Abstract
Meibomian glands play a significant role in tear production by contributing lipids to the superficial tear film.1 Dysfunction of the meibomian glands destabilizes tears resulting in evaporative dry eye.2,3 Historically, the meibomian glands were assessed in an ex vivo fashion through histologic studies. However, innovations in ocular imaging have advanced significantly in recent decades to include meibography. Meibography is an imaging study developed 35 years ago exclusively for the purpose of observing the morphology of meibomian glands in vivo.4,5 In this review of meibography, we briefly describe the etiology of meibomian gland dysfunction and then discuss various meibography techniques, technologies, and methods of image analysis. We close with a review of the literature, crediting various studies for the significant contributions made toward our current understanding of the meibomian glands.
Keywords: Meibography, Infrared meibography, Meibomian glands, Meibomian gland dysfunction, Dry eye
Meibomian gland dysfunction
Description of the meibomian glands
The posterior lamella of the eyelid hosts a fleet of meibomian glands situated between the palpebral conjunctiva and tarsal plate. A normal meibomian gland is approximately linear and 3–4 mm in length, traversing the posterior eyelid perpendicularly from the lid margin to the opposite edge of the tarsus.5 Closer inspection of a meibomian gland demonstrates a tubulo-acinar architecture with saccular arrangements of acini and a ductal system that communicates with orifices near the mucocutaneous junction of the eyelid.6 Glandular acini contain clusters of modified sebaceous cells called meibocytes.
The functional unit of a meibomian gland is the meibocyte which synthesizes and secretes lipids (meibum) into the precorneal tear film. Meibum permeates the tear surface where it serves several important functions. It prevents tear evaporation and thus desiccation of the ocular surface; it acts as a physical and hydrophobic barrier to the inward movement of environmental and organic agents; and it lubricates the ocular surface to prevent irritation while promoting a clear ocular image. Consequently, tear physiology is dependent upon the proper functioning of the meibomian glands.7–9
A stable tear film is essential to a healthy ocular surface. Tears are composed of three distinct layers: the superficial lipid layer prevents tear evaporation and is produced by the meibomian glands in the eyelid; the intermediate aqueous layer hydrates the ocular surface and is produced by the lacrimal (and accessory) gland in the superolateral orbit; and the deep mucinous layer promotes adhesion of tears to the ocular surface and is produced by goblet cells embedded in the conjunctiva.1–4 Eyelid blink disburses tears along the ocular surface that ultimately drain through the nasolacrimal system.10,11
Definition of meibomian gland dysfunction
The International Workshop on Meibomian Gland Dysfunction formally defined it as a chronic, diffuse abnormality of the meibomian glands, commonly characterized by terminal duct obstruction and/or qualitative/quantitative changes in the glandular secretion.12 It may result in alteration of the tear film, symptoms of eye irritation, clinically apparent inflammation, and ocular surface disease.12 An optimum level of meibum expression is required for tear stability which allows for the division of MGD into two types:12–14
-
1.
Low delivery or obstructive-type MGD: Absence or hyposecretion of meibum results in a lipid-deficient tear film and evaporative dry eye. Histopathologic studies of obstructive-type MGD demonstrate squamous metaplasia and keratinized plugs at gland orifices, inspissation of lipids and cell debris within dilated ducts, gland hypertrophy, lysis of meibocyte cell junctions, and gland atrophy.15–20
-
2.
High delivery or seborrheic-type MGD: Hypersecretion of meibum into the tear film incites an inflammatory reaction at the ocular surface resulting in symptoms of eye irritation.21,22 This form of MGD is less common and studies are limited; however, Mathers et al.17 and Arita et al.21 showed that changes in meibomian gland morphology are not characteristic of seborrheic-type MGD.
The epidemiology of meibomian gland dysfunction
MGD is purported to be the most common cause of evaporative dry eye syndrome and is frequently encountered in ophthalmic practices.3,23–26 The prevalence of MGD is estimated to range from 0.39% to 69.3% in population- and clinical-based studies.27–38 Such a wide variation in the prevalence of MGD is likely due to lack of a common definition, different diagnostic methods, and normal variation in the prevalence of MGD with age and ethnicity.25 A comparison of studies suggests that MGD is most prevalent in Asian populations (46.2–69.3%) and least prevalent in Caucasian populations (3.5–19.9%).28–32,38 To our knowledge, there have been no studies reporting the prevalence of MGD by age group.
Methods for evaluating the meibomian glands
The multifactorial nature of dry eye syndrome necessitates several tests in diagnosing the underlying cause(s). Dry eye is classically divided into aqueous deficient-type and evaporative-type but they frequently co-occur.3 Investigation of the meibomian glands is warranted when clinical presentation and/or workup suggests evaporative dry eye which is most commonly caused by MGD.3,12,25,39 Numerous techniques exist for directly and indirectly assessing the structure and function of meibomian glands. Here, we briefly discuss commonly used techniques in both research and clinical scenarios for evaluating the meibomian glands with an emphasis on meibography.
Measurements of tear production and evaporation rate
The Schirmer test was first described in 1903 and is an invasive method for measuring tear production.40 By measuring the rate of tear production, the Schirmer test can indirectly measure the function of meibomian glands. The Schirmer test differentiates MGD from aqueous-deficient dry eye because obstructive-type MGD results in activation of reflex tearing secondary to ocular irritation whereas aqueous-deficient dry eye features a reduced capacity for any reflex tearing.17,41,42 The Schirmer test should be interpreted with caution because there are many causes of abnormal reflex tearing aside from MGD. As a result, the Schirmer test cannot diagnose MGD.
The tear film breakup time (TBUT) was first described in 1969 by Norn and is a noninvasive method for assessing tear stability.43,44 TBUT represents the time elapsed from the last complete eyelid blink until appearance of the first dry spot on the cornea. By measuring tear film stability, the TBUT can indirectly assess the function of meibomian glands. MGD leads to a lipid-deficient tear film that is unstable and rapidly evaporates.45 Studies have shown that MGD leads to decreased TBUT; however, several causes of aqueous-deficient and evaporative dry eye lead to decreased TBUT and it is therefore not diagnostic of MGD.17,46–50
Biomicroscopic examination of the eyelid and digital pressure meibum expression
Slit-lamp biomicroscopic examination of the eyelid is a common method for assessing the meibomian glands. This is a non-invasive examination of the eyelid for evidence of irregular lid margins as evidenced by hyperemia, telangiectasias, vascular engorgement, meibum orifice plugging, and provocation of meibum expression through application of digital pressure to the eyelid.51–53 These changes in eyelid appearance and meibum expression have been shown to correlate with dysfunction of the meibomian glands.54 While this method is frequently used in the clinical setting as part of the standard slit-lamp examination of the eyelid, it is limited as an indirect measure of meibomian gland structure and function.
Vital stains of the ocular surface
Topical dyes reveal certain features of the ocular surface in a relatively non-invasive, rapid, and economical manner. Commonly used topical dyes include fluorescein, rose bengal, and lissamine green. Fluorescein is a mildly invasive stain that marks the tear film and defects in the corneal and conjunctival epithelium.55 The tear film staining properties of fluoroscein also make it useful in determining the TBUT. Rose bengal and lissamine green are conjunctival stains, but rose bengal is considerably more invasive than lissamine green due to its greater toxicity to the ocular surface.56,57 Topical stains are useful for their ability to detect tear and ocular surface abnormalities associated with MGD; however, there is currently no stain for meibomian glands that can be used in vivo.
Lipid layer interferometry
Lipid layer interferometry is a technique predominantly utilized in research that measures tear stability by imaging the surface contour of the tear film and analyzing the depth or “thickness” of the lipid layer.58,59 On interferometry, an appropriately thick lipid layer spans the tear surface in a continuous manner whereas a thin lipid layer degenerates into discontinuous patchy regions denoting an unstable tear film.58,59 Previous studies have found interferometry to be useful in diagnosing the presence and severity of dry eye, but not at further defining the cause of dry eye such as MGD.60,61
Tear osmolarity
Tear osmolarity depends on multiple factors including the function of meibomian glands. Studies have shown that meibum maintains tear osmolarity by preventing evaporation of the aqueous layer.17,62 Loss of meibum expression in patients with obstructive MGD leads to increased evaporation of water resulting in increased tear osmolarity.17,63 Hyperosmolar tears are directly irritative to the ocular surface which provides one explanation for the irritative symptoms of MGD.3,64 Technical difficulty and sample analysis are the two major drawbacks of the tear osmolarity test. Significant care must be taken to minimize reflex tearing during sample collection which could alter tear osmolarity. Furthermore, tear osmolarity cannot be determined clinically, but requires laboratory analysis. Determination of tear osmolarity is limited as a test for MGD because hyperosmolar tears are a hallmark characteristic of virtually any cause of dry eye syndrome.3,64
Meibometry
Meibometry is a technique developed to measure basal meibum levels at the eyelid margin.65–67 Essentially, a sample of meibum at the lid margin is transferred to specialized tape whose transparency is altered by exposure to meibum. The degree of change in tape transparency is analyzed photometrically for the purpose of quantifying the amount of basal meibum levels at the lid margin. Studies have shown basal meibum levels to be altered in MGD.65 Unfortunately, MGD can be focal or diffuse, rendering meibometric analysis vulnerable to inconsistent measurements within a given eyelid depending on the site of meibum collection along the lid margin.65
Meibography
Meibography is a specialized imaging study developed exclusively for the purpose of directly visualizing the morphology of meibomian glands in vivo (Fig. 1).4,5,18,48,63 There are several advantages to using meibography when evaluating patients with dry eye. While the methods previously discussed are capable of measuring some parameter of meibomian gland function, they are incapable of directly evaluating meibomian gland structure. Methods available for directly observing the architecture of meibomian glands are meibography and posterior eyelid biopsy. Biopsy of the posterior eyelid demonstrates the microscopic structure of meibomian glands, but it is an invasive ex vivo study and many patients are reluctant to consent to such a procedure.16,68 By comparison, meibography is a non-invasive in vivo study that permits gross and microscopic examination of the structure of meibomian glands.15–20,68 An experienced meibographer can complete the study in minutes with minimal discomfort to the patient.69 Meibographical images are scrutinized using any of several previously described techniques for quantitating gland architecture.70–72 Many studies have verified the utility of meibography in the diagnosis and evaluation of MGD (Fig. 2).4,5,14,17,18,48,49,63,70,73–80 We now turn to a more complete discussion of meibography.
Figure 1.
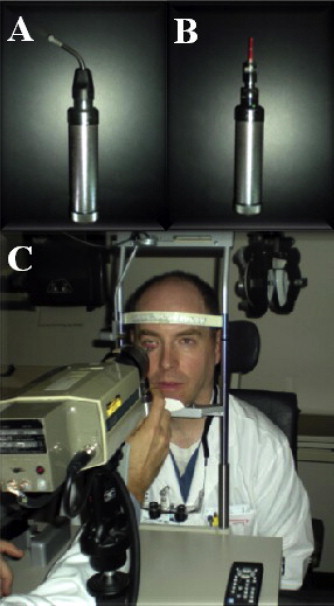
Contact IR meibography. Lower eyelid probe (A) is T-shaped on end. Upper eyelid probe (B) is linear. IR camera (C) films the everted and transilluminated eyelid.
Figure 2.
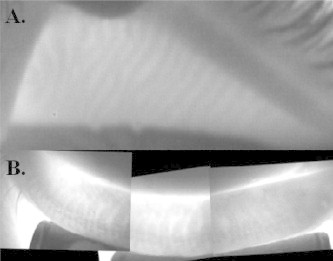
Contact IR meibography of a normal upper eyelid (A) and normal lower eyelid (B).
Meibography
A brief history of meibography
Meibography was first described in 1977 by Tapie who used UV Woods light to fluoresce meibomian ducts on biomicroscopy and infrared light to illuminate the meibomian glands on IR photography.4 In 1982, Jester et al. improved upon Tapie’s IR photography while documenting MGD in rabbits.5 In 1991, Mathers et al. was the first to refer to IR photography of meibomian glands as “meibography” in a study demonstrating several changes in the meibomian glands of patients with chronic blepharitis.17 In 1994, Mathers et al. introduced video IR meibography which represented a major advancement in the field by reducing dependence on the comparatively cumbersome and inefficient technique of photographic meibography.75 Since its inception 35 years ago, the evolution of meibography has recently accelerated with improvements to IR meibography as well as the advent of non-IR technologies including laser confocal meibography and, most recently, OCT meibography.81 A description of various meibographical techniques, technologies, and scoring systems is provided in the sections to follow.
Techniques in meibography
There are two meibography techniques: contact and non-contact. Contact meibography is the traditional technique developed in the late 1970’s, involving direct application of a light probe to the skin for eversion and transillumination of the eyelid followed by imaging with a specialized camera (Fig. 1).4,5,75 Contact meibography systems have enjoyed great success over the years, but there are disadvantages. First, operator expertise is required to properly use the equipment and attain good images. Second, eyelids have unique physical characteristics that are not always amenable to manipulation with the light probe. Partial lid eversion is a common and time-consuming problem that requires several images to be taken and merged to form a composite panoramic image of the eyelid. Third, patients may complain of discomfort due to heat, pressure, brightness, and sharpness of the probe.76 To address these limitations, a newly designed “oblique T-shaped probe” was introduced in 2007 and claimed to improve lid manipulation and image quality while reducing patient discomfort.76 Although advancements in contact meibography such as the oblique T-shaped probe are well-intentioned, they have largely been overshadowed by the introduction of non-contact meibography.
Introduced in 2008 by Arita et al., non-contact meibography is the latest meibographic technique.82 It uses a slit-lamp biomicroscope with IR filter and an IR charge-coupled device video camera to image a digitally everted eyelid. A light probe is unnecessary in non-contact meibography and distinguishes it from the contact technique. By eliminating the need for a light probe, non-contact meibography addresses the challenges of lid manipulation and patient discomfort commonly encountered in contact meibography. Consequently, non-contact meibography claims to be faster, more patient friendly, and easier to use than contact techniques.82 Another advantage of non-contact meibography is its ability to view a greater surface area of the everted eyelid, requiring fewer images and less time to merge images to create a panoramic view of the lid for evaluation.82
Perhaps the most convenient advancement in meibography techniques was described in 2012 by Arita et al. who introduced the mobile pen-shaped meibography system.83 The mobile pen-shaped meibography system is a subset of non-contact meibography that uses an infrared LED fixed to a handheld pen-shaped camera capable of capturing video or photographs of the meibomian glands which are comparable in quality to previous meibography systems.83 Thus, the mobile pen-shaped meibography system effectively eliminates the need for a slit-lamp biomicroscope typically used in non-contact meibography. A simple, fast, and portable meibography device has the potential to transform meibography from an academic center study to a test used in common clinical practice.
Meibographic technologies
Infrared meibography
Although it was the first technology utilized, infrared meibography remains the mainstay of most meibomian gland imaging studies due to its historical familiarity and reliability in producing high-quality gross images of the meibomian glands (Figs. 1 and 2).4,5,75,82,83 Infrared meibography is the technology most commonly utilized in contact and non-contact techniques which were described above and, therefore, would be redundant to discuss further. Until recently, however, infrared meibography was the only technology available, but novel technologies with the capacity to resolve microscopic features of meibomian glands have recently been proposed.
Laser confocal meibography
Laser confocal microscopy is an imaging modality utilized in many areas of medicine, but has recently found favor in the field of ophthalmology for its ability to evaluate various ocular structures and evaluate ocular surface disease and anterior segment disorders.84 In 2005, Kobayashi et al. was the first to demonstrate the palpebral conjunctiva and subconjunctival meibomian glands using in vivo laser confocal meibography (CM).85 The major advantage of CM over IR meibography is its ability to resolve and characterize the microenvironment and microscopic structures of the meibomian glands.45,49,85 However, CM is more invasive than IR meibography, involving alignment of the lens directly against the posterior aspect of the everted eyelid which requires a topical anesthetic.
Matsumoto et al. studied patients with MGD using CM and described two new meibomian gland parameters: glandular acinar unit diameter and acinar unit density.45 This study showed that patients with MGD had an increased acinar unit diameter and decreased acinar unit density, reflecting inspissation of lipids and gland dropout, respectively. A subsequent study by Matsumoto et al. used CM to follow patients with obstructive-type MGD and described two more meibomian gland parameters: periglandular inflammatory cell infiltrates and periglandular fibrosis.49 This study demonstrated that patients with MGD have periglandular fibrosis as well as increased periglandular inflammatory infiltrates that respond favorably to topical anti-inflammatory agents. Findings of periglandular inflammatory infiltrates and fibrosis were also observed using CM in patients with chronic graft-versus-host disease involving the eyelid.86 The ability to assess glandular acinar unit density, acinar unit diameter, periglandular inflammatory infiltrates and fibrosis was shown to have good sensitivity and specificity in diagnosing obstructive-type MGD.87 Therefore, as an in vivo technique, CM is noteworthy for its ability to measure microscopic parameters of the meibomian glands that were once only measurable in an ex vivo fashion through eyelid biopsy. As such, CM represents a significant advancement in the field of meibography.
Optical coherence tomographic meibography
Representing the most recent type of meibography, optical coherence tomography was first described in 2010 by Bizheva et al.81 OCT meibography (OCTM) is a non-invasive method capable of obtaining 2-D and 3-D tomograms of the meibomian glands in vivo. The distinguishing feature of OCTM from other forms of meibography is the capability to quantify meibomian gland morphology volumetrically. Volumetric measurements of the meibomian glands were previously made possible only through ex vivo histologic studies.81 Theoretically, prospective studies using OCTM could document the volumetric changes in the meibomian glands thought to occur with progression of MGD, including early gland hypertrophy and late gland atrophy.15,17,18 While OCTM adds a new dimension to meibographic analysis, the application of such a technology is in its infancy and its true usefulness to the field of meibography is yet to be defined.81,88
Meibographic image analysis
Remarkable images of the meibomian glands are generated using meibography. On IR meibography, normal meibomian glands are hypoilluminescent grape-like clusters while ducts and underlying tarsus are hyperilluminescent (Fig. 2).5 Abnormal meibomian glands show features consistent with histopathologic studies in which ducts appear dilated and glands become enlarged, tortuous, and eventually dropout (Fig. 3). Detailed evaluation of meibographic images is required to quantitatively assess and compare eyelids. Grading meibomian gland structure can be used to document the presence, progression, and treatment response in MGD.5,17,49 To date, meibomian gland grading is a largely informal and poorly defined process making comparison between studies difficult. The meiboscore and meibograde systems represent methodical approaches to quantifying meibomian gland morphology on IR meibography. Brief descriptions of these methods are provided below.
Figure 3.
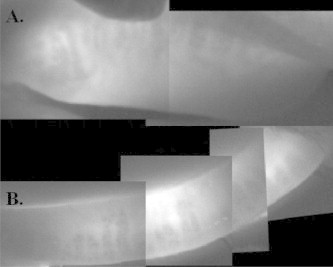
Contact IR meibography of meibomian gland dysfunction involving the upper eyelid (A) and lower eyelid (B).
The meiboscore method
Officially termed the “meiboscore” in 2008 by Arita et al., this method represents a variant of older grading schemes that examine meibographs for evidence of gland dropout.17,54,63,70,82,89 First, meibographs are inspected for the presence of partial or absent meibomian glands and assigned a numerical score proportional to the area of involved eyelid. Evaluation: (0): lid has no partial or missing glands; (1): involve lid area is <33%; (2): involved lid area is 33–66%; (3): involved lid area is >66%. Second, meiboscores for the upper and lower eyelids are summed by side to derive a total meiboscore from 0 through 6 per eye. Scores are used to compare eyes for differences in meibomian gland morphology which can support a diagnosis of MGD.
Interestingly, Arita et al. showed that meiboscores correlate with lid margin abnormality scores, effectively verifying the meiboscore as a valid measure of meibomian gland structure.69 Further, the same study showed that meiboscores correlate with meibum scores, supporting the meiboscores’ ability to infer meibomian gland function.69 The meiboscore method is appealing because it involves a single scoring category which streamlines image analysis and data quantification. The simplicity of the meiboscore, however, also represents its greatest limitation because it fails to account for features of meibomian gland architecture that may precede gland dropout.82
The meibograde method
First described in 2012 by Call et al., the meibograde method represents the latest and most comprehensive scoring system available for evaluating meibomian gland morphology.72 Essentially, the meibograde comprises three distinct categories based on previously described histopathologic changes in the meibomian glands: gland distortion, gland shortening, and gland dropout.15,17–20 It conserves the meiboscore point system, assigning a score of 0 through 3 to each of the three categories and then sums the categories to obtain a meibograde from 0 through 9 per eyelid. Eyelid meiboscores can be compared for differences in meibomian gland morphology which may be used to diagnose MGD.
Limitations of the meiboscore as an incomplete scoring system prompted the development of the meibograde method. In theory, the meibograde should be able to detect minor changes in the meibomian glands leading up to and including dropout. However, determining the meibograde is more laborious than previously described scoring systems, but the added attention to detail may provide the information necessary to identify subtle pathologic changes in the meibomian glands before becoming irreversible.
Review of the literature
The contribution of meibography to our understanding of meibomian glands cannot be overstated. Studies commonly employ meibography as part of a battery of tests to identify changes in meibomian glands associated with certain variables. In doing so, meibography studies attempt to identify risk factors for MGD. Here, we review some of the findings of these studies.
Meibum synthesis is thought to be under neuroendocrine control. Histologic studies previously identified retinoic acid, androgen, estrogen, and progestin receptors on meibocytes.24 Meibography has been used in some studies to show that fluctuations in hormone levels could underlie the association between MGD and age,54,82 Accutane therapy,63 androgen deficiency90–92 and hormone replacement therapy.93 Furthermore, the presence of acetylcholine receptors on meibocytes suggests a role in the neural regulation of meibum synthesis.94–96 However, we were unable to identify any meibography studies attempting to find an association between cholinergic activity and changes in meibomian gland morphology.
Eyelid muscle contraction compresses the meibomian glands and propels meibum into the tear film.24 In the seventeenth century, Jean Riolan first described a set of muscle fibers encircling the meibomian glands collectively known as the muscle of Riolan.97 In 2002, Lipham et al. subsequently showed through histologic analysis of the muscle of Riolan that it was a component of the orbicularis muscle, relating eyelid blink to meibum expression.98 It follows that orbicularis weakness due to a number of causes (e.g. Bell’s palsy, myotonic dystrophy, status post botulinum toxin injection) could lead to stagnation of meibum resulting in obstructive-type MGD. Indeed, this theory was recently supported in the literature by Call et al.72 and Shah et al.50 using IR meibography to demonstrate an association between MGD and both the duration and severity of orbicularis weakness secondary to seventh nerve palsy.
In summary, the risk factors for MGD are numerous and meibomian gland images have helped elucidate many of them. These include age, gender, hormonal imbalance (e.g. androgen deficiency, menopause), contact lens wear, medications (e.g. Accutane, anti-glaucoma drops, hormone replacement therapy), eyelid anatomy, eyelid surgery, eyelid tattooing, eye trauma, ocular radiation, orbicularis weakness (e.g. CN VII palsy, status post botulinum toxin injection), allergic conjunctivitis, trachoma, Sjogren’s Syndrome, Stevens Johnson syndrome, atopic dermatitis, seborrheic dermatitis, acne rosacea, chronic blepharitis, and graft-versus-host disease.13,17,50–52,54,63,68,72,77,78,82,86,89,92,93,99–113 Clearly, a myriad of factors deserve attention when treating MGD, which may represent a final common pathway to evaporative dry eye syndrome.
Conclusion
Our understanding of the meibomian glands and their relationship to dry eye syndrome has progressed in parallel with advances in meibography. The future of meibography is bright. IR meibography is currently the technology of choice for studying the morphology of meibomian glands. The development of portable non-contact IR meibography has the potential to popularize the field resulting in widespread clinical use. Further, a renewed enthusiasm in the field of meibography is owed to the addition of laser confocal meibography and OCT meibography. These novel technologies show promise in their ability to measure unique features of the meibomian glands and will likely motivate future studies in the field of meibography.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Bergeron C.M., Moe K.S. The evaluation and treatment of upper eyelid paralysis. Facial Plast Surg. 2008;24:220–230. doi: 10.1055/s-2008-1075838. [DOI] [PubMed] [Google Scholar]
- 2.Green-Church K.B., Butovich I., Willcox M. The international workshop on meibomian gland dysfunction: report of the subcommittee on tear film lipids and lipid–protein interactions in health and disease. Invest Ophthalmol Vis Sci. 2011;52:1979–1993. doi: 10.1167/iovs.10-6997d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 2007;5:75–92. [DOI] [PubMed]
- 4.Tapie R. Etude biomicroscopique des glandes de meibomius. Ann Oculistique. 1977;210:637–648. [Google Scholar]
- 5.Jester J.V., Rife L., Nii D., Luttrull J.K., Wilson L., Smith R.E. In vivo biomicroscopy and photography of meibomian glands in a rabbit model of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 1982;22:660–677. [PubMed] [Google Scholar]
- 6.Sirigu P., Shen R.L., Pinto da Silva P. Human meibomian glands: the ultrastructure of acinar cells as viewed by thin section and freeze-fracture transmission electron microscopies. Invest Ophthalmol Vis Sci. 1992;33:2284–2292. [PubMed] [Google Scholar]
- 7.Holly F.J., Lemp M.A. Tear physiology and dry eyes. Surv Ophthalmol. 1977;22:69–87. doi: 10.1016/0039-6257(77)90087-x. [DOI] [PubMed] [Google Scholar]
- 8.Mishima S., Maurice D.M. The oily layer of the tear film and evaporation from the corneal surface. Exp Eye Res. 1961;1:39–45. doi: 10.1016/s0014-4835(61)80006-7. [DOI] [PubMed] [Google Scholar]
- 9.Tiffany J.M. The lipid secretion of the meibomian glands. Adv Lipid Res. 1987;22:1–62. doi: 10.1016/b978-0-12-024922-0.50005-9. [DOI] [PubMed] [Google Scholar]
- 10.Doane M.G. Interactions of eyelids and tears in corneal wetting and the dynamics of the normal human eyeblink. Am J Ophthalmol. 1980;89:507–516. doi: 10.1016/0002-9394(80)90058-6. [DOI] [PubMed] [Google Scholar]
- 11.Doane M.G. Blinking and the mechanics of the lacrimal drainage system. Ophthalmology. 1981;88:844–851. doi: 10.1016/s0161-6420(81)34940-9. [DOI] [PubMed] [Google Scholar]
- 12.Nelson J.D., Shimazaki J., Benitez-del-Castillo J.M. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011;52:1930–1937. doi: 10.1167/iovs.10-6997b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bron A.J., Tiffany J.M. The contribution of meibomian disease to dry eye. Ocul Surf. 2004;2:149–165. doi: 10.1016/s1542-0124(12)70150-7. [DOI] [PubMed] [Google Scholar]
- 14.Foulks G.N., Bron A.J. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf. 2003;1:107–126. doi: 10.1016/s1542-0124(12)70139-8. [DOI] [PubMed] [Google Scholar]
- 15.Jester J.V., Nicolaides N., Smith R.E. Meibomian gland studies: histologic and ultrastructural investigations. Invest Ophthalmol Vis Sci. 1981;20:537–547. [PubMed] [Google Scholar]
- 16.Lee S.H., Tseng S.C. Rose bengal staining and cytologic characteristics associated with lipid tear deficiency. Am J Ophthalmol. 1997;124:736–750. doi: 10.1016/s0002-9394(14)71690-3. [DOI] [PubMed] [Google Scholar]
- 17.Mathers W.D., Shields W.J., Sachdev M.S., Petroll W.M., Jester J.V. Meibomian gland dysfunction in chronic blepharitis. Cornea. 1991;10:277–285. doi: 10.1097/00003226-199107000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Robin J.B., Jester J.V., Nobe J., Nicolaides N., Smith R.E. In vivo transillumination biomicroscopy and photography of meibomian gland dysfunction. A clinical study. Ophthalmology. 1985;92:1423–1426. doi: 10.1016/s0161-6420(85)33848-4. [DOI] [PubMed] [Google Scholar]
- 19.Gutgesell V.J., Stern G.A., Hood C.I. Histopathology of meibomian gland dysfunction. Am J Ophthalmol. 1982;94:383–387. doi: 10.1016/0002-9394(82)90365-8. [DOI] [PubMed] [Google Scholar]
- 20.Jester J.V., Nicolaides N., Kiss-Palvolgyi I., Smith R.E. Meibomian gland dysfunction. II. The role of keratinization in a rabbit model of MGD. Invest Ophthalmol Vis Sci. 1989;30:936–945. [PubMed] [Google Scholar]
- 21.Arita R., Itoh K., Maeda S. Proposed diagnostic criteria for seborrheic meibomian gland dysfunction. Cornea. 2010;29:980–984. doi: 10.1097/ICO.0b013e3181cc7b1b. [DOI] [PubMed] [Google Scholar]
- 22.Dhaliwal U., Arora V.K., Singh N., Bhatia A. Cytopathology of chalazia. Diagn Cytopathol. 2004;31:118–122. doi: 10.1002/dc.20092. [DOI] [PubMed] [Google Scholar]
- 23.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye Workshop (2007). Ocul Surf 2007;5:93–107. [DOI] [PubMed]
- 24.Knop E., Knop N., Millar T., Obata H., Sullivan D.A. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52:1938–1978. doi: 10.1167/iovs.10-6997c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaumberg D.A., Nichols J.J., Papas E.B., Tong L., Uchino M., Nichols K.K. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for MGD. Invest Ophthalmol Vis Sci. 2011;52:1994–2005. doi: 10.1167/iovs.10-6997e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Buskirk E.M. Corneal anesthesia after timolol maleate therapy. Am J Ophthalmol. 1979;88:739–743. doi: 10.1016/0002-9394(79)90675-5. [DOI] [PubMed] [Google Scholar]
- 27.Schein O.D., Munoz B., Tielsch J.M., Bandeen-Roche K., West S. Prevalence of dry eye among the elderly. Am J Ophthalmol. 1997;124:723–728. doi: 10.1016/s0002-9394(14)71688-5. [DOI] [PubMed] [Google Scholar]
- 28.Lekhanont K., Rojanaporn D., Chuck R.S., Vongthongsri A. Prevalence of dry eye in Bangkok, Thailand. Cornea. 2006;25:1162–1167. doi: 10.1097/01.ico.0000244875.92879.1a. [DOI] [PubMed] [Google Scholar]
- 29.Lin P.Y., Tsai S.Y., Cheng C.Y., Liu J.H., Chou P., Hsu W.M. Prevalence of dry eye among an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology. 2003;110:1096–1101. doi: 10.1016/S0161-6420(03)00262-8. [DOI] [PubMed] [Google Scholar]
- 30.Uchino M., Dogru M., Yagi Y. The features of dry eye disease in a Japanese elderly population. Optom Vis Sci. 2006;83:797–802. doi: 10.1097/01.opx.0000232814.39651.fa. [DOI] [PubMed] [Google Scholar]
- 31.Jie Y., Xu L., Wu Y.Y., Jonas J.B. Prevalence of dry eye among adult Chinese in the Beijing Eye Study. Eye (Lond) 2009;23:688–693. doi: 10.1038/sj.eye.6703101. [DOI] [PubMed] [Google Scholar]
- 32.McCarty C.A., Bansal A.K., Livingston P.M., Stanislavsky Y.L., Taylor H.R. The epidemiology of dry eye in Melbourne, Australia. Ophthalmology. 1998;105:1114–1119. doi: 10.1016/S0161-6420(98)96016-X. [DOI] [PubMed] [Google Scholar]
- 33.Doughty M.J., Fonn D., Richter D., Simpson T., Caffery B., Gordon K. A patient questionnaire approach to estimating the prevalence of dry eye symptoms in patients presenting to optometric practices across Canada. Optom Vis Sci. 1997;74:624–631. doi: 10.1097/00006324-199708000-00023. [DOI] [PubMed] [Google Scholar]
- 34.Schein O.D., Hochberg M.C., Munoz B. Dry eye and dry mouth in the elderly: a population-based assessment. Arch Intern Med. 1999;159:1359–1363. doi: 10.1001/archinte.159.12.1359. [DOI] [PubMed] [Google Scholar]
- 35.Moss S.E., Klein R., Klein B.E. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118:1264–1268. doi: 10.1001/archopht.118.9.1264. [DOI] [PubMed] [Google Scholar]
- 36.Yazdani C., McLaughlin T., Smeeding J.E., Walt J. Prevalence of treated dry eye disease in a managed care population. Clin Ther. 2001;23:1672–1682. doi: 10.1016/s0149-2918(01)80136-3. [DOI] [PubMed] [Google Scholar]
- 37.Chia E.M., Mitchell P., Rochtchina E., Lee A.J., Maroun R., Wang J.J. Prevalence and associations of dry eye syndrome in an older population: the Blue Mountains Eye Study. Clin Experiment Ophthalmol. 2003;31:229–232. doi: 10.1046/j.1442-9071.2003.00634.x. [DOI] [PubMed] [Google Scholar]
- 38.Schaumberg D.A., Sullivan D.A., Buring J.E., Dana M.R. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318–326. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 39.Tomlinson A., Bron A.J., Korb D.R. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011;52:2006–2049. doi: 10.1167/iovs.10-6997f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schirmer O. Studien zur physiologie und pathologie der trenabsonderung und trenabfuhr. Arch Klin Exp Ophthalmol. 1903;56:197–291. [Google Scholar]
- 41.Shimazaki J., Sakata M., Tsubota K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch Ophthalmol. 1995;113:1266–1270. doi: 10.1001/archopht.1995.01100100054027. [DOI] [PubMed] [Google Scholar]
- 42.Arita R., Itoh K., Maeda S., Maeda K., Tomidokoro A., Amano S. Efficacy of diagnostic criteria for the differential diagnosis between obstructive meibomian gland dysfunction and aqueous deficiency dry eye. Jpn J Ophthalmol. 2010;54:387–391. doi: 10.1007/s10384-010-0858-1. [DOI] [PubMed] [Google Scholar]
- 43.Norn M.S. Desiccation of the precorneal film. I. Corneal wetting-time. Acta Ophthalmol (Copenh) 1969;47:865–880. doi: 10.1111/j.1755-3768.1969.tb03711.x. [DOI] [PubMed] [Google Scholar]
- 44.Lemp M.A., Hamill J.R., Jr. Factors affecting tear film breakup in normal eyes. Arch Ophthalmol. 1973;89:103–105. doi: 10.1001/archopht.1973.01000040105007. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto Y., Sato E.A., Ibrahim O.M., Dogru M., Tsubota K. The application of in vivo laser confocal microscopy to the diagnosis and evaluation of meibomian gland dysfunction. Mol Vis. 2008;14:1263–1271. [PMC free article] [PubMed] [Google Scholar]
- 46.Zengin N., Tol H., Gunduz K., Okudan S., Balevi S., Endogru H. Meibomian gland dysfunction and tear film abnormalities in rosacea. Cornea. 1995;14:144–146. [PubMed] [Google Scholar]
- 47.Rolando M., Refojo M.F., Kenyon K.R. Tear water evaporation and eye surface diseases. Ophthalmologica. 1985;190:147–149. doi: 10.1159/000309511. [DOI] [PubMed] [Google Scholar]
- 48.Mathers W.D. Ocular evaporation in meibomian gland dysfunction and dry eye. Ophthalmology. 1993;100:347–351. doi: 10.1016/s0161-6420(93)31643-x. [DOI] [PubMed] [Google Scholar]
- 49.Matsumoto Y., Shigeno Y., Sato E.A. The evaluation of the treatment response in obstructive meibomian gland disease by in vivo laser confocal microscopy. Graefes Arch Clin Exp Ophthalmol. 2009;247:821–829. doi: 10.1007/s00417-008-1017-y. [DOI] [PubMed] [Google Scholar]
- 50.Shah C.T., Blount A.L., Nguyen E.V., Hassan A.S. Cranial nerve seven palsy and its influence on meibomian gland function. Ophthal Plast Reconstr Surg. 2012;28:166–168. doi: 10.1097/IOP.0b013e31823f2f82. [DOI] [PubMed] [Google Scholar]
- 51.Bron A.J., Benjamin L., Snibson G.R. Meibomian gland disease. Classification and grading of lid changes. Eye (Lond) 1991;5(Pt 4):395–411. doi: 10.1038/eye.1991.65. [DOI] [PubMed] [Google Scholar]
- 52.Hykin P.G., Bron A.J. Age-related morphological changes in lid margin and meibomian gland anatomy. Cornea. 1992;11:334–342. doi: 10.1097/00003226-199207000-00012. [DOI] [PubMed] [Google Scholar]
- 53.Norn M. Expressibility of meibomian secretion. Relation to age, lipid precorneal film, scales, foam, hair and pigmentation. Acta Ophthalmol (Copenh) 1987;65:137–142. doi: 10.1111/j.1755-3768.1987.tb06991.x. [DOI] [PubMed] [Google Scholar]
- 54.Den S., Shimizu K., Ikeda T., Tsubota K., Shimmura S., Shimazaki J. Association between meibomian gland changes and aging, sex, or tear function. Cornea. 2006;25:651–655. doi: 10.1097/01.ico.0000227889.11500.6f. [DOI] [PubMed] [Google Scholar]
- 55.Pfluger Zur Ernahrung der Cornea. Klin Monatsbl Augenheilkd. 1882;20:69–81. [Google Scholar]
- 56.Sjogren Zur Kenntnis der Keratoconjuctivitis sicca (Keratitis filiformis bei Hypofunktion der Tranendrusen) Acta Ophthalmol Scand Suppl. 1933;13:40–45. [Google Scholar]
- 57.Norn M.S. Lissamine green. Vital staining of cornea and conjunctiva. Acta Ophthalmol (Copenh) 1973;51:483–491. doi: 10.1111/j.1755-3768.1973.tb06027.x. [DOI] [PubMed] [Google Scholar]
- 58.Yokoi N., Takehisa Y., Kinoshita S. Correlation of tear lipid layer interference patterns with the diagnosis and severity of dry eye. Am J Ophthalmol. 1996;122:818–824. doi: 10.1016/s0002-9394(14)70378-2. [DOI] [PubMed] [Google Scholar]
- 59.Norn M.S. Semiquantitative interference study of fatty layer of precorneal film. Acta Ophthalmol (Copenh) 1979;57:766–774. doi: 10.1111/j.1755-3768.1979.tb01842.x. [DOI] [PubMed] [Google Scholar]
- 60.Paugh J.R., Louie D.J., Guzman E. White light tear film interferometry in dry eye sub-types. Invest Ophthalmol. 2004;45:93. [Google Scholar]
- 61.Yokoi N., Komuro A., Maruyama K., Kinoshita S. New instruments for dry eye diagnosis. Semin Ophthalmol. 2005;20:63–70. doi: 10.1080/08820530590931124. [DOI] [PubMed] [Google Scholar]
- 62.Gilbard J.P., Rossi S.C., Heyda K.G. Tear film and ocular surface changes after closure of the meibomian gland orifices in the rabbit. Ophthalmology. 1989;96:1180–1186. doi: 10.1016/s0161-6420(89)32753-9. [DOI] [PubMed] [Google Scholar]
- 63.Mathers W.D., Shields W.J., Sachdev M.S., Petroll W.M., Jester J.V. Meibomian gland morphology and tear osmolarity: changes with Accutane therapy. Cornea. 1991;10:286–290. doi: 10.1097/00003226-199107000-00002. [DOI] [PubMed] [Google Scholar]
- 64.Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye Workshop (2007). Ocul Surf 2007;5:108–52. [DOI] [PubMed]
- 65.Yokoi N., Mossa F., Tiffany J.M., Bron A.J. Assessment of meibomian gland function in dry eye using meibometry. Arch Ophthalmol. 1999;117:723–729. doi: 10.1001/archopht.117.6.723. [DOI] [PubMed] [Google Scholar]
- 66.Chew C.K., Jansweijer C., Tiffany J.M., Dikstein S., Bron A.J. An instrument for quantifying meibomian lipid on the lid margin: the Meibometer. Curr Eye Res. 1993;12:247–254. doi: 10.3109/02713689308999470. [DOI] [PubMed] [Google Scholar]
- 67.Komuro A., Yokoi N., Kinoshita S., Tiffany J.M., Bron A.J., Suzuki T. Assessment of meibomian gland function by a newly-developed laser meibometer. Adv Exp Med Biol. 2002;506:517–520. doi: 10.1007/978-1-4615-0717-8_73. [DOI] [PubMed] [Google Scholar]
- 68.Alsuhaibani A.H., Carter K.D., Abramoff M.D., Nerad J.A. Utility of meibography in the evaluation of meibomian glands morphology in normal and diseased eyelids. Saudi J Ophthalmol. 2011;25:61–66. doi: 10.1016/j.sjopt.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arita R., Itoh K., Maeda S. Proposed diagnostic criteria for obstructive meibomian gland dysfunction. Ophthalmology. 2009;116:2058–2063 e1. doi: 10.1016/j.ophtha.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 70.Nichols J.J., Berntsen D.A., Mitchell G.L., Nichols K.K. An assessment of grading scales for meibography images. Cornea. 2005;24:382–388. doi: 10.1097/01.ico.0000148291.38076.59. [DOI] [PubMed] [Google Scholar]
- 71.Powell D.R., Nichols J.J., Nichols K.K. Inter-examiner reliability in meibomian gland dysfunction assessment. Invest Ophthalmol Vis Sci. 2012;53:3120–3125. doi: 10.1167/iovs.12-9600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Call CB, Wise RF, Hansen MR, Carter KD, Allen RC. In vivo examination of meibomian gland morphology in patients with facial nerve palsy using infrared meibography. Ophthal Plast Reconstr Surg 2012. Epub ahead of print. [DOI] [PubMed]
- 73.Mathers W.D., Billborough M. Meibomian gland function and giant papillary conjunctivitis. Am J Ophthalmol. 1992;114:188–192. doi: 10.1016/s0002-9394(14)73983-2. [DOI] [PubMed] [Google Scholar]
- 74.Goto E., Endo K., Suzuki A., Fujikura Y., Matsumoto Y., Tsubota K. Tear evaporation dynamics in normal subjects and subjects with obstructive meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2003;44:533–539. doi: 10.1167/iovs.02-0170. [DOI] [PubMed] [Google Scholar]
- 75.Mathers W.D., Daley T., Verdick R. Video imaging of the meibomian gland. Arch Ophthalmol. 1994;112:448–449. doi: 10.1001/archopht.1994.01090160022008. [DOI] [PubMed] [Google Scholar]
- 76.Yokoi N., Komuro A., Yamada H., Maruyama K., Kinoshita S. A newly developed video-meibography system featuring a newly designed probe. Jpn J Ophthalmol. 2007;51:53–56. doi: 10.1007/s10384-006-0397-y. [DOI] [PubMed] [Google Scholar]
- 77.Arita R., Itoh K., Maeda S. Comparison of the long-term effects of various topical antiglaucoma medications on meibomian glands. Cornea. 2012 doi: 10.1097/ICO.0b013e31823f8e7d. [DOI] [PubMed] [Google Scholar]
- 78.Arita R., Itoh K., Maeda S., Maeda K., Tomidokoro A., Amano S. Association of contact lens-related allergic conjunctivitis with changes in the morphology of meibomian glands. Jpn J Ophthalmol. 2012;56:14–19. doi: 10.1007/s10384-011-0103-6. [DOI] [PubMed] [Google Scholar]
- 79.McCulley J.P., Shine W.E. Meibomian gland function and the tear lipid layer. Ocul Surf. 2003;1:97–106. doi: 10.1016/s1542-0124(12)70138-6. [DOI] [PubMed] [Google Scholar]
- 80.Matsuoka T., Tsumura T., Ueda H. Video-meibographic observations of the meibomian gland. Jpn J Clin Ophthalmol. 1996;50:351–354. [Google Scholar]
- 81.Bizheva K., Lee P., Sorbara L., Hutchings N., Simpson T. In vivo volumetric imaging of the human upper eyelid with ultrahigh-resolution optical coherence tomography. J Biomed Opt. 2010;15:040508. doi: 10.1117/1.3475957. [DOI] [PubMed] [Google Scholar]
- 82.Arita R., Itoh K., Inoue K., Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology. 2008;115:911–915. doi: 10.1016/j.ophtha.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 83.Arita R., Itoh K., Maeda S., Maeda K., Amano S. A newly developed noninvasive and mobile pen-shaped meibography system. Cornea. 2012 doi: 10.1097/ICO.0b013e31825425ef. [DOI] [PubMed] [Google Scholar]
- 84.Kaufman S.C., Musch D.C., Belin M.W. Confocal microscopy: a report by the American Academy of Ophthalmology. Ophthalmology. 2004;111:396–406. doi: 10.1016/j.ophtha.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 85.Kobayashi A., Yoshita T., Sugiyama K. In vivo findings of the bulbar/palpebral conjunctiva and presumed meibomian glands by laser scanning confocal microscopy. Cornea. 2005;24:985–988. doi: 10.1097/01.ico.0000160976.88824.2b. [DOI] [PubMed] [Google Scholar]
- 86.Ban Y., Ogawa Y., Ibrahim O.M. Morphologic evaluation of meibomian glands in chronic graft-versus-host disease using in vivo laser confocal microscopy. Mol Vis. 2011;17:2533–2543. [PMC free article] [PubMed] [Google Scholar]
- 87.Ibrahim O.M., Matsumoto Y., Dogru M. The efficacy, sensitivity, and specificity of in vivo laser confocal microscopy in the diagnosis of meibomian gland dysfunction. Ophthalmology. 2010;117:665–672. doi: 10.1016/j.ophtha.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 88.Hwang H.S., Park C.W., Joo C.K. Novel noncontact meibography with anterior segment optical coherence tomography: Hosik meibography. Cornea. 2012 doi: 10.1097/ICO.0b013e318247b2fd. [DOI] [PubMed] [Google Scholar]
- 89.Shimazaki J., Goto E., Ono M., Shimmura S., Tsubota K. Meibomian gland dysfunction in patients with Sjogren syndrome. Ophthalmology. 1998;105:1485–1488. doi: 10.1016/S0161-6420(98)98033-2. [DOI] [PubMed] [Google Scholar]
- 90.Sullivan B.D., Evans J.E., Cermak J.M., Krenzer K.L., Dana M.R., Sullivan D.A. Complete androgen insensitivity syndrome: effect on human meibomian gland secretions. Arch Ophthalmol. 2002;120:1689–1699. doi: 10.1001/archopht.120.12.1689. [DOI] [PubMed] [Google Scholar]
- 91.Sullivan B.D., Evans J.E., Dana M.R., Sullivan D.A. Impact of androgen deficiency on the lipid profiles in human meibomian gland secretions. Adv Exp Med Biol. 2002;506:449–458. doi: 10.1007/978-1-4615-0717-8_63. [DOI] [PubMed] [Google Scholar]
- 92.Sullivan D.A., Sullivan B.D., Evans J.E. Androgen deficiency, Meibomian gland dysfunction, and evaporative dry eye. Ann N Y Acad Sci. 2002;966:211–222. doi: 10.1111/j.1749-6632.2002.tb04217.x. [DOI] [PubMed] [Google Scholar]
- 93.Schaumberg D.A., Buring J.E., Sullivan D.A., Dana M.R. Hormone replacement therapy and dry eye syndrome. JAMA. 2001;286:2114–2119. doi: 10.1001/jama.286.17.2114. [DOI] [PubMed] [Google Scholar]
- 94.Perra M.T., Serra A., Sirigu P., Tumo F. Histochemical demonstration of acetyl cholinesterase activity in human meibomian gland. Eur J Histochem. 1996;40:39–44. [PubMed] [Google Scholar]
- 95.Aisa J., Lahoz M., Serrano P. Acetylcholinesterase-positive and paraformaldehyde-induced-fluorescence-positive innervation in the upper eyelid of the sheep (Ovis aries) Histol Histopathol. 2001;16:487–496. doi: 10.14670/HH-16.487. [DOI] [PubMed] [Google Scholar]
- 96.Chung C.W., Tigges M., Stone R.A. Peptidergic innervation of the primate meibomian gland. Invest Ophthalmol Vis Sci. 1996;37:238–245. [PubMed] [Google Scholar]
- 97.Riolan J. Anthropographia et osteologia. Paris, France 1626;Moreau.
- 98.Lipham W.J., Tawfik H.A., Dutton J.J. A histologic analysis and three-dimensional reconstruction of the muscle of Riolan. Ophthal Plast Reconstr Surg. 2002;18:93–98. doi: 10.1097/00002341-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 99.McCulley J.P., Dougherty J.M., Deneau D.G. Classification of chronic blepharitis. Ophthalmology. 1982:891173–891180. doi: 10.1016/s0161-6420(82)34669-2. [DOI] [PubMed] [Google Scholar]
- 100.Karp L.A., Streeten B.W., Cogan D.G. Radiation-induced atrophy of the Meibomian gland. Arch Ophthalmol. 1979;97:303–305. doi: 10.1001/archopht.1979.01020010155013. [DOI] [PubMed] [Google Scholar]
- 101.al-Rajhi A.A., Hidayat A., Nasr A., al-Faran M. The histopathology and the mechanism of entropion in patients with trachoma. Ophthalmology. 1993;100:1293–1296. doi: 10.1016/s0161-6420(93)31485-5. [DOI] [PubMed] [Google Scholar]
- 102.Lambert R.W., Smith R.E. Pathogenesis of blepharoconjunctivitis complicating 13-cis-retinoic acid (isotretinoin) therapy in a laboratory model. Invest Ophthalmol Vis Sci. 1988;29:1559–1564. [PubMed] [Google Scholar]
- 103.Lambert R.W., Fan J., Smith R.E. Ocular surface abnormalities in an animal model of retinoid-induced blepharoconjunctivitis. Invest Ophthalmol Vis Sci Suppl. 1989;30:471. [Google Scholar]
- 104.Arita R., Itoh K., Maeda S. Meibomian gland duct distortion in patients with perennial allergic conjunctivitis. Cornea. 2010;29:858–860. doi: 10.1097/ICO.0b013e3181ca3668. [DOI] [PubMed] [Google Scholar]
- 105.McCann L.C., Tomlinson A., Pearce E.I., Diaper C. Tear and meibomian gland function in blepharitis and normals. Eye Contact Lens. 2009;35:203–208. doi: 10.1097/ICL.0b013e3181a9d79d. [DOI] [PubMed] [Google Scholar]
- 106.Kojima T., Dogru M., Matsumoto Y., Goto E., Tsubota K. Tear film and ocular surface abnormalities after eyelid tattooing. Ophthal Plast Reconstr Surg. 2005;21:69–71. doi: 10.1097/01.iop.0000153028.08506.47. [DOI] [PubMed] [Google Scholar]
- 107.Arita R., Itoh K., Inoue K., Kuchiba A., Yamaguchi T., Amano S. Contact lens wear is associated with decrease of meibomian glands. Ophthalmology. 2009;116:379–384. doi: 10.1016/j.ophtha.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 108.Sullivan D.A., Yamagami H., Liu M. Sex steroids, the meibomian gland and evaporative dry eye. Adv Exp Med Biol. 2002;506:389–399. doi: 10.1007/978-1-4615-0717-8_56. [DOI] [PubMed] [Google Scholar]
- 109.Sullivan D.A., Schaumberg D.A., Suzuki T. Sex steroids, meibomian gland dysfunction and evaporative dry eye in Sjogren’s syndrome. Lupus. 2002;11:667. doi: 10.1191/0961203302lu275oa. [DOI] [PubMed] [Google Scholar]
- 110.Yamagami H., Richards S.M., Sullivan B.D., Liu M., Steagall R.J., Sullivan D.A. Gender-associated differences in gene expression of the meibomian gland. Adv Exp Med Biol. 2002;506:459–463. doi: 10.1007/978-1-4615-0717-8_64. [DOI] [PubMed] [Google Scholar]
- 111.Steagall R.J., Yamagami H., Wickham L.A., Sullivan D.A. Androgen control of gene expression in the rabbit meibomian gland. Adv Exp Med Biol. 2002;506:465–476. doi: 10.1007/978-1-4615-0717-8_65. [DOI] [PubMed] [Google Scholar]
- 112.Yamagami H., Schirra F., Liu M., Richards S.M., Sullivan B.D., Sullivan D.A. Androgen influence on gene expression in the meibomian gland. Adv Exp Med Biol. 2002;506:477–481. doi: 10.1007/978-1-4615-0717-8_66. [DOI] [PubMed] [Google Scholar]
- 113.Richards S.M., Yamagami H., Schirra F., Suzuki T., Jensen R.V., Sullivan D.A. Sex-related effect on gene expression in the mouse meibomian gland. Curr Eye Res. 2006;31:119–128. doi: 10.1080/02713680500514644. [DOI] [PubMed] [Google Scholar]



