptic externalization which is a modification of our earlier procedure especially for foldable IOL.
Another major change is the combined procedures like optical penetrating keratoplasty, iridoplasty, trabeculectomy, descemet stripping & endothelial keratoplasty and strabismus surgery performed along with glued IOL, which not only reduces the surgical trauma but also the expenditure and time.
Since the introduction of glued IOL procedure, there are many patients who are satisfied subjectively and are at regular follow up. Moreover the frequent complications of secondary IOL implantation like secondary glaucoma, cystoid macular edema or bullous keratopathy are not seen in any of our patients. Another important advantage of this technique is the prevention of suture related complications like suture erosion, suture knot exposure or dislocation of IOL after suture disintegration or broken suture. IOL explantation in malpositioned PC IOL is totally removed as the same IOL is repositioned with this method. We believe this method of PC IOL implantation is appropriate for eyes with deficient or absent posterior capsule and this can be performed easily with the available IOL designs, instruments and with less surgical time. However, evaluation of the clinical importance of the technique requires a clinical study with greater number of patients and longer duration follow up to judge the long term functional and anatomical results of the procedure.
7. Conclusion
Glued IOL procedure can be done as a primary procedure or secondary IOL implantation in eyes with deficient capsular support. Monofocal (single piece/multipiece), multifocal or aniridia IOL’s can be implanted. The suture related and corneal endothelial complications are prevented via this procedure. Recently introduced modifications in the glued IOL (Handshake technique, use of 23G infusion, foldable glued IOL) help in easy learning and wider use of the technique for various indications.
8. Method of literature search
The authors performed a Medline search with Pubmed. The search was restricted to publications related to humans, in English and other-language publications with English abstracts. Articles and book chapters obtained from the reference lists of other articles were reviewed and included when considered appropriate.
References
- Agarwal A., Kumar D.A., Jacob S., Baid C., Agarwal A., Srinivasan S. Fibrin glue-assisted sutureless posterior chamber intraocular lens implantation in eyes with deficient posterior capsules. J. Cataract Refract. Surg. 2008;34:1433–1438. doi: 10.1016/j.jcrs.2008.04.040. [DOI] [PubMed] [Google Scholar]
- Agarwal A., Kumar D.A., Prakash G. Fibrin glue-assisted sutureless posterior chamber intraocular lens implantation in eyes with deficient posterior capsules [Reply to letter] J. Cataract Refract. Surg. 2009;35:795–796. doi: 10.1016/j.jcrs.2008.04.040. [DOI] [PubMed] [Google Scholar]
- Agarwal A., Kumar D.A., Jacob S., Prakash G., Agarwal A. Reply: Fibrin glue-assisted sutureless scleral fixation. J. Cataract Refract. Surg. 2009;35:795–796. doi: 10.1016/j.jcrs.2008.12.043. [DOI] [PubMed] [Google Scholar]
- Agarwal A., Kumar D.A., Nair V. Cataract surgery in the setting of trauma. Curr Opin Ophthalmol. 2010;21(1):65–70. doi: 10.1097/ICU.0b013e3283331579. [DOI] [PubMed] [Google Scholar]
- Asadi R., Kheirkhah A. Long-term results of scleral fixation of posterior chamber intraocular lenses in children. Ophthalmology. 2008;115(1):67–72. doi: 10.1016/j.ophtha.2007.02.018. (Epub 2007 May 3) [DOI] [PubMed] [Google Scholar]
- Bergman M., Laatikainen L. Long-term evaluation of primary anterior chamber intraocular lens implantation in complicated cataract surgery. Int. Ophthalmol. 1996–1997;20(6):295–299. doi: 10.1007/BF00176881. [DOI] [PubMed] [Google Scholar]
- Bergman M.A., Nieminen H., Laatikainen L.T. Corneal endothelial cell density after complicated cataract surgery and implantation of an anterior chamber intraocular lens. Eur. J. Implant. Ref. Surg. 1993;5:237–241. [Google Scholar]
- Biro Z. Results and complications of secondary intraocular lens implantation. J. Cataract Refract. Surg. 1993;19:64–67. doi: 10.1016/s0886-3350(13)80284-2. [DOI] [PubMed] [Google Scholar]
- Collins J.F., Gaster R.N., Krol W.F., Colling C.L., Kirk G.F., Smith T.J., Department of Veterans Affairs Cooperative Cataract Study A comparison of anterior chamber and posterior chamber intraocular lenses after vitreous presentation during cataract surgery: the Department of Veterans Affairs Cooperative Cataract Study. Am. J. Ophthalmol. 2003;136(1):1–9. doi: 10.1016/s0002-9394(02)01924-4. [DOI] [PubMed] [Google Scholar]
- Donaldson K.E., Gorscak J.J., Budenz D.L., Feuer W.J., Benz M.S., Forster R.K. Anterior chamber and sutured posterior chamber intraocular lenses in eyes with poor capsular support. J. Cataract Refract. Surg. 2005;31(5):903–909. doi: 10.1016/j.jcrs.2004.10.061. [DOI] [PubMed] [Google Scholar]
- Ferguson A.W., Malik T.Y. Pseudophakic posterior iris chafing syndrome. Eye. 2003;17:451–452. doi: 10.1038/sj.eye.6700322. [DOI] [PubMed] [Google Scholar]
- Gabor S.G., Pavilidis M.M. Sutureless intrascleral posterior chamber intraocular lens fixation. J. Cataract Refract. Surg. 2007;33(11):1851–1854. doi: 10.1016/j.jcrs.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Grewing R., Mester U. Fibrin sealant in the management of complicated hypotony after trabeculectomy. Ophthalmic Surg. Lasers. 1997;28:124–127. [PubMed] [Google Scholar]
- Hayashi K., Hayashi H., Nakao F., Hayashi F. Intraocular lens tilt and decentration, anterior chamber depth, and refractive error after trans-scleral suture fixation surgery. Ophthalmology. 1999;106:878–882. doi: 10.1016/S0161-6420(99)00504-7. [DOI] [PubMed] [Google Scholar]
- Heilskov T., Joondeph B.C., Olsen K.R., Blankenship G.W. Late endophthalmitis after transscleral fixation of a posterior chamber intraocular lens. Arch. Ophthalmol. 1989;107:1427. doi: 10.1001/archopht.1989.01070020501017. [DOI] [PubMed] [Google Scholar]
- Jacob S., Prakash G., Ashok Kumar D., Nair V., Agarwal A., Agarwal A. Anterior segment transplantation with a novel biosynthetic graft. Eye Contact Lens. 2010;36(2):130–136. doi: 10.1097/ICL.0b013e3181cd1b14. [DOI] [PubMed] [Google Scholar]
- Jongebloed W.L., Worst J.F.G. Degradation of polypropylene in the human eye: a SEM-study. Doc. Ophthalmol. 1986;64:143–152. doi: 10.1007/BF00166695. [DOI] [PubMed] [Google Scholar]
- Kumar D.A., Agarwal A., Prakash G., Jacob S., Saravanan Y., Agarwal A. Glued posterior chamber IOL in eyes with deficient capsular support: a retrospective analysis of 1-year post-operative outcomes. Eye (Lond) 2010;24(7):1143–1148. doi: 10.1038/eye.2010.10. [DOI] [PubMed] [Google Scholar]
- Kumar D.A., Agarwal A., Jacob S., Prakash G., Agarwal A., Sivagnanam S. Repositioning of the dislocated intraocular lens with sutureless 20-gauge vitrectomy. Retina. 2010;30(4):682–687. doi: 10.1097/iae.0b013e3181cd4929. [DOI] [PubMed] [Google Scholar]
- Kumar D.A., Agarwal A., Jacob S., Prakash G. Agarwal AUse of 23-gauge or 25-gauge trocar cannula for globe maintenance in glued intraocular lens surgery. J. Cataract Refract. Surg. 2010;36(4):690–691. doi: 10.1016/j.jcrs.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Kumar D.A., Agarwal A., Prakash G., Jacob S. Managing total aniridia with aphakia using a glued iris prosthesis. J. Cataract Refract. Surg. 2010;36(5):864–865. doi: 10.1016/j.jcrs.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Lagoutte F.M., Gauthier L., Comte P.R.M. A fibrin sealant for perforated and preperforated corneal ulcers. Br. J. Ophthalmol. 1989;73:757–761. doi: 10.1136/bjo.73.9.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.H., Kim M.S., Hahn T.W., Kim J.H. Comparison of histologic findings in wound healing of rabbit scleral homografts with fibrin glue (Tisseel) and suture material. J. Refract. Surg. 1995;11:397–401. doi: 10.3928/1081-597X-19950901-18. [DOI] [PubMed] [Google Scholar]
- Lorencova V., Rozsival P., Urminsky J. Clinical results of the aphakia correction by means of secondary implantation of the iris-fixated anterior chamber intraocular lens. Cesk. Slov. Oftalmol. 2007;63(4):285–291. [PubMed] [Google Scholar]
- Maggi R., Maggi C. Sutureless scleral fixation of intraocular lenses. J. Cataract Refract. Surg. 1997;23(9):1289–1294. doi: 10.1016/s0886-3350(97)80104-6. [DOI] [PubMed] [Google Scholar]
- Nair V., Kumar D.A., Prakash G., Jacob S., Agarwal A. Bilateral spontaneous in-the-bag anterior subluxation of PC IOL managed with glued IOL technique: A case report. Eye Contact Lens. 2009;35(4):215–217. doi: 10.1097/ICL.0b013e3181ac3706. [DOI] [PubMed] [Google Scholar]
- Numa A., Nkamura J., Takashima M. Long-term corneal endothelial changes after intraocular lens implantation. Anterior vs posterior chamber lenses. Jpn. J. Ophthalmol. 1993;37(1):78–87. [PubMed] [Google Scholar]
- Parekh P., Green W.R., Stark W.J., Akpek E.K. Subluxation of suture-fixated posterior chamber intraocular lenses: a clinicopathologic study. Ophthalmology. 2007;114:232–237. doi: 10.1016/j.ophtha.2006.10.037. [DOI] [PubMed] [Google Scholar]
- Prakash G., Kumar D.A., Jacob S., Kumar K.S., Agarwal A., Agarwal A. Anterior segment optical coherence tomography–aided diagnosis and primary posterior chamber intraocular lens implantation with fibrin glue in traumatic phacocele with scleral perforation. J. Cataract Refract. Surg. 2009;35:782–784. doi: 10.1016/j.jcrs.2008.09.031. [DOI] [PubMed] [Google Scholar]
- Prakash G., Jacob S., Kumar D.A., Narsimhan S., Agarwal A., Agarwal A. Femtosecond assisted keratoplasty with fibrin glue-assisted sutureless posterior chamber lens implantation: a new triple procedure. J. Cataract Refract. Surg. 2009;35(6):973–979. doi: 10.1016/j.jcrs.2008.12.049. [DOI] [PubMed] [Google Scholar]
- Prakash G., Agarwal A., Jacob S., Kumar D.A., Chaudhary P., Agarwal A. Femtosecond-assisted descemet stripping automated endothelial keratoplasty with fibrin glue-assisted sutureless posterior chamber lens implantation. Cornea. 2010;29(11):1315–1319. doi: 10.1097/ICO.0b013e3181cb4120. [DOI] [PubMed] [Google Scholar]
- Prakash G., Agarwal A., Kumar D.A., Saleem A., Jacob S., Agarwal A. Translocation of malpositioned posterior chamber intraocular lens from anterior to posterior chamber along with fibrin glue-assisted transscleral fixation. Eye Contact Lens. 2010;36(1):45–48. doi: 10.1097/ICL.0b013e3181c786c2. [DOI] [PubMed] [Google Scholar]
- Price M.O., Price F.W., Jr., Werner L. Late dislocation of scleral-sutured posterior chamber intraocular lenses. J. Cataract Refract. Surg. 2005;31(7):1320–1326. doi: 10.1016/j.jcrs.2004.12.060. [DOI] [PubMed] [Google Scholar]
- Rattigan S.M., Ellerton C.R., Chitkara D.K., Smerdon D.L. Flexible open-loop anterior chamber intraocular lens implantation after posterior capsule complications in extracapsular cataract extraction. J. Cataract Refract. Surg. 1996;22(2):243–246. doi: 10.1016/s0886-3350(96)80226-4. [DOI] [PubMed] [Google Scholar]
- Solomon K., Gussler J.R., Gussler C., Van Meter W.S. Incidence and management of complications of transsclerally sutured posterior chamber lenses. J. Cataract Refract. Surg. 1993;19:488–493. doi: 10.1016/s0886-3350(13)80612-8. [DOI] [PubMed] [Google Scholar]
- Teichmann K.D., Teichmann I.A.M. The torque and tilt gamble. J. Cataract Refract. Surg. 1997;23:413–418. doi: 10.1016/s0886-3350(97)80186-1. [DOI] [PubMed] [Google Scholar]
- Tilanus M.A.D., Deutman T., Deutman A.F. Full-thickness macular holes treated with vitrectomy and tissue glue. Int. Ophthalmol. 1994/1995;18:355–358. doi: 10.1007/BF00930314. [DOI] [PubMed] [Google Scholar]
- Zeh W.G., Price F.W. Iris fixation of posterior chamber intraocular lenses. J. Cataract Refract. Surg. 2000;26:1028–1034. doi: 10.1016/s0886-3350(00)00322-9. [DOI] [PubMed] [Google Scholar]


